Abstract
Aims
A challenge of pulmonary vein isolation (PVI) in catheter ablation for paroxysmal atrial fibrillation (PAF) is electrical reconnection of the PV. EFFICAS I showed correlation between contact force (CF) parameters and PV durable isolation but no prospective evaluation was made. EFFICAS II was a multicentre study to prospectively assess the impact of CF guidance for an effective reduction of PVI gaps.
Methods and results
Pulmonary vein isolation using a radiofrequency (RF) ablation catheter with an integrated force sensor (TactiCath™) was performed in patients with PAF. Operators were provided EFFICAS I-based CF guidelines [target 20 g, range 10–30 g, minimum 400 g s force-time integral (FTI)]. Conduction gaps were assessed by remapping of PVs after 3 months, and gap rate was compared with EFFICAS I outcome. At follow up, 24 patients had 85% of PVs remaining isolated, compared with 72% in EFFICAS I (P = 0.037) in which CF guidelines were not used. The remaining 15% of gaps correlated to the number of catheter moves at creating the PVI line, quantified as Continuity Index. For PV lines with contiguous lesions and low catheter moves, durable isolation was 81% in EFFICAS I and 98% in EFFICAS II (P = 0.005). At index procedure, the number of lesions was reduced by 15% in EFFICAS II vs. EFFICAS I.
Conclusion
The use of CF with the above guidelines and contiguous deployment of RF lesions in EFFICAS II study resulted in more durable PVI in catheter ablation of PAF.
Keywords: Catheter ablation, Atrial fibrillation, Contact force, Conduction gaps, Pulmonary vein isolation
What's new?
Maintenance of adequate contact of the ablation catheter with the tissue using contact force (CF) sensing technology results in higher percentage of durable pulmonary venous isolation at 3 months of follow-up.
Durable pulmonary venous isolation appears to be further improved when ablation lesions are deployed point-by-point continuously along the circumferential isolation line.
The number of lesions necessary to complete pulmonary vein isolation is reduced when maintaining adequate CF parameters during ablation.
Introduction
Pulmonary vein isolation (PVI) using radiofrequency (RF) current has become the most accepted approach for symptomatic paroxysmal atrial fibrillation (PAF).1–4 However, long-term clinical efficacy is limited and arrhythmia recurrences are frequent (from 20 to 55%).2,5,6 Although the mechanisms of recurrences are not fully understood, conduction gaps within the initial isolation line have been documented in the majority of cases.
Recently, contact force (CF) between the catheter tip and the myocardium has been identified as one of the important determinants influencing RF lesion size.7–13 The Toccata clinical trial14 using the TactiCath™ catheter (St Jude Medical GVA, Geneva, Switzerland) was the first study to demonstrate the importance of CF sensing for improved clinical outcome. Besides CF, also the Force-Time Integral (FTI™, unit in gram seconds10,15,16) showed positive correlation with better patient outcome at 12 months.14 Until recently, no clear guidance was formulated to quantify the optimal amount of CF in a multi-centric setting.17 EFFICAS I was the first attempt to define simple CF guidelines based on a retrospective correlation between occurrence of PVI conduction gaps at 3 months and the CF parameters used during ablation.18 The objective of EFFICAS II was to prospectively evaluate clinical utility of these guidelines for effective reduction of PVI gap rates in patients with PAF.
Methods
Study protocol
EFFICAS II was conducted in the same three European centres as the EFFICAS I study and consisted of two procedures: primary PVI ablation (index procedure), and remapping 3 months later to assess conduction gaps in PV lines using a circular diagnostic catheter, assuming that isolation is considered durable after 3 months healing. The protocol was approved at local Ethics Committees.
Study endpoints were a reduction in the variability of CF applied during ablation, and a reduction in reconnections (gaps) at 3-month follow-up (FU) compared with EFFICAS I. Complications were assessed during the entire study.
Patient groups
Patients with documented PAF refractory or intolerant to at least one Class I–IV anti-arrhythmic drug according to ACC/AHA/ESC consensus5 were enrolled. All subjects signed informed consent forms prior to enrolment.
Study device
The TactiCath ablation catheter was used for ablation during index procedures. It is a 7 Fr RF catheter with 3.5 mm saline-irrigated tip (six holes) and ability to measure real-time CF at catheter–tissue interface.7,8 The TactiCath system detects onset and end of RF current delivery that is used for FTI computation for each ablation. Real-time CF and FTI are displayed on the same screen.10
Index procedure
Pulmonary vein isolation was achieved by wide antral ablation encircling each pair of ipsilateral PVs supported by a 3D mapping system (EnSite™ NavX™, St Jude Medical, St Paul, MN, USA). The periprocedural parameters during the index procedure were not changed from local hospital practice. Investigators followed the CF guidelines from EFFICAS I: target CF of 20 g (with range of 10–30 g) and minimum FTI of 400 g s. As in EFFICAS I, the location of each RF application was assigned to 1 of 8 predefined positions around each ipsilateral pair of PV: numbered 1–8 around the left pulmonary veins and 9–16 around the right pulmonary veins19 (Figure 1A).
Figure 1.
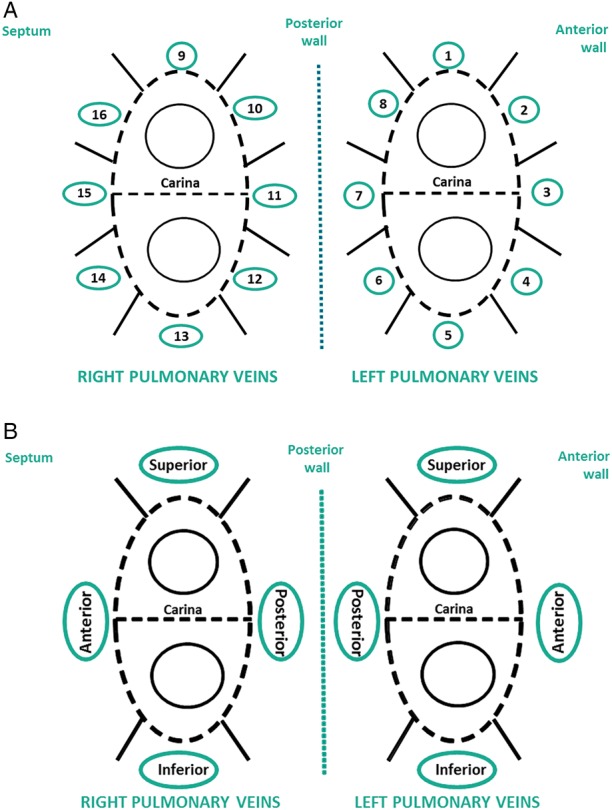
Positions and segments around pulmonary veins. (A) Sixteen numbered positions: 1, 9: superior; 2, 16: anterior-superior; 3, 15: anterior-middle; 4, 14: anterior-inferior; 5, 13: inferior; 6, 12: posterior-inferior; 7, 11: posterior-middle; 8, 10: posterior-superior. (B) Positions 2–4 and 14–16 were grouped respectively into left and right anterior segments and positions 6–8 and 10–12 were grouped into respectively left and right posterior segments.
Pulmonary vein isolation was achieved point-to-point to ensure strict correlation between each lesion and its CF characteristics. A circular diagnostic catheter was used to confirm isolation by entrance block at the end of each ipsilateral pair of PVs ablation. Procedure parameters (position, CF, FTI, start time of ablation, RF power, number of ablations, and sequence of ablation positions) were recorded during the index procedure. Variability in CF and FTI was compared with EFFICAS I.
Remapping at 3 months
As in EFFICAS I, all patients who agreed to participate in 3-month FU underwent an invasive remapping procedure that was performed following similar preparatory steps as the index procedure. Durable PVI was checked using a circular catheter to determine isolation or reconnection status for each vein, and to localize the gap(s) in case of reconnection. Gaps were correlated with the corresponding CF and FTI applied at index procedure.
To minimize positioning error when correlating the location of the catheter at index procedure vs. gap at FU, the anterior and posterior positions (numbered 2–4, 14–16 and respectively 6–8, 10–12 in Figure 1A) were grouped into segments per ipsilateral pairs of veins (Figure 1B). This resulted in a more common anatomical representation for anterior, posterior, superior, and inferior segments.
Study endpoint and lesion analysis
To assess the impact of applied CF guidelines, rate of reconnected veins in EFFICAS II was compared with EFFICAS I. Since not every operator from EFFICAS I participated in EFFICAS II, only data from patients who were treated by operators participating in both studies were included for analysis to avoid study bias.
Beyond the study endpoint, the remaining gaps were further analysed in a post hoc analysis to determine if ‘Minimum FTI’ was still the predictive factor (as it was in EFFICAS I), or if secondary confounding parameters were discriminative for those gaps. Since CF and FTI influence mainly lesion size for transmurality, gaps in EFFICAS II were further evaluated for potential impact of continuity in deployment of lesions around ipsilateral PVs. It was assessed using a new metric, ‘Continuity Index’ (CI) that is quantified through the number of positions the catheter tip has moved over, when subsequent ablations were performed in non-adjacent positions (Figure 2) during the index procedure. The CI was incremented only until the PV line was fully encircled and does not include moves related to the closing for individual remaining gaps. A low value of the aggregated CI indicates contiguous catheter movement; a high value indicates that many consecutive ablations were performed in positions not adjacent to one another, reflecting frequent movements of the catheter tip in between ablations. The aggregate CI at index procedure was correlated to remaining gap occurrence in PV pairs to assess impact of lesion contiguity as a secondary parameter.
Figure 2.
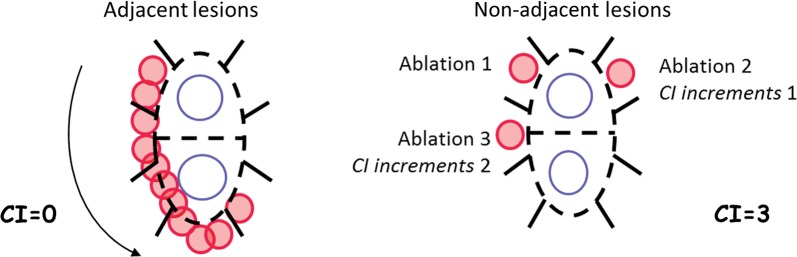
Continuity Index assessment. Continuity Index calculation for two examples of consecutive ablations. Arrow and numbers show order of RF applications.
Statistical analysis
All continuous parameters are presented as means ± standard deviations, unless specified otherwise. Normality tests were performed to verify if a Gaussian approximation was valid. Whenever parametric test was suitable, Student's two-tailed t-tests were performed with or without assumption of equal variance according to the result of the F-test for homoscedasticity. A Mann–Whitney test was performed for non-normally distributed data. Categorical variables were assessed by Fisher's exact test. A P-value of <0.05 was considered significant. All calculations were done using Excel 2007 or GraphPad Prism 5 (2008).
Results
Patient population for EFFICAS II vs. EFFICAS I
Five investigators treated patients in both EFFICAS I and EFFICAS II. Twenty-four EFFICAS II patients completed the remapping at 3 months and were compared with the 26 patients with 3-month remapping in EFFICAS I. Baseline characteristics of subjects were not different in the two studies (Table 1, P = NS).
Table 1.
Patient demographics—EFFICAS I vs. EFFICAS II (all P = NS)
| Average (range/percentage) |
||
|---|---|---|
| EFFICAS I (n = 26) | EFFICAS II (n = 24) | |
| Age (year) | 58 ± 11 (18–72) | 57 ± 11 (36–71) |
| Gender (males) | 20 (76.9%) | 16 (66.7%) |
| PAF | 26 (100%) | 24 (100%) |
| Persistent atrial fibrillation—out of protocol | 0 (0%) | 0 (0%) |
| Previous AADa | 26 (100%) | 23 (95.8%) |
| Other cardiac disease | 19 (73.1%) | 17 (70.8%) |
| Including hypertension | 16 (61.5%) | 14 (58.3%) |
aAnti-arrhythmic drug.
Study outcome: pulmonary vein isolation gap rate EFFICAS II vs. EFFICAS I
Using the proposed CF guidelines in patients with PAF resulted in an effective reduction of PVI gaps: durable PV isolation at 3 months increased from 72% in EEFFICAS I (73/102) to 85% in EFFICAS II (77/91) (P = 0.037, Figure 3A). This reduction of PVI gaps ensures the primary study endpoint was met.
Figure 3.
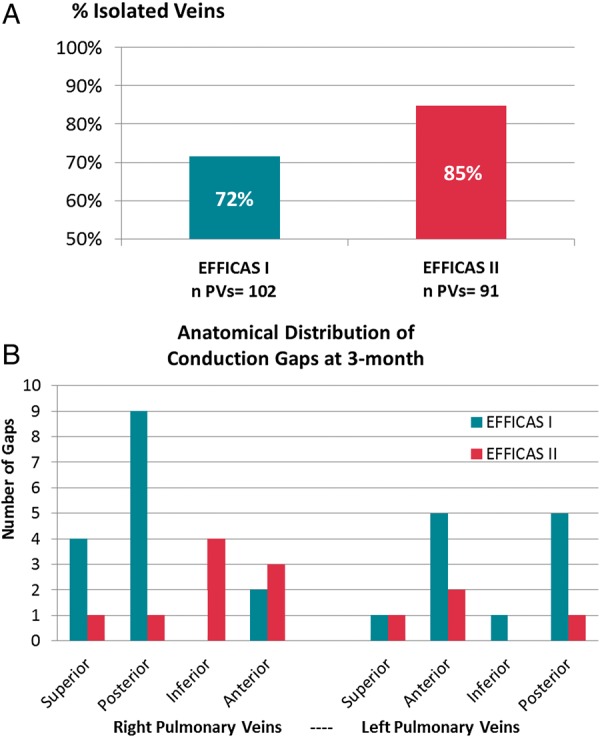
Pulmonary vein isolation at 3-month remapping. (A) Comparison of durable isolation rates per vein in EFFICAS I, without CF guidelines, and EFFICAS II, with CF guidelines. n PVs, total number of veins. (B) The number of conduction gaps is given per PV segment for both studies.
In EFFICAS I, three of the patients had gaps only in the LPV, and six patients had gaps only in the RPV. Seven patients had gaps in both LPV and RPV. In EFFICAS II, two of the patients had gaps only in the LPV, and five patients had gaps only in the RPV. Two patients had gaps in both LPV and RPV. Gaps were distributed among different sites around PVs (Figure 3B). The distribution is coherent with previously published data.20
Remaining pulmonary vein isolation gaps: confounding parameter
Application of minimum FTI > 400 g s was the main parameter to reduce gaps from EFFICAS I to EFFICAS II. However, in EFFICAS II, still 15% of re-conduction gaps remained in the PVI lines. Minimum CF and minimum FTI were not significantly different when comparing the ablation lines with gap or no-gap in EFFICAS II (Table 2). As a secondary parameter, assessment of contiguity of RF lesions along the ablation line using CI showed that CI for durably isolated lines was significantly lower at 4.1 ± 2.4 than CI for PV lines with gap at 8.4 ± 4.1 (P < 0.0001, Figure 4B). All gaps but 1 in EFFICAS II were associated with PV lines that had a high CI. To understand the importance of the new discriminating parameter, CI was also evaluated on the EFFICAS I data. Continuity Index was a significant confounding factor that could also have predicted gap or success in EFFICAS I with CI for durably isolated lines at 5.3 ± 4.0 vs. 9.1 ± 5.0 for PV lines with gap (P = 0.004, Figure 4A).
Table 2.
Median values in EFFICAS II segments with gap vs. successful isolation
| Gap segments (n = 13) | Successfully isolated segments (n = 169) | P-Value | |
|---|---|---|---|
| Minimum CF (g) | 13.1 | 11.4 | 0.22 |
| Minimum FTI (g s) | 223 | 290 | 0.48 |
| Average CF (g) | 28.8 | 19.2 | <0.01 |
| Average FTI (g s) | 868 | 704 | 0.03 |
| Total number of ablations | 6.0 | 6.0 | 0.52 |
Figure 4.
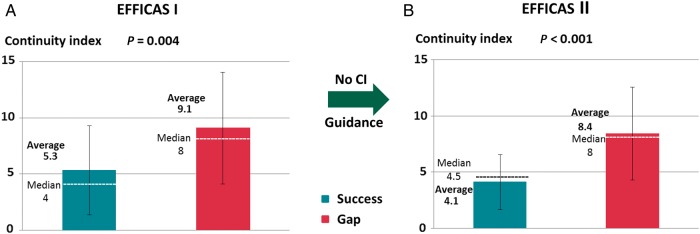
Evolution of CI from EFFICAS I to EFFICAS II. Continuity Index for ‘Success’ and ‘Gap’ segments is represented with average, standard deviation, and median values. (A) In EFFICAS I, besides minimum FTI, CI was also a significant predictive factors for ‘Gap’ or ‘Success’ in the pulmonary vein isolation line. (B) In EFFICAS II, guidance was prospectively given on CF and FTI, whereas no guidance on CI was given to operators. As a result, CI for ‘Gap’ and ‘Success’ remained unaffected and CI appeared to be the only remaining predictive factor for gaps in EFFICAS II.
In EFFICAS II, PV lines isolated initially with a CI <6 (low CI) had a 98% (56/57) chance of remaining isolated, a significantly higher chance compared with PV lines with a CI ≥6 (high CI) which had only 62% (21/34) chance of remaining isolated (P < 0.001). When comparing the two studies for PVs treated with low CI <6, the PV isolation rate increased to 98% (56/57 PV) in EFFICAS II from 81% (39/48 PV) in EFFICAS I (P = 0.005).
Comparative outcomes of EFFICAS II vs. EFFICAS I—index procedure data
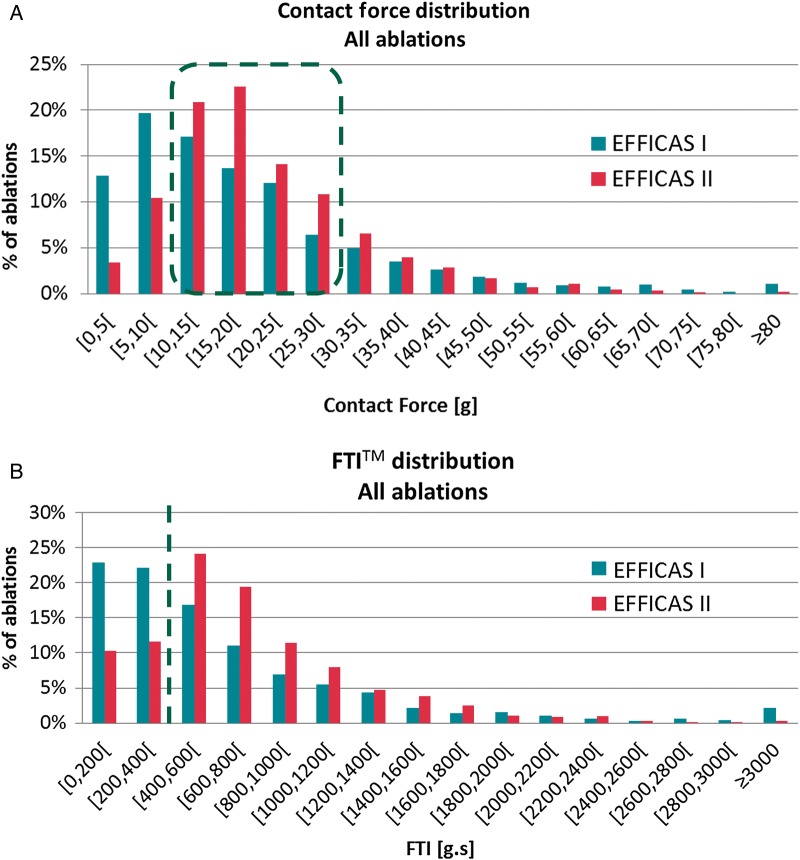
Application of CF guidelines in EFFICAS II resulted in lower variability in both CF and FTI compared with EFFICAS I in which the operator was blinded to CF reading. Achieved CF ranged between 10 and 30 g in 68% of the ablations in EFFICAS II, compared with only 49% in EFFICAS I [Figure 5A, odds ratio (OR) = 2.2, P < 0.001], and the percentage of ablations with FTI > 400 g s was 78% in EFFICAS II vs. 55% in EFFICAS I (Figure 5B, OR = 2.9, P < 0.001).
Figure 5.
Distribution of CF and FTI. (A) Comparison of CF distribution in EFFICAS I (1856 ablations) and EFFICAS II (1372 ablations). Red dots highlight the global percentage of ablations within EFFICAS II recommended CF range (10–30 g). This percentage significantly increased from 49 to 68% between both trials (P < 0.001). Proportion of low CF ablations (<10 g) reduced from 33 to 14%, and high CF ablations (>30 g) was 18% for both trials. (B) Analogous distribution of FTI. The red dots segregate low FTI (<400 g s) vs. EFFICAS II recommended range (>400 g s). Proportion of low FTI decreased from 45 to 22% of ablations between both trials (P < 0.001).
For equal setting of RF power (23.4 ± 2.2 W in EFFICAS II vs. 24.4 ± 2.7 W in EFFICAS I, P = NS), there was a reduction of number of ablations by 15% (1372 ablations in EFFICAS II vs. 1818 in EFFICAS I, P = 0.05).
Complications
There were two cases of tamponade related to the index procedure in EFFICAS II, compared with zero in EFFICAS I. In one patient delayed tamponade occurred, while in one patient the event prevented isolation of one pair of PV. One patient required pericardiocentesis, and one patient required surgery but the site of perforation could not be identified. Both patients made a full recovery. Extensive analysis of the procedures was performed and adjudicated at Investigator Meetings. No excessive CF was recorded in any of these subjects when compared with the rest of the patient cohort. In general, ablation with CF >30 g was not higher in EFFICAS II vs. EFFICAS I (18 vs. 18%, P = NS)
Discussion
The main result of EFFICAS II study is the confirmation that adequate catheter tip–tissue contact during the ablation procedure can be achieved using simple CF guidelines (target CF 20 g and minimum FTI 400 g s). Maintaining adequate CF levels resulted in a high proportion of durable PVI at 3-month FU. This figure reached 85% in EFFICAS II when compared with 71% in EFFICAS I, in which the operator was blinded to CF reading. High average CF, as suggested in previous publications, may not be sufficient to predict success. In the EFFICAS study series, we showed that the minimum CF/FTI is more important, although it is clear that for most operators there is a correlation between their average procedure CF levels and the minimum values applied. Furthermore, the narrower distribution of CF and FTI in EFFICAS II indicates a better control of the catheter manipulation and resulted in a reduction of the number of RF application by 15% in EFFICAS II vs. EFFICAS I. Finally, assessment of continuity of RF deliveries along circumferential ablation line revealed that durable PVI rate further increased significantly when CI was low and thus, when individual lesions were applied successively next to each other. The additional potential impact of CI should be demonstrated in further studies.
The results of EFFICAS II study are in line with pre-clinical evidence about lesion size being proportionally greater with higher and stable CF and FTI.7,10,15,16 In the clinical arena, EFFICAS II observation on the value of CF assessment for creation of durable PVI appears to be reflected by the first prospective study on utility of CF vs. non-CF catheter for efficacious AF ablation.21 In this study, 30 patients were enrolled in each group, with a standardized 12-month FU, free of anti-arrhythmic therapy. Although complete PVI was eventually achieved in all cases in both groups, success using an exclusive anatomic approach was 80.0% in CF group vs. 36.7% in control group (P < 0.0001). The use of CF was associated with significant reductions in fluoroscopy exposure (P < 0.01) and RF time (P = 0.01). The incidence rates of AF recurrence were 10.5% (95% CI, 1.38–22.4) in the CF group and 35.9% (95% CI, 12.4–59.4) in the control group (log rank test, P = 0.04). After adjustment of potential confounders, the use of CF catheter was found to be associated with a lower AF recurrence (OR 0.18, 95% CI 0.04–0.94, P = 0.04). Interestingly, the mean total CF during PVI in the CF group was 21.7 ± 4 g. In another prospective study,22 CF-guided ablation was compared with ablation using standard non-CF sensing catheter. After PVI, all patients received adenosine to test dormant conduction. The study found significant difference in the presence of dormant conduction between both patient groups. While it was observed only in four PV pairs (8%; 16% of patients) in the CF group, control group presented with dormant conduction in 35 PV pairs (35%; 52% of patients) (P = 0.0004 per PV pair and P = 0.0029 per patient). The single procedure off anti-arrhythmic drug freedom from recurrent atrial arrhythmias at 1 year was 88% in the CF group vs. 66% in the standard group (P = 0.047).
EFFICAS II demonstrated another important piece of evidence for catheter ablation practice. The analysis of CI revealed that even with effective use of optimized CF, 15% of pulmonary veins reconnected after ablation due to non-contiguity between point-by-point lesions along ablation line. Importantly, durable PVI rate increased to 98% when CI was low (<6) and thus, lesions were deployed successively without moving to an entirely different position along the circumferential line. On the contrary, movements of catheter to different segments of the line appear to increase risk for imperfect catheter positioning. Such findings are coherent with a recently published study.23 The alternative explanation for higher occurrence of gaps when catheter moves from segment to segment could be reflected by rapid edema formation around each lesion. Edema after deployment of RF lesion has been previously described using intracardiac echocardiography.24 Rapid development of edema may prevent subsequent transmural or contiguous lesion formation at adjacent positions or could significantly alter or conceal local electrograms in the region of stunned tissue. Contact force/FTI and CI are physically independent mechanisms and both should be taken into account in daily clinical practice. The study showed that after application of the correct CF/FTI guidelines, continuity in the PV line appeared to be the major contributor for remaining gaps.
The event rate with two tamponades seems high compared with the number of patients enrolled. There was no correlation found with high CF or high FTI. In the recently published Smart AF study,17 2.5% of cardiac effusions occurred. Although the EFFICAS I data indicate that an ablation with FTI < 400 g s has a probability of failure four times higher compared with FTI > 400 g s (21 vs. 5%), the relative success rate is still 79% with FTI < 400 g s. Therefore from a precautionary standpoint, one might consider accepting FTI < 400 g s, especially when taking into consideration indicators for potential safety issues (i.e. impedance drop or temperature rise). When using the CF information, the tip to tissue contact has improved stability—translated into a high FTI, while RF power is not yet systematically lowered and not yet always sufficiently balanced vs. the increased CF. This might be one of the reasons for local over heating of the tissue and its disruption.
A few further elements are to be emphasized regarding the study. Both EFFICAS I and EFFICAS II were uniquely designed to be sequential, allowing a retrospective evaluation of CF parameters and a prospective evaluation of conclusions (guidelines) on CF from the first study. Therefore, the studies were intentionally non-randomized. Invasive FU at 3 months represents durable isolation and allowed for a more precise localization of conduction gaps and best possible correlation with CF parameters used at the index procedure.
Limitations
First, data analysis for EFFICAS studies required a point-to-point ablation, which may deviate from standard clinical practice.
Second, the unique protocol with an electrophysiology endpoint at 3 months was chosen to maximize the precision for assessment of the durability of the lesion. Translation into long-term clinical outcome can only be inferred based on the common understanding that most clinical recurrences are related to gaps in the PVI line.
Conclusion
EFFICAS II is the first study that prospectively evaluated a set of CF guidelines for ensuring durable isolation of the PV. These comprised a target CF of 20 g, range of 10 –30 g, and a minimum FTI of 400 g s per RF delivery spot. Their use resulted in a superior rate of durable PVI when compared with the similar protocol of EFFICAS I study without guidelines. Besides CF guidance, continuity in deployment of RF lesions along the ablation line is another determinant factor for durable PVI.
Funding
This study was supported by a research grant from Endosense SA. After the end of the study, Endosense was acquired by St Jude Medical, St Paul, MN, USA. Funding to pay the Open Access publication charges for this article was provided by St Jude Medical.
Conflict of interest: J.K.: scientific advisor and speaker for Biosense Webster, Boston, Scientific Corp./EP Technologies, GE Healthcare, St Jude Medical, Siemens Healthcare and speaker for Biotronik GmbH, Medtronic. K.-H.K.: Scientific advisor and shareholder for Stereotaxis Inc., Biotronik GmbH, St Jude Medical. H.L. and A.Y.: St Jude Medical employees. P.N.: consultant for Ev3, St Jude Medical, CryoCath, Technologies. P.P.: speaker for St Jude Medical. E.W.: speaker for Biosense Webster, Biotronik GmbH, CardioFocus, Medtronic. All other co-authors have nothing to disclose.
Acknowledgements
Data were analysed by Mr Olivier Frémont from the Engineering Department of the Ecole Polytechnique Fédérale, Lausanne (EPFL), Switzerland.
References
- 1.Santini M, Ricci RP. The worldwide social burden of atrial fibrillation: what should be done and where do we go? J Interv Card Electrophysiol 2006;17:183–8. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 2010;122:2368–77. [DOI] [PubMed] [Google Scholar]
- 3.Hussein AA, Saliba WI, Martin DO, Bhargava M, Sherman M, Magnelli-Reyes C, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:271–8. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt C, Kisselbach J, Schweizer PA, Katus HA, Thomas D. The pathology and treatment of cardiac arrhythmias: focus on atrial fibrillation. Vasc Health Risk Manag 2011;7:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raviele A, Natale A, Calkins H, Camm JA, Cappato R, Ann Chen S, et al. Venice Chart international consensus document on atrial fibrillation ablation: 2011 update. J Cardiovasc Electrophysiol 2012;23:890–923. [DOI] [PubMed] [Google Scholar]
- 6.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama K, Nakagawa H, Shah DC, Lambert H, Leo G, Aeby N, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ Arrhythm Electrophysiol 2008;1:354–62. [DOI] [PubMed] [Google Scholar]
- 8.Thiagalingam A, D'Avila A, Foley L, Guerrero JL, Lambert H, Leo G, et al. Importance of catheter contact force during irrigated radiofrequency ablation: evaluation in a porcine ex vivo model using a force-sensing catheter. J Cardiovasc Electrophysiol 2010;21:806–11. [DOI] [PubMed] [Google Scholar]
- 9.Okumura Y, Johnson SB, Bunch TJ, Henz BD, O'Brien CJ, Packer DL. A systematical analysis of in vivo contact forces on virtual catheter tip/tissue surface contact during cardiac mapping and intervention. J Cardiovasc Electrophysiol 2008;19:632–40. [DOI] [PubMed] [Google Scholar]
- 10.Shah DC, Lambert H, Nakagawa H, Langenkamp A, Aeby N, Leo G. Area under the real-time contact force curve (force-time integral) predicts radiofrequency lesion size in an in vitro contractile model. J Cardiovasc Electrophysiol 2010;21:1038–43. [DOI] [PubMed] [Google Scholar]
- 11.Kimura M, Sasaki S, Owada S, Horiuchi D, Sasaki K, Itoh T, et al. Comparison of lesion formation between contact force-guided and non-guided circumferential pulmonary vein isolation: a prospective, randomized study. Heart Rhythm 2014;11:984–91. [DOI] [PubMed] [Google Scholar]
- 12.Providência R, Marijon E, Combes S, Bouzeman A, Jourda F, Khoueiry Z, et al. Higher contact-force values associated with better mid-term outcome of paroxysmal atrial fibrillation ablation using the SmartTouch™ catheter. Europace 2015;17:56–63. [DOI] [PubMed] [Google Scholar]
- 13.Stabile G, Solimene F, Calò L, Anselmino M, Castro A, Pratola C, et al. Catheter-tissue contact force for pulmonary veins isolation: a pilot multicentre study on effect on procedure and fluoroscopy time. Europace 2014;16:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy VY, Shah D, Kautzner J, Schmidt B, Saoudi N, Herrera C, et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm 2012;9:1789–95. [DOI] [PubMed] [Google Scholar]
- 15.Squara F, Latcu DG, Massaad Y, Mahjoub M, Bun SS, Saoudi N. Contact force and force-time integral in atrial radiofrequency ablation predict transmurality of lesions. Europace 2014;16:660–7. [DOI] [PubMed] [Google Scholar]
- 16.le Polain de Waroux JB, Weerasooriya R, Anvardeen K, Barbraud C, Marchandise S, De Meester C, et al. Low contact force and force-time integral predict early recovery and dormant conduction revealed by adenosine after pulmonary vein isolation. Europace 2015;17:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HT, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol 2014;64:647–56. [DOI] [PubMed] [Google Scholar]
- 18.Neuzil P, Reddy VY, Kautzner J, Petru J, Wichterle D, Shah D, et al. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol 2013;6:327–33. [DOI] [PubMed] [Google Scholar]
- 19.Wang XH, Shi HF, Sun YM, Gu JN, Zhou L, Liu X. Circumferential pulmonary vein isolation: the role of key target sites. Europace 2008;10:197–204. [DOI] [PubMed] [Google Scholar]
- 20.Sotomi Y, Kikkawa T, Inoue K, Tanaka K, Toyoshima Y, Oka T, et al. Regional difference of optimal contact force to prevent acute pulmonary vein reconnection during radiofrequency catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2014;25:130–7. [DOI] [PubMed] [Google Scholar]
- 21.Marijon E, Fazaa S, Narayanan K, Guy-Moyat B, Bouzeman A, Providencia R, et al. Real-time contact force sensing for pulmonary vein isolation in the setting of paroxysmal atrial fibrillation: procedural and 1-year results. J Cardiovasc Electrophysiol 2014;25:130–7. [DOI] [PubMed] [Google Scholar]
- 22.Andrade JG, Monir G, Pollak SJ, Khairy P, Dubuc M, Roy D, et al. Pulmonary vein isolation using “contact force” ablation: The effect on dormant conduction and long-term freedom from recurrent atrial fibrillation—a prospective study. Heart Rhythm 2014;11:1919–24. [DOI] [PubMed] [Google Scholar]
- 23.Park CI, Lehrmann H, Keyl C, Weber R, Schiebeling J, Allgeier J, et al. Mechanisms of pulmonary vein reconnection after radiofrequency ablation of atrial fibrillation: the deterministic role of contact force and interlesion distance. J Cardiovasc Electrophysiol 2014;25:701–8. [DOI] [PubMed] [Google Scholar]
- 24.Ren JF, Callans DJ, Schwartzman D, Michele JJ, Marchlinski FE. Changes in local wall thickness correlate with pathologic lesion size following radiofrequency catheter ablation: an intracardiac echocardiographic imaging study. Echocardiography 2001;18:503–7. [DOI] [PubMed] [Google Scholar]