Abstract
The effects of fenoxycarb, a Juvenile hormone analogue, at sublethal concentrations were tested on some biological parameters of Plutella xylostella (L.) in two consecutive generations. The calculated LC10, LC25, and LC50 values of the insecticide were 21.58, 43.25, and 93.62 mg/liter on third-instar larvae, respectively. Fenoxycarb significantly reduced pupal weight and oviposition period in parent generation. In addition, the fecundity of treated groups (LC10 = 71.06, LC25 = 40.60 eggs per female) in parents was significantly lower than control (169.40 eggs per female). Although fenoxycarb could not affect gross reproductive rate and death rate, it decreased net reproductive rate, intrinsic rate of increase, finite rate of increase, and birth rate in offspring generation. Also, mean generation time and doubling time of treated insects was significantly longer than control at LC10 level. Therefore, the data from this study suggested that fenoxycarb could adversely cause population decline in the subsequent generation.
Keywords: Plutella xylostella, sublethal, fenoxycarb, biological and oviposition parameter
The diamondback moth (DBM), Plutella xylostella (L.) (Lepidoptera: Plutellidae), is the most destructive insect pest of Brassicaceae worldwide (Talekar and Shelton 1993). In the last two decades, its damage has increased in Tehran and other areas of Iran. Verkerk and Wright (1996) reported that the DBM produces 20 or more generations in tropical regions and cause up to 90% of yield loss. Although, there are many novel methods for insect control, chemical control using insecticides remained as a dependable method (Lee 2000). Up to now, DBM shows the significant resistance to several groups of insecticides (Talekar and Shelton 1993). Following the rules of resistance management, it is necessary to make a follow-up treatment with a pesticide of different mode of action. IGRs are new group of pesticides that is effective even at sublethal concentrations. They are effective on physiological or behavioral parameters of exposed treatments (Haynes 1988, Desneux et al. 2006, Alizadeh et al. 2012). Some of these changes are developmental time (Kumar and Chapman 1984, Coppen and Jepson 1996, Mahmoudvand et al. 2011b), larval and pupal weight (Abro et al. 1993, Jun et al. 1999, Yin et al. 2008), pupal rate and adult emergence (Sial and Brunner 2010, Han et al. 2012), fecundity (Elzen 2001, Cho et al. 2002), egg size and hatching (Yin et al. 2008, Han et al. 2012), adult longevity (Ergin et al. 2007, Hamedi et al. 2010, Mahmoudvand et al. 2012) as well as other biological parameters such as net reproductive rate and intrinsic rate of increase (Mahmoudvand et al. 2011a; Ahmad et al. 2012). Juvenile Hormone Analogues (JHAs) can alter the endocrine balance and therefore cause to abnormal development in some insects especially after metamorphosis (Retnakaran et al. 1985, Dhadialla et al. 1998). Fenoxycarb, [2(phenoxy–phenoxy)-ethyl carbamate], is one of these insecticides that was discovered by the Roche-Socar and Maag societies. It was the first compound from JHAs that was introduced to agricultural pest control (Masner et al. 1980, Miyamoto et al. 1993). It is recognized to have ovicidal and larvicidal activity and have impact on population control of Lepidoptera (Masner et al. 1987). In addition, fenoxycarb has low soil mobility, it does not accumulate, and it breaks down relatively quickly in the environment (Bicchi et al. 1990, Sullivan 2000, Michel et al. 2001). Therefore, this insecticide could be a rational candidate for integrated pest management of DBM. Fenoxycarb as a source of juvenilizing agent can have remarkable impact by disrupting growth, development, and behavior of insect pests. It may affect population size even in the subsequent generation. However, the adverse effects of this pesticide at sublethal concentrations on parents and subsequent generation of DBM have not been studied. In this study, we first, tried to determine lethal and sublethal concentrations of fenoxycarb against DBM. Thereafter, the experiments were established to investigate the sublethal effects on biological and population growth parameters of the insect in two consecutive generations.
Materials and Methods
Insect Rearing
The initial colonies of DBM were established by collection of larvae from infested leaves of cauliflower, Brassica oleracea L. (Brassicaceae) in a pesticide-free plantation located in the fields of Tarbiat Modares University, Tehran, Iran, in July 2013. Adults were introduced to cabbage leaves for egg laying in a plastic cage (58 by 20 by 28 cm) given access to 10% sugar solution. Insect stock was maintained at 25 ± 1°C and 65 ± 5% relative humidity under a photoperiod of 16:8 (L:D) h. The experiments were started after three generations.
Bioassay and Insecticide
Fenoxycarb (Insegar 25 WG) was supplied by Syngenta, Basel, Swaziland. Circular leaf disks (4.5 cm diameter) dipped in aqueous solutions of insecticide (0.02% Tween-20) for 10 s. Water containing 0.02% Tween-20 was used as control (Larew et al. 1985). Leaf disks dried at room temperature put in a plastic cup (3 by 5.5 cm) containing 10 third-instar larvae. The experiment was replicated four times and the mortality was recorded 96 h after treatment. The mortality data were subjected to probit analysis using SAS Ver. 9.1 (SAS Institute 2002) to calculate lethal (LC50) and sublethal concentrations (LC10 and LC25).
Effects of Sublethal Doses of Fenoxycarb.
Sublethal Effects on Parent of P. xylostella
Leaf disks dipped in solution equivalent to LC10 and LC25 for 10 s as described above. Then 25 third-instar larvae put on air-dried leaves and allowed to feed for 96 h. Then survived larvae let to feed and pupate on untreated leaves. Then pupae were maintained individually until adult emergence. The weight of each pupa was recorded on second day of pupation. Fifteen pairs of adults from each treatment were introduced into a mating box (8.5 by 6.5 by 4 cm) containing a piece of cabbage leaf for oviposition. The leaves were collected daily and replaced by a new one. The fecundity and egg hatch was recorded every day until death. The pre-oviposition, oviposition, and post-oviposition periods were recorded in parents. The experiment was conducted with eight replications in a growth chamber similar to stock culture condition.
Sublethal Effects on Offspring Generation
The study of sublethal effects was continued on the subsequent generation by rearing the insect from egg to adult in untreated leaves. In this experiment, numbers of 100 eggs were transferred to a plastic cage by a fine brush separately and the pre-embryonic development time and the hatching rate were recorded. The post-embryonic development was studied until adult fecundity and ultimately death as described in the previous section.
Data Analysis
Biological parameters were calculated based on Carey (1993). Jackknife technique (Maia et al. 2000) which is similar to bootstrapping was used to estimate the sample mean and standard error of biological parameters. Intrinsic rate of increase (rm) that is the immediate rate of increase of a population under marked condition in discrete time (Birch 1948) was calculated by the following equation: , where “x” is the age, “mx” the age-specific fecundity, and “lx” is the survival rate. Gross reproductive rate and net reproductive rate are the age-specific fecundity and the average number of female offspring that produced by a females through their lifetime (Pressat 1985). Finite rate of increase is the multiplication of increase per unit time. Doubling time and mean generation time are defined as the period that a population needs to increase to two and R0 fold of initial size, respectively (Carey 1993). Birth rate and death rate () were calculated in this study. The terms of pre-oviposition period (TPOP) is the time between birth to first oviposition and the adult pre-oviposition period (APOP) is referred to the time from adult emergence to first oviposition. Post-oviposition period is the time from last egg laying to death (Yin et al. 2008).
One-way analysis of variance (ANOVA) was performed to check significant differences among treatments after checking for normality. Post-test ANOVA was used to separate means by Tukey’s Studentized Range test at P < 0.05. SAS software was used for all analyses (SAS Institute 2002).
Results
Toxicity of Fenoxycarb
The data indicated that the fenoxycarb had high toxicity against third-instar larvae. The estimated LC50 was 93.92 mg/liter after 96 h. Also, the LC10 and LC25 values were 21.58 and 43.25 mg/liter, respectively (Table 1).
Table 1.
Toxicity of fenoxycarb on third-instar larvae of P. xylostella
| Treatment | na | df | LC10 (mg/liter)b | LC25 (mg/liter)b | LC50 (mg/liter)b | Slope ± SE | χ2 | P-value |
|---|---|---|---|---|---|---|---|---|
| Fenoxycarb | 280 | 5 | 21.58 (8.11–33.92) | 43.25 (24.52–57.48) | 93.62 (74.85–115.65) | 2.01 ± 0.39 | 2.04 | 0.84 |
aNumber of larvae.
b95% confidence limits in parenthesis.
Development Time
The development time of various stages in offspring generation are shown in Table 2. Sublethal concentrations at LC10 and LC25 level extended embryonic development for 2.88 and 3.21 days in the offspring, respectively (F = 120.37, df = 2, 291, P < 0.0001). Also fenoxycarb prolonged larval development time significantly (1st: F = 4.46, df = 2, 204, P = 0.0127; 2nd: F = 9.95, df = 2, 188, P < 0.0001; 3rd: F = 1.29, df = 2, 178, P = 0.2962; 4th: F = 33.14, df = 2, 162, P < 0.0001). Although the pupal developmental time was increased (F = 24.87, df = 2, 141, P < 0.0001), but prepupal period was not statistically different (F = 0.91, df = 2, 159, P = 0.4055). The results also indicated that the developmental time of pre-adult stage increased when fenoxycarb treated (F = 88.88, df = 2, 140, P < 0.0001). The total life span of males and females did not affect by fenoxycarb at LC10 and LC25 concentrations (Male: F = 1.70, df = 2, 72, P = 0.1906; Female: F = 1.82, df = 2, 67, P = 0.1697).
Table 2.
Effect of fenoxycarb on development time of P. xylostella offspring when treated in parents third-instar larvae
| Entries | Development time (Mean ± SE) (day)a |
F | P | dft,e | ||
|---|---|---|---|---|---|---|
| Control | LC10 | LC25 | ||||
| Egg | 2.18 ± 0.03 c | 2.88 ± 0.05 b | 3.21 ± 0.05 a | 120.37 | <0.0001 | 2, 291 |
| First-instar larvae | 2.98 ± 0.05 b | 3.34 ± 0.09 a | 3.07 ± 0.10 ab | 4.46 | 0.0127 | 2, 204 |
| Second-instar larvae | 1.61 ± 0.08 b | 1.82 ± 0.08 b | 2.19 ± 0.10 a | 9.95 | <0.0001 | 2, 188 |
| Third-instar larvae | 1.25 ± 0.07 a | 1.34 ± 0.06 a | 1.21 ± 0.06 a | 1.22 | 0.2962 | 2, 178 |
| Fourth-instar larvae | 1.51 ± 0.06 b | 2.40 ± 0.08 a | 2.28 ± 0.12 a | 33.14 | <0.0001 | 2, 162 |
| All larvae | 7.44 ± 0.23 c | 8.89 ± 0.16 a | 8.25 ± 0.13 b | 17.54 | <0.0001 | 2, 159 |
| Prepupa | 0.40 ± 0.06 a | 0.35 ± 0.06 a | 0.48 ± 0.08 a | 0.91 | 0.4055 | 2, 159 |
| Pupa | 3.70 ± 0.10 b | 4.98 ± 0.16 a | 4.71 ± 0.16 a | 24.87 | <0.0001 | 2, 141 |
| Pre adult stages | 13.61 ± 0.19 b | 17.22 ± 0.21 a | 16.62 ± 0.24 a | 88.88 | <0.0001 | 2, 140 |
| Total life span (male) | 30.78 ± 1.26 a | 32.38 ± 1.39 a | 28.76 ± 1.33 a | 1.70 | 0.1906 | 2, 72 |
| Total life span (female) | 31.30 ± 1.18 a | 32.20 ± 1.57 a | 28.12 ± 1.47 a | 1.82 | 0.1697 | 2, 67 |
aMeans followed by the same letters within a row are not significantly different (Tukey's test; P < 0.05).
Oviposition Period in Parent and Offspring
Fenoxycarb at LC10 and LC25 extended the APOP significantly (F = 5.40, df = 2, 42, P = 0.0082) but decreased the oviposition period in a dose-dependent manner in parents (F = 14.63, df = 2, 42, P < 0.0001); however, it had no effect on the post-oviposition period (F = 1.01, df = 2, 42, P = 0.3719) as well as the adult longevity (both male and female) of parents (Male: F = 0.61, df = 2, 42, P = 0.5477; Female: F = 1.51, df = 2, 42, P = 0.2326). In offspring generation, APOP was not significantly affected (F = 3.06, df = 2, 67, P = 0.0534). In addition, total pre-oviposition period (TPOP) was extended significantly at sublethal concentrations (F = 27.37, df = 2, 67, P < 0.0001), but oviposition and post-oviposition periods did not affect in the offspring (oviposition period: F = 0.27, df = 2, 67, P = 0.7608; post-oviposition period: F = 1.02, df = 2, 67, P = 0.3675). At LC25, the male and female longevity were diminished (Male: F = 4.34, df = 2, 70, P = 0.0168, and Female: F = 3.48, df = 2, 67, P = 0.0366) (Table 3).
Table 3.
Effect of sublethal concentrations of fenoxycarb on pre-oviposition, oviposition, post-oviposition periods and adult longevity of P. xylostella in parents and offspring
| Generations | Stages | Mean ± SE (day)a |
F | P | dft,e | ||
|---|---|---|---|---|---|---|---|
| Control | LC10 | LC25 | |||||
| Parent | APOPb | 2.13 ± 0.66 b | 7.53 ± 1.80 a | 8.33 ± 2.14 a | 5.40 | 0.0082 | 2, 42 |
| Oviposition | 11.26 ± 0.95 a | 7.46 ± 1.04 b | 3.26 ± 1.13 c | 14.63 | <0.0001 | 2, 42 | |
| Post-Oviposition | 0.86 ± 0.30 a | 1.46 ± 0.40 a | 0.80 ± 0.38 a | 1.01 | 0.3719 | 2, 42 | |
| Male longevity | 20.07 ± 1.98 a | 18.27 ± 1.79 a | 17.00 ± 2.13 a | 0.61 | 0.5477 | 2, 42 | |
| Female longevity | 14.26 ± 1.16 a | 16.46 ± 1.39 a | 12.40 ± 2.22 a | 1.51 | 0.2326 | 2, 42 | |
| Offspring | APOPb | 4.13 ± 0.78 a | 2.70 ± 0.46 a | 2.75 ± 1.61 a | 3.06 | 0.0534 | 2, 67 |
| TPOPc | 13.17 ± 0.26 b | 16.79 ± 0.32 a | 17.31 ± 1.06 a | 27.37 | <0.0001 | 2, 67 | |
| Oviposition | 10.65 ± 0.89 a | 10.37 ± 1.35 a | 9.43 ± 0.93 a | 0.27 | 0.7608 | 2, 67 | |
| Post-oviposition | 2.06 ± 0.57 a | 1.29 ± 0.43 a | 1.25 ± 0.44 a | 1.02 | 0.3675 | 2, 67 | |
| Male longevity | 16.60 ± 1.27 a | 14.65 ± 1.32 ab | 11.76 ± 1.33 b | 4.34 | 0.0168 | 2, 70 | |
| Female longevity | 16.62 ± 1.20 a | 14.33 ± 1.52 ab | 11.17 ± 1.50 b | 3.48 | 0.0366 | 2, 67 | |
aMeans followed by the same letters within a row are not significantly different (Tukey's test; P < 0.05).
bAdult pre-oviposition period, time between adult emergence and first oviposition.
cTotal pre-oviposition period, time from birth to first reproduction in female.
Pupal Weight in Parent and Offspring
The pupal weight was significantly decreased compared with control in parents. Also LC25 was more effective than LC10 (F = 8.09, df = 2, 72, P = 0.0007) (Fig. 1). Pupal weight of offspring was significantly decreased only at LC25 (F = 7.03, df = 2, 72, P = 0.0016).
Fig. 1.
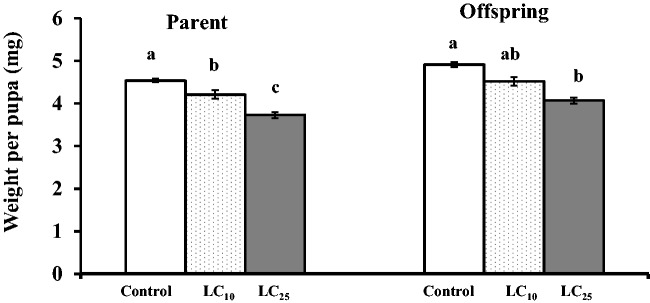
Effects of fenoxycarb on pupal weight of P. xylostella in the parent and offspring when third-instar larvae were fed on fenoxycarb treated leaves for 96 h in the first generation.
Sublethal Effects on Fecundity and Population Growth Parameters
The fecundity and population growth parameters of DBM were significantly affected by fenoxycarb (Tables 4 and 5). In parent generation, fenoxycarb reduced the fecundity in a dose-dependent manner significantly (F = 24.63, df = 2, 42, P < 0.0001), however, it had no significant impact on fecundity of offspring (LC25: F = 1.51, df = 2, 67, P = 0.2287). In addition, the daily fecundity and fertility did not affect by the LC10 and LC25 (fecundity: F = 1.70, df = 2, 67, P = 0.1911; fertility: F = 1.99, df = 2, 67, P = 0.1452) (Table 4).
Table 4.
Comparison of fecundity and fertility of P. xylostella treated with sublethal doses of fenoxycarb and control in parents and offsprings
| Entries | Parent (mean ± SE) (day)a |
Offsprings (mean ± SE) (day)a |
||
|---|---|---|---|---|
| Fecundity (eggs/female) | Fecundity (eggs/female) | Fecundity (egg/female/day) | Fertility (fertile egg/female/day) | |
| Control | 169.40 ± 12.84 a | 148.00 ± 12.22 a | 4.33 ± 0.42 a | 4.33 ± 0.42 a |
| LC10 | 71.06 ± 14.34 b | 120.25 ± 15.25 a | 3.28 ± 0.43 a | 3.18 ± 0.41 a |
| LC25 | 40.60 ± 13.46 b | 153.06 ± 15.11 a | 4.20 ± 0.35 a | 4.07 ± 0.34 a |
| F | 24.63 | 1.51 | 1.70 | 1.99 |
| P | <0.0001 | 0.2287 | 0.1911 | 0.1452 |
| dft,e | 2, 42 | 2, 67 | 2, 67 | 2, 67 |
aMeans followed by the same letters within a column are not significantly different (Tukey's test; P < 0.05).
Table 5.
Effect of fenoxycarb on biological parameters of P. xylostella offspring when treated in parents third-instar larvae
| Entries | Biological parameters (mean ± SE)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| GRR | R0 | rm (day−1) | λ (day−1) | T (day) | Dt (day) | b (birth rate) | d (death rate) | |
| Control | 98.68 ± 12.02 a | 50.76 ± 4.00 a | 0.190 ± 0.008 a | 1.21 ± 0.01 a | 20.73 ± 0.71 b | 3.61 ± 0.18 b | 0.26 ± 0.01 a | 0.07 ± 0.003 a |
| LC10 | 91.39 ± 12.84 a | 31.31 ± 3.95 b | 0.148 ± 0.008 b | 1.16 ± 0.01 b | 23.27 ± 0.78 a | 4.68 ± 0.26 a | 0.21 ± 0.01 b | 0.06 ± 0.002 a |
| LC25 | 112.09 ± 14.15 a | 32.20 ± 2.13 b | 0.164 ± 0.006 ab | 1.18 ± 0.08 b | 21.26 ± 0.54 ab | 4.17 ± 0.20 ab | 0.22 ± 0.01 ab | 0.06 ± 0.001 a |
| F | 0.42 | 6.75 | 8.59 | 7.79 | 4.05 | 6.72 | 7.45 | 0.14 |
| P | 0.6761 | 0.0021 | 0.0005 | 0.0009 | 0.0218 | 0.0022 | 0.0012 | 0.8708 |
| dft,e | 2, 67 | 2, 67 | 2, 67 | 2, 67 | 2, 67 | 2, 67 | 2, 67 | 2, 67 |
aMeans followed by the same letters within a column are not significantly different (Tukey's test; P < 0.05). See ‘Materials and methods’ section for abbreviations.
Although the net reproductive rate (R0) was significantly decreased by LC10 and LC25 (F = 6.75, df = 2, 67, P = 0.0021) (Fig. 2), however, no significant differences was observed in GRR (F = 0.42, df = 2, 67, P = 0.6167). In contrast to LC25, the LC10 had a significant effect on the intrinsic rate of increase (rm) (F = 8.59, df = 2, 67, P = 0.0005). Increasing the fecundity at LC25 dose is the main reason of this difference. The finite rate of increase (λ) at two sublethal concentrations was significantly decreased (F = 7.79, df = 2, 67, P = 0.0009). The doubling time (Dt) and mean generation time (T) were significantly extended only by the LC10. Dt and T are related to rm. Hence, similar to rm, these parameters were affected only by LC10 (Dt: F = 6.72, df = 2, 67, P = 0.0022; T: F = 4.05, df = 2, 67, P = 0.0218). The birth rate (b) at LC10 was obviously lower than those of control (F = 7.45, df = 2, 67, P = 0.0026), but no significant differences was observed in death rate of treatments (d) (F = 0.14, df = 2, 67, P = 0.8708) (Table 5).
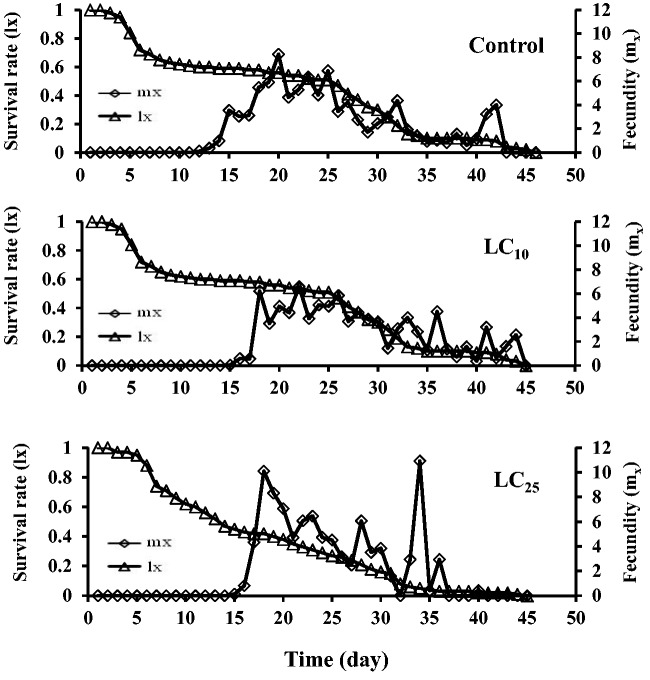
Fig 2.
Age-specific survival rate (lx) and age-specific fecundity (mx) of P. xylostella in offspring when third-instar larvae were fed on fenoxycarb treated leaves for 96 h in the previous generation.
Discussion
There are several studies showing the toxicity of fenoxycarb on a number of insects (Chandler et al. 1992, Singh and Tiwari 2015), however, this is the first study reporting the sublethal effects of fenoxycarb on DBM. In this study, fenoxycarb was detrimental to DBM in parent and offspring generation. However, it should be caution that fenoxycarb may enhance the fecundity of some insects such as Cacopsylla pyricola (Forster) (Homoptera: Psyllidae) (Solomon and Fitzgerald 1987, Horton and Lewis 1996). Other insecticides such as pyriproxyfen, a JH analogue (Oouchi 2005) and methomyl and fanvalerate (Sota et al. 1998, Fujiwara et al. 2002) may also promote reproduction of DBM. Similar to neemarin (Ahmad et al. 2012) and metaflumizone (Zhang et al. 2012), fenoxycarb could reduce the reproductive and biological parameters of DBM. The disruption in reproduction has been reported when insects were treated by JHAs such as fenoxycarb at larval stage (Retnakaran et al. 1985, Rumpf et al. 1998). Impaired reproduction may occur as a result of production of underweight pupae by failure of food uptake or disturbing somatic physiology (Grosch and Hoffman 1973). In this study, fenoxycarb caused underweight pupae in parent and offspring. In contrast, Mauchamp et al. (1989) have reported an increase in body weight of tobacco budworm, Heliothis virescens (F.) (Lepidoptera: Noctuidae) fenoxycarb-treated insects. However, this study is accordant with studies of Biddinger and Hull (1999), who reported the production of small pupae as a consequence of fenoxycarb treatment. We found that developmental time of eggs, larvae, and pupae prolonged significantly in the offspring generation. Extending the development time of pre-adult stages may increase exposure risk to natural enemies (Charleston 2004). It seems that fenoxycarb, like JHs, can usually maintain juvenalizing character by extending the immature stages as stated by other researchers (Mauchamp et al. 1989; Reid et al. 1990, 1994; Letellier et al. 1995; Singh and Johnson 2013). It could be concluded that JHAs act in the same manner as JHs but they are much more chemically stable (Matolcsy et al. 1988). Therefore, fenoxycarb may cause the adult insects to maintain larval characteristics with ultimate failure of normal reproduction. Our findings revealed the adult life span was affected by fenoxycarb. The pre-oviposition period in parents was significantly increased when last instar larvae were fed with treated leaves. Similarly, female longevity of C. pyricola (F.) (Homoptera: Psyllidae) (Horton and Lewis 1996) and DBM (Yin et al. 2008) has extended when they treated by fenoxycarb and spinosad in larval stage, respectively. In contrast, adult’s longevity was diminished when DBM larvae was treated with hexaflumuron an IGR (Mahmoudvand et al. 2011b).
In this study, we found that fenoxycarb was highly toxic to third-instar larvae of DBM. Larval death might be caused by abnormal amounts of JHA. We also demonstrated that feeding the third-instar larvae with fenoxycarb-treated leaves for 96 h may cause a significant decrease in pupal weight and fecundity in subsequent generation, even the insects were fed with untreated leaves.
High levels of JHAs such as fenoxycarb, when applied to later instars, may cause the final adult stage to maintain larval characteristics and these insects generally cannot reproduce (Sullivan 2000). Fenoxycarb had toxic effects by decreasing the fecundity but increasing the development time. In the other hand, fenoxycarb can effectively influence reproductive physiology, such as intrinsic rate of increase and other biological parameters. However, further studies are necessary to reveal long-term effects of fenoxycarb on DBM.
Acknowledgements
This research is a part of PH.D. thesis that was supported by Faculty of Agriculture, Tarbiat Modares University, Tehran, Iran.
References Cited
- Abro G. H., Corbitt T. S., Christie P. T., Wright D. J. 1993. Sub-lethal effects of abamectin on Plutella xylostella L. and Spodoptera littoralis Boisduval larvae. Crop Prot. 12: 39–44. [Google Scholar]
- Ahmad N., Ansari M. S., Nazrussalam 2012. Effect of neemarin on life table indices of Plutella xylostella (L.). Crop. Prot. 38: 7–14. [Google Scholar]
- Alizadeh M., Karimzadeh J., Rassoulian G. R., Farazmand H., Hoseini-Naveh V., Pourian H. R. 2012. Sublethal effects of pyriproxyfen, a juvenile hormone analogue, on Plutella xylostella (Lepidoptera: Plutellidae): life table study. Arch. Phytopathol. Plant Prot. 45: 1741–1763. [Google Scholar]
- Bicchi C. D., Amato A., Tonutti I., Cantamessa L. 1990. Simultaneous determination of clofentezine, fenoxycarb and hexythiazox by hplc on apples, pears and their pulps. Pestic. Sci. 30: 13–19. [Google Scholar]
- Biddinger D. J., Hull L. A. 1999. Sublethal effects of selected insecticides on growth and reproduction of a laboratory susceptible strain of tufted apple bud moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 92: 314–324. [Google Scholar]
- Birch L. C. 1948. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 17: 15–26. [Google Scholar]
- Carey J. R., 1993. Applied demography for biologists with special emphasis on insects. Oxford University Press, New York. [Google Scholar]
- Chandler L. D., Pair S. D., Raulston J. R. 1992. Effects of selected insect growth regulators on longevity and mortality of corn earworm and fall armyworm (Lepidoptera: Noctuidae) larvae. J. Econ. Entomol. 85: 1972–1978. [Google Scholar]
- Charleston D. S. 2004. Integrating biological control and botanical pesticides for management of Plutella xylostella. Wageningen University, The Netherlands. [Google Scholar]
- Cho J. R., Kim Y. J., Kim H. S., Yoo J. K. 2002. Some biochemical evidence on the selective insecticide toxicity between the two aphids, Aphis citricola and Myzus malisuctus (Homoptera: Aphididae), and their predator, Harmonia axyridis (Coleoptera: Coccinellidae). J. Asia-Pac. Entomol. 5: 49–53. [Google Scholar]
- Coppen G. D. A., Jepson P. C. 1996. The effects of the duration of exposure on the toxicity of diflubenzuron, hexaflumuron and teflubenzuron to various stages of II instar Schistocerca gregaria. Pestic. Sci. 46: 191–197. [Google Scholar]
- Desneux N., Decourtye A., Delpuech J. 2006. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52: 81–106. [DOI] [PubMed] [Google Scholar]
- Dhadialla T. S., Carlson G. R., Le D. P. 1998. New insecticides with ecdysteroidal and juvenile hormone activity. Annu. Rev. Entomol. 43: 545–569. [DOI] [PubMed] [Google Scholar]
- Elzen G. W. 2001. Lethal and sublethal effects of insecticide residues on Orius insidiosus (Hemiptera: Anthocoridae) and Geocoris punctipes (Hemiptera: Lygaeidae). J. Econ. Entomol. 94: 55–59. [DOI] [PubMed] [Google Scholar]
- Ergin E., Er A., Uçkan F., Rivers D. B. 2007. Effect of cypermethrin exposed hosts on egg-adult developmental time, number of offspring, sex ratio, longevity, and size of Apanteles galleriae wilkinson (Hymenoptera: Braconidae). Bel. J. Zool. 137: 27–31. [Google Scholar]
- Fujiwara Y., Takahashi T., Yoshioka T., Nakasuji F. 2002. Changes in egg size of the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae) treated with fenvalerate at sublethal doses and viability of the eggs. Appl. Entomol. Zool. 37: 103–109. [Google Scholar]
- Grosch D. S., Hoffman A. C. 1973. The vulnerability of specific cells in the oogenetic sequence of bracon hebetor say to some degradation products of carbamate pesticides. Environ. Entomol. 2: 1029–1032. [Google Scholar]
- Hamedi N., Fathipour Y., Saber M. 2010. Sublethal effects of fenpyroximate on life table parameters of the predatory mite Phytoseius plumifer. BioControl 55: 271–278. [Google Scholar]
- Han W., Zhang S., Shen F., Liu M., Ren C., Gao X. 2012. Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 68: 1184–1190. [DOI] [PubMed] [Google Scholar]
- Haynes K. F., 1988. Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 33: 149–168. [DOI] [PubMed] [Google Scholar]
- Horton D. R., Lewis T. M. 1996. Effects of fenoxycarb on ovarian development, spring fecundity and longevity in winter form pear psylla. Entomol. Exp. Appl. 81: 181–187. [Google Scholar]
- Jun W., Daqiang Y., Genfa L., Fengfan Z. 1999. Effects of dimehypo (disodium 2-methylaminotrimethylene dithiosulfonate) on growth and cocooning of the silkworm, Bombyx mori (Lepidoptera: Saturnidae). Pestic. Sci. 55: 1070–1076. [Google Scholar]
- Kumar K., Chapman R. B. 1984. Sublethal effects of insecticides on the diamondback moth Plutella xylostella (L.). Pestic. Sci. 15: 344–352. [Google Scholar]
- Larew H. G., Knodel-Montz J. J., Webb R. E., Warthen D. J. 1985. Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) control on Chrysanthemum by neem seed extract applied to soil. J. Econ. Entomol. 78: 80–84. [Google Scholar]
- Lee C. Y. 2000. Sublethal effects of insecticides on longevity, fecundity and behaviour of insect pests: a review. J. Biosci. 11: 107–112. [Google Scholar]
- Letellier C., Haubruge E., Gaspar C. 1995. Biological activity of fenoxycarb against Sitophilus zeamais Motsch. (Coleoptera: Curculionidae). J. Stored Prod. Res. 31: 37–42. [Google Scholar]
- Mahmoudvand M., Abbasipour H., Sheikhi Garjan A., Bandani A. R. 2011a. Sublethal effects of indoxacarb on the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 46: 75–80. [Google Scholar]
- Mahmoudvand M., Abbasipour H., Sheikhi Garjan A., Bandani A. R. 2011b. Sublethal effects of hexaflumuron on development and reproduction of the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Insect Sci. 18: 689–696. [Google Scholar]
- Mahmoudvand M., Abbasipour H., Sheikhi Garjan A., Bandani A. R. 2012. Change in life expectancy and stable age distribution of the diamondback moth, Plutella xylostella (L.) after indoxacarb treatment. J. Plant Prot. Res. 52: 342–346. [Google Scholar]
- Maia A. D. H., Alfredo J. B., Campanhola C. 2000. Statistical inference on associated fertility life table parameters using jackknife technique: computational aspects. J. Econ. Entomol. 93: 511–518. [DOI] [PubMed] [Google Scholar]
- Masner P., Angst M., Dorn S. 1987. Fenoxycarb, an insect growth regulator with juvenile hormone activity: a candidate for Heliothis virescens (F.) control on cotton. Pestic. Sci. 18: 89–94. [Google Scholar]
- Masner P., Dorn S., Vogel W., Kalin M., Graf O., Gunthart E. 1980. Types of response of insects to a new igr and to proven standards, pp. 809–818. In Sehnal F., et al. (ed.), Regulation of insect development and behavior. Technical University Wroclaw Press. [Google Scholar]
- Matolcsy G., Nadasy M., Andriska V. 1988. Pesticide chemistry: studies in environmental science, Elsevier; New York. [Google Scholar]
- Mauchamp B., Malosse C., Saroglia P., 1989. Biological effects and metabolism of fenoxycarb after treatment of the fourth and the fifth instars of the tobacco budworm, Heliothis virescens F. Pestic. Sci. 26: 283–301. [Google Scholar]
- Michel M., Krause A., Buszewski B. 2001. Column switching and liquid chromatographic technique for the rapid determination of fenoxycarb insecticide residues in apples. Polish J. Environ. Studies 10: 283–287. [Google Scholar]
- Miyamoto J., Hirano M., Takimoto Y., Hatakoshi M. 1993. Insect growth regulators for pest control with emphasis on juvenile hormone analogs: present status and future prospects. Pest Control Enhanc. Environ. Saf. 524: 144–168. [Google Scholar]
- Oouchi H. 2005. Insecticidal properties of a juvenoid, pyriproxyfen, on all life stages of the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 40: 145–149. [Google Scholar]
- Pressat R. 1985. The dictionary of demography. Bell and Bain, Ltd., Glasgow. [Google Scholar]
- Reid B. L., Bennett G. W., Yonker J. W. 1990. Influence of fenoxycarb on german cockroach (dictyoptera: Blattellidae) populations in public housing. J. Econ. Entomol. 83: 444–450. [DOI] [PubMed] [Google Scholar]
- Reid B. L., Brock V. L., Bennett G. W. 1994. Development, morphogenetic and reproductive effects of four polycyclic nonisoprenoid juvenoids in the German cockroach (Dictyoptera: Blattellidae). J. Entomol. Sci. 29: 31–42. [Google Scholar]
- Retnakaran A., Granett J., Ennis T. 1985. Insect growth regulators, pp. 529–601. In Kerkut G. A., Gilbert L. I., (eds.), Comprehensive insect physiology biochemistry and pharmacology. Pergamon Press, Oxford, UK. [Google Scholar]
- Rumpf S., Frampton C., Dietrich D. R. 1998. Effects of conventional insecticides and insect growth regulators on fecundity and other life-table parameters of Micromus tasmaniae (Neuroptera: Hemerobiidae). J. Econ. Entomol. 91: 34–40. [Google Scholar]
- SAS Institute. 2002. SAS/STAT. Ver. 9.1. SAS Institute, Cary, NC. [Google Scholar]
- Sial A. A., Brunner J. F. 2010. Lethal and sublethal effects of an insect growth regulator, pyriproxyfen, on obliquebanded leafroller (Lepidoptera: Tortricidae). J. Econ. Entomol. 103: 340–347. [DOI] [PubMed] [Google Scholar]
- Singh N., Johnson D. T. 2013. Baseline responses of Alphitobius diaperinus (Coleoptera: Tenebrionidae) to insect growth regulators. J. Agric. Urban Entomol. 29: 35–54. [Google Scholar]
- Singh A., Tiwari S. K. 2015. Effects of fenoxycarb, on the biology of rice moth, Corcyra cephalonica Stainton (Lepidoptera: Pyralidae) exposed as first In-star larvae. Front. Biol. Life Sci. 3: 14–18. [Google Scholar]
- Solomon M. G., Fitzgerald J. D. 1987. Fenoxycarb for control of pear sucker, Cacopsylla pyricola. Ann. Appl. Biol. 110: 22–23. [Google Scholar]
- Sota N., Motoyama N., Fujisaki K., Nakasuji F. 1998. Possible amplification of insecticide hormoligosis from resistance in the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 33: 435–440. [Google Scholar]
- Sullivan J. 2000. Environmental fate of fenoxycarb. Environmental monitoring fate reviews. Environmental Monitoring Branch, Department of Pesticide Regulation, California EPA, Sacramento, CA, USA. [DOI] [PubMed] [Google Scholar]
- Talekar N. S., Shelton A. M. 1993. Biology, ecology and management of the diamondback moth. Annu. Rev. Entomol. 38: 275–301. [DOI] [PubMed] [Google Scholar]
- Verkerk R. H. J., Wright D. J. 1996. Multitrophic interactions and management of the diamondback moth: a review. Bull. Entomol. Res. 86: 205–216. [Google Scholar]
- Yin X. H., Wu Q. J., Li X. F., Zhang Y. J., Xu B. Y., 2008. Sublethal effects of spinosad on Plutella xylostella (Lepidoptera: Yponomeutidae). Crop Prot. 27: 1385–1391. [Google Scholar]
- Zhang Z., Li J. X., Gao W. 2012. Sublethal effects of metaflumizone on Plutella xylostella (Lepidoptera: Plutellidae). J. Integr. Agric. 11: 1145–1150. [Google Scholar]