Abstract
BACKGROUND AND PURPOSE
The Acclino is a laser-cut closed-cell microstent composed of nitinol. It was developed for stent-assisted coiling of wide-neck intracranial aneurysms. The key feature of the stent is its deployability via low-profile microcatheters with an inner diameter of 0.0165 inch, which are also suited for coil deployment. The objective of this study was to evaluate the safety and feasibility as well as the immediate and mid-term results of this new device.
MATERIALS AND METHODS
Our database was screened for all Acclino-based stent-assisted intracranial coil embolizations since its introduction to the European market in June 2012. Case files and imaging data were retrospectively analyzed for angiographical and clinical outcome parameters, including immediate and mid-term modified Raymond-Roy aneurysm occlusion classification (RROC) rates and procedural complications.
RESULTS
Fourteen patients comprising 14 aneurysms (9 unruptured and 5 ruptured) were treated with the Acclino. All except for a dissecting one were wide-neck saccular aneurysms. Immediate complete occlusion (RROC1) was observed in 8/14 cases (57%), a residual neck (RROC2) in 4/14 (29%), and a persistent filling of the dome (RROC 3) in 1/14 cases (7%). An in-stent thrombus formation in one case (7%) was medically resolved without neurological deficit. Follow-up was available in 9/14 cases (64%) after a mean of 137 days (SD ± 50). All followed cases depicted a complete occlusion (RROC1).
CONCLUSIONS
The Acclino microstent showed a satisfactory safety profile and a promising rate of immediate and mid-term complete aneurysm occlusion for stent-assisted coil embolization in wide-neck intracranial aneurysms, warranting further investigation of the device.
INTRODUCTION
Endovascular coil embolization of ruptured and unruptured intracranial aneurysms has evolved into the treatment of choice in the majority of cases when compared to surgical clipping [1–4]. Recently, Molyneux et al., showed that it yields superior long-term results concerning death or dependency in comparison to clipping [5]. However, so-called complex aneurysms, being either wide-necked, fusiform or giant ones remain challenging entities; their recurrence rates are reported to be as high as 30% after coil embolization with GDC coils alone [6]. The balloon remodeling technique was introduced in 1990s to tackle these problems and has shown to be effective within anatomical limitations posed by the neck diameter of the aneurysm [7]. It remains to date a valuable tool, especially in acutely ruptured aneurysms [8, 9]. Nevertheless, the advent of flexible intracranial stents such as Neuroform (Boston Scientific, Natick, MA), Enterprise (Cordis, Bridgewater, NJ), and LEO (Balt Extrusion, Montmorency, France), which serve as bridging-neck devices when combined with coiling, broadened the range of aneurysms that can be occluded by endovascular techniques [10, 11]. These stents support the coils in the aneurysm sac, especially in wide-necked aneurysms with unfavorable neck/sac ratios of >0.7 and allow for higher coil-packing densities [12, 13].
Recently, a new generation of highly flexible intracranial microstents has been introduced, for example, the low-profile visualized intraluminal support (LVIS) devices (MicroVention, Tustin, CA), LEO+ Baby (Balt Extrusion, Montmorency, France), and Acclino (Acandis, Pforzheim, Germany).
Acclino gained CE marking for the European Union in June 2012. The distinctive feature of the aforementioned new generation of microstents is their compatibility with the very small inner diameter of microcatheters that are normally used for coiling, for example, 0.0165 ” for the Acclino, rendering microcatheter exchange maneuvers obsolete for the majority of cases. They can be deployed in small cerebral arteries down to a diameter of 2.0 mm, allowing for technically safe stenting and assisting in coiling anatomically unfavorable aneurysms in more distal vessel locations [14]. The Acclino can be resheathed even when it has been deployed between 50–90% of its maximum length, offering more versatility during the placement.
Our aim is to report the initial technical and clinical experience with the new Acclino stent in the treatment of ruptured and unruptured intracranial wide-necked aneurysms.
MATERIALS AND METHODS
We searched our longitudinal neurointerventional database for all patients with wide-neck aneurysms that were treated with stent-assisted coil embolization comprising the Acandis Acclino microstent on an intention-to-treat basis between June 2012 and June 2014 and conducted a retrospective analysis. According to institutional guidelines, no ethics committee approval was required for this observational study. According to our institutional protocol, all patients underwent postinterventional cranial computed tomography (CT) or magnetic resonance imaging (MRI) 12–24h after the procedure. All imaging and patient data were reviewed by two experienced interventional neuroradiologists.
Acandis Acclino Stent System
Acclino is a laser-cut closed-cell microstent (Fig. 1). Each end of the stent is flared and marked by three radio-opaque tips, made of gold. A middle marker on the transport wire improves visibility especially in the mid-portion of the stent to enhance visualization during stent deployment across the aneurysm neck. The microstent is available in two different diameters (3.5 mm and 4.5 mm), each of them offered in different lengths (e.g., 15 mm, 25 mm, and 35 mm). The stent is compatible with any 0.0165” microcatheter, which is typically navigated distal to the aneurysm neck with a standard microguidewire. After removing the microguidewire, the stent is introduced into the hub of the microcatheter, pushed through the catheter and unsheathed and deployed across the aneurysm neck by a typical push-and-pull maneuver. In case of an incorrect or unsatisfactory placement, the Acclino stent allows for a resheathing even if it was deployed between 50–90% of its total length. The mesh pore size is 1.8 mm which makes an exchange for a coiling catheter obsolete; the target aneurysm can be probed immediately with the same microcatheter in order to ultimately embolize it with coils.
Figure 1. Acclino microstent in vitro.

Endovascular Technique
Every intervention was performed via the transfemoral approach under general anesthesia. A 6F guiding catheter (Envoy MPC, Codman, Raynham, MA) was introduced through a short femoral sheath into the internal carotid or vertebral artery, respectively. In addition to standard bi-plane angiographic imaging, a three-dimensional rotational angiography was obtained in each case for the procedural planning. We then selected an appropriate Acclino microstent according to the parent vessel diameter and the width of the aneurysm neck. The manufacturer recommends the smaller diameter stent (3.5 mm) for parent vessel diameters between 2 mm and 3 mm and the larger one (4.5 mm) for parent vessel diameters between 3 mm and 4 mm. We selected the length of the utilized stent in a way that it ensured a coverage of at least 7 mm before and beyond the proximal and distal limit of the individual aneurysm neck. Different types of microcatheters were used for stent delivery (up to the respective operator's preference): NeuroSlider 17 (Acandis, Pforzheim, Germany), Headway 17 (MicroVention, Tustin, CA), Nautica and Rebar 18 (ev3, Irvine, CA).
Antiplatelet Regimen
In case of an elective coiling procedure, patients started 5 days prior to the intervention with ASA 100 mg and clopidogrel 75 mg daily after an initial loading dose of 300 mg clopidogrel. Platelet function testing was performed on all elective patients immediately before the intervention by using ASA and P2Y12 assays (VerifyNow, Accumetrics, San Diego, CA). A platelet inhibition level between 30–60% for P2Y12 and 350–550 ARU for ASA was required before stenting. A life-long treatment with ASA 100 mg/d was prescribed for the postinterventional period and clopidogrel 75 mg/d for 4 months.
In case of an emergency treatment of an acutely ruptured wide-neck aneurysm, we administered either 500 mg ASA or tirofiban hydrochloride intravenously during the procedure before stent deployment. After excluding a progression of the subarachnoid hemorrhage (SAH) in a cranial control CT 12–24h after the procedure patients were loaded with 300 mg clopidogrel in addition to 100 mg of ASA; any tirofiban hydrochloride infusion was discontinued beyond 24h. Next day, the regimen was continued with life-long ASA 100 mg/d and clopidogrel 75 mg/d for 4 months.
RESULTS
Procedural Data
A total of 14 aneurysms in 14 patients, 12 female and 2 male (median age 48 years, range 33-79 years) were treated. Five of 14 cases (36%) presented with ruptured aneurysms and SAH and were emergency treatments. All aneurysms except for one (ruptured dissecting PICA-aneurysm, case No. 14) were saccular wide-necked ones. The Y-stenting technique was utilized in 3/14 procedures. Overall, an Acclino microstent delivery was attempted 17 times (11 single microstent and 3 double microstent procedures); it was technically feasible in 16/17 attempts (94%). In one elective procedure (case No. 2), the Acclino microstent could not be unsheathed; it was not possible to withdraw the microcatheter with reasonable force. Consequently, we withdraw the microcatheter with the Acclino stent and switched to an Enterprise stent for that procedure, which proceeded uneventfully. Table 1 gives an overview of the anatomical and technical data of the procedures. The “jailing” technique with a second microcatheter was performed in 2/14 cases; in the remainder the stent-delivering microcatheter was used with a microwire for directly probing the aneurysm sac through the stent interstices.
Table 1. Procedural data.
| Case | Modality | Location | Geometry | Neck (mm) | Dome width (mm) | Treatment | Jailing | AE | Sequelae | RROC I (mm). | RROC FU (days) | Size (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Elective | MCA | wn | 3 | 2.5 | S | No | IIIa | I (101) | 3.5 × 25 | ||
| 2 | Elective | MCA | wn | 4 | 4 | S | No | Del. failure | None | I | ENTP | |
| 3 | SAH | BA | wn | 7 | 6 | S | No | II | I (169) | 4.5 × 25 | ||
| 4 | Elective | MCA | wn | 5 | 5 | S | Yes | I | I (97) | 3.5 × 25 | ||
| 5 | Elective | BA | wn | 4 | 3 | S | No | I | I (96) | 3.5 × 25 | ||
| 6 | Elective | MCA | wn | 3 | 2 | Y | No | Thrombus | None | I | I (96) | 3.5 × 25 |
| 7 | Elective | BA | wn | 3 | 2 | S | No | II | I (38) | 4.5 × 35 | ||
| 8 | Elective | Acom | wn | 4 | 3 | S | No | II | I (183) | 3.5 × 25 | ||
| 9 | Elective | MCA | wn | 3 | 2 | S | Yes | I | I (56) | 3.5 × 25 | ||
| 10 | SAH | Acom | wn | 4 | 3 | S | No | I | 3.5 × 15 | |||
| 11 | Elective | MCA | wn | 6 | 5 | S | No | II | I (188) | 3.5 × 20 | ||
| 12 | SAH | Acom | wn | 4 | 3 | Y | No | I | 3.5 × 25 | |||
| 13 | SAH | BA | wn | 4 | 3 | Y | No | I | 3.5 × 25 | |||
| 14 | SAH | PICA | dis | 3 | 2 | S | No | I | 3.5 × 25 |
wn: wide-neck; AE: adverse event; del. failure: delivery failure of the Acclino microstent; ENTP: Enterprise stent
Complications
In case No. 6, an elective procedure, a thrombus formation was detected inside the first Acclino stent during Y-stenting, although the patient had been prepared regularly with double antiplatelets and had also shown a favorable response in the preinterventional testing. It could be resolved without clinical sequelae by administration of i.v. tirofiban (maintained for 24h).
Immediate Imaging Results
Pertaining to the Acclino microstent, a complete occlusion (RROC1) at the end of the procedure was observed in 8/14 cases (57%). We saw a residual neck (RROC2) in 4/14 (29%) and a persistent filling of the dome (RROC 3) in 1/14 cases (7%). The case with the Enterprise stent was RROC 1. The wall adaption of the Acclino was satisfactory in all cases without a need for any adjunctive in-stent PTA. All aneurysms were embolized with either Axium (eV3, Irvine, CA) or Target coils (Stryker, Fremont, CA).
No patient showed signs of new cerebral ischemia or hemorrhage in the postinterventional cranial CT or MRI imaging.
Angiographical Follow-Up
DSA-based angiographical follow-up was available in 9/14 cases (64%), performed after a mean of 137 days (SD ± 50). In all followed cases, DSA depicted a complete occlusion (RROC1). We observed no in-stent stenosis/intimal hyperplasia in these controls. Clinically, none of the patients developed any new transient or permanent neurological deficit during follow-up.
Illustrative Case
Acclino-assisted coil embolization of an acutely ruptured BA-tip aneurysm.
DISCUSSION
The Acandis Acclino is a laser-cut, closed-cell microstent deliverable via low-lumen microcatheters, offering the potential to facilitate the stent-assisted intracranial aneurysm coiling procedure. Its closed-cell design allows thereby for a retrieval and repositioning as long as it is not fully deployed, which is a substantial advantage compared to other available intracranial stents.
In the current study, the device delivery and placement turned out to be technically feasible with a technical success rate of >90%. It can be safely utilized in the anterior and posterior cerebral circulation, offering a viable alternative to the first generation of intracranial stents for the treatment of wide-neck aneurysms, especially in parent arteries with small lumen diameters (e.g., down to 2 mm). We were also able to treat acutely ruptured wide-neck aneurysms successfully with the Acclino.
The complication rate of 1/14 cases (7%) was acceptably low and did not lead to a permanent neurological deficit. The complication rate was lower compared to the one reported recently for the LVIS Jr. (15%) in a series of 32 patients with a similar proportion of ruptured cases and Y-stenting [14]. In our series, we observed no treatment-related postinterventional morbidity and mortality, neither in the electively nor in the acutely treated patients. All postinterventional imaging studies excluded procedure-related ischemia or hemorrhage.
Follow-up DSA imaging was available for the majority of patients and showed an unusually high rate of complete occlusions (100% RROC 1). Although promising, this result has to be interpreted cautiously as it is most probably attributable to the small size of our cohort and needs verification in larger studies. Five patients of our cohort have not yet been followed because they were recently treated; all of them featured though a total occlusion (RROC1) at the end of the procedure. Poncyljusz et al., reported a rate of 82% RROC 1 occlusions during FU in their LVIS series [15]; Fiorella et al., were able to confirm these results in the US Humanitarian Device Exemption Study of the LVIS (75% RROC 1 during FU) [16]. Data on the LEO+ Baby and the Acclino is scarce; to date there are no larger case-series available (January 2015, Pubmed query). A broad range of data is available for the first-generation intracranial stent devices Neuroform and Enterprise, which are potential alternatives, depending on the individual anatomy and diameter of the parent vessel of the wide-neck aneurysm to be treated. Recently, King et al., presented a comprehensive review of the available literature about stent-assisted coiling in wide-neck aneurysms with the Neuroform and Enterprise stents [17]. In more than 2000 procedures with each stent, the Enterprise demonstrated statistically significant superior results compared to the Neuroform in terms of less deployment failures (0.2% vs. 2.3%), less peri-procedural intracranial hemorrhage (1.6% vs. 3.4%) and a higher rate of complete occlusions (74.7% vs. 61.1%) during a mean FU of 14.1 months (SD ± 10). A comparison of these results with the performance of the newer intracranial microstents has to be conducted carefully, as the range of indications is not totally the same. The microstents can be placed more distally in the intracranial vasculature, which may give rise to new challenges in terms of technical and clinical complications and long-term results. If we compare the initial results of the Acclino and the LVIS with their predecessors, we should compare them with the Enterprise, which is also a closed-cell stent in contrast to the open-cell design of the Neuroform. Accordingly, our preliminary FU occlusion rates of the Acclino and the available rates for the LVIS seem to be in the same range with the Enterprise, which in our opinion warrants further investigation of these new microstents in larger, preferably multicentric studies as endovascular coiling has become the standard therapy for the majority of intracranial aneurysms.
Conclusion
In this initial clinical study of the Acandis Acclino microstent for stent-assisted endovascular coil embolization in wide-neck intracranial aneurysms, we observed a satisfactory safety-profile and a promising rate of DSA-confirmed immediate and mid-term complete aneurysm occlusion, warranting further investigation of the device.
ABBREVIATIONS
- ASA
Acetylsalicylic acid
- Acom
Anterior communicating artery
- BA
Basilar artery
- CT
Computed tomography
- DSA
Digital subtraction angiography
- GDC
Guglielmi detachable coils
- MCA
Middle cerebral artery
- MRI
Magnetic resonance imaging
- PICA
Posterior inferior cerebellar artery
- RROC
Raymond-Roy occlusion classification
- SAH
Subarachnoid hemorrhage
Figure 2. Initial subtracted image depicting a bi-lobed BA-tip aneurysm.

Figure 3. Unsubtracted image; Acclino stent markers from left P1-Segment into BA, covering the aneurysm neck. 1St microcatheter jailed in the right sided lobe of the aneurysm while the microstent delivery catheter has been navigated into the aneurysm, ready to deliver the 1st coil.
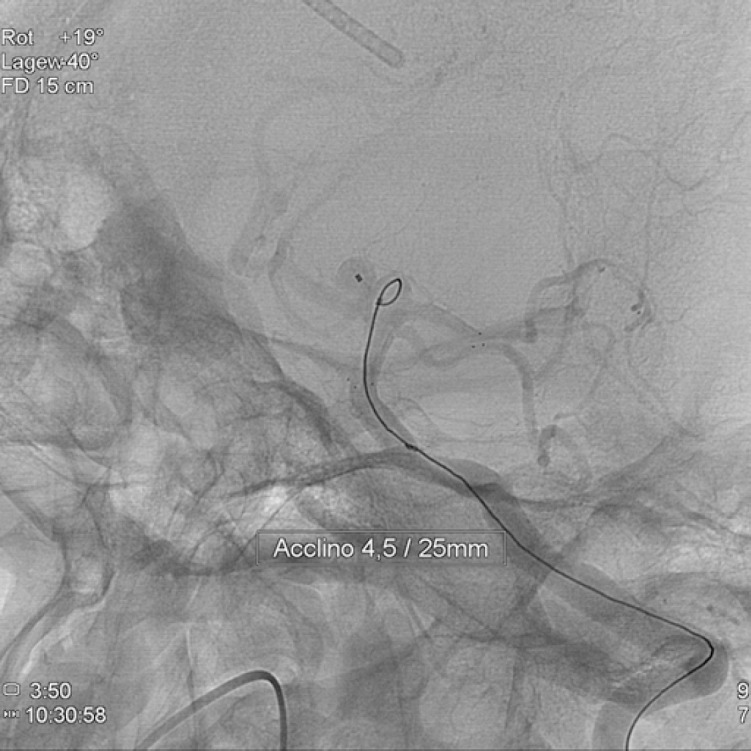
Figure 4. Subtracted image of final result, showing RROC 1 occlusion.

Footnotes
Funding: No private or public funding.
Conflict of Interest: The authors declare that there is no conflict of interest.
REFERENCES
- Alshekhlee A, Mehta S, Edgell RC, Vora N, Feen E, Mohammadi A, Kale SP, Cruz-Flores S. Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. [Dec 23;2014 ];Stroke [Internet] 2010 Jul;41(7):1471–1476. doi: 10.1161/STROKEAHA.110.580647. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20522817. [DOI] [PubMed] [Google Scholar]
- Brinjikji W, Rabinstein AA, Nasr DM, Lanzino G, Kallmes DF, Cloft HJ. Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001-2008. [Dec 23;2014 ];AJNR Am J Neuroradiol [Internet] Jan;32(6):1071–1075. doi: 10.3174/ajnr.A2453. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21511860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetzler RF, McDougall CG, Albuquerque FC, Zabramski JM, Hills NK, Partovi S, Nakaji P, Wallace RC. The barrow ruptured aneurysm trial: 3-year results. [Dec 23;2014 ];J Neurosurg [Internet] 2013 Jul;119(1):146–157. doi: 10.3171/2013.3.JNS12683. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23621600. [DOI] [PubMed] [Google Scholar]
- Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. [2014 Dec 13;];Lancet [Internet] 2002 Oct 26;360(9342):1267–1274. doi: 10.1016/s0140-6736(02)11314-6. http://www.ncbi.nlm.nih.gov/pubmed/12414200 Available from: [DOI] [PubMed] [Google Scholar]
- Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RSC. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18-year follow-up of the UK cohort of the international subarachnoid aneurysm trial (ISAT) [Oct 28;2014 ];Lancet [Internet] 2014 Oct 28; doi: 10.1016/S0140-6736(14)60975-2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25465111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J, Debrun GM, Aletich VA, Bashir Q, Charbel FT, Ausman J. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. [Dec 19;2014 ];Neurosurgery [Internet] 2002 Feb;50(2):239–249. 249–250. doi: 10.1097/00006123-200202000-00003. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11844258. [DOI] [PubMed] [Google Scholar]
- Moret J, Cognard C, Weill A, Castaings L, Rey A. [Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases] [Dec 23;2014 ];J Neuroradiol [Internet] 1997 Jun;24(1):30–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9303942. [PubMed] [Google Scholar]
- Kessler IM, Mounayer C, Piotin M, Spelle L, Vanzin JR, Moret J. The use of balloon-expandable stents in the management of intracranial arterial diseases: a 5-year single-center experience. [Dec 23;2014 ];AJNR Am J Neuroradiol [Internet] 2005 Oct;26(9):2342–2348. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16219843. [PMC free article] [PubMed] [Google Scholar]
- Pierot L, Cognard C, Spelle L, Moret J. Safety and efficacy of balloon remodeling technique during endovascular treatment of intracranial aneurysms: critical review of the literature. [Dec 23;2014 ];AJNR Am J Neuroradiol [Internet] 2012 Jan;33(1):12–15. doi: 10.3174/ajnr.A2403. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21349960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W, Bendszus M, Kis B, Boulanger T, Solymosi L, Kühne D. A new self-expanding nitinol stent (Enterprise) for the treatment of wide-necked intracranial aneurysms: initial clinical and angiographic results in 31 aneurysms. [Dec 23;2014 ];Neuroradiology [Internet] 2007 Jul;49(7):555–561. doi: 10.1007/s00234-007-0232-2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17476494. [DOI] [PubMed] [Google Scholar]
- Felber S, Henkes H, Weber W, Miloslavski E, Brew S, Kühne D. Treatment of extracranial and intracranial aneurysms and arteriovenous fistulae using stent grafts. [Dec 23;2014 ];Neurosurgery [Internet] 2004 Sep;55(3):631–638. 638–639. doi: 10.1227/01.neu.0000134455.02947.1f. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15335430. [DOI] [PubMed] [Google Scholar]
- Vanninen R, Manninen H, Ronkainen A. Broad-based intracranial aneurysms: Thrombosis induced by stent placement. [Dec 23;2014 ];AJNR Am J Neuroradiol [Internet] 2003 Feb;24(2):263–266. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12591645. [PMC free article] [PubMed] [Google Scholar]
- Tähtinen OI, Manninen HI, Vanninen RL, Rautio R, Haapanen A, Seppänen J, Niskakangas T, Rinne J, Keski-Nisula Stent-assisted embolization of recurrent or residual intracranial aneurysms. [Dec 23;2014 ];Neuroradiology [Internet] 2013 Oct;55(10):1221–1231. doi: 10.1007/s00234-013-1234-x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23861213. [DOI] [PubMed] [Google Scholar]
- Behme D, Weber A, Kowoll A, Berlis A, Burke TH, Weber W. Low-profile visualized intraluminal support device (LVIS Jr) as a novel tool in the treatment of wide-necked intracranial aneurysms: Initial experience in 32 cases. [Dec 23;2014 ];J Neurointerv Surg [Internet] 2014 Apr 10; doi: 10.1136/neurintsurg-2014-011157. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24699567. [DOI] [PubMed]
- Poncyljusz W, Biliński P, Safranow K, Baron J, Zbroszczyk M, Jaworski M, Bereza S, Burke TH. The LVIS/LVIS Jr. stents in the treatment of wide-neck intracranial aneurysms: multicentre registry. [Jan 20;2015 ];J Neurointerv Surg [Internet] 2014 May 14; doi: 10.1136/neurintsurg-2014-011229. http://www.ncbi.nlm.nih.gov/pubmed/24827067 Available from: [DOI] [PubMed]
- Fiorella D, Derdeyn C, Turk A, Boulos A, Diaz O, Pride G, Jabbour P, Woo H. O-004 Final results of the US humanitarian device exemption study of the low-profile visualised intraluminal support (LVIS) device. [Jan 20;];J Neurointerv Surg [Internet] 2014 Jul;6(Suppl 1):A2. doi: 10.1136/neurintsurg-2015-011937. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25064881. [DOI] [PubMed] [Google Scholar]
- King B, Vaziri S, Singla A, Fargen KM, Mocco J. Clinical and angiographic outcomes after stent-assisted coiling of cerebral aneurysms with Enterprise and Neuroform stents: a comparative analysis of the literature. [Jan 13;2015 ];J Neurointerv Surg [Internet] 2014 Oct 28; doi: 10.1136/neurintsurg-2014-011457. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25352581. [DOI] [PubMed]


