Abstract
Background
Laser Doppler flowmetry (LDF) can reliably reflect brain perfusion in experimental stroke by monitoring both the degree and the duration of relative regional cerebral blood flow (rCBF). Variation in rCBF was continuously monitored in 68 mice undergoing middle cerebral artery occlusion (MCAO) and 25 mice undergoing sham-operation and documented as LDF (%). Transcranial LDF changes in the territory of right middle cerebral artery during MCAO procedure were correlated with corrected infarct volume (CIV) and neurological deficit score (NDS).
Methods
Ninety-three C57BL/6 mice (Harlan Laboratories, Indianapolis, IN) between 9 and 11 weeks old were randomly selected and assigned to either MCAO for 45 minutes (n = 68) or sham group (n = 25). Ischemia was induced using the transient intraluminal filament model of MCAO based on Koizumi’s method and transcranial LDF was used to measure CBF during the procedure. Neurological deficits were measured at 2 and 23 hours after MCA reperfusion with NDS and 2% triphenyltetrazolium chloride (TTC) staining of carefully dissected brains was performed at 23 hours after reperfusion to determine infarct area.
Results
After common carotid artery occlusion (CCAO), there was a negative association between LDF drop from base line and NDS at 2 hours (r = −0.43, P = 0.038) and 23 hours (r = −0.61, P = 0.003). Also, a negative correlation was noted between MCA reperfusion LDF and NDS at 23 hours (r = −0.53, P = 0.001). Moreover, post-MCA reperfusion LDF had a positive association with initial CCAO LDF (r = 0.761, P = 0.000) and MCA occlusion LDF (r = 0.31, P = 0.036) in predicting neurological outcome. NDS at 23 hours corresponded well with the infarct volume (r = 0.31, P = 0.005).
Conclusions
Greater augmentation of rCBF after MCA reperfusion was associated with improved neurological deficit scoring. Interestingly, greater reduction of regional cerebral blood flow after CCAO was also associated with improved neurological outcomes. The favorable neurological outcome is possibly due to interplay of factors such as vascular reserve, collaterals, and autoregulation mechanisms. We propose LDF changes as an additional noninvasive prognosticator of stroke outcome in the setting of experimental brain ischemia.
Keywords: intraluminal middle cerebral artery occlusion, ischemic stroke, laser doppler flowmetry, middle cerebral artery, murine stroke, neurological deficit
BACKGROUND
The ability to predict outcomes after stroke is relevant for helping to evaluate and determine treatment options in both experimental studies and patients. Currently, the most accurate method for evaluating the degree of brain ischemia is by direct infarct volume measurement using pathology or in vivo imaging such as magnetic resonance imaging (MRI) [1]. Objective neurological assessments and behavioral examinations, despite having variable accuracy, are also integral for animal experimental ischemic models due to their ability to reflect the extent of stroke damage and serve as functional outcome measures. Recently developed techniques, such as magnetic resonance spectroscopy, also aid in noninvasively assessing the severity of brain damage by measuring alteration in the metabolite content of biomarkers such as N-acetyl aspartate, glutamate, and taurine in the tissue [2, 3]. A simple and effective noninvasive measure that predicts outcome would be beneficial for improving standardization and evaluating interventions across different murine stroke models.
Currently, laser Doppler flowmetry (LDF) is used in the animal stroke model to monitor regional cerebral blood flow (rCBF) dynamics in a particular vascular territory following targeted vessel occlusion. Using this technique, experimenters can reliably detect the presence of brain ischemia and quantify rCBF [4]. Because of the interconnected nature of the cerebral vasculature, distal LDF changes after iatrogenic common carotid artery occlusion (CCAO) may represent the patency of the mouse circle of Willis (CW) and the amount of collateral blood flow. Mouse models are extensively used in experimental stroke despite known variability between mouse and human CW [5]. In stroke patients, effective CW, leptomeningeal collaterals, and vascular reserve can play a significant role in attaining favorable outcome. Poor collateral vasculature results in the development of brain infarction within a few minutes following an acute vessel occlusion, while others with good collaterals can initially maintain a large penumbra. The presence of collaterals to the CW and retrograde filling of pial arteries (an indicator of functional leptomeningeal collateral vessels) can sustain perfusion following occlusion [6–10]. Approximately, half of all individuals have complete and patent CW, while many others live with anatomical variations, such as atresia or string-like arteries, duplication or triplication or even fetal origin of vessels [9]. Understanding the pathophysiology of reperfusion following an ischemic insult is especially clinically relevant in patients who undergo recanalization (Fig. 1).
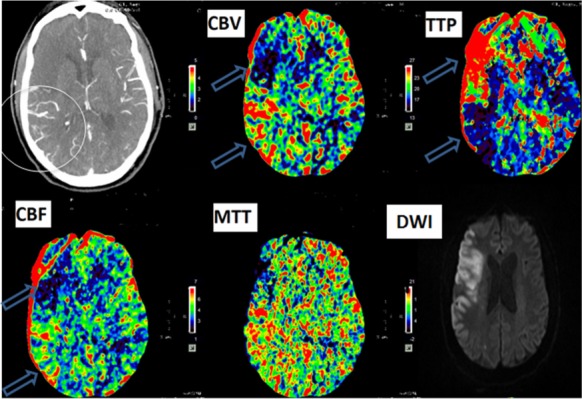
Figure 1. Hyperperfusion after recanalization in right MCA occlusion patient. Post-thrombolysis CT perfusion show dilated blood vessels (white circle) due to successful recanalization corresponding to decrease time to peak (TTP), increase cerebral blood flow (CBF) and cerebral blood volume (CBV) in the posterior aspect of the frontal lobe (upper blue arrow) due to hyperperfusion postrecanalization. In the anterior aspect of the frontal lobe, an increase in TTP and reduced CBF and CBV secondary to failed recanalization implies completed infarct. The diffusion-weighted imaging (DWI) confirms the corresponding infarct (cytotoxic edema) in the area with reduced perfusion (upper blue arrow), whereas the lower aspect of the frontal lobe (lower blue arrow) was spared from infarction due to hyperperfusion from successful recanalization.
When monitoring rCBF using transcranial LDF in the intraluminal middle cerebral artery occlusion (MCAO) stroke model, the degree and duration of percent LDF change should theoretically impact the final infarct volume and neurological outcome. Some argue that changes in LDF associated with CCAO directly impacts final stroke volume and outcome and may reflect both auto-regulatory capability and collateral flow status of the CW. Others suggest that hemodynamic responses to MCAO may serve as an indication of the performance of both the anterior cerebral artery (ACA) and leptomeningeal collaterals to perfuse the MCA territory [8]. However, while some animal studies have shown a correlation between outcome and rCBF reduction, others fail to find such a relationship [11, 12]. Because of this controversy in the literature, there is a need for further studies to clarify the relationship between cerebral blood flow and outcome.
The purpose of this study was to monitor relative changes in rCBF by LDF during experimental ischemia, and the subsequent relationship with NDS at 2 and 23 hours, and infarct volume at 23 hours to test the hypothesis that LDF correlates with acute poststroke outcomes in the intraluminal MCAO model. LDF percent changes were analyzed after CCAO, MCAO, and MCA reperfusion, for their ability to predict the neurological outcome.
METHODS
Animals Groups
A total of ninety-three C57BL/6 mice (Harlan Laboratories, Indianapolis, IN) between 9 and 11 weeks old were randomly selected and assigned to one of the two groups. In MCAO group, 68 mice underwent MCAO for 45 minutes. In sham group, 25 mice underwent sham operation. Animals were housed under a 12:12 light/dark cycle with free access to water and rodent chow. In our final analysis, mice with hemorrhagic transformation or subarachnoid hemorrhage caused by rupture of the intracranial artery and mice without observable neurological deficits following MCAO were excluded. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Florida and in compliance with both the "Guide for the Care and Use of Laboratory Animals" (eighth edition, National Academy of Sciences, 2011) and the EC Directive 86/609/EEC for animal experiments.
Surgical Procedure
Anesthesia was induced with 4% isoflurane and maintained with 1.5–2% isoflurane in 30% O2 and 70% air mixture via a nose cone. Core body temperature was maintained at 37±0.5°C with a heating pad and monitored using a rectal probe. Ischemia was induced using the transient intraluminal filament model of MCAO based on Koizumi’s method as described previously [33, 34]. After positioning the animal in a supine position, a 1 cm midline vertical incision was made between the manubrium and jaw. After dissecting the underlying structures, the CCA was secured with three loose collar sutures. Next, the most proximal collar suture around the CCA was tightened to permanently occlude blood flow. An arteriotomy was performed between the CCA bifurcation and the ligator, and a heat-blunted 7–0 monofilament nylon suture (Ethicon, Inc., Somerville, NJ, USA; tip diameter: 0.20–0.25 mm; heat-blunted length: 1–2 mm; silicone rubber coating length: 2–3 mm; overall suture length: 11–13 mm) was introduced and advanced into the CCA until the MCA was occluded. The two remaining collar sutures were tightened to prevent retrograde bleeding. After 45 minutes of ischemia, reperfusion was simulated by withdrawing the occluding micro suture. Finally, the distal two collar sutures were further tightened and trimmed. Transcranial LDF (Moor VMS-LDF, Moor Instruments, United Kingdom) was used to measure CBF in the MCA territory (1 mm posterior and 5 mm lateral to the bregma on the right parietal skull), and only mice with ≥80% flow reduction during the ischemic period were included in this study in order to exclude incomplete ischemia [33]. For sham group animals, vessels were visualized and cleared of overlying connective tissue as would be done in normal surgical dissection, but no additional manipulations were made.
Neurological Evaluation
Neurological deficits were measured at 2 and 23 hours after MCA reperfusion. Neurological deficit scoring (NDS) was performed with a 4-point scale modified from Bederson’s neurological scoring scale [31]: 0—No observable deficits; 1—Failure to extend contralateral forepaw (mild focal neurologic deficit); 2—Circling in a direction contralateral to infarct (moderate focal neurologic deficit); 3—Falling in a direction contralateral to infarct or/and depressed level of consciousness without spontaneous movement (severe focal neurologic deficit).
Staining and Quantitative Measurement of Infarct Volume
At 23 hours after reperfusion, animals were euthanized with 4% isoflurane according to approved protocol. The brain was carefully dissected, sectioned into 2 mm coronal sections with a mouse brain matrix slicer, and incubated in 2% triphenyltetrazolium chloride (TTC) in phosphate buffered saline at 37°C for 20 minutes to allow for visualization of the infarct. Infarcted tissue was unstained in comparison with viable tissue that stained brick-red. Images of individual sections were digitized and infarct area was measured with the ImageJ software (NIH). The area of infarct, ipsilateral hemisphere, and contralateral hemisphere were measured for each section. Volume for each region was calculated by summing the areas in all sections and multiplying by the slice thickness (2 mm). In order to correct for edema, which can distort the ipsilateral hemisphere and infarct areas, infarct volume as a percentage of edema-corrected hemispheric volume was calculated as follows [33]:
Corrected Infarct Volume (CIV); Contralateral hemisphere volume (CHV); Ipsilateral hemisphere volume (IHV); Infarct volume (IV)
Statistical Analysis
All values are presented as mean±SD and P < 0.05 was considered significant. All statistical analysis was performed using IBM SPSS Statistic 20 software. Kolmogronove–Smirnov test was used to check normality of parameters. The distribution of CIV (%) between ordered groups of neurological scoring was tested using Jonckheere–Terpstra with hypothesis order from smallest to largest. Measures of correlation were obtained using Spearman correlation coefficient (r). Independent samples median test was used to compare treatment group medians. Finally, the Wilcoxon signed ranks test was used to compare paired data means.
RESULTS
Preoperative weights in MCAO and sham groups were 25.76±1.87 g and 24.69±1.64 g and weights before sacrificing were 22.87±1.61 g and 24.32±1.64 g, respectively. There were no significant differences in body weight between the two groups before (P = 0.01) or after intervention (P = 0.00).
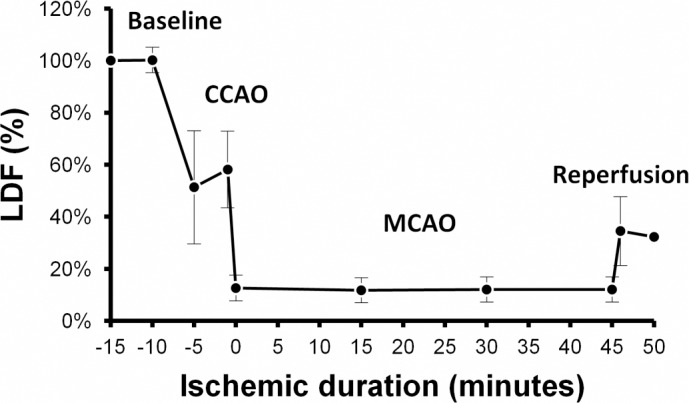
The change in LDF was measured in comparison to the baseline LDF before occlusion, which was considered to be 100%. The mean LDF levels for CCAO and MCAO were 57.03±14.91% and 11.71±4.36%, while the mean LDF increased to 32.71±12.90% after MCA reperfusion. Figure 2 demonstrates the trend of relative CBF, as measured by LDF changes during CCAO, MCAO, and MCA reperfusion.
Figure 2. Laser Doppler flowmetry changes through intraluminal MCAO procedure. The baseline blood flow is considered to be 100% for all animals. Change in LDF after occlusion of CCA and MCA and also reperfusion is demonstrated. Bars represent the 95% confidence interval of each value. CCAO: Common Carotid Artery Occlusion; MCAO: Middle Cerebral Artery Occlusion; LDF: Laser Doppler flowmetry.
The mean percent of CIV was 38.43±21.83% following 45 minutes of MCAO. Sham-operated animals demonstrated no visible infarction. Details of CIV for each subgroup of neurologic scores at 2 and 23 hours are presented in Table 1.
Table 1. NDS at 2 and 23 hours following reperfusion with corresponding infarct volume percentage.
| Neurological deficit score | Corrected infarct volume (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 45 min MCAO | Sham operated | 45 min MCAO | Sham operated | ||||||
| N | % | N | % | Mean±SD | Median | Mean±SD | Median | ||
| NDS 2 hours after reperfusion | 0 | 0 | 0.0% | 25 | 100% | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 | 19 | 27.9% | 0 | 0.0% | 36.60±22.88 | 35.00 | 0.00 | 0.00 | |
| 2 | 41 | 60.3% | 0 | 0.0% | 36.29±20.10 | 41.00 | 0.00 | 0.00 | |
| 3 | 8 | 11.8% | 0 | 0.0% | 53.75±24.54 | 53.00 | 0.00 | 0.00 | |
| NDS 23 hours after reperfusion | |||||||||
| 0 | 6 | 8.8% | 25 | 100% | 26.16±24.22 | 26.50 | 0.00 | 0.00 | |
| 1 | 25 | 36.8% | 0 | 0.0% | 36.64±21.63 | 40.00 | 0.00 | 0.00 | |
| 2 | 26 | 38.2% | 0 | 0.0% | 31.87±14.88 | 35.50 | 0.00 | 0.00 | |
| 3 | 11 | 16.2% | 0 | 0.0% | 64.72±.91 | 64.00 | 0.00 | 0.00 | |
Correlation of Laser Doppler Flowmetry with Neurological Deficit and Infarct Volume
Changes in laser Doppler flowmetry and neurological deficit score at 2 hours postreperfusion
We considered the relationship between LDF drop at CCAO, MCAO, and MCA reperfusion with the NDS at 2 hours after reperfusion. There was a negative correlation between CCAO LDF drop and NDS at 2 hours (r = −0.43, P = 0.038). A greater drop in LDF after CCAO was associated with lower NDS, or improved neurological outcome. No significant correlation was observed between either MCAO LDF drop (r = −0.03, P = 0.429) or MCA reperfusion LDF (r = −0.14, P = 0.217) and NDS at 2 hours.
Changes in laser Doppler flowmetry and neurological deficit score at 23 hours postreperfusion
We considered the relationship between LDF drop at CCAO, MCAO, and MCA reperfusion with the NDS at 23 hours after reperfusion. There was a negative correlation between CCAO LDF drop and NDS at 23 hours (r = −0.61, P = 0.003), a similar finding as seen at 2 hours. A greater drop in LDF after CCAO was associated with lower neurological deficits. No significant correlation was observed between MCAO LDF drop and NDS at 23 hours (r = −0.08, P = 0.315). Interestingly, there was also a negative correlation between MCA reperfusion LDF and NDS at 23 hours (r = −0.53, P = 0.001). Increased LDF after MCA reperfusion was associated with lower neurological deficits.
Association of middle cerebral artery reperfusion laser Doppler Flowmetry with common carotid artery occlusion and middle cerebral artery occlusion laser Doppler flowmetry
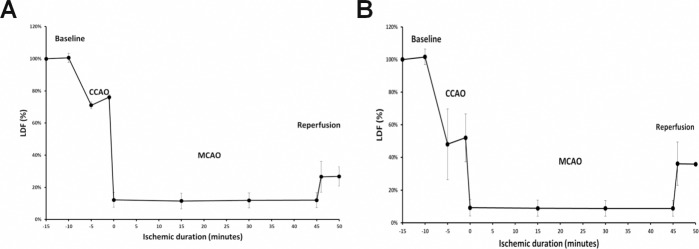
We considered the relationship between the degree of MCA reperfusion after suture removal with the degree of CCAO and MCAO. A significant correlation was detected between MCA reperfusion and both CCAO LDF drop (r = 0.76, P = 0.000) and MCAO LDF drop (r = 0.31, P = 0.036). Increased LDF drop during CCAO and MCAO was associated with increased MCA reperfusion (Fig. 3).
Figure 3. Large drop in CCAO LDF (%) was associated with large MCA reperfusion LDF (%). A: In mice with smaller drop in CCAO LDF (%), there was a smaller reperfusion LDF (%) after withdrawal of the filament from the MCA. B: In mice with larger drop in CCAO LDF (%), there was a larger MCA reperfusion LDF (%).
Association of laser Doppler flowmetry changes and corrected infarct volume
We also considered the relationship between infarct volume percentage and changes in LDF during CCAO, MCAO, and MCA reperfusion. No correlation was detected between stroke volume (CIV) and CCAO LDF drop (r = −0.20, P = 0.207), MCAO LDF drop (r = 0.05, P = 0.376), or MCA reperfusion LDF (r = −0.045, P = 0.400).
Correlations Between Neurological Deficits and Infarct Volume
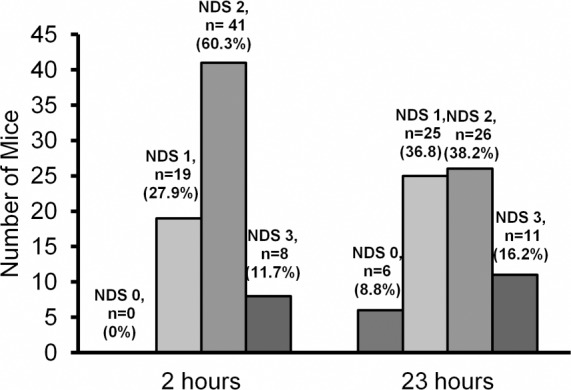
Detailed NDS values are presented in Table 1. The mode of NDS at 2 and 23 hours was 2 (60.3% and 38.2%, respectively) (Fig. 4). Sham-operated mice showed no observable neurological deficit at either time point (NDS = 0) (Table 1). Mice with NDS of 1 and 2 at the 2 hour time point were subject to greater change in their neurological status during the first 23 hours following reperfusion.
Figure 4. Neurological deficit scoring (NDS) when evaluated 2 and 23 hours after reperfusion. NDS scoring: 0—No observable deficits; 1—Failure to extend contralateral forepaw (mild focal neurologic deficit); 2—Circling in a direction contralateral to infarct (moderate focal neurologic deficit); 3—Falling in a direction contralateral to infarct and/or depressed level of consciousness without spontaneous movement (severe focal neurologic deficit).
We also considered the relationship between the percent infarct volume and early NDS. No correlation was found between CIV and NDS at 2 hours (r = 0.15, P = 0.103). According to the Jonckeheere–Tarpstra test for ordered alternatives, the distribution of the CIV was not similar through the categories of NDS after 23 hours following the reperfusion (P = 0.01). There was a positive correlation between NDS at 23 hours post-reperfusion and CIV (r = 0.31, P = 0.005). An increased CIV was associated with increased NDS, or 23 hours post reperfusion NDS is a good predictor of final anatomical outcome after stroke.
DISCUSSION
Because murine models of cerebral ischemia are important for investigating stroke pathophysiology, rCBF changes and neurobehavioral assessments can be noninvasive alternatives for evaluating outcome and estimating the final infarct volume. By analyzing the relationship between LDF changes and neurological score or infarct volume, we found several important meaningful associations between LDF (%), NDS, and CIV (%). Our findings suggest that both an increased CCAO LDF drop and an increased MCA reperfusion LDF was correlated with improved NDS. In addition, increased CCAO LDF drop and MCAO LDF drop was associated with increased MCA reperfusion LDF. We also found that NDS at 23 hours post-reperfusion directly related to the extent of CIV.
Correlation of laser Doppler flowmetry with Neurological Outcome
According to our data, large drop in LDF after CCAO showed predilection for better NDS at both 2 and 23 hours. Because larger LDF drop should reflect poor compensatory rCBF, this relationship is contrary to what we initially expected. During CCAO, blood flow to the ipsilateral hemisphere can be compensated through the CW, leptomeningeal vessels, autoregulation or vasomotor reactivity (vascular reserve) to counteract hypoperfusion. Since vascular imaging is not part of this study we cannot comment on the collaterals and CW. Also, studies have shown increase in oxygen extraction fraction while CBF decreases (misery perfusion syndrome) helping the tissue to survive [13].
Our study also showed a positive correlation between MCA reperfusion and CCAO (P = 0.00) and a negative correlation between MCA reperfusion and NDS at 23 hours (P = 0.001). These findings imply that a greater drop in LDF after CCAO can predict higher MCA reperfusion (higher rebound velocity) and favorable neurological outcomes (Fig. 3). Bonnin et al suggested that vasodilation and decrease in blood pressure distal to the occlusion causes an increased shift of blood flow from surrounding vessels, such as the patent arterial segments of CW (especially the anterior and posterior communicating arteries) and leptomeningeal vessels (cortical anastomoses between anterior, middle, and posterior cerebral arteries), toward the injured side and compensates ischemia during occlusion [7]. According to their data, rats with greater compensated blood flow and increase in blood-flow velocity through the adjacent and contralateral vessels in CW showed smaller ischemic lesion. They also noted that occlusion of both carotids resulted in lower blood flow in the MCA territory with fewer animals without lesion, suggesting that even with a greater ischemic insult collaterals play an important role in compensation of occlusion-induced ischemia.
Stability of cerebral perfusion pressure is maintained by the effects of autoregulation irrespective of changes in blood pressure hemodynamics [14]. Since autoregulation mainly functions within the threshold of innate vasomotor reactivity, inefficient vascular reserve can lead to a failure in preserving cerebral perfusion homeostasis. However, the correlation between greater occlusion and better neurological outcomes cannot be completely explained by autoregulation alone. We believe this phenomenon can additionally be explained by flow regulatory mechanisms involved in acute cerebral ischemia. Mechanisms like carbon dioxide (CO2) pressure changes, pressure gradient and hyperperfusion can all have bearing on outcome.
During ischemic phase, hypoxia from ipsilateral CCAO can lead to CO2 pressure changes which have reflection on vascular resistance since CO2 is a potent vasodilator. Because cerebrovascular resistance is mainly regulated by CO2 pressure (PaCO2), for each 1 mmHg increase in PaCO2 within physiological range, CBF also increases 1 – 2 ml/100g/min due to vasodilation [15]. In the reperfusion phase, there are two other mechanisms that may play a role in vasodilation. First, those related to the hyperactivity of cellular metabolism such as oxidative phosphorylation, ATP formation, synthesis of transcript factors or growth factors. Second, those excreted from the cells undergoing irreversible damage, such as massive release of excitatory amino acids [16].
In addition to autoregulation, reactive vasodilation develops a “pressure gradient” between the ischemic zone and adjacent normal area, resulting in redistribution of blood flow from non-ischemic zone to ischemic regions [15, 17–19]. This phenomenon, in which more blood is received by ischemic tissue, is called the “Robin Hood Phenomenon” or “inverse-steal” [15]. In the presence of ischemia without occlusion, hypercapnia causes vasodilation (increased vessel radius) and increased blood flow to the region of ischemia as compensation. When ischemia results from an occlusion, increased pressure in the nonischemic region causes vasodilation and overall increased blood flow to that region (reversed Robin Hood). Hyperperfusion in the nonischemic region causes hyperoxia, which causes a vasoconstriction and decreased blood flow. After the occlusion is removed, there is increased blood flow to the ischemic region because of the vasoconstriction and increased resistance that occurred in the nonischemic region.
Our LDF data demonstrated that there is more favorable neurological outcome with higher reperfusion velocity. We suggest higher reperfusion velocity might have led to early post ischemic hyperperfusion (EPIH) syndrome yielding better outcomes. PET scan has been used extensively in both experimental and clinical setting to study the pathophysiologic mechanism of this syndrome and its effect on clinical outcome. EPIH may effectively result in patchy grey matter damage, abnormal vasodilation, and luxury perfusion. This can lead to varying cerebral blood flow and oxygen extraction causing post ischemic rebound of cellular metabolism in structurally preserved tissue. Tissue exhibiting EPIH had better outcome with subsequent imaging not showing significant infarction [16]. Hyperperfusion after ischemic occlusion is clinically relevant in ischemic stroke patients who undergo recanalization (Fig. 5). Since we used temporary occlusion in our model, we assume EPIH and vasodilation helped improve outcome.
There was a poor correlation between MCAO LDF drop and the NDS, at both 2 and 23 hours. These results were supported by previous studies using simultaneous probes at two different locations in the ipsilateral hemisphere. Some experts have also suggested that perfusion deficit in the ipsilateral MCA territory might predict ischemia but not necessarily correlate with infarct size or neurological score [8].
Correlations Between Neurological Deficits and Infarct Volume
We analyzed the relationship between stroke size and neurological deficits using CIV and NDS. As demonstrated in Table 1, there is an increasing trend in the median CIV with increased NDS. The correlation is more pronounced when evaluated 23 hours after reperfusion (r = 0.31, P = 0.005) in comparison to 2 hours. The higher degree of correlation at 23 hours suggests that very early neurologic scoring may have low ability to predict anatomical infarct size. We hypothesize that this may be due to the unstable condition of animal, recovery process and underlying stress coping mechanisms in the hyperacute period following MCAO. As shown in Table 2, a large number of animals had improved NDS upon evaluation at 23 hours in comparison to 2 hours NDS. In our study at 2 hours, 60% of animals had an NDS of 2 and 27.9% had an NDS of 1. At 23 hours, 38% of animals had an NDS of 2, and 36.8% had an NDS of 1. At 2 hours, none of the animals exhibited an NDS of 0, while a small percentage of animals (8.8%) were free of any observable deficit(s) at 23 hours. Among animals with an NDS of 3 at 2 hours, 50% maintained this degree of impairment at 23 hours. These results suggest that if the initial neurological deficit from MCAO is severe (NDS = 3), it is less likely for that animal to demonstrate improved neurological function at a later time point. Extending the assessment time points may be needed to more accurately analyze the correlation between NDS and stroke volume. Xi et al also presented a significant positive correlation (r > 0.7) in three study groups (MCAO via the ECA, MCAO via the CCA, and direct ligation of the MCA) between infarct volume and 7 day neurological score [20]. Similar to our findings, this suggests that increased infarct volume may be associated with increased neurological deficits.
Previous studies utilizing final infarct volume to predict neurological outcome in experimental stroke model have had varying results [20–24]. Parts of these inconsistencies are due to diverse evaluation techniques, strain-related differences [25], size of suture tip [26], duration of occlusion [27], core body temperature [28], anesthesia [29], and timing of evaluation [21, 30, 31]. As observed in this study with NDS at 2 and 23 hours postreperfusion, the length of time between reperfusion and assessment of the neurologic deficit can also influence the result [23, 24, 32]. Neurological evaluations immediately following intervention may not be reliable and may subsequently change over time. Reglodi et al stated that the maximum scoring in their study was at 2 hours with no change during the first 6 hours [32]. Neurological scoring at 12 and 24 hours showed improved neurological function without significant difference between these two time points. Wahl et al suggested that neurologic scoring is maximized during the first day and remains constant in the following 48 hours [23]. Zhang et al described no significant correlation between infarct size and a four-point neurological score evaluated at 90 days following MCAO [24]. They suggested that NDS can detect sensorimotor deficits only up to 30 days after ischemia. As a result, both early assessments and very late neurologic evaluations may fail to correlate with infarct size. According to these studies, the 23 hours postreperfusion time point that we used is both late enough to be reliable and early enough before major recovery.
CONCLUSIONS
In transient intraluminal MCAO murine stroke model, we propose using LDF as a noninvasive measurement tool for assessment of pathologic insult. As expected, greater augmentation of CBF after MCA reperfusion was associated with improved neurological outcomes and higher infarct volume was associated with worse neurological outcomes. Interestingly, we discovered that increased ischemia during CCAO was also associated with improved neurological outcomes, possibly due to the association between greater ischemia during occlusion and increased MCA reperfusion. Normal autoregulatory mechanisms and pressure gradients (Robin Hood phenomenon) directing blood flow toward the ischemic penumbra are possible mechanisms for our findings. Understanding the hemodynamics involved in ischemic occlusion and reperfusion are critical for the proper design and analysis of future studies on treatment modalities for ischemic stroke.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Hedna, Ansari, Shahjouei, Cai, Ahmad, Mocco, and Qureshi contributed to study concept and design. Acquisition of data was provided by Ansari, Cai. Hedna, Ansari, Shahjouei, Cai, Ahmad, Mocco, and Qureshi provided their contribution to analysis and interpretation of data. Hedna, Ansari, Shahjouei, Cai, Ahmad, Mocco, and Qureshi drafted and revised the manuscript. Statistical analysis is provided by Hedna, Ansari, Shahjouei, and Cai. Administrative, technical, and material support are provided by Hedna, Ahmad, Mocco, Qureshi. Study supervision was done by Hedna, Ahmad, Mocco, Qureshi.
ACKNOWLEDGMENTS
None
References
- Durand A, Chauveau F, Cho TH, Bolbos R, Langlois JB, Hermitte L, Wiart M, Berthezene Y, Nighoghossian N. Spontaneous reperfusion after in situ thromboembolic stroke in mice. PLoS One. 2012;7:e50083. doi: 10.1371/journal.pone.0050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet C, Lei H, Gruetter R, Hirt L. Early predictive biomarkers for lesion after transient cerebral ischemia. Stroke. 2011;42:799–805. doi: 10.1161/STROKEAHA.110.603647. [DOI] [PubMed] [Google Scholar]
- Lei H, Berthet C, Hirt L, Gruetter R. Evolution of the neurochemical profile after transient focal cerebral ischemia in the mouse brain. J Cereb Blood Flow Metab. 2009;29:811–819. doi: 10.1038/jcbfm.2009.8. [DOI] [PubMed] [Google Scholar]
- Ohshima M, Tsuji M, Taguchi A, Kasahara Y, Ikeda T. Cerebral blood flow during reperfusion predicts later brain damage in a mouse and a rat model of neonatal hypoxic-ischemic encephalopathy. Exp Neurol. 2011. [DOI] [PubMed]
- Okuyama S, Okuyama J, Okuyama J, Tamatsu Y, Shimada K, Hoshi H, Iwai J. The arterial circle of Willis of the mouse helps to decipher secrets of cerebral vascular accidents in the human. Med Hypotheses. 2004;63:997–1009. doi: 10.1016/j.mehy.2003.12.055. [DOI] [PubMed] [Google Scholar]
- Herz R, Hillen B, Versteeg D, De Wildt DJ. Collateral hemodynamics after middle cerebral artery occlusion in Wistar and Fischer-344 rats. Brain Res. 1998;793:289. doi: 10.1016/s0006-8993(98)00187-5. [DOI] [PubMed] [Google Scholar]
- Bonnin P, Leger PL, Deroide N, Fau S, Baud O, Pocard M, Charriaut-Marlangue C, Renolleau S. Impact of intracranial blood-flow redistribution on stroke size during ischemia–reperfusion in 7-day-old rats. J Neurosci Methods. 2011;198:103–109. doi: 10.1016/j.jneumeth.2011.02.030. [DOI] [PubMed] [Google Scholar]
- Riva M, Pappadà GB, Papadakis M, Cuccione E, Carone D, Menendez VR, Sganzerla EP, Beretta S. Hemodynamic monitoring of intracranial collateral flow predicts tissue and functional outcome in experimental ischemic stroke. Exp Neurol. 2011. [DOI] [PubMed]
- Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Kasahara Y, Nakagomi T, Stern DM, Fukunaga M, Ishikawa M, Matsuyama T. A reproducible and simple model of permanent cerebral ischemia in CB-17 and SCID mice. J Exp Stroke Transl Med. 2010;3:28–33. doi: 10.6030/1939-067x-3.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano MA, Sanz O, Ferrer I, Planas AM. Cortical infarct volume is dependent on the ischemic reduction of perifocal cerebral blood flow in a three-vessel intraluminal MCA occlusion/reperfusion model in the rat. Brain Res. 1997;747:273–278. doi: 10.1016/s0006-8993(96)01285-1. [DOI] [PubMed] [Google Scholar]
- Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P. Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke. 1981;12:454–459. doi: 10.1161/01.str.12.4.454. [DOI] [PubMed] [Google Scholar]
- Bhalla A, Tilling K, Kolominsky-Rabas P, Heuschmann P, Megherbi SE, Czlonkowska A, Kobayashi A, Mendel T, Giroud M, Rudd A, Wolfe C. Variation in the management of acute physiological parameters after ischaemic stroke: a European perspective. Eur J Neurol. 2003;10:25–33. doi: 10.1046/j.1468-1331.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- Mishra LD. Cerebral blood flow and anaesthesia: a review. Indian J Anaesth. 2002;46:87–95. [Google Scholar]
- Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from positron emission tomography. J Cereb Blood Flow Metab. 1999;19:467–482. doi: 10.1097/00004647-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Baron JC. Positron tomography in cerebral ischemia. A review. Neuroradiology. 1985;27:509–516. doi: 10.1007/BF00340846. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Sharma VK, Lao AY, Tsivgoulis G, Malkoff MD, Alexandrov AW. Reversed Robin Hood syndrome in acute ischemic stroke patients. Stroke. 2007;38:3045–3048. doi: 10.1161/STROKEAHA.107.482810. [DOI] [PubMed] [Google Scholar]
- Milakovic B, Dimitrijevic I, Malenkovic V, Markovic D, Pantic-Palibrk V, Gvozdenovic L. Preoperative assessment and preparation of patients with diseases affecting the central nervous system. Acta Chir Iugosl. 2011;58:83–90. doi: 10.2298/aci1102083m. [DOI] [PubMed] [Google Scholar]
- Xi GM, Wang HQ, He GH, Huang CF, Wei GY. Evaluation of murine models of permanent focal cerebral ischemia. Chin Med J (Engl) 2004;117:389–394. [PubMed] [Google Scholar]
- Boyko M, Ohayon S, Goldsmith T, Novack L, Novack V, Perry ZH, Gruenbaum BF, Gruenbaum SE, Steiner O, Shapira Y. Morphological and neuro-behavioral parallels in the rat model of stroke. Behav Brain Res. 2011. [DOI] [PubMed]
- Nedelmann M, Wilhelm-Schwenkmezger T, Alessandri B, Heimann A, Schneider F, Eicke BM, Dieterich M, Kempski O. Cerebral embolic ischemia in rats: correlation of stroke severity and functional deficit as important outcome parameter. Brain Res. 2007;1130:188–196. doi: 10.1016/j.brainres.2006.10.087. [DOI] [PubMed] [Google Scholar]
- Wahl F, Allix M, Plotkine M, Boulu R. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke. 1992;23:267–272. doi: 10.1161/01.str.23.2.267. [DOI] [PubMed] [Google Scholar]
- Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- Barone FC, Knudsen DJ, Nelson AH, Feuerstein GZ, Willette RN. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab. 1993;13:683–692. doi: 10.1038/jcbfm.1993.87. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Vemuganti R, Sailor KA, Dempsey RJ. Ideal suture diameter is critical for consistent middle cerebral artery occlusion in mice. Neurosurgery. 2005;56:196–200. doi: 10.1227/01.neu.0000144490.92966.59. discussion 196-200. [DOI] [PubMed] [Google Scholar]
- Clark WM, Lessov NS, Dixon MP, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19:641–648. doi: 10.1080/01616412.1997.11740874. [DOI] [PubMed] [Google Scholar]
- Barber PA, Hoyte L, Colbourne F, Buchan AM. Temperature-regulated model of focal ischemia in the mouse: a study with histopathological and behavioral outcomes. Stroke. 2004;35:1720–1725. doi: 10.1161/01.STR.0000129653.22241.d7. [DOI] [PubMed] [Google Scholar]
- Kapinya KJ, Prass K, Dirnagl U. Isoflurane induced prolonged protection against cerebral ischemia in mice: a redox sensitive mechanism? Neuroreport. 2002;13:1431–1435. doi: 10.1097/00001756-200208070-00017. [DOI] [PubMed] [Google Scholar]
- Rickels E. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1993;32:479. doi: 10.1097/00006123-199303000-00030. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Tamas A, Lengvari I. Examination of sensorimotor performance following middle cerebral artery occlusion in rats. Brain Res Bull. 2003;59:459–466. doi: 10.1016/s0361-9230(02)00962-0. [DOI] [PubMed] [Google Scholar]
- Ansari S, Azari H, McConnell DJ, Afzal A, Mocco J. Intraluminal middle cerebral artery occlusion (MCAO) model for ischemic stroke with laser doppler flowmetry guidance in mice. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed]
- Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. I. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]


