Abstract
BACKGROUND
Bow Hunter’s Syndrome is a mechanical occlusion of the vertebral artery which leads to a reduction in blood flow in posterior cerebral circulation resulting in transient reversible symptomatic vertebrobasilar insufficiency.
CASE DESCRIPTION
We present a case of Bow Hunter’s syndrome in a 53-year-old male that occurred after the patient underwent surgical correction of a proximal left subclavian artery aneurysm. Shortly after the surgery, the patient began to complain of transient visual changes, presyncopal spells, and dizziness upon turning his head to the left. A transcranial doppler ultrasound confirmed the diagnosis of Bow Hunter’s syndrome.
SYSTEMIC REVIEW
We analyzed the data on 153 patients with Bow Hunter’s syndrome from the literature. An osteophyte was the most common cause of vertebral artery occlusion, and left vertebral artery was more commonly involved in patients with Bow Hunter’s syndrome. Dynamic angiography was the definitive imaging modality to confirm the diagnosis, and surgery was most successful in alleviating symptoms.
CONCLUSION
We believe that this is the first case of iatrogenic Bow Hunter’s syndrome after surgical intervention for an aneurysm repair, and the largest review of literature of Bow Hunter’s syndrome. Dynamic angiography is the gold standard for the diagnosis of Bow Hunter’s syndrome. Surgery should be considered as the primary treatment approach in these patients, especially those who have bony compression as the etiology.
Keywords: Bow Hunter’s syndrome, rotational vertebrobasilar insufficiency, iatrogenic, surgery, dynamic angiography
INTRODUCTION
Bow Hunter’s syndrome (BHS), also known as rotational vertebral artery syndrome is a form of vertebrobasilar insufficiency (VBI) in which the vertebral artery (VA) is mechanically compressed by the transverse process of a cervical vertebrae or surrounding structures when the head is turned more than 45° to the right or left [1]. Normally, the vertebral arteries course through the transverse foramina of the cervical vertebrae, entering the foramina of the C6 vertebra and exiting the C1 vertebra caudally. Compression in BHS has been most commonly observed at or above C2 [2]. The unilateral compression of one of the two vertebral arteries can be symptomatic; however, symptoms are usually transient and may be relieved when the head is returned to the neutral position. The condition is manifested by a constellation of symptoms including vertigo, nausea, dysarthria, dysphagia, transient blurring of vision in one or both eye fields, gait disturbance, headaches, and other sensorimotor findings such as tinnitus, hearing loss, syncope, or drop attacks [2].
In 1957, Tatlow and Bammer hypothesized that symptomatic head rotation may be due to mechanical compression of the vertebral arteries and observed compressions at C1–C2 and C5–C6 in subsequent studies done on cadavers [3]. In 1960, cervical spondylosis [4] and compressions by the anterior scalene muscle [5] were thought to be the two most common etiologic factors contributing to the condition.
In 1978, the term Bow Hunter’s syndrome was coined by Sorensen in order to define the reproducible symptoms associated with VBI upon head rotation [6]. Since that time osteophytes, fibrous bands, cervical disc herniation, C1 or C2 instability, chiropractic manipulation, surgical positioning, a multitude of physical activities, and sports have been implicated in various patient cases [7, 8]. An additional hypothesis is that of a thrombus formation within the lumen of the VA can precipitate by flow abnormalities during compressive periods. These thrombi may then embolize, which would result in transient ischemic attacks or cerebral vascular accidents [9]. In 1995, a case report described a syndrome in which a patient suffered from vertigo, dizziness, and blurred vision with head rotation [10]. Since then many typical and atypical cases of BHS have been documented in literature [11].
In this study, we aim to highlight a case of BHS at our center, which presented after surgical intervention to fix a proximal subclavian aneurysm. We further focus on all the cases that have been documented in literature, in order to ascertain the demographics of BHS. We also wish to look at all the imaging studies that have been used for the diagnosis of BHS along with the treatment approaches that have been considered for its cure. We intend to determine the prognosis in BHS patients.
Case Presentation
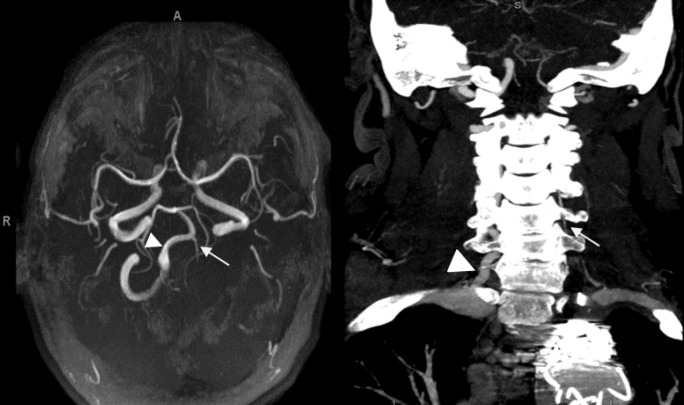
A 53-year-old Hispanic male with a history of diabetes mellitus, hypertension, hyperlipidemia, and a remote history of Bell’s palsy was presented to our emergency department with chest pain and shortness of breath. The initial investigation confirmed a left subclavian artery aneurysm and coarctation of the aorta just distal to the origin of the left subclavian artery. Computed tomography angiogram (CTA) of the neck showed that the diminutive left VA originated from the apex of the aneurismal portion of the left subclavian artery. The hypoplastic distal left VA was reconstituted via collaterals approximately at the C3 level (Fig. 1).
Figure 1. Magnetic resonance angiogram of the patient’s brain (left) and computerized tomography angiogram (CTA) of the patient’s neck (right) showing hypoplastic left vertebral artery (straight arrow) and dominant right vertebral artery (arrowhead) in comparison.
The patient underwent thoracic endovascular aortic repair involving a left subclavian artery to carotid artery transposition and left subclavian artery embolization. The surgeons elected to ligate the diminutive left VA and not reanastomose it. He had no postoperative complications and was started on aspirin, atorvastatin, and metoprolol.
Two weeks later, he returned to the emergency department with complaints of mild pain and swelling at the right groin incision site, as well as intermittent fevers. While in the emergency department, the patient experienced 30 seconds of transient left binocular visual loss. The patient states that this has only happened once, although he has had episodes of dizziness when he turns his head to the left since the surgery. Repeat CTA of the head and neck showed the graft and an occlusion of the left VA.
The patient was admitted for the evaluation of acute stroke. A head CT did not reveal any acute abnormalities. Magnetic resonance angiogram (MRA) of the brain at that time showed an area of restricted diffusion in the right occipital white matter and punctate foci of restricted diffusion in the right occipital lobe consistent with small emboli and some evidence of subcortical white matter disease (not shown). Along with the expected postoperative changes from the recent treatment of the left subclavian artery aneurysm, a 6.5 mm right VA aneurysm and a 17 mm right subclavian artery aneurysm were also found. Head CTA findings were suggestive of focal ectasia or early fusiform aneurysm formation of the right intradural VA with minimal early mass effects on right anterolateral medulla. Neck CTA revealed right VA dominance, nonvisualization of the proximal and mid left VA, and hypoplastic distal left VA reconstituted via collateralization (Fig. 1). An echocardiogram showed no evidence of significant valve dysfunction, and no cardiac source of embolus.
After 8 months, the patient presented to the cognitive clinic with complaints of memory problems following repair of the left subclavian artery aneurysm. Additionally, the patient continued to complain of dizziness when turning his head to the left and when standing. The patient was not found to have any evidence of neurodegenerative dementia, and the amount of white matter disease present on the brain magnetic resonance imaging (MRI) did not appear to be substantial enough to cause vascular dementia.
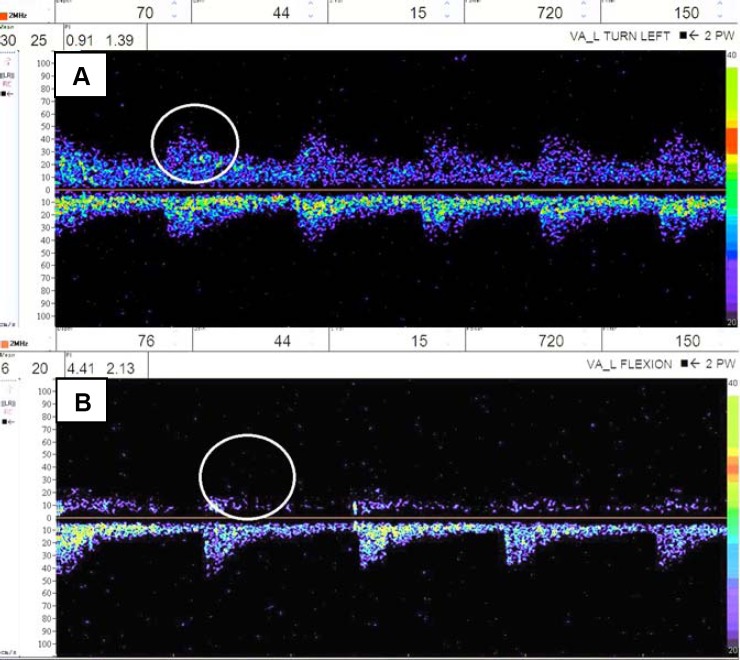
He was then referred to the stroke clinic to rule out VA aneurysm as the source of his dizziness. The patient stated that his symptoms are only when he turns his head to the left and his chin is up and denied any other transient ischemic attack or stroke-like symptoms. If his head is continually positioned to the left he feels as though he will pass out, but denies any syncope or presyncopal falls. Along with dizziness, a sustained head position to the left leads to his vision going “out of focus,” but he denied loss of vision, blurred vision, and diplopia. These sensations were reproducible in clinic, although without any associated pulse changes in left arm or presyncopal episodes. At this point, the differential diagnosis included BHS versus mild subclavian steal phenomenon. He was advised to avoid extreme rotation or excessive manipulation of his neck to the left side. Transcranial Doppler ultrasound (TCD) confirmed a reduction in blood flow in the left VA to the extent of 35% when patient turned head to the left, and also exhibited typical symptoms at the blood flow drop (Fig. 2). Thus, the diagnosis of BHS was confirmed via TCD results.
Figure 2. A: Transcranial Doppler ultrasound (TCD) of left vertebral artery with head in neutral position showing adequate flow (circle) through the left vertebral artery and mean velocity of 30 cm/s along with absence of symptoms. B: TCD of left vertebral artery with neck turned to left and in flexion showing decrease in flow within the left vertebral artery (circle) and mean velocity of 6 cm/s in comparison to top along with occurrence of symptoms of dizziness, presyncope, and visual changes.
Patient was re-evaluated with cervical spine MRI which revealed degenerative spondylosis, with the most significant disease between C4 and C7, and persistent occlusion of left VA. No neurosurgical intervention was warranted at that time.
SYSTEMIC LITERATURE REVIEW
Patients and Techniques: A systematic search was performed for publications in Medline from 1966–2013 with keywords “Bow Hunter’s syndrome, Bow Hunter’s stroke, rotational vertebral artery occlusion, rotational vertebral artery syndrome” in subject headings and keywords with no language restrictions. A total of 30 results were obtained for the keywords “Bow Hunter’s Syndrome,” 35 results were obtained with keywords “Bow Hunter’s Stroke,” 42 results were obtained with keywords “Rotational Vertebral Artery Syndrome,” and 11 results were obtained with keywords “Rotational Vertebral artery Occlusion.”. The authors then reviewed all the studies to check whether they contained information about BHS. Those studies where only abstracts were available and those that were in any language except English, such as Japanese, were excluded from the review. Overall 71 relevant studies were identified [1, 2, 8, 11–78].
Clinical Variables and Measurement of Outcome: The following items were included in the analysis: total number of patients, sex, mean age, etiology, symptoms, laterality, vertebral level of occlusion, imaging modality, treatment modality and outcome. Multiple imaging modalities have been used for the diagnosis of BHS which include TCD, CTA, MRA, dynamic digital subtraction angiography (dDSA), and dynamic angiography. We grouped dynamic cerebral angiography, dynamic vertebral angiography, dynamic aortic arch angiography, and dynamic angiography together under one heading, “Dynamic Angiography.” For outcome analysis, we broadly classified the patients into favorable and unfavorable outcomes depending on the recovery of symptoms to the baseline since their presentation in the initial visit. The favorable outcome group involved patients who showed complete recovery from their symptoms, whereas the unfavorable outcome group included patients who had zero, modest, or partial recovery of their symptoms over different time periods.
Statistical Analysis: All analyses were performed by the authors using Microsoft Excel and JMP software (version 10.0).
RESULTS
Baseline demographics: The total number of patients in this review was 153. The overall mean age of the patients was 53 years, after excluding the pediatric patients (<18 years) which accounted for 10 patients in the review. Some baseline demographics were not available for eight patients. The occurrence of BHS was seen in 100 males and 45 females and this difference was determined significant (p<0.0001).
Anatomical Information: The site of VA occlusion was noted to be at the level of the Atlanto-axial (C1–C2) joint in 99 cases out of total 142 cases, which was significantly (p < 0.0001) higher than any other level of VA occlusion. Osteophytes, or bone spurs, was the most common etiology for BHS. Involvement of the left VA in BHS was seen in 80 patients, while the right VA was involved in 56 cases, and bilateral VA involvement was seen in 11 cases. Overall, the left VA was involved in a significantly (p < 0.0001) higher number of BHS patients than the right VA or even the bilateral VA involvement.
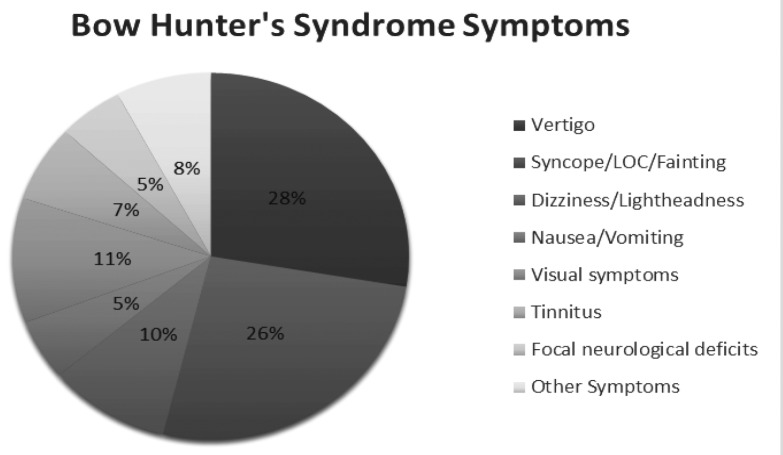
Clinical Differences: It was noted that BHS was more commonly (p < 0.05) seen on turning the head to right side (55 patients) as compared to the left (44 patients) or both sides (2 patients). Upon analyzing the symptoms at the time of presentation, it was observed that vertigo and syncope were present in 28% and 26% of the cases respectively and were the most prevalent symptoms noticed in patients with BHS (Fig. 3). Other prevalent symptoms documented included visual symptoms such as diplopia, blurred vision, dizziness or lightheadedness, tinnitus, nausea/vomiting and focal neurological deficits (ataxia, focal numbness, weakness, or paresthesia).
Figure 3. Distribution of the symptoms in patients with Bow Hunter’s Syndrome in the literature.
Imaging Characteristics: Out of all the imaging modalities, dynamic angiography was more commonly used to confirm the diagnosis of BHS, as 88 patients out of total 144 patients had dynamic angiography used for the confirmation of underlying etiology (p < 0.0001). Interpretation of the imaging led to a diagnosis of acute or subacute cerebrovascular accidents in 10 patients. A total of 21 patients had stenosis, 13 had hypoplasia, and three had dissection of the ipsilateral, contralateral, or both vertebral arteries.
Treatment Modalities: Huge diversity in treatment approaches were noted involving surgical, conservative or the combination of both. Treatment data were missing for seven patients. A surgical approach was considered in 105 cases out of total 146 cases, a conservative approach in 39 patients and a combination of surgical and conservative treatments in two patients (p < 0.0001). Thus, there was a significantly higher emphasis on surgery as the primary treatment approach in BHS patients.
An outcome analysis revealed that surgery was associated with favorable outcomes. In the cases where surgery was the primary treatment approach, 80 patients out of total 96 patients had favorable outcomes, whereas in cases of conservative approach, favorable outcomes were only noticed in 37% of the patients (p < 0.0001). Hence, surgery was associated with a significantly higher number of favorable outcomes in BHS patients.
DISCUSSION
In this large systemic review of BHS, we analyzed data of 153 patients. We found that men were significantly more prone to develop BHS and left VA was more commonly involved. The significantly higher involvement in the left VA might be attributed to fact that the left VA is dominant in 50% of the people, whereas right dominance is found in only 25% [79]. As VA [80] is the major blood supply of the posterior circulation and this circulation is responsible for the symptoms observed in BHS patients, there are more chances of getting symptoms on occlusion of dominant VA rather than the non-dominant VA.
The cause of VA occlusion in most of the cases was the anatomical compression by the cervical vertebrae provoked by head turning to either the ipsilateral or contralateral side. Osteophytes were the most common underlying abnormality responsible for this compression. Compression was significantly more common on right head rotations and at the level of atlanto-axial (C1–C2) joint. At the atlanto-axial joint, the VA is relatively immobilized at the transverse foramen [8], thus providing an easy target for occlusion. The atlanto-axial joint plays a vital role in neck rotation. Some cadaver studies have shown that during the neck rotation, the contralateral C1–C2 joint rotates asymmetrically forward and downward [81]. This motion may lead to the lengthening of the VA under the atlanto-axial ligament along the posterior C1 ring or between the C1 and C2 foramen [8]. This lengthening of the VA can cause its thinning or occlusion [82]. So, right head turn can cause the left joint movement and can cause left VA occlusion. Thus, both right head turn and left VA are more commonly involved in the BHS. BHS is a relatively rare disease, which indicates that there are other factors that might play a role in the development of BHS. These may include the presence of an isolated posterior circulation that has no collateral supply from any other arteries and the presence of hypoplasia or stenosis or dissection of the contralateral VA [8].
In the patient prior to the correction of the subclavian artery aneurysm, a CT angiogram of the neck showed that hypoplastic left VA was still patent and contributed minimally to left posterior inferior cerebellar artery (PICA). During the operation, the left VA was electively ligated. Shortly thereafter, the patient began experiencing dizziness upon turning his head to the left. We propose that compression of the right VA may have cause decreased perfusion of left VA territory. Flow to the left PICA was also supplemented by a retrograde flow from the right VA. During the surgical correction of the left subclavian artery, the left VA was electively ligated, and not reconnected. Postoperatively, the left PICA began receiving all of its blood supply via a retrograde flow from the opposite VA. As described in the previous paragraph, the right VA could be compressed when the patient turns his head to the left. If the right VA is compressed in this way, it would preferentially preserve its core territory. Therefore, retrograde perfusion of the left VA and left PICA would decrease, contributing to the BHS symptoms observed in this patient.
The patient complained specifically feeling dizzy when turning his head to the left, pre-syncope, and transient visual changes only after the surgical correction of his subclavian artery aneurysm. These symptoms were similar to the BHS symptoms that we observed in our literature cohort such as diplopia, blurred vision, dizziness or lightheadedness, tinnitus, nausea/vomiting and focal neurological deficits (ataxia, focal numbness, weakness or paresthesia). Vertigo and syncope were the most common symptoms in the literature. Dizziness was reported in 11% patients of our cohort; however, it was the main presenting symptom in our patient. The dizziness could be associated with decreased flow to the PICA, although the ischemic changes seen here are likely transient.
Other factors that could be contributing to his BHS symptoms are his vasculopathic issues, including uncontrolled diabetes and long-standing hypertension. The patient also has degenerative spondylosis, which is most significant between C4 and C7, and is the one of the most common inciting factors in BHS [5]. We believe that the patient’s issues regarding the BHS are multifactorial in nature, and stem from both the compression of the left-sided collaterals and the right VA compression, leading to a decrease in retrograde flow via the vertebrobasilar system. One potential life-threatening risk of this syndrome is posterior circulation infarction [17].
Imaging studies play a crucial role in the diagnosis of BHS. Some of the common imaging studies employed in BHS diagnosis include TCD, CTA, MRA, dynamic angiography, and dDSA. The term dynamic signifies that the study is conducted in different head positions including neutral position, head turning in either direction at different angles. This helps in the identification of the occlusion position along with the compression source in correlation to the symptoms. In our analysis, we found that dynamic angiography was most commonly used in order to confirm the diagnosis of BHS. It is also considered to be the gold standard for the diagnosis of BHS [17]. Netuka et al also advocated the use of dynamic angiography after surgery to confirm the degree of decompression [8]. TCD is another commonly used imaging study, it is a cheap, noninvasive ultrasound technique used to visualize the intracranial vessels. Intracranial vessel stenosis, vasospasm, spontaneous emboli detection, brain death confirmation, right to left shunt detection, collateral circulation detection, and facilitation of stroke thrombolysis are few of the indications for TCD. TCD can save unnecessary investigations, and radiation as proved in this case report [83].
Treatment options in BHS depend on its etiology: Intrinsic vascular disease, such as atherosclerosis or dissection; conversely, mechanical external compression of the VA during head rotation from muscular or tendinous insertions, osteophytes, or degenerative changes secondary to cervical spondylosis [20, 84]. Conservative management includes anticoagulation therapy, cervical collars, and avoidance of prolonged head rotation [17]. Surgical options include cervical decompression, cervical spine fusion, and endovascular stent placement within the unaffected VA to increase blood flow during head turning [20].
In regards to our patient, since BHS symptoms were mild, conservative treatment and continued stroke prophylaxis was pursued at this time with no surgical intervention. However, our literature search revealed that surgery was the most commonly used treatment approach in BHS patients. There was a significantly higher number of patients who underwent surgery as part of their treatment plan for BHS in comparison to the conservative treatment only or a combination of both.
On analyzing the prognosis of BHS patients after various treatments had been used, we found that surgery was associated with a significantly higher number of favorable outcomes as compared to the other treatment modalities. As described previously that anatomical compression by the bony structures is the most prevalent etiology in BHS, surgery can rapidly resolve the compression and thus the symptoms.
CONCLUSION
In the literature, this is the first case of iatrogenic Bow Hunter’s syndrome after surgical intervention for an aneurysm repair. Vascular surgeons should be aware of this potential complication that was observed in our patient. Dynamic angiography is the gold standard for diagnosis of BHS. Surgery should be used as the primary treatment approach in these patients, especially where bony compression is the main etiology.
Consent
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
List of Abbreviations
- BHS
Bow Hunter’s syndrome
- CT
computed tomography
- CTA
computerized tomography angiogram
- MRI
magnetic resonance imaging
- MRA
magnetic resonance angiogram
- PICA
posterior inferior cerebellar artery
- TCD
transcranial Doppler ultrasound
- VA
vertebral artery
- dDSA
dynamic digital subtraction angiography
Competing Interests
There are no competing interests or disclosures.
Authors’ Contributions
VR, AR, and OM were involved in drafting the manuscript and revising it critically for intellectual content. VSH helped conceive the study, participated in its design and coordination, and helped review the manuscript for intellectual content. BV, PS, SM, SK, AK, PR, and SB revised the paper critically for intellectual content.
Table 1. Summary of the case series and case reports used in the analysis.
| Demographics and characteristics | Value (n) | p value (if applicable) |
|---|---|---|
| Total number of patients | 153 | – |
| Mean age | 53 ± 13 (143) | – |
| Sex (M vs. F) | 100 vs. 45 | p<0.0001 |
| Etiology (most common) | Osteophytes | – |
| Level of occlusion (C1–C2) | 99 (142) | – |
| Laterality (L vs. R) | 80 vs. 56 (147) | p<0.0001 |
| Head rotation side (R vs. L) | 55 vs. 44 (101) | p<0.05 |
| Confirmatory imaging modality (most common) | Dynamic angiography | – |
| Patients affected by stroke | 10 | – |
| Treatment (surgery vs. conservative) | 68% vs. 27% | p<0.0001 |
| Favorable uutcome (surgery vs. conservative) | 83% vs. 37% | p<0.0001 |
REFERENCES
- Horowitz M, Jovin T, Balzar J, Welch W, Kassam A. Bow Hunter’s syndrome in the setting of contralateral vertebral artery stenosis: evaluation and treatment options. Spine (Phila Pa 1976) 2002;27:E495–E498. doi: 10.1097/00007632-200212010-00015. [DOI] [PubMed] [Google Scholar]
- Lee V, Riles TS, Stableford J, Berguer R. Two case presentations and surgical management of Bow Hunter’s syndrome associated with bony abnormalities of the C7 vertebra. J Vasc Surg. 2011;53:1381–1385. doi: 10.1016/j.jvs.2010.11.093. [DOI] [PubMed] [Google Scholar]
- Tissingtom Tatlow WF, Bammer HG. Syndrome of vertebral artery compression. Neurology. 1957;7:331–340. doi: 10.1212/wnl.7.5.331. [DOI] [PubMed] [Google Scholar]
- Sheehan Sheila BRB, Meyer John S. Vertebral artery compression in cervical spondylosis Arteriographic demonstration during life of vertebral artery insufficiency due to rotation and extension. Neurology. 1960;10:968–968. [Google Scholar]
- Powers SR, Drislane TM, Nevins S. Intermittent vertebral artery compression; a new syndrome. Surgery. 1961;49:257–264. [PubMed] [Google Scholar]
- Sorensen BF. Bow Hunter’s stroke. Neurosurgery. 1978;2:259–261. doi: 10.1227/00006123-197805000-00013. [DOI] [PubMed] [Google Scholar]
- George B, Carpentier A. In: Compression of and by the vertebral artery. Spetzler R, editor. Vol. 4. Operative Techniques in Neurosurgery, Saunders; Orlando: 2001. pp. 202–218. [Google Scholar]
- Netuka D, Benes V, Mikulík R, Kuba R. Symptomatic rotational occlusion of the vertebral artery—case report and review of the literature. Zentralbl Neurochir. 2005;66:217–222. doi: 10.1055/s-2005-836600. [DOI] [PubMed] [Google Scholar]
- Sullivan HG, Harbison JW, Vines FS, Becker D. Embolic posterior cerebral artery occlusion secondary to spondylitic vertebral artery compression. Case report. J Neurosurg. 1975;43:618–622. doi: 10.3171/jns.1975.43.5.0618. [DOI] [PubMed] [Google Scholar]
- Ryan GM, Cope S. Cervical vertigo. Lancet. 1955;269:1355–1358. doi: 10.1016/s0140-6736(55)93159-7. [DOI] [PubMed] [Google Scholar]
- Kuether TA, Nesbit GM, Clark WM, Barnwell SL. Rotational vertebral artery occlusion: a mechanism of vertebrobasilar insufficiency. Neurosurgery. 1997;41:427–432. doi: 10.1097/00006123-199708000-00019. discussion 432–423. [DOI] [PubMed] [Google Scholar]
- Ding D, Mehta GU, Medel R, Liu KC. Utility of intraoperative angiography during subaxial foramen transversarium decompression for Bow Hunter’s syndrome. Interv Neuroradiol. 2013;19:240–244. doi: 10.1177/159101991301900215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darkhabani MZ, Thompson MC, Lazzaro MA, Taqi MA, Zaidat OO. Vertebral artery stenting for the treatment of bow hunter’s syndrome: report of 4 cases. J Stroke Cerebrovasc Dis. 2012;21:e901–e905. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Dargon PT, Liang CW, Kohal A, Dogan A, Barnwell SL, Landry GJ. Bilateral mechanical rotational vertebral artery occlusion. J Vasc Surg. 2013;58:1076–1079. doi: 10.1016/j.jvs.2012.12.044. [DOI] [PubMed] [Google Scholar]
- Dadsetan MR, Skerhut HE. Rotational vertebrobasilar insufficiency secondary to vertebral artery occlusion from fibrous band of the longus coli muscle. Neuroradiology. 1990;32:514–515. doi: 10.1007/BF02426468. [DOI] [PubMed] [Google Scholar]
- Dabus G, Gerstle RJ, Parsons M, Cross DT, 3rd, Moran CJ, Thompson R, Derdeyn CP. Rotational vertebrobasilar insufficiency due to dynamic compression of the dominant vertebral artery by the thyroid cartilage and occlusion of the contralateral vertebral artery at C1-2 level. J Neuroimaging. 2008;18:184–187. doi: 10.1111/j.1552-6569.2007.00177.x. [DOI] [PubMed] [Google Scholar]
- Cornelius JF, George B, N’dri Oka D, Spiriev T, Steiger HJ, Hanggi D. Bow-Hunter’s syndrome caused by dynamic vertebral artery stenosis at the cranio-cervical junction—a management algorithm based on a systematic review and a clinical series. Neurosurg Rev. 2012;35:127–135. doi: 10.1007/s10143-011-0343-4. discussion 135. [DOI] [PubMed] [Google Scholar]
- Chough CK, Cheng BC, Welch WC, Park CK. Bow Hunter’s stroke caused by a severe facet hypertrophy of C1-2. J Korean Neurosurg Soc. 2010;47:134–136. doi: 10.3340/jkns.2010.47.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Shin HY, Kim JS, Kim SH, Kwon OK, Koo JW, Park SH, Yoon BW, Roh JK. Rotational vertebral artery syndrome: oculographic analysis of nystagmus. Neurology. 2005;65:1287–1290. doi: 10.1212/01.wnl.0000180405.00560.51. [DOI] [PubMed] [Google Scholar]
- Choi KD, Choi JH, Kim JS, Kim HJ, Kim MJ, Lee TH, Lee H, Moon IS, Oh HJ, Kim JI. Rotational vertebral artery occlusion: mechanisms and long-term outcome. Stroke. 2013;44:1817–1824. doi: 10.1161/STROKEAHA.113.001219. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Chung HM, Yang CH, Chen DL. Vertebrobasilar artery anomaly presenting with transient Bow Hunter’s syndrome. Tzu Chi Medical Journal. 2009;22:149–152. [Google Scholar]
- Anaizi AN, Sayah A, Berkowitz F, McGrail K. Bow Hunter’s syndrome: the use of dynamic magnetic resonance angiography and intraoperative fluorescent angiography. J Neurosurg Spine. 2014;20:71–74. doi: 10.3171/2013.9.SPINE121019. [DOI] [PubMed] [Google Scholar]
- Andereggen L, Arnold M, Andres RH, Raabe A, Reinert M, Gralla J. Bow Hunter’s stroke due to prominent degenerative spinal disorder. Clin Neuroradiol. 2012;22:355–358. doi: 10.1007/s00062-012-0154-1. [DOI] [PubMed] [Google Scholar]
- Inamasu J, Nakatsukasa M. Rotational vertebral artery occlusion associated with occipitoatlantal assimilation, atlantoaxial subluxation, and basilar impression. Clin Neurol Neurosurg. 2013;115:1520–1523. doi: 10.1016/j.clineuro.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Ikeda DS, Villelli N, Shaw A, Powers C. Bow Hunter’s syndrome unmasked after contralateral vertebral artery sacrifice for aneurysmal subarachnoid hemorrhage. J Clin Neurosci. 2014;21:1044–1046. doi: 10.1016/j.jocn.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Iguchi Y, Kimura K, Shibazaki K, Iwanaga T, Ueno Y, Inoue T. Transcranial doppler and carotid duplex ultrasonography findings in Bow Hunter’s syndrome. J Neuroimaging. 2006;16:278–280. doi: 10.1111/j.1552-6569.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- Helton TJ, Bavry AA. Images in cardiovascular medicine. Don’t turn your head! Circulation. 2009;120:e162. doi: 10.1161/CIRCULATIONAHA.109.896043. [DOI] [PubMed] [Google Scholar]
- Greiner HM, Abruzzo TA, Kabbouche M, Leach JL, Zuccarello M. Rotational vertebral artery occlusion in a child with multiple strokes: a case-based update. Childs Nerv Syst. 2010;26:1669–1674. doi: 10.1007/s00381-010-1299-3. [DOI] [PubMed] [Google Scholar]
- Go G, Hwang SH, Park IS, Park H. Rotational vertebral artery compression:Bow Hunter’s syndrome. J Korean Neurosurg Soc. 2013;54:243–245. doi: 10.3340/jkns.2013.54.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MW, Piepgras DG, Bartleson JD. Anterolateral decompression of the atlantoaxial vertebral artery for symptomatic positional occlusion of the vertebral artery. Case report. J Neurosurg. 1995;83:737–740. doi: 10.3171/jns.1995.83.4.0737. [DOI] [PubMed] [Google Scholar]
- Fleming JB, Vora TK, Harrigan MR. Rare case of bilateral vertebral artery stenosis caused by C4-5 spondylotic changes manifesting with bilateral Bow Hunter’s syndrome. World Neurosurg. 2013;79:799.E791–795. doi: 10.1016/j.wneu.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Kim HA, Yi HA, Lee CY, Lee H. Origin of isolated vertigo in rotational vertebral artery syndrome. Neurol Sci. 2011;32:1203–1207. doi: 10.1007/s10072-011-0667-4. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Fujita S, Hosoda K, Shibata Y, Iwakura M, Tamaki N. Rotational occlusion of the vertebral artery caused by transverse process hyperrotation and unilateral apophyseal joint subluxation. Case report. J Neurosurg. 1997;86:1031–1035. doi: 10.3171/jns.1997.86.6.1031. [DOI] [PubMed] [Google Scholar]
- Lu DC, Gupta N, Mummaneni PV. Minimally invasive decompression of a suboccipital osseous prominence causing rotational vertebral artery occlusion. Case report. J Neurosurg Pediatr. 2009;4:191–195. doi: 10.3171/2009.3.PEDS08270. [DOI] [PubMed] [Google Scholar]
- Lu DC, Zador Z, Mummaneni PV, Lawton MT. Rotational vertebral artery occlusion-series of 9 cases. Neurosurgery. 2010;67:1066–1072. doi: 10.1227/NEU.0b013e3181ee36db. discussion 1072. [DOI] [PubMed] [Google Scholar]
- Kan P, Yashar P, Langer DJ, Siddiqui AH, Levy EI. Posterior inferior cerebellar artery to posterior inferior cerebellar artery in situ bypass for the treatment of Bow Hunter’s-type dynamic ischemia in holovertebral dissection. World Neurosurg. 2012;78:553.e515–557. doi: 10.1016/j.wneu.2011.09.050. [DOI] [PubMed] [Google Scholar]
- Miele VJ, France JC, Rosen CL. Subaxial positional vertebral artery occlusion corrected by decompression and fusion. Spine (Phila Pa 1976) 2008;33:E366–E370. doi: 10.1097/BRS.0b013e31817192a1. [DOI] [PubMed] [Google Scholar]
- Matsuyama T, Morimoto T, Sakaki T. Comparison of C1-2 posterior fusion and decompression of the vertebral artery in the treatment of bow hunter’s stroke. J Neurosurg. 1997;86:619–623. doi: 10.3171/jns.1997.86.4.0619. [DOI] [PubMed] [Google Scholar]
- Matsuyama T, Morimoto T, Sakaki T. Bow Hunter’s stroke caused by a nondominant vertebral artery occlusion: case report. Neurosurgery. 1997;41:1393–1395. doi: 10.1097/00006123-199712000-00030. [DOI] [PubMed] [Google Scholar]
- Mapstone T, Spetzler RF. Vertebrobasilar insufficiency secondary to vertebral artery occlusion froma fibrous band. Case report. J Neurosurg. 1982;56:581–583. doi: 10.3171/jns.1982.56.4.0581. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Kaido T, Uchiyama Y, Tokunaga H, Sakaki T, Iwasaki S. Rotational obstruction of nondominant vertebral artery and ischemia. Case report. J Neurosurg. 1996;85:507–509. doi: 10.3171/jns.1996.85.3.0507. [DOI] [PubMed] [Google Scholar]
- Anene-Maidoh TI, Vega RA, Fautheree GL, Reavey-Cantwell JF. An unusual case of pediatric Bow Hunter’s stroke. Surg Neurol Int. 2013;4:148. doi: 10.4103/2152-7806.121647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh Y, Kwon OK, Kim HJ, Kim JS. Rotational vertebral artery syndrome due to compression of nondominant vertebral artery terminating in posterior inferior cerebellar artery. J Neurol. 2011;258:1775–1780. doi: 10.1007/s00415-011-6005-1. [DOI] [PubMed] [Google Scholar]
- Schelfaut S, Verhasselt S, Carpentier K, Moke L. Subaxial rotational vertebral artery syndrome: resection of the uncinate process and anterior fusion can be sufficient! J Spinal Disord Tech. 2013. [DOI] [PubMed]
- Saito K, Hirano M, Taoka T, Nakagawa H, Kitauchi T, Ikeda M, Tanizawa E, Kichikawa K, Ueno S. Juvenile Bow Hunter’s stroke without hemodynamic changes. Clin Med Insights Case Rep. 2010;3:1–4. [PMC free article] [PubMed] [Google Scholar]
- Safain MG, Talan J, Malek AM, Hwang SW. Spontaneous atraumatic vertebral artery occlusion due to physiological cervical extension: case report. J Neurosurg Spine. 2014;20:278–282. doi: 10.3171/2013.12.SPINE13653. [DOI] [PubMed] [Google Scholar]
- Park SH, Kim SJ, Seo JD, Kim DH, Choi JH, Choi KD, Kim JS. Upbeat nystagmus during head rotation in rotational vertebral artery occlusion. J Neurol. 2014. [DOI] [PubMed]
- Petridis AK, Barth H, Buhl R, Mehdorn HM. Vertebral artery decompression in a patient with rotational occlusion. Acta Neurochir (Wien) 2008;150:391–394. doi: 10.1007/s00701-008-1502-4. discussion 394. [DOI] [PubMed] [Google Scholar]
- Puca A, Scogna A, Rollo M. Craniovertebral junction malformation and rotational occlusion of the vertebral artery. Br J Neurosurg. 2000;14:361–364. doi: 10.1080/026886900417405. [DOI] [PubMed] [Google Scholar]
- Saito K, Hirano M, Taoka T, Nakagawa H, Kitauchi T, Tanizawa E, Yoshida K, Sakurai Y, Tamura K, Nakase H, Yoshioka A, Sakaki T, Kichikawa K, Ueno S. Artery-to-artery embolism with a mobile mural thrombus due to rotational vertebral artery occlusion. J Neuroimaging. 2010;20:284–286. doi: 10.1111/j.1552-6569.2008.00309.x. [DOI] [PubMed] [Google Scholar]
- Panchal RR, Hutton DS, Kim KE. Bilateral cerebellar infarcts from vertebral artery insufficiency caused by cervical osteophytes. J Spine. 2012;1 [Google Scholar]
- Sakai K, Tsutsui T. Bow Hunter’s stroke associated with atlantooccipital assimilation—case report. Neurol Med Chir (Tokyo) 1999;39:696–700. doi: 10.2176/nmc.39.696. [DOI] [PubMed] [Google Scholar]
- Owolabi MO, Ogah OS, Ogunniyi A. Episodic vertigo resulting from vascular risk factors, cervical spondylosis and head rotation: two case reports. Neuropsychiatr Dis Treat. 2007;3:675–678. [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y, Kimura K, Iguchi Y, Iwanaga T, Toi H, Matsubara S, Uno M. An embolic Bow Hunter’s stroke associated with anomaly of cervical spine. Neurology. 2011;77:1403–1404. doi: 10.1212/WNL.0b013e31823152f9. [DOI] [PubMed] [Google Scholar]
- Ogino M, Kawamoto T, Asakuno K, Maeda Y, Kim P. Proper management of the rotational vertebral artery occlusion secondary to spondylosis. Clin Neurol Neurosurg. 2001;103:250–253. doi: 10.1016/s0303-8467(01)00168-8. [DOI] [PubMed] [Google Scholar]
- Strupp M, Planck JH, Arbusow V, Steiger JH, Bruckmann H, Brandt T. Rotational vertebral artery occlusion syndrome with vertigo due to "labyrinthine excitation.". Neurology. 2000;54:1376–1379. doi: 10.1212/wnl.54.6.1376. [DOI] [PubMed] [Google Scholar]
- Shetty SR, Shankaraiah BA, Hegde T, Nagarajaiah RK. Bow Hunter’s stroke—a rare presentation of CV junction anomaly: case report. Neurol India. 2012;60:520–521. doi: 10.4103/0028-3886.103204. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Yamada M, Takagi H, Fujii K, Kan S. Bow Hunter’s stroke associated with an aberrant course of the vertebral artery—case report. Neurol Med Chir (Tokyo) 1999;39:867–869. doi: 10.2176/nmc.39.867. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Waga S, Kojima T, Niwa S. Decompression of the vertebral artery for Bow-Hunter’s stroke. Case report. J Neurosurg. 1988;69:127–131. doi: 10.3171/jns.1988.69.1.0127. [DOI] [PubMed] [Google Scholar]
- Sugiu K, Agari T, Tokunaga K, Nishida A, Date I. Endovascular treatment for bow hunter’s syndrome: case report. Minim Invasive Neurosurg. 2009;52:193–195. doi: 10.1055/s-0029-1239501. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Kaneko S, Asaoka K, Harada T. Rotational occlusion of the vertebral artery at the atlantoaxial joint: is it truly physiological? Neuroradiology. 1994;36:273–275. doi: 10.1007/BF00593258. [DOI] [PubMed] [Google Scholar]
- Taylor WB, Vandergriff CL, Opatowsky MJ, Layton KF. Bow Hunter’s syndrome diagnosed with provocative digital subtraction cerebral angiography. Proc (Bayl Univ Med Cent) 2012;25:26–27. doi: 10.1080/08998280.2012.11928776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Takahashi T, Shimizu H, Yoshimoto T. Rotational vertebral artery occlusion from occipital bone anomaly: a rare cause of embolic stroke. Case report. J Neurosurg. 2002;97:1456–1459. doi: 10.3171/jns.2002.97.6.1456. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Ito M, Yasumoto Y. Simultaneous bilateral vertebral artery occlusion in the lower cervical spine manifesting as Bow Hunter’s syndrome. Neurol Med Chir (Tokyo) 2008;48:90–94. doi: 10.2176/nmc.48.90. [DOI] [PubMed] [Google Scholar]
- Sell JJ, Rael JR, Orrison WW. Rotational vertebrobasilar insufficiency as a component of thoracic outlet syndrome resulting in transient blindness. Case report. J Neurosurg. 1994;81:617–619. doi: 10.3171/jns.1994.81.4.0617. [DOI] [PubMed] [Google Scholar]
- Seki T, Hida K, Akino M, Iwasaki Y. Anterior decompression of the atlantoaxial vertebral artery to treat bow hunter’s stroke: technical case report. Neurosurgery. 2001;49:1474–1476. doi: 10.1097/00006123-200112000-00037. [DOI] [PubMed] [Google Scholar]
- Zaidi HA, Albuquerque FC, Chowdhry SA, Zabramski JM, Ducruet AF, Spetzler RF. Diagnosis and management of Bow Hunter’s syndrome: 15-year experience at Barrow neurological institute. World Neurosurg. 2014. [DOI] [PubMed]
- Yoshimura K, Iwatsuki K, Ishihara M, Onishi Y, Umegaki M, Yoshimine T. Bow Hunter’s stroke due to instability at the uncovertebral C3/4 joint. Eur Spine J. 2011;20(Suppl 2):S266–S270. doi: 10.1007/s00586-010-1669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PJ, Latack JT, Gabrielsen T, Knake J, Gebarski S, Chandler W. Rotational vertebral artery occlusion at C1-C2. AJNR Am J Neuroradiol. 1985;6:96–100. [PMC free article] [PubMed] [Google Scholar]
- Wakayama K, Murakami M, Suzuki M, Ono S, Shimizu N. Ischemic symptoms induced by occlusion of the unilateral vertebral artery with head rotation together with contralateral vertebral artery dissection—case report. J Neurol Sci. 2005;236:87–90. doi: 10.1016/j.jns.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Whitmore RG, Simon SL, Hurst RW, Nisenbaum HL, Kasner SE, Zager EL. Bow Hunter’s syndrome caused by accessory cervical ossification: posterolateral decompression and the use of intraoperative Doppler ultrasonography. Surg Neurol. 2007;67:169–171. doi: 10.1016/j.surneu.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Vilela MD, Goodkin R, Lundin DA, Newell DW. Rotational vertebrobasilar ischemia: hemodynamic assessment and surgical treatment. Neurosurgery. 2005;56:36–43. doi: 10.1227/01.neu.0000146441.93026.ce. discussion 43–35. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Nagasawa H, Yamakawa T, Kato T. Bow Hunter’s syndrome after contralateral vertebral artery dissection. J Stroke Cerebrovasc Dis. 2012;21:916.e917–919. doi: 10.1016/j.jstrokecerebrovasdis.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Velat GJ, Reavey-Cantwell JF, Ulm AJ, Lewis SB. Intraoperative dynamic angiography to detect resolution of Bow Hunter’s syndrome: technical case report. Surg Neurol. 2006;66:420–423. doi: 10.1016/j.surneu.2006.03.040. discussion 423. [DOI] [PubMed] [Google Scholar]
- Choi KH, Lee SH, Kim JM, Oh DS, Kim JT, Park MS, Cho KH. Rotational vertebral artery syndrome in moyamoya disease: a sign of unilateral vertebral artery stenosis. Clin Neurol Neurosurg. 2013;115:1900–1902. doi: 10.1016/j.clineuro.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Kim K, Isu T, Morimoto D, Kominami S, Kobayashi S, Teramoto A. Anterior vertebral artery decompression with an ultrasonic bone curette to treat Bow Hunter’s syndrome. Acta Neurochir (Wien) 2008;150:301–303. doi: 10.1007/s00701-008-1491-8. discussion 303. [DOI] [PubMed] [Google Scholar]
- Kimura T, Sako K, Tohyama Y, Hodozuka A. Bow Hunter’s stroke caused by simultaneous occlusion of both vertebral arteries. Acta Neurochir (Wien) 1999;141:895–896. doi: 10.1007/s007010050394. [DOI] [PubMed] [Google Scholar]
- Koonce JD, Neal MT, Heck D, Wilson JA, Morris PP. Angiographic assessment of rotational vertebral artery syndrome (Bow Hunter’s syndrome): a case series. Neurographics. 2013;3:100–107. [Google Scholar]
- Cloud GC, Markus HS. Diagnosis and management of vertebral artery stenosis. QJM. 2003;96:27–54. doi: 10.1093/qjmed/hcg003. [DOI] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia AS, McNamara JO, Williams SM. Neuroscience. Sinauer Associates; 2001. The Blood Supply of the Brain and Spinal Cord. [Google Scholar]
- Toole JF, Tucker SH. Influence of head position upon cerebral circulation. Studies on blood flow in cadavers. Arch Neurol. 1960;2:616–623. doi: 10.1001/archneur.1960.03840120022003. [DOI] [PubMed] [Google Scholar]
- Barton JW, Margolis MT. Rotational obstructions of the vertebral artery at the atlantoaxial joint. Neuroradiology. 1975;9:117–120. doi: 10.1007/BF00332957. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Ghosh S, Ghosh SK, Collier A. Role of transcranial Doppler ultrasonography in stroke. Postgrad Med J. 2007;83:683–689. doi: 10.1136/pgmj.2007.058602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayman A, Nogueira RG, Gupta R. Diagnosis and management of vertebrobasilar insufficiency. Curr Treat Options Cardiovasc Med. 2013;15:240–251. doi: 10.1007/s11936-013-0228-7. [DOI] [PubMed] [Google Scholar]