Abstract
In June 2012, Food and Drug Administration (FDA) issued a warning about the risk of catheter entrapment associated with Onyx embolization. We used our experience, literature review, and FDA Manufacturer and User Facility Device Experience (MAUDE) data review to identify five strategies to address catheter entrapment: 1/. Surgical resection of vessel at point of entrapment of catheter and retraction from exterior portion at the femoral region; 2/. Advancing and closing the loop of snare over the entrapped catheter followed by retraction; 3/. Advancing the distal access catheter over the entrapped catheter and retraction with forward movement of the distal access catheters; 4/. Inflation of balloon catheter coaxial to the entrapped catheter with subsequent retraction; and 5/. Intravascular retention and internalization of microcatheter. In the MAUDE data, there were 77 reports of catheter entrapment with Onyx embolization; microcatheter was retracted by surgical excision in 15, endovascular snare or other retriever devices in 5, deliberately entrapped inside the vessel using stent in 1, and left without intervention within intravascular compartment in 27 patients.
Keywords: Catheter entrapment, liquid embolic system, Onyx, copolymer, microcatheter, intravascular
BACKGROUND
The onyx liquid embolic system (Onyx) was approved in the European Union in 1999. The Food and Drug Administration (FDA) provided premarket approval application (PMA) approval for the Onyx® Liquid Embolic System (Micro Therapeutics, Inc) for presurgical embolization of brain arteriovenous malformations (AVMs) in July 2005. Onyx is comprised of EVOH (ethylene vinyl alcohol) copolymer dissolved in DMSO (dimethyl sulfoxide), and suspended micronized tantalum powder to provide contrast for visualization under fluoroscopy. Subsequently, several studies have reported the use of Onyx® embolization for treatment of brain AVMs [1–4]. Onyx has nonadhesive properties, which allow longer intranidal progression of the embolic agent before solidification and presumably a low or no risk of catheter entrapment [5, 6]. No occurrence of catheter entrapment and one event of difficult catheter removal was reported in 54 patients randomized to the Onyx embolization in the multicenter trial comparing Onyx with N-butyl-cyanoacrylate (NBCA) for presurgical embolization of brain AVMs [7]. No occurrence of catheter entrapment was reported in 117 patients analyzed in a prospective European registry of Onyx embolization for brain AVMs [1].
With wide spread use, anectdotal reports of catheter entrapment have been published where the microcatheter is encased within a cast of Onyx and retrieval of catheter after completion of Onyx injection is not possible [8, 9]. Presumably, Onyx reflux around the microcatheter can form a highly viscous plug entrapping the microcatheter during its retrieval [5]. In June 2012, FDA issued a warning about the risk of catheter entrapment associated with the use of Onyx® [10]. We reviewed the literature and data collected by FDA as part of its surveillance methodology since such reports are usually underreported and single center studies can only identify and report upon a very small number of patients.
METHODS AND RESULTS
Case Report
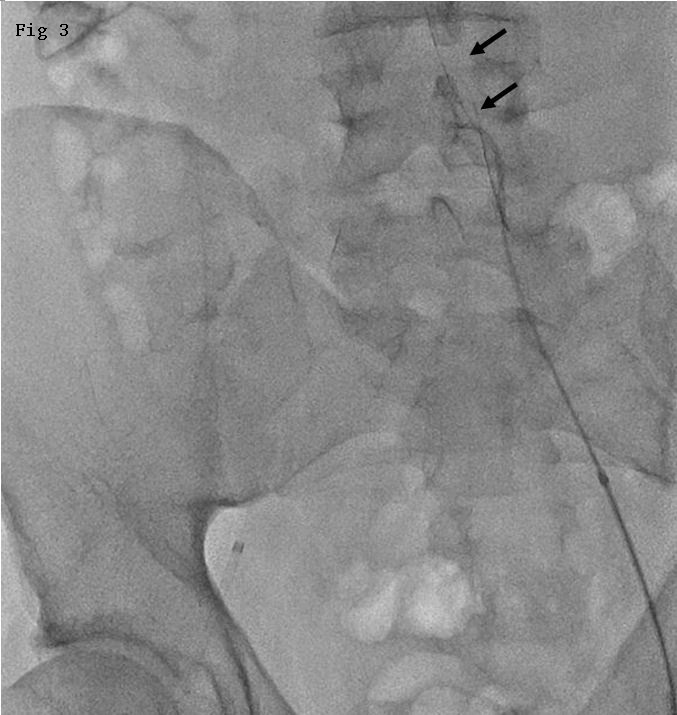
In our experience, a 37-year-old man presented with gradual worsening of headaches, nausea, vomiting, and left sided ataxia for 2 months. A hemangioblastoma was identified in right cerebellar hemisphere based on magnetic resonance imaging (MRI) appearance and diagnosis was confirmed with cerebral angiography. Preoperative endovascular embolization was performed with selective microcatheterization of arterial feeders from the right posterior inferior cerebellar artery (Fig. 1A). A 6F Envoy guidecatheter was placed in the right vertebral artery. Onyx-18 embolization of the arterial feeder was performed using Echelon (Covidien ev3 Neurovascular, CA) microcatheter placed through the guidecatheter. Onyx was intentionally allowed to reflux to build a cast of about 5-mm around the distal end of Echelon microcatheter. However, due to possible strictures in the vasculature and small size of the vessels within the tumor, no significant penetration of Onyx within the tumor bed was noted. The arterial feeder and its tributaries were completely obliterated. When Onyx reflux was noted to be more than 1-cm (1.2 cm) proximal to the microcatheter tip, embolization was discontinued and retrieval of catheter was attempted. Catheter entrapment was noticed and catheter was left in place for about 1 hour under slight tension to dislodge from Onyx cast. Attempts were continued for another hour to retrieve the catheter, with a maximum pull of about 4 cm from its relaxed position. Further tension on the vasculature was not considered safe and the catheter was left embedded in the Onyx cast. The external portion of Echlon microcatheter including hub was transected at the femoral region and guidecatheter was removed with the introducer sheath and external portion of microcatheter left in place in the right common femoral artery. The tumor was surgically excised and microcatheter was left in position within the arterial feeders (Fig. 2). After 1 day following surgical resection, the patient underwent introducer sheath placement in left common femoral artery. A SIMMONS II diagnostic catheter was introduced through the left femoral sheath and a cerebral angiogram was performed to document patency of arteries involved. No arterial occlusion, dissection, or vasospasm was noted. The SIMMONS II catheter was subsequently placed into the abdominal aorta. Rotational movement of the catheter allowed entanglement of the existing microcatheter and retraction of proximal internal and external segments into the left iliac artery (Fig. 3). The femoral sheath on right side was removed followed by manual compression to achieve hemostasis. Patient did not develop any new symptoms throughout the hospital stay. At four months follow-up, patient did not have new neurological deficits and reported residual pain in right femoral region.
Figure 1. Angiographic images of the hemangioblastoma and arterial feeders into the vascular tumor bed. A. Pre-embolization lateral view and feeding arteries from posterior inferior cerebellar artery (arrow indicating the feeder of interest). B. The entrapped microcatheter during retraction in lateral view (arrow indicates straightening of posterior inferior cerebellar artery loop and lack of arterial flow). C. The entrapped microcatheter in relaxed position in lateral view (arrow indicates reconstitution of posterior inferior cerebellar artery loop and resteroration of arterial flow).
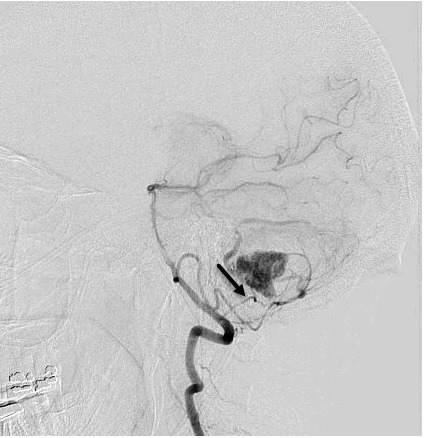
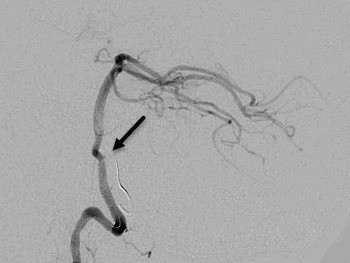
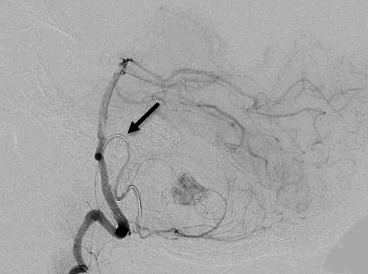
Figure 2. Postsurgical resection T2-weighted MRI transverse section. The void anterior to the cerebellum is the intravascular component of the entrapped microcatheter.
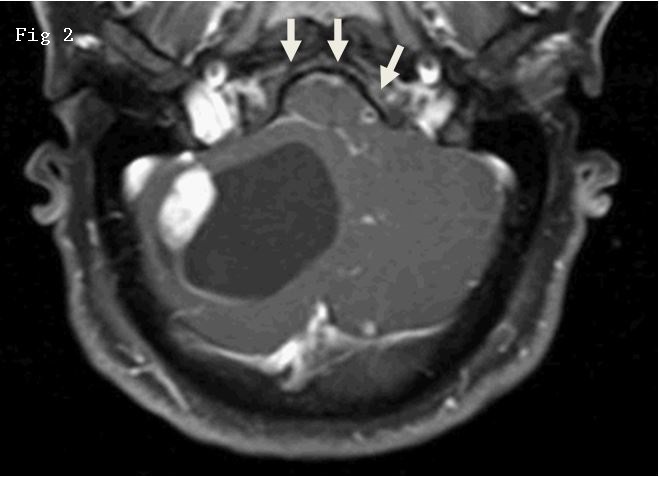
Figure 3. Rotational movement of the catheter allowed entanglement of the existing microcatheter and retraction into the left iliac artery.
LITERATURE REVIEW
We performed a search on PubMed from January 2000 to April 2014 with the keywords “retrieval,” “microcatheter,” and “Onyx.” A total of 11 articles were retrieved of which five were pertinent. We performed another search using the key words “catheter entrapment” and “Onyx.” Two articles were identified of which one was pertinent. Additional articles were identified through bibliography of the select articles. There were four strategies that were identified: 1. Surgical resection of vessel at point of catheter entrapment and retraction from exterior portion at the femoral region; 2. Advancing the loop of snare over the entrapped catheter until the distal most portion is reached and then retract the ensnared entrapped catheter; 3. Advancing the distal access catheter over the entrapped catheter and retraction of the microcatheter with slight forward movement of the distal access catheters; and 4. Advancing a balloon catheter coaxial to the entrapped catheter with inflation of the balloon and retraction of the microcatheter. The second and third approach may require transection of the exterior portion and hub of entrapped microcatheter.
Walcott et al.[8] reported upon entrapment of the microcatheter (Marathon flow directed microcatheter; eV3 Neurovascular, Inc., Irvine, CA, USA) in the Onyx plug. After AVM resection, a single remaining feeding vessel originating from the distal anterior cerebral artery suspected to contain the entrapped microcatheter was identified using stereotactic guidance (BrainLab, Munich, Germany). The vessel was then sectioned, and the microcatheter (102 cm) was then extracted cranially using gentle traction. Pandey et al.[11] reported upon a patient in whom UltraFlow microcatheter entrapment was associated with intraprocedural rupture of brain AVM. During emergent surgical excision, the microcatheter was identified as a hard substance within the soft vessel by palpation of the feeder with a forceps. After coagulation of the feeder close to the nidus, the nidus was removed. The groin was then exposed and gentle traction was applied from the groin and the microcatheter was retrieved. Kelly et al.[12] reported upon an Echelon-10 microcatheter (eV3 Neurovascular, Inc.) entrapment in the right occipital artery because of reflux and vessel tortuosity. The hub of the indwelling Echelon microcatheter was cut off and a 2-mm Amplatz Goose Neck microsnare (Microvena Corp., White Bear Lake, MN) and rapid transit microcatheter were then advanced over the indwelling Echelon microcatheter. The snare and rapid transit microcatheter were advanced distally into the occipital artery and the snare was retracted to engage and remove the microcatheter. Alamri et al.[13] reported the use of Amplatz 4-mm Gooseneck Microsnare(TM) (ev3, Plymouth, MN) in Excelsior 1018(TM) microcatheter (Boston Scientific, Natick, MA) in 14 brain AVM feeders. The Marathon microcatheter was cut just distal to the hub, and the Amplatz/Excelsior combination was introduced along the length of the Marathon microcatheter to ensnare and retract the embedded catheter. Microcatheter tip removal was successful in all cases, except in one procedure where microcatheter tip becoming detached and needing no further intervention. Newman et al.[14] reported upon two cases of catheter entrapment; in both instances, the hubs of the microcatheters were transected and Concentric Medical Outreach distal access catheters were then advanced over the microcatheters and positioned at the proximal aspects of the microcatheter-Onyx plugs. Using the distal access catheter for countertraction, the Echelon-10 microcatheters were then successfully released from the Onyx plugs under fluoroscopic visualization without significant distortion of the arteries. Santillan et al.[15] reported a case of Marathon microcatheter (eV3) entrapment within M3 branch of the middle cerebral artery during Onyx-18 embolization. A balloon microcatheter (Hyperform(TM), eV3) was advanced distally and inflated to provide distal counter tension, allowing microcatheter retrieval without prominent distortion of the arteries [16].
Review of Manufacturer and User Facility Device Experience Data
We analyzed data collected as part of MAUDE [17], which consists of medical device reports (MDRs) of suspected device-associated deaths, serious injuries, and malfunctions. The FDA uses MDRs to monitor device performance, detect potential device-related safety issues, and assess benefit-risk assessments of these products. The data consists of voluntary reports since June 1993, user facility reports since 1991, distributor reports since 1993, and manufacturer reports since August 1996. The MAUDE database [17] is based on MDRs submitted to the FDA by mandatory reporters (manufacturers, importers, and device user facilities) and voluntary reporters such as healthcare professionals, patients, and consumers. Manufacturers and importers must submit reports when they become aware of information that reasonably suggests that one of their marketed devices may have caused or contributed to a death or serious injury or has malfunctioned. Device user facilities, including hospitals, outpatient diagnostic or treatment facilities, nursing homes and ambulatory surgical facilities, must submit reports when they become aware of information that reasonably suggests that a device may have caused or contributed to a death or serious injury of a patient in their facility. Death reports must be sent to the FDA and the manufacturer, if known. We searched the MAUDE database reports on Onyx embolic agent that may have malfunctioned or caused a death or serious injury until March 2013. All reports were reviewed by one of the two reviewers’ who abstracted relevant data regarding devices and patients involved in the event. Additional data were abstracted from textual information provided on MEDWATCH forms.
There were 435 reports of ONYX embolization procedures and complications associated with suspected device-associated deaths, serious injuries, and malfunctions. The indication of treatment was brain AVM in 317, aneurysm in 38, and unspecified in 85 reports. Catheter entrapment was associated with intracerebral or subarachnoid hemorrhage in 7, subdural hematoma in 2, ischemic stroke in 3, and minor neurologic deficits in 6 patients. The microcatheter was retracted by surgical excision in 15, retrieved with assistance using endovascular ensnare or other retriever device in 5, deliberately entrapped inside the vessel using stent in 1 , and left without intervention within intravascular compartment in 27 patients. Among the 27 patients in whom no intervention was performed, 2 intracerebral hemorrhages, 2 subdural hemorrhages, 2 deaths, 1 ischemic stroke, and 2 minor neurologic deficits were reported. The overall outcome of patients was classified in the reports was death in 6, disability in 40, and/or requiring further intervention or hospitalization in 25 patients.
DISCUSSION
We report characteristics of catheter entrapment during Onyx embolization, and strategies used by physicians to address the issue, and associated clinical adverse events. We used our experience, literature review, and FDA MAUDE data review to acquire the information. The literature review provided technical details regarding the strategies used for retraction of entrapped catheter. In most occurrences reported in the MAUDE data, the catheter was left in vivo without any additional intervention similar to our experience. Surgical excision of microcatheter was performed in 15 of 74 catheter entrapment occurrences. Catheter entrapment was associated with intracerebral or subarachnoid hemorrhage (n = 7), subdural hematoma (n = 2), ischemic stroke (n = 3), and minor neurologic deficits (n = 6) patients. Therefore, catheter entrapment appears to be a clinically significant event. It should be noted that MDR data alone cannot be used to establish rate of events, evaluate a change in event rates over time or compare event rates between devices. The passive surveillance system has limitations, including the potential submission of incomplete, inaccurate, untimely, unverified, or biased data.
Although infrequent, institutions, neurosurgeons, and neurointerventionalists must be aware of the risk of catheter entrapment with onyx embolization of brain AVMs and strategies to retract the entrapped catheter from intravascular compartment. Intravascular retention and internalization of microcatheter’s external portion may be an acceptable option in many patients.
Acknowledgments
None
Footnotes
Funding: none
Disclosure: None
Conflict of Interest: None
The results were partially submitted at the "International Stroke Conference", Nashvillie, TN, February 2015
REFERENCES
- Pierot L, Cognard C, Herbreteau D, Fransen WJ, Van Rooij E, Boccardi A, Beltramello N, Sourour K, Kupcs A, Biondi A, Bonafe W, Reith A, Casasco Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: results of a prospective, multicentre study (BRAVO) Eur Radiol. 2013;23(10):2838–2845. doi: 10.1007/s00330-013-2870-6. [DOI] [PubMed] [Google Scholar]
- Sobh K, Hegazy A. Feasibility and outcomes of endovascular embolization of cerebral arteriovenous malformations at a low-volume centre. J Vasc Interv Neurol. 2013;5(2):4–8. [PMC free article] [PubMed] [Google Scholar]
- Strauss I, Frolov V, Buchbut D, Gonen L, Malmon S. Critical appraisal of endovascular treatment of brain arteriovenous malformation using onyx in a series of 92 consecutive patients. Acta Neurochir (Wien) 2013;155(4):611–617. doi: 10.1007/s00701-013-1633-0. [DOI] [PubMed] [Google Scholar]
- van Rooij WJ, Jacobs S, Sluzewski M, van der Pol B, Beute GN. Curative embolization of brain arteriovenous malformations with onyx: patient selection, embolization technique, and results. AJNR Am J Neuroradiol. 2012;33(7):1299–1304. doi: 10.3174/ajnr.A2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abud DG, Abud TG, Nakiri GS. Management of brain AVM procedural hemorrhagic complication by the “security” catheter technique. J Neuroradiol. 2013;40(1):45–49. doi: 10.1016/j.neurad.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Ayad M, Eskioglu E, Mericle RA. Onyx: a unique neuroembolic agent. Expert Rev Med Devices. 2006;3(6):705–715. doi: 10.1586/17434440.3.6.705. [DOI] [PubMed] [Google Scholar]
- Loh Y, Duckwiler GR, Onyx trial I. A prospective, multicenter, randomized trial of the onyx liquid embolic system and N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations. Clinical article. J Neurosurg. 2010;113(4):733–741. doi: 10.3171/2010.3.JNS09370. [DOI] [PubMed] [Google Scholar]
- Walcott BP, Gerrard JL, Nogueira RG, Nahed BV, Terry AR, Ogilvy CS. Microsurgical retrieval of an endovascular microcatheter trapped during onyx embolization of a cerebral arteriovenous malformation. J Neurointerv Surg. 2011;3(1):77–79. doi: 10.1136/jnis.2010.002733. [DOI] [PubMed] [Google Scholar]
- Huk W, Becker H. [Complication after embolization of an AVM with onyx] Klin Neuroradiol. 2009;19(2):145–152. doi: 10.1007/s00062-009-1001-x. [DOI] [PubMed] [Google Scholar]
- FDA safety communication: catheter entrapment with the ev3 onyx liquid embolic system. 2013. In: Administration UFaD, ed: FDA.
- Pandey P, Shetty R, Sabharwal P, Aravinda HR. Retrieval of a microcatheter from arteriovenous malformations after hemorrhage following Onyx embolization. Neurol India. 2013;61(5):523–525. doi: 10.4103/0028-3886.121934. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Turner Rt, Gonugunta V, Rasmussen PA, Woo HH, Fiorella D. Monorail snare technique for the retrieval of an adherent microcatheter from an onyx cast: technical case report. Neurosurgery. 2008;63(1 Suppl 1):ONSE89. doi: 10.1227/01.neu.0000335018.68369.e8. discussion ONSE89. [DOI] [PubMed] [Google Scholar]
- Alamri A, Hyodo A, Suzuki K, Tanaka Y, Uchida T, Takano I, Kowata K, Iwatate K, Suzuki R. Retrieving microcatheters from Onyx casts in a series of brain arteriovenous malformations: a technical report. Neuroradiology. 2012;54(11):1237–1240. doi: 10.1007/s00234-011-0971-y. [DOI] [PubMed] [Google Scholar]
- Newman CB, Park MS, Kerber CW, Levy ML, Barr JD, Pakbaz RS. Over-the-catheter retrieval of a retained microcatheter following Onyx embolization: a technical report. J Neurointerv Surg. 2012;4(4):e13. doi: 10.1136/neurintsurg-2011-010040. [DOI] [PubMed] [Google Scholar]
- Santillan A, Zink W, Knopman J, Riina H, Gobin YP. Balloon-assisted technique for trapped microcatheter retrieval following onyx embolization. A case report. Interv Neuroradiol. 2009;15(4):453–455. doi: 10.1177/159101990901500414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arat A, Cil BE, Vargel I, Turkbey B, Canyigit M, Peynircioglu B, Arat YA. Embolization of high-flow craniofacial vascular malformations with onyx. AJNR Am J Neuroradiol. 2007;28(7):1409–1414. doi: 10.3174/ajnr.A0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUDE - Manufacturer and User Facility Device Experience


