Abstract
A 57-year-old woman with National Institutes of Health Stroke Scale (NIHSS) score of 26 was found to have an acute left carotid occlusion with tandem left M1 thrombus within 1.5 hours of symptom onset. After no neurologic improvement following standard-dose intravenous (IV) recombinant tissue plasminogen activator (rtPA), emergent neuroendovascular revascularization with carotid stenting and intracranial thrombectomy were performed under conscious sedation. Thrombolysis in myocardial infarction (TIMI)-3 flow restoration and symptom resolution were achieved postprocedure; however, complete carotid stent thrombosis was noted on final angiographic runs (25 minutes later), correlating with neurologic decline. Rapid administration of an intraarterial (IA) bolus dose of eptifibatide resulted in TIMI-3 flow restoration, with neurologic improvement. The patient was discharged three days postrevascularization on dual antiplatelet therapy with an NIHSS score of 1. Intraarterial (IA) eptifibatide can be an effective option for acute stent occlusion during emergent neuroendovascular revascularization after IV rtPA administration.
ABBREVIATIONS
- CLEAR
Combined approach to lysis utilizing eptifibatide and RtPA
- CT
computed tomographic
- Fr
French
- GP
glycoprotein
- IA
intraarterial
- ICA
internal carotid artery
- IV
intravenous
- MCA
middle cerebral artery
- NIHSS
National Institutes of Health Stroke Scale
- rtPA
recombinant tissue plasminogen activator
- TIMI
thrombolysis in myocardial infarction
Keywords: carotid stent thrombosis, intraarterial eptifibatide, intravenous recombinant tissue, plasminogen activator
INTRODUCTION
Carotid stent thrombosis is an uncommon source of profound morbidity and mortality that has been described in the setting of elective carotid stenting for atherosclerotic disease [1–5]. We report a case of hyperacute carotid stent occlusion immediately following emergent revascularization that was treated successfully with intraarterial (IA) eptifibatide in a patient who had received intravenous (IV) recombinant tissue plasminogen activator (rtPA) in the setting of acute ischemic stroke.
CASE PRESENTATION
A 57-year-old woman with no major past medical history or precipitating trauma was brought to the emergency department after being found unresponsive at home by family members. The patient was last seen normal 1.5 hours prior to arrival. On examination, the patient had a dense right-sided hemiparesis, global aphasia, left gaze preference, severe right-sided neglect, and sensory loss (National Institutes of Health Stroke Scale [NIHSS] score of 26).
INVESTIGATIONS
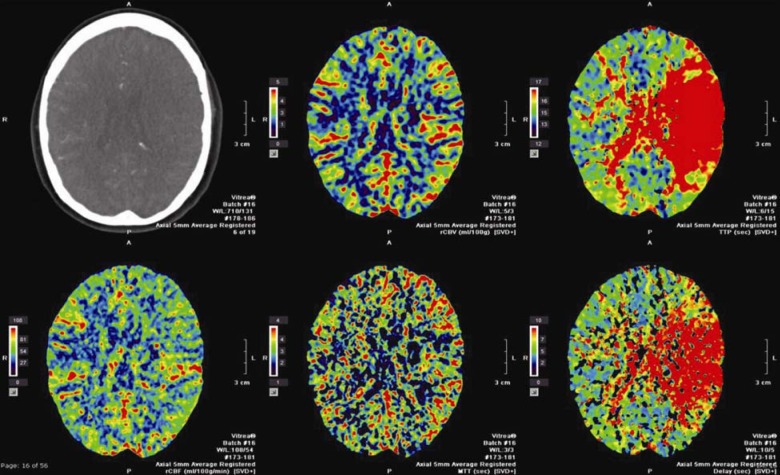
A computed tomographic (CT) stroke study, consisting of CT angiography of the head and neck, as well as CT perfusion imaging of the head was consistent with a large left-sided middle cerebral artery (MCA) territory penumbra and no significant completed infarct core, correlating with acute tandem occlusions of the left extracranial internal carotid artery (ICA) and the left M1 segment (Figure 1).
Figure 1. Computed tomographic (CT) perfusion images of the head showing increased time-to-peak with preserved cerebral blood volume and flow suggesting penumbra in the left middle cerebral artery (MCA) territory.
TREATMENT AND OUTCOME
Given these findings and symptom onset within the 3-hour window for thrombolytic therapy, standard-dose IV recombinant tissue plasminogen activator (rtPA) (0.9 mg/kg) was administered. In accordance with our institution’s protocol, 45 minutes after rtPA without neurological improvement and no hemorrhage observed on noncontrast head CT imaging, the patient was taken to the angiography suite for emergent IA revascularization.
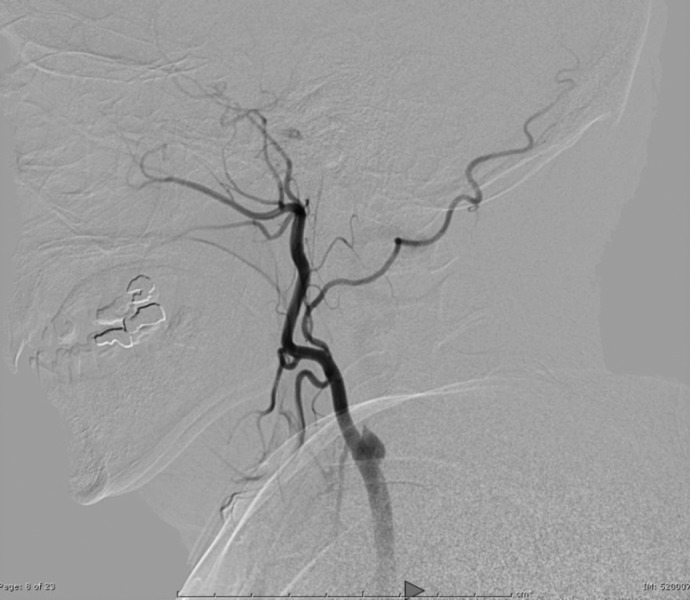
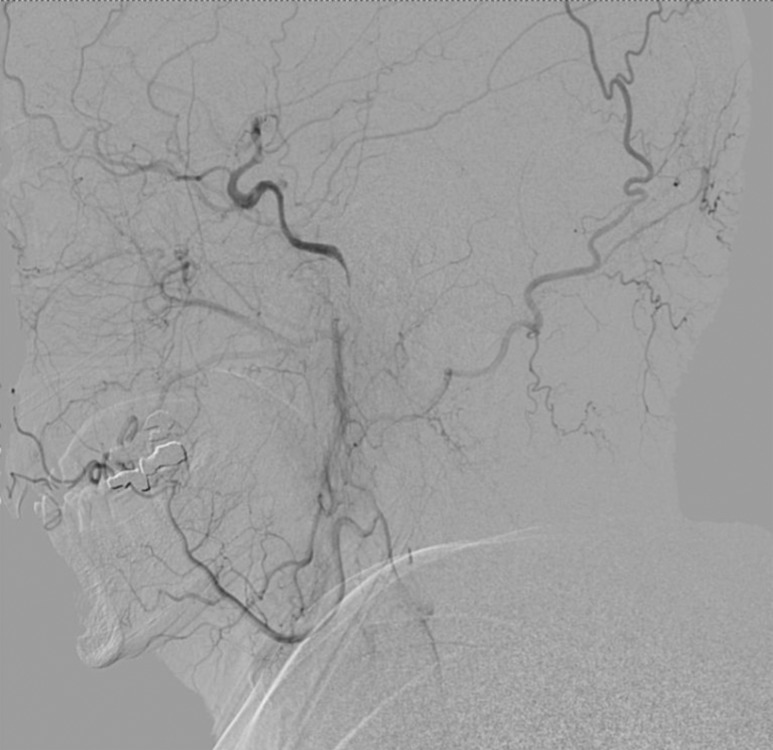
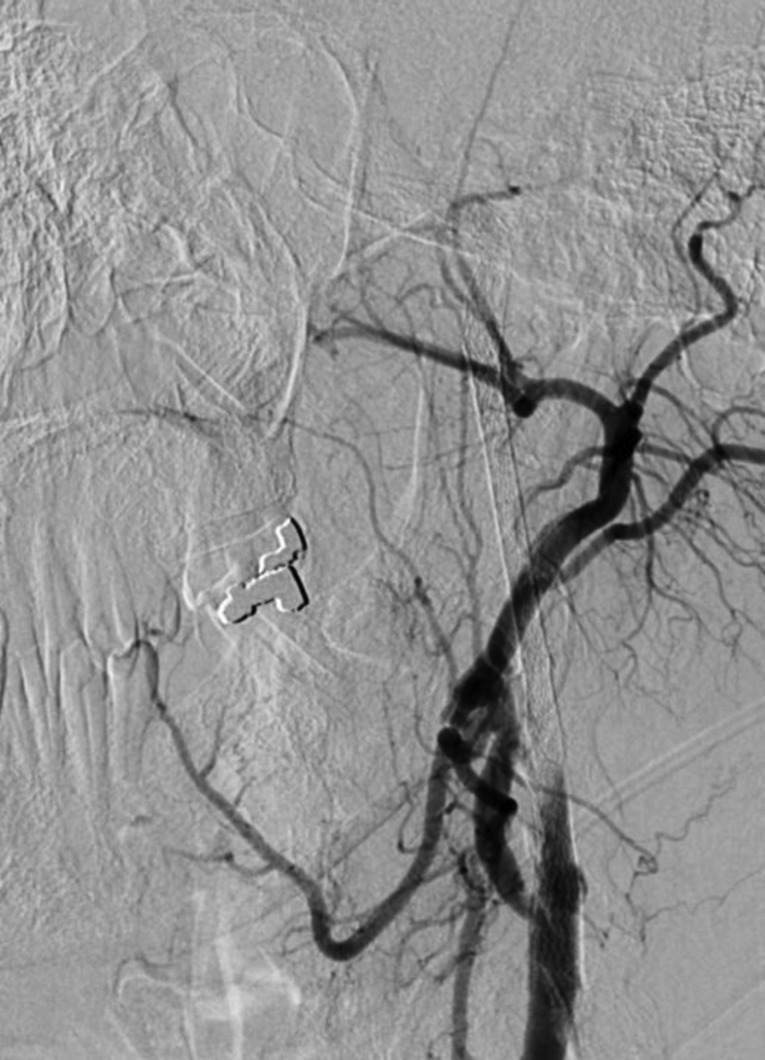
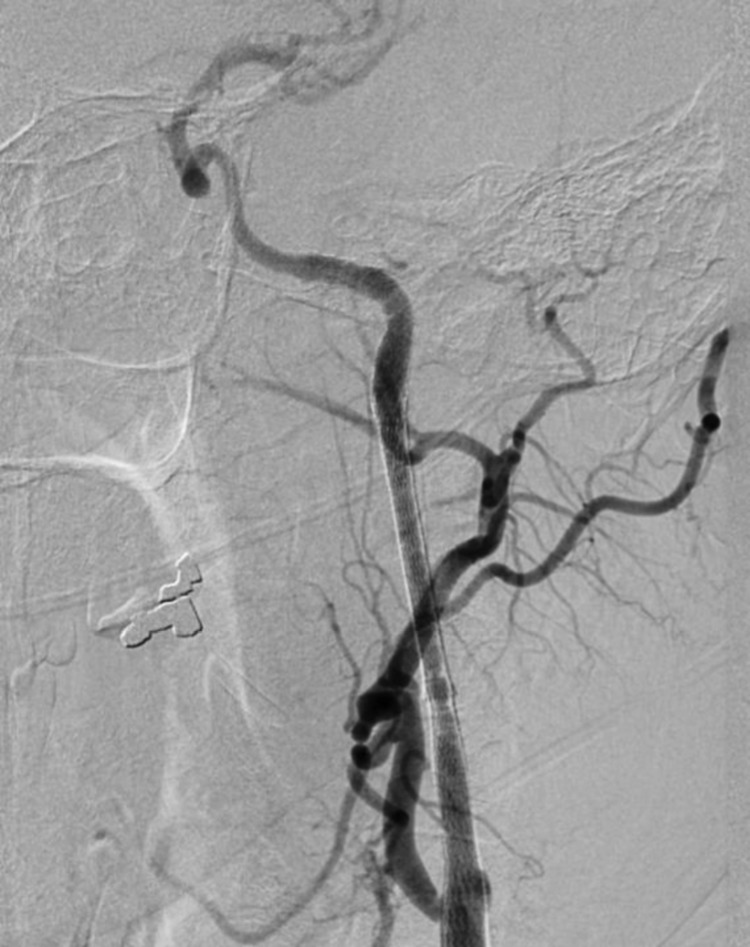
Right femoral artery access was obtained via a 6-French (Fr) sheath. A bilateral common carotid and left vertebral diagnostic cerebral angiogram was performed to confirm tandem occlusion of the left ICA and M1 segment, with poor collateral circulation through the anterior and posterior communicating vessels isolating the left MCA territory (Figure 2). After heparinization to achieve an activated coagulation time between 250 and 300 seconds, an Amplatz Super Stiff Exchange Wire (Cook, Bloomington, IN, USA) was placed into the external carotid artery allowing a 9-Fr sheath exchange, followed by positioning of a Mo.Ma Ultra proximal protection device (Invatec, Roncadelle, Italy). After unsuccessful attempts at crossing the ICA thrombus with Guide Wire M (Terumo, Tokyo, Japan) and Balanced Middle Weight (Abbott, Santa Clara, CA, USA) microwires, a V18 control wire (Boston Scientific, Natick, MA, USA) was successful in navigating a Nautica microcatheter (ev3/Covidien, Irvine, CA, USA) through the thrombus up to the cavernous carotid artery. Microangiographic runs confirmed patency of the ICA beyond the petrous segment (Figure 3). Given the high level of concern for dissection as the precipitating pathology, stenting was performed in a distal-to-proximal fashion utilizing a 6 mm × 40 mm Xpert Biliary Stent (Abbott), a 6–8 mm × 40 mm Xact stent (Abbott), and a 7–9 mm × 40 mm Xact stent (Abbott), resulting in thrombolysis in myocardial infarction (TIMI) 3 flow (Figure 4).
Figure 2. Lateral common carotid artery injection showing left ICA occlusion.
Figure 3. Lateral common carotid artery injection showing reconstitution of the ICA beyond the petrous segment via ophthalmic collaterals.
Figure 4. Lateral common carotid artery injection revealing restoration of flow in the ICA after stenting.
Attention was then turned to the M1 thrombus, which migrated to a superior M2 trunk of an MCA trifurcation after ICA flow was restored. Successful recanalization was achieved using a 5MAX reperfusion catheter (Penumbra, Alameda, CA, USA) in conjunction with the Solitaire FR thrombectomy device (ev3/Covidien) delivered via a velocity delivery microcatheter (Penumbra). Because we were performing the procedure under conscious sedation, we were able to confirm immediate and complete resolution of the patient’s aphasia and right hemiparesis on the angiography table. This improvement was correlated with TIMI 3 flow through the stented segments and the left MCA territory.
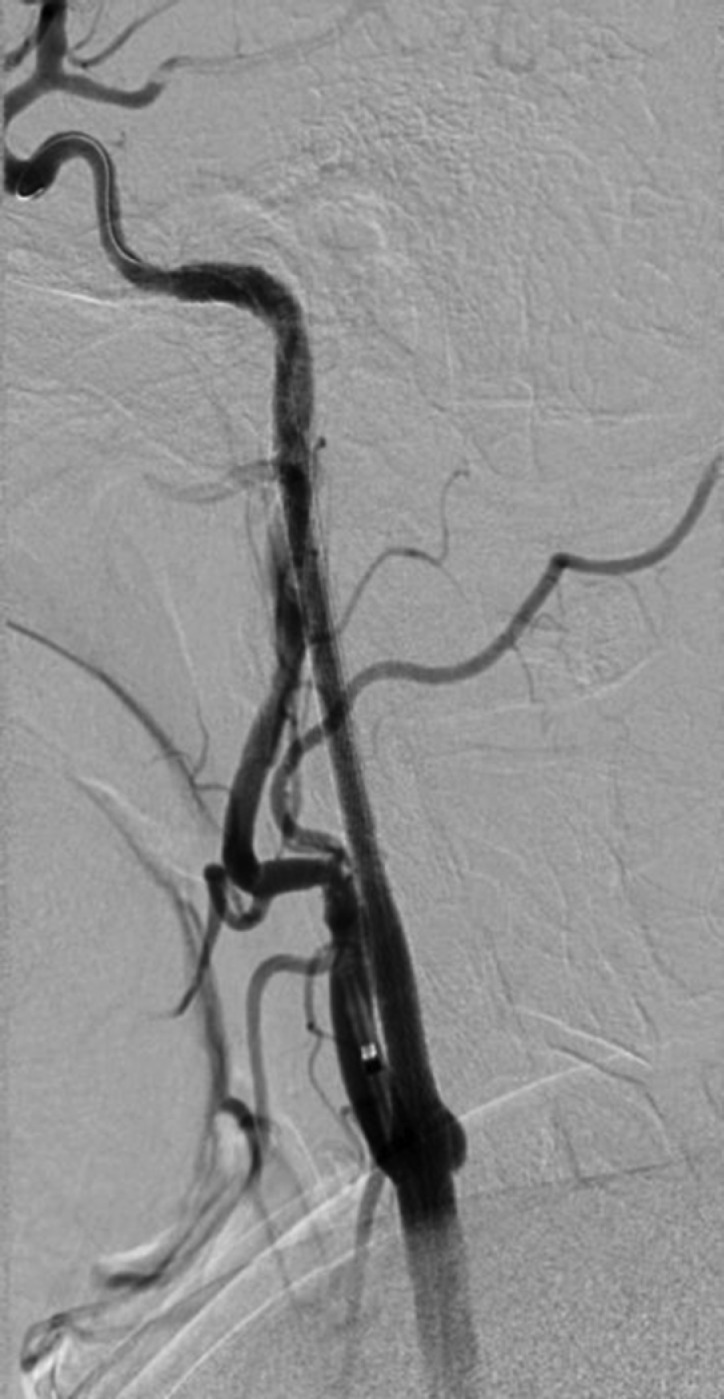
However, after removing the intracranial delivery and reperfusion catheters, but before removal of the cervical 9Fr Mo.Ma guide, cervical angiographic runs were obtained that showed initially mild and then progressively increasing delay in intracranial ICA flow. This rapidly progressed to complete ICA occlusion with fresh thrombus in the stented segments (Figure 5). This finding correlated with acute recurrent deterioration in the neurologic examination. The time lapse from the original cervical carotid revascularization to reocclusion was 25 minutes. After recognition of failure to preoperatively load antiplatelet medications, an intraarterial (IA) bolus dose of eptifibatide (180 μg/kg) was rapidly administered intraarterially through the guide catheter in the left common carotid artery. The patient’s neurologic examination immediately and steadily started to improve. Repeat cervical angiographic runs revealed complete restoration of flow 13 minutes after eptifibatide administration (Figure 6). There was no evidence of any new intracranial embolization. Given the complete revascularization and clinical improvement noted upon examination, an eptifibatide infusion was not administered. The patient received an oral bolus dose of aspirin (650 mg) and clopidogrel (600 mg) in the angiosuite; she was subsequently transferred to the neurointensive care unit in excellent condition, with the resolution of right hemiparesis and aphasia. Postprocedural CT scan revealed no intracranial hemorrhage. The patient was discharged 3 days after the intervention with mild dysarthria (NIHSS 1) on a dual antiplatelet regimen of daily aspirin (325 mg) and clopidogrel (75 mg). The patient had no neurologic deficits at the 30-day follow-up examination (NIHSS 0), with excellent flow on Duplex studies through the cervical left ICA.
Figure 5. Lateral common carotid artery injection revealing complete occlusion of the stented ICA 25 minutes after revascularization.
Figure 6. Lateral common carotid artery injection revealing restoration of flow 13 minutes after intraarterial administration of an eptifibatide bolus.
DISCUSSION
Carotid stent thrombosis is an uncommon source of profound morbidity and mortality. Most literature to date describes acute thrombosis beyond 24 hours in the setting of elective carotid stenting for atherosclerotic disease [1–5]. We found one case report describing hyperacute thrombosis 15 minutes after elective carotid stenting for chronic disease [6]. The case presented here is unique in that it occurred in a hyperacute fashion during emergency revascularization after the patient had received standard-dose IV rtPA for stroke and was systemically anticoagulated with heparin confirmed by an activated coagulation time of greater than 250 seconds.
The combined use of glycoprotein (GP) IIb/IIIa inhibitors and thrombolytic agents is not novel. Each targets different phases of thrombus formation, making concurrent use relevant to revascularization. Thrombolytic therapy targets the later stages of the coagulation cascade in disrupting the fibrin and thrombin network required for the formation of a hemostatic plug. In doing so, transient thrombin and platelet activation can cause a paradoxical hypercoagulable phase conducive for vessel reocclusion [7–10]. GP IIb/IIIa inhibitors block expressed GP IIb/IIIa receptors on activated platelets, thereby preventing important cross-bridging required for platelet aggregation [10–11]. This mechanism of inhibition is important in preventing platelet-rich thrombi that contribute to early vessel reocclusion, particularly after implantation of intravascular metal devices such as stents [12–13]. The lack of antiplatelet loading prior to placing the long stent construct described previously contributed to the hyperacute reocclusion and illustrates the importance of antiplatelet therapy despite recent rtPA administration when contemplating stent implantation-assisted revascularization.
The combined effects of GP IIb/IIIa inhibitor and thrombolytic therapy are recognized in the cardiac literature for the management of acute coronary syndrome; [14–17] however, there is less consensus in the neurological literature for acute ischemic stroke. The combined approach to lysis utilizing eptifibatide and RtPA (CLEAR) stroke trial showed that combination IV low-dose rtPA and IV eptifibatide had a safe intracranial hemorrhage profile when used for ischemic stroke within 3 hours of presentation but lacked revascularization efficacy compared to standard-dose alteplase [18]. Qureshi et al.[9] used IA reteplase and IV abciximab in 20 patients with acute ischemic stroke during a phase I safety investigation with a 20% mortality rate and 33% favorable modified Rankin Scale scores at 30 days (one symptomatic intracranial hemorrhage). Memon et al. [12] reported a 77% TIMI 2 or 3 recanalization rate and a 14% intracranial hemorrhage rate in a case series of 35 patients who received IA eptifibatide as a bail-out strategy for reocclusion during endovascular intervention in the setting of acute ischemic stroke, of which 14 patients received concurrent IV or IA tPA. Lastly, Eckert et al. [19] reported a 40-patient case series in which neurological and survival outcomes improved in patients with acute vertebrobasilar occlusion treated with combined IV abciximab, IA rtPA, and angioplasty with or without stenting but at an increased risk of intracranial hemorrhage.
In summary, the optimum combination of thrombolytic and GP IIb/IIIa inhibitor therapy continues to be a source of study for neurological revascularization. An eptifibatide bolus can represent an effective means for the treatment of acute vessel reocclusion after stent-assisted revascularization despite recent rtPA administration; however, this combination should be used with caution in conjunction with combined antiplatelet and anticoagulant therapy.
FINANCIAL RELATIONSHIPS/POTENTIAL CONFLICTS OF INTEREST
Dr. Dumont, Dr. Eller, Dr. Natarajan, and Dr. Sorkin report no financial relationships. Dr. Levy receives research grant support, other research support (devices), and honoraria from Boston Scientific and research support from Codman and Shurtleff, Inc., and ev3/Covidien Vascular Therapies; has ownership interests in Intratech Medical Ltd. and Mynx/Access Closure; serves as a consultant on the board of Scientific Advisors to Codman and Shurtleff, Inc.; serves as a consultant per project and/or per hour for Codman and Shurtleff, Inc., ev3/Covidien Vascular Therapies, and TheraSyn Sensors, Inc.; and receives fees for carotid stent training from Abbott Vascular and ev3/Covidien Vascular Therapies. Dr. Levy receives no consulting salary arrangements. All consulting is per project and/or per hour. Dr. Mokin has received an educational grant from Toshiba Medical Systems. Dr. Siddiqui has received research grants from the National Institutes of Health (co-investigator: NINDS 1R01NS064592-01A1, Hemodynamic induction of pathologic remodeling leading to intracranial aneurysms; not related to present manuscript) and the University at Buffalo (Research Development Award); holds financial interests in Hotspur, Intratech Medical, StimSox, and Valor Medical; serves as a consultant to Codman & Shurtleff, Inc., Concentric Medical, ev3/Covidien Vascular Therapies, GuidePoint Global Consulting, and Penumbra; belongs to the speakers’ bureaus of Codman and Shurtleff, Inc. and Genentech; serves on an advisory board for Codman and Shurtleff; and has received honoraria from Abbott Vascular, American Association of Neurological Surgeons’ courses, an emergency medicine conference, Genentech, Neocure Group LLC, Peripheral Angioplasty and All That Jazz Conference, and from Abbott Vascular and Codman and Shurtleff, Inc. for training other neurointerventionists in carotid stenting and for training physicians in endovascular stenting for aneurysms. Dr. Siddiqui receives no consulting salary arrangements. All consulting is per project and/or per hour.
ETHICS
Treatment was performed at Gates Vascular Institute in Buffalo, New York, USA. The University at Buffalo institutional review board does not require approval for a case report or technical note.
FINANCIAL AND MATERIAL SUPPORT
None in conjunction with this submission/study.
CONTRIBUTORS
Sorkin provided his contribution to conception and design of the study; acquisition of data was provided by all authors; Sorkin, Dumont, Mokin, Siddiqui, and Natarajan were involved in the analysis and interpretation of data; Sorkin drafted this manuscript; this manuscript was revised and approved by all authors;
ACKNOWLEDGMENTS
The authors thank Paul H. Dressel BFA for assistance with the preparation of the images and Debra J Zimmer for editorial assistance.
REFERENCES
- Iancu A, Grosz C, Lazar A. Acute carotid stent thrombosis: review of the literature and long-term follow-up. Cardiovasc Revasc Med. 2010;11:110–113. doi: 10.1016/j.carrev.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Markatis F, Petrosyan A, Abdulamit T, Bergeron P. Acute carotid stent thrombosis: a case of surgical revascularization and review of treatment options. Vascular. 2012;20:217–220. doi: 10.1258/vasc.2011.cr0303. [DOI] [PubMed] [Google Scholar]
- Setacci C, de Donato G, Setacci F, Chisci E, Cappelli A, Pieraccini M, Castriota F, Cremonesi A. Surgical management of acute carotid thrombosis after carotid stenting: a report of three cases. J Vasc Surg. 2005;42:993–996. doi: 10.1016/j.jvs.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Steiner-Boker S, Cejna M, Nasel C, Minar E, Kopp CW. Successful revascularization of acute carotid stent thrombosis by facilitated thrombolysis. AJNR Am J Neuroradiol. 2004;25:1411–1413. [PMC free article] [PubMed] [Google Scholar]
- Xiromeritis K, Dalainas I, Stamatakos M, Katsikas V, Martinakis V, Stamatelopoulos K, Psarros V. Acute carotid stent thrombosis after carotid artery stenting. Eur Rev Med Pharmacol Sci. 2012;16:355–362. [PubMed] [Google Scholar]
- Bush RL, Bhama JK, Lin PH, Lumsden AB. Transient ischemic attack due to early carotid stent thrombosis: successful rescue with rheolytic thrombectomy and systemic abciximab. J Endovasc Ther. 2003;10:870–874. doi: 10.1177/152660280301000504. [DOI] [PubMed] [Google Scholar]
- Kawano K, Aoki I, Aoki N, Homori M, Maki A, Hioki Y, Hasumura Y, Terano Y, Arai T, Mizuno H, Ishikawa K. Human platelet activation by thrombolytic agents: effects of tissue-type plasminogen activator and urokinase on platelet surface P-selectin expression. Am Heart J. 1998;135:268–271. doi: 10.1016/s0002-8703(98)70092-4. [DOI] [PubMed] [Google Scholar]
- Nordt TK, Moser M, Kohler B, Ruef J, Peter K, Kubler W, Bode C. Augmented platelet aggregation as predictor of reocclusion after thrombolysis in acute myocardial infarction. Thromb Haemost. 1998;80:881–886. [PubMed] [Google Scholar]
- Qureshi AI, Harris-Lane P, Kirmani JF, Janjua N, Divani AA, Mohammad YM, Suarez JI, Montgomery MO. Intra-arterial reteplase and intravenous abciximab in patients with acute ischemic stroke: an open-label, dose-ranging, phase I study. Neurosurgery. 2006;59:789–797. doi: 10.1227/01.NEU.0000232862.06246.3D. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Luft AR, Sharma M, Guterman LR, Hopkins LN. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: Part I—pathophysiological and pharmacological features. Neurosurgery. 2000;46:1344–1359. doi: 10.1097/00006123-200006000-00012. [DOI] [PubMed] [Google Scholar]
- Manoharan G, Adgey AA. Considerations in combination therapy: fibrinolytics plus glycoprotein IIb/IIIa receptor inhibitors in acute myocardial infarction. Clin Cardiol. 2004;27:381–386. doi: 10.1002/clc.4960270703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon MZ, Natarajan SK, Sharma J, Mathews MS, Snyder KV, Siddiqui AH, Hopkins LN, Levy FI. Safety and feasibility of intraarterial eptifibatide as a revascularization tool in acute ischemic stroke. J Neurosurg. 2011;114:1008–1013. doi: 10.3171/2010.8.JNS10318. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Ali Z, Ringer AJ, Boulos AS, Nakada MT, Alberico RA, Martin LB, Guterman LR, Hopkins LN. Intraarterial reteplase and intravenous abciximab for treatment of acute ischemic stroke. A preliminary feasibility and safety study in a non-human primate model. Neuroradiology. 2005;47:845–854. doi: 10.1007/s00234-003-1097-7. [DOI] [PubMed] [Google Scholar]
- ESPRIT investigators (enhanced suppression of the platelet IIb/IIIa receptor with integrilin therapy): novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. Lancet. 2000;356:2037–2044. doi: 10.1016/S0140-6736(00)03400-0. [DOI] [PubMed] [Google Scholar]
- Antman EM, Giugliano RP, Gibson CM, McCabe CH, Coussement P, Kleiman NS, Vahanian A, Adgey AA, Menown I, Ruppercht HJ, Van der Wieken R, Ducas J, Scherer J, Anderson K, Ven de Werf F, Braunwald E. Abciximab facilitates the rate and extent of thrombolysis: results of the thrombolysis in myocardial infarction (TIMI) 14 trial. The TIMI 14 Investigators. Circulation. 1999;99:2720–2732. doi: 10.1161/01.cir.99.21.2720. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaffer JW, Smith EE, 3rd, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Smith SC., Jr ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction--summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002;40:1366–1374. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- Goa KL, Noble S. Eptifibatide: a review of its use in patients with acute coronary syndromes and/or undergoing percutaneous coronary intervention. Drugs. 1999;57:439–462. doi: 10.2165/00003495-199957030-00015. [DOI] [PubMed] [Google Scholar]
- Pancioli AM, Broderick J, Brott T, Tomsick T, Khoury J, Bean J, del Zoppo G, Kleindorfer D, Woo D, Khatri P, Castaldo J, Frey J, Gebel J, Jr, Kasner S, Kidwell C, Kwiatkowski T, Libman R, Mackenzie R, Scott P, Starkman S, Thurman RJ. The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial. Stroke. 2008;39:3268–3276. doi: 10.1161/STROKEAHA.108.517656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert B, Koch C, Thomalla G, Kucinski T, Grzyska U, Roether J, Alfke NA, Jansen O, Zeumer H. Aggressive therapy with intravenous abciximab and intra-arterial rtPA and additional PTA/stenting improves clinical outcome in acute vertebrobasilar occlusion: combined local fibrinolysis and intravenous abciximab in acute vertebrobasilar stroke treatment (FAST): results of a multicenter study. Stroke. 2005;36:1160–1165. doi: 10.1161/01.STR.0000165918.80812.1e. [DOI] [PubMed] [Google Scholar]