Abstract
Xanthones are a class of heterocyclic compounds characterized by a dibenzo-γ-pyrone nucleus. Analysis of their mode of action in cells, namely uncovering alterations in gene expression, is important because these compounds have potential therapeutic applications. Thus, we studied the transcriptional response of the filamentous fungus Neurospora crassa to a group of synthetic (thio)xanthone derivatives with antitumor activity using high throughput RNA sequencing. The induction of ABC transporters in N. crassa, particularly atrb and cdr4, is a common consequence of the treatment with xanthones. In addition, we found a group of genes repressed by all of the tested (thio)xanthone derivatives, that are evocative of genes downregulated during oxidative stress. The transcriptional response of N. crassa treated with an acetophenone isolated from the soil fungus Neosartorya siamensis shares some features with the (thio)xanthone-elicited gene expression profiles. Two of the (thio)xanthone derivatives and the N. siamensis-derived acetophenone inhibited the growth of N. crassa. Our work also provides framework datasets that may orientate future studies on the mechanisms of action of some groups of xanthones.
Keywords: Neurospora crassa, Transcriptional profiling, Xanthones, Acetophenone, ABC transporters, CZT-1
Introduction
Xanthones are a class of heterocyclic compounds characterized by a dibenzo-γ-pyrone nucleus [1], [2], [3], [4], [5], [5], [6]. They are composed by a unique chemical scaffold, the tricyclic aromatic system (C6–C3–C6) named xanthene-9-one [2], [5], [5], [7]. They are commonly found as secondary metabolites in higher plants, fungi and lichens [8], and can be obtained by synthetic methods [9]. This class of compounds is composed by a rigid heteroaromatic tricyclic platform which may be considered as a ‘privileged structure’ since it can provide different ligands through modification of functional groups, allowing them to interact with a large variety of biological targets [10]. The biological activity of xanthones and their derivatives is based on their tricyclic scaffold but depends on the nature and position of the different substituents [2]. Since xanthones may play a role as anti-proliferative and pro-apoptotic agents [2], [11], [12], [13], [14], several derivatives, including (thio)xanthone derivatives, have been synthesized and tested in human cancer cell lines [14], [15], but not in fungi. It is thus important to add data from different organisms to the current knowledge on the mechanism of action of xanthones.
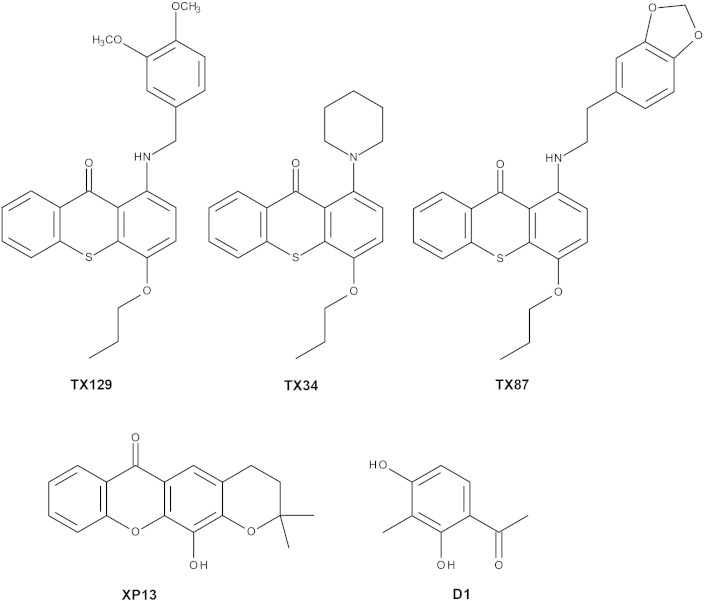
XP13 (3,4-dihydro-12-hydroxy-2,2-dimethyl-2H,6H-pyrano [3,2-b]-xanthen-6-one) (Fig. 1) is a prenylated analogue of 3,4-dihydroxyxanth-9-one [11], [13]. XP13 was shown to possess very potent anticancer activity in leukemia [11] and breast cancer [13] cells. XP13 was also shown to reduce cell proliferation, arrest the cell cycle at the S phase and induce apoptosis [11]. In addition, XP13 was shown to inhibit the interaction of p53 with MDM2 and mimicked the activity of known p53 activators, leading to the activation of p53-dependent transcriptional activity [16].
Fig. 1.
Chemical structures of the compounds used in this study. TX129: 1-[(3,4-dimethoxybenzyl)amino]-4-propoxy-9H-thioxanthen-9-one; TX34: 1-(piperidin-1-yl)-4-propoxy-9H-thioxanthen-9-one; TX87: 1-{[2-(1,3-benzodioxol-5-yl)ethyl]amino}-4-propoxy-9H-thioxanthen-9-one; XP13: 3,4-dihydro-12-hydroyi-2,2-dimethyl-2H,6Hpyrano [3,2-b]-xanthen-6-one, D1: 2,4-dihydroxy-3-methylacetophenone.
In this work, we have also tested the effects of three thioxanthone derivatives on N. crassa—TX129 (1-[(3,4-dimethoxybenzyl)amino]-4-propoxy-9H-thioxanthen-9-one), TX34 (1-(piperidin-1-yl)-4-propoxy-9H-thioxanthen-9-one) and TX87 (1-{[2-(1,3-benzodioxol-5-yl)ethyl]amino}-4-propoxy-9H-thioxanthen-9-one) (Fig. 1). These compounds are aminated thioxanthones and were obtained by synthesis after being designed to inhibit cancer cell proliferation and to block the human P-glycoprotein [15], [17]. These compounds were obtained by merging the structural skeleton of thioxanthones with moieties important for P-glycoprotein modulation [17]. TX34 has been shown to behave as a non-competitive inhibitor of the P-glycoprotein and to block the growth of cancer cells in vitro [15], [17].
Here we used the filamentous fungus N. crassa as a model to study the transcriptional response to a group of synthetic (thio)xanthone derivatives using high-throughput RNA sequencing (RNA-seq) to assess alterations in gene expression. Our data suggest that, in N. crassa, some ABC transporter-encoding genes and the transcription factor czt-1 are induced by different (thio)xanthone derivatives and that a group of genes – the majority of which encode currently uncharacterized proteins – are repressed by all of the tested compounds. XP13 (3,4-dihydro-12-hydroxy-2,2-dimethyl-2H,6H-pyrano [3,2-b]-xanthen-6-one) led to the strongest transcriptional response and growth impairment of N. crassa. The present study is the first to describe the effects of (thio)xanthones in fungi and will be important to understand their mechanism of action.
Material and methods
Strains, culture media and compounds
Standard procedures for the handling of wild type N. crassa cells (FGSC 2489) were employed [18]. Cells were grown in Vogel's MM supplemented with 1.5% (w/v) sucrose [19]. Agar at 1.5% (w/v) concentration was added to obtain solid medium. Conidia were harvested by the addition of sterile dH2O, agitation and filtration through cheesecloth in 7-day cultures on Vogel's MM with agar at 26 °C.
The xanthone XP13 and the thioxanthones TX34, TX87, and TX129 were synthesized according to previously described procedures [11], [17]. The purity of each compound was determined by HPLC–DAD analysis using an isocratic elution of MeOH:H2O basified with TEA (1%) or acidified with CH3COOH (1%) at a constant flow rate of 1.0 ml/min. All tested compounds possessed a purity of at least 95%. (Thio)xanthone derivatives and 2,4-dihydroxy-3-methylacetophenone (D1) were dissolved in DMSO (Sigma-Aldrich).
RNA sequencing (RNA-seq)
Conidial suspensions at 1 × 106 cells/ml were incubated in minimal medium for 6 h (26 °C, 140 rpm, constant light), followed by the addition of the indicated compounds (or the DMSO control solvent) for 1 more hour. Cells were harvested using 0.45 μm filters and immediately frozen in liquid nitrogen. Total RNA was isolated by the Trizol–Phenol–Chloroform method, three times for control samples and once for the treatments with (thio)xanthones and the acetophenone. After digestion of 25 μg RNA with TURBO DNAse (Life Technologies), mRNA was purified using Dynabeads oligo(dT) magnetic beads (Life Technologies). The mRNA was chemically fragmented using the Ambion RNA fragmentation kit (Life Technologies). First and second strand cDNA synthesis was achieved using appropriate kits (Life Technologies). The Illumina TruSeq kit was employed to generate the cDNA libraries with indexing adapters essentially following the manufacturer's protocol. After purification of the libraries with AMPure XP beads (Roche), the quality of the libraries was analyzed in an Agilent 2100 Bioanalyzer. The cDNA libraries were sequenced in an Illumina HiSeq2000 and single reads of 50 bp were obtained. Sequencing data was handled essentially with Tophat, Cufflinks and Cuffdiff [20]. Expression levels are presented as Fragments/Reads Per Kilobase of transcript per Million mapped reads (FPKM/RPKM). The resulting dataset is available at the NCBI GEO database (http://www.ncbi.nlm.nih.gov/geo/; series record: GSE53040). Functional enrichment of sets of genes was assessed with FunCat [21] and Venn diagrams were obtained using Venny [22].
Evaluation of growth in microplates
For growth assessment in liquid Vogel's MM, 1 × 104 conidia/ml were incubated at 26 °C, 100 rpm, under constant light in 96-well plates (200 μl/well) and absorbance at 620 nm [23] was followed (Thermo Electron Corporation Multiskan EX) during 24 h. At least three independent experiments were performed in each case. The Mann–Whitney statistical test was used to make comparisons between conditions.
Results and discussion
Transcriptional profiling and functional enrichment analyses of the N. crassa response to (thio)xanthone derivatives
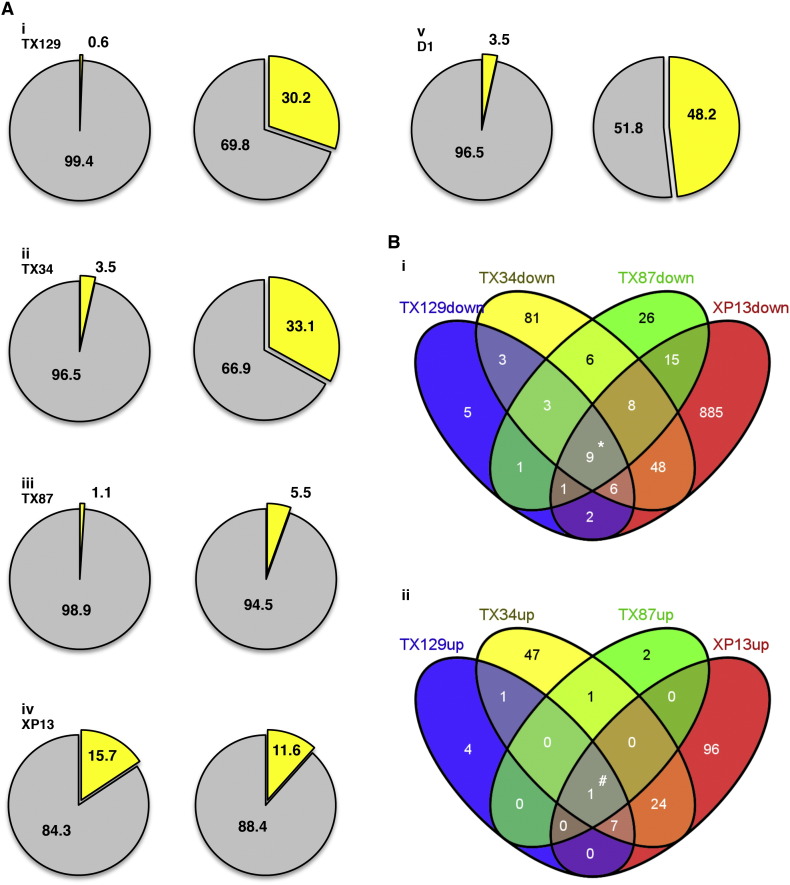
N. crassa cells were grown for 6 h in liquid Vogel's medium, treated for 1 h with 20 μM of each of the (thio)xanthone derivatives TX129, TX34, TX87 and XP13 (Fig. 1), and processed for RNA-seq. Treatment with the different compounds led to an alteration of the expression levels of a significantly different fraction of genes (Supplemental File 1). XP13 was the xanthone derivative eliciting the most active transcriptional response, altering the expression of 15.7% of the genes (Fig. 2A, i–iv, left panels). TX34, TX87 and TX129 modified the expression of 3.5, 1.1 and 0.6% of the genes, respectively. As a comparison, a 1-hour treatment with 20 μM staurosporine, a protein kinase inhibitor that induces cell death in N. crassa, causes the alteration of approximately 20% of the genes [24]. Interestingly, all the (thio)xanthone derivatives caused a large downregulation of gene expression, particularly TX87 and XP13, with 94.5% and 88.4% of repressed genes among those with altered expression (Fig. 1A, i–iv, right panels). These data indicate that treatment with (thio)xanthone derivatives mainly leads to the decrease in expression levels of N. crassa genes, which is considerably different from the fungal response to staurosporine, which causes a similar percentage of gene induction and repression [24].
Fig. 2.
Overview of the N. crassa transcriptional response to (thio)xanthone derivatives and a secondary metabolite from N. siamensis. (A) The percentage of genes with altered expression upon treatment with each of the indicated compounds (left panel; yellow: altered genes; grey: unaltered genes) and the fraction of induced and repressed genes (right panel; yellow: induced genes; grey: repressed genes) was calculated for cells treated with TX129 (i), TX34 (ii), TX87 (iii), XP13 (iv) and D1 (v). (B) Venn diagrams were used to assess the amount of (thio)xanthone-specific repressed (i) and induced (ii) genes. * and # indicate genes that are either repressed or induced by all of the tested (thio)xanthone derivatives, respectively.
FunCat [21] was employed to evaluate if there were enriched functional categories among datasets of genes which showed increased or reduced expression levels for each compound (Supplemental File 2 and Table 1). The dataset of TX129-induced genes was enriched in molecules involved in the response to oxidative stress and in resistance and detoxification. In turn, TX34 induced genes involved in metabolism (carbon, carbohydrate and lipid metabolism) and in ion transport and homeostasis. The FunCat analysis was particularly useful in the case of XP13-altered genes. The XP13-induced dataset was highly enriched in genes involved in cell rescue, defense and virulence (namely genes related with detoxification by members of the ABC transporter family and genes implicated in the response to oxidative stress) and in genes implicated in metabolism and energy. On the other hand, XP13-repressed genes seem to be related to cell cycle and RNA synthesis and processing. The latter feature is shared with staurosporine [24], suggesting that some pathways may be disturbed by both compounds.
Table 1.
Functional enrichment analysis of genes whose expression was altered by the (thio)xanthone derivatives (P-value < 0.0001).
| FunCat ID | Category | P-value |
|---|---|---|
| TX129 (induced genes) | ||
| 32.01.01 | Oxidative stress response | 1.99E − 05 |
| 32.05.01 | Resistance proteins | 4.82E − 05 |
| 32.07 | Detoxification | 1.28E − 05 |
| TX34 (induced genes) | ||
| 1 | Metabolism | 2.21E − 14 |
| 01.05 | C-compound and carbohydrate metabolism | 2.84E − 05 |
| 01.06 | Lipid, fatty acid and isoprenoid metabolism | 2.01E − 08 |
| 20.01.01 | Ion tranport | 8.37E − 05 |
| 20.01.01.01 | Cation transport (H+, Na+, K+, Ca2 +, NH4 +, etc.) | 2.28E − 05 |
| 20.03.22 | Transport ATPases | 2.48E − 07 |
| 20.09.16.01 | Type I protein secretion system (ABC-type transport systems) | 4.96E − 06 |
| 32.05.01 | Resistance proteins | 1.51E − 05 |
| 34.01 | Homeostasis | 5.09E − 05 |
| 34.01.01 | Homeostasis of cations | 2.33E − 05 |
| 34.01.01.01 | Homeostasis of metal ions (Na, K, Ca etc.) | 1.96E − 06 |
| XP13 (induced genes) | ||
| 1 | Metabolism | 1.29E − 07 |
| 01.05 | C-compound and carbohydrate metabolism | 6.58E − 06 |
| 2 | Energy | 1.42E − 06 |
| 20.01.01 | Ion transport | 6.58E − 06 |
| 20.01.27 | Drug/toxin transport | 1.07E − 05 |
| 20.03.22 | Transport ATPases | 4.32E − 07 |
| 20.03.25 | ABC transporters | 7.67E − 05 |
| 20.09.16.01 | Type I protein secretion system (ABC-type transport systems) | 3.16E − 05 |
| 32 | Cell rescue, defense and virulence | 3.78E − 09 |
| 32.01.01 | Oxidative stress response | 2.01E − 07 |
| 32.07 | Detoxification | 1.92E − 13 |
| 32.07.05 | Detoxification by export | 5.63E − 06 |
| 32.07.07 | Oxygen and radical detoxification | 2.07E − 05 |
| XP13 (repressed genes) | ||
| 10 | Cell cycle and DNA processing | 2.54E − 07 |
| 10.03 | Cell cycle | 1.57E − 05 |
| 10.03.01 | Mitotic cell cycle and cell cycle control | 8.94E − 06 |
| 11 | Transcription | 4.17E − 13 |
| 11.02 | RNA synthesis | 9.80E − 08 |
| 11.02.03 | mRNA synthesis | 4.04E − 06 |
| 11.02.03.04 | Transcriptional control | 4.06E − 07 |
| 11.04 | RNA processing | 1.47E − 05 |
| 11.04.01 | rRNA processing | 1.43E − 07 |
| 16.03 | Nucleic acid binding | 3.42E − 05 |
| 18.02.05 | Regulator of G-protein signaling | 9.64E − 05 |
| 20.09 | Transport routes | 6.46E − 05 |
Venn diagrams were built in order to evaluate whether repressed or induced genes were shared between the (thio)xanthone derivatives. For most of the genes, the alteration in expression levels elicited by the (thio)xanthone derivatives was drug-specific (Fig. 2A–B). However, nine genes (Fig. 2B, i, marked with *) were repressed by all of the (thio)xanthone derivatives – NCU04907, NCU00999, NCU07235, NCU11307, NCU09020, NCU09428, NCU04910, NCU11922, and NCU02098 – and one gene (Fig. 2B, ii, marked with #) was induced by all of them—NCU09830. Table 2 presents the FPKMs for these genes in control- as well as in (thio)xanthone-treated cells and Table 3 describes their annotation. The function of the majority of these proteins is still unknown and they are all annotated as ‘hypothetical proteins’. However, based on previous transcription profiling work, we noticed a clear trend that indicates that some of these genes are involved in oxidative stress because, as in the case of the (thio)xanthone derivatives, they were either repressed – NCU04907, NCU09428, NCU04910 and NCU02098 – or induced – NCU09830 – by different oxidative stress inducers, namely staurosporine, phytosphingosine and menadione [24], [25], [26], [27]. Thus, the results suggest that xanthone derivatives affect the cellular machinery that is implicated in the response to oxidative stress. In addition, we observed that among the nine genes repressed by all of the tested (thio)xanthone derivatives, two encode proteins that harbor a methyltransferase domain, suggesting that proteins that possess this functional domain may be involved in the mechanism of action of this type of compounds.
Table 2.
Genes that are repressed (*) and induced (#) by all of the tested (thio)xanthone derivatives and the respective FPKM.
| Shared genes | FPKM |
|||||
|---|---|---|---|---|---|---|
| DMSO | TX129 | TX34 | TX87 | XP13 | D1 | |
| NCU04907 ⁎ | 56.17 | 22.52 (0.40) | 13.86 (0.25) | 23.56 (0.42) | 14.85 (0.26) | 16.02 (0.29) |
| NCU00999 ⁎ | 65.75 | 25.78 (0.39) | 13.65 (0.21) | 24.39 (0.37) | 25.60 (0.39) | 50.77 (0.77, ns) |
| NCU07235 ⁎ | 14.72 | 4.89 (0.33) | 1.36 (0.09) | 5.06 (0.34) | 1.22 (0.08) | 4.57 (0.31) |
| NCU11307 ⁎ | 65.40 | 29.14 (0.45) | 20.47 (0.31) | 15.30 (0.23) | 20.00 (0.31) | 26.42 (0.40) |
| NCU09020 ⁎ | 11.89 | 4.13 (0.35) | 3.96 (0.33) | 4.06 (0.34) | 0.93 (0.08) | 6.41 (0.54, ns) |
| NCU09428 ⁎ | 5.67 | 0.89 (0.16) | 1.16 (0.20) | 1.50 (0.26) | 0.54 (0.10) | 1.02 (0.18) |
| NCU04910 ⁎ | 85.13 | 25.19 (0.30) | 26.93 (0.32) | 26.09 (0.31) | 34.94 (0.41) | 31.90 (0.37) |
| NCU11922 ⁎ | 35.84 | 8.82 (0.25) | 4.41 (0.12) | 14.70 (0.41) | 5.61 (0.16) | 10.08 (0.28) |
| NCU02098 ⁎ | 48.30 | 17.72 (0.37) | 9.38 (0.19) | 22.13 (0.46) | 6.04 (0.13) | 37.14 (0.77, ns) |
| NCU09830 # | 2.31 | 342.31 (148.19) | 427.86 (185.22) | 99.49 (43.07) | 357.96 (154.96) | 11.46 (4.96) |
Fold change versus control (DMSO) is indicated between parentheses. ns: not significant.
Table 3.
Annotation details of genes that are repressed (*) and induced (#) by all of the tested (thio)xanthone derivatives.
| Gene | Annotation a | Conserved domains a | Observations a | STS [24]b | PHS [25]c | MENA [26]d |
|---|---|---|---|---|---|---|
| NCU04907 | Hypothetical protein | Methyltransferase domain | Oxidative stress | Down | Down | Down |
| NCU00999 | Hypothetical protein | |||||
| NCU07235 | Hypothetical protein | Down | ||||
| NCU11307 | CCC1 | VIT family | Iron homeostasis; vacuolar iron transporter; oxidative stress | Down | Up | |
| NCU09020 | Hypothetical protein | C4-dicarboxylate transporter/malic acid transport protein | Down | |||
| NCU09428 | Hypothetical protein | Oxidative stress | Down | Down | ||
| NCU04910 | Hypothetical protein | Methyltransferase domain | Oxidative stress | Down | Down | Down |
| NCU11922 | Hypothetical protein | Also annotated as NCU16555 | Down | Down | ||
| NCU02098 | G/U mismatch-specific DNA glycosylase | Uracil DNA glycosylase superfamily; Mismatch-specific thymine-DNA glycosylate | Base-excision repair; oxidative stress | Down | ||
| NCU09830 | ABC multidrug transporter | ABC transporter | Oxidative stress | Up e |
STS: staurosporine, PHS: phytosphingosine, MENA: menadione.
N. crassa database at the Broad Institute (www.broadinstitute.org/annotation/genome/neurospora/MultiHome.html).
The dataset was obtained by RNA-seq.
The dataset was obtained by microarray analysis.
The dataset was obtained by digital gene expression profiling.
NCU09830 was the gene with the highest induction by menadione.
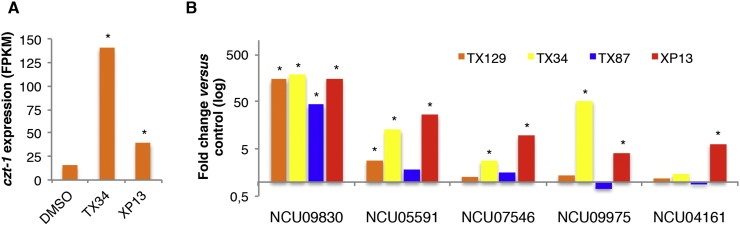
We have recently characterized CZT-1, a transcription factor encoded by NCU09974 that is involved in staurosporine-induced cell death in N. crassa [24]. Treatment with TX34 and XP13 also led to a strong induction of czt-1 (Fig. 3A), further emphasizing its role in the response to cellular stress. In addition, NCU07235, NCU11307, NCU09020 and NCU11992, genes whose expression is repressed by all of the (thio)xanthone derivatives, are strongly repressed in a Δczt-1 deletion mutant [24], suggesting that these genes are under the regulatory control of the CZT-1 transcription factor.
Fig. 3.
(Thio)xanthone derivatives induce the expression of czt-1 and of different members of the ABC transporter family. (A) The levels of expression (in FPKMs) of NCU09974 (czt-1) in N. crassa cells treated with TX34, XP13 (or DMSO) are presented. (B) The levels of expression (in FPKMs) of NCU09830 (atrb), NCU05591 (cdr4), NCU07546, NCU09975 (abc-3) and NCU04161 in N. crassa cells treated with the (thio)xanthone derivatives were obtained and the fold change versus the control (DMSO) was calculated. *, P-value < 0.05.
Analysis of ABC transporter-encoding genes in the (thio)xanthone-treated gene expression datasets
We compiled a list of described and predicted ABC transporter-encoding genes in N. crassa and analyzed their expression levels upon treatment with the (thio)xanthone derivatives. Interestingly, we found that some genes encoding ABC transporters increased in expression level upon treatment with different (thio)xanthone derivatives. Besides NCU09830 (induced by all of the tested (thio)xanthone derivatives), we also observed that NCU05591 (induced by TX129, TX34 and XP13), NCU07546 and NCU09975 (induced by TX34 and XP13) and NCU04161 (induced by XP13) were induced by more than one of the compounds (Fig. 3B). Thus, our data indicate that NCU09830, NCU05591 and other ABC transporter-encoding genes appear to be an important target for xanthones in N. crassa.
NCU09830 and NCU05591 encode ATRB and CDR4, respectively, and share high similarity with ABC transporters of the pleiotropic drug resistance (PDR) subfamily [28]. In N. crassa, ATRB was found in a group of menadione-induced genes [27] while CDR4 is an important mediator of resistance to azole drugs [28]. NCU09975 and NCU07546 are ABC transporters that belong to the multidrug resistance (MDR) subfamily. NCU09975 encodes ABC-3, an ABC transporter that performs the efflux of staurosporine in N. crassa [29], [30]. NCU04161 is an ABC transporter that belongs to the multidrug resistance-associated protein (MRP) subfamily. Interestingly, the transcription factor CZT-1 regulates the expression of NCU09975, NCU07546 and NCU05591 [24], indicating that it may have an important role during the fungal response to (thio)xanthones.
The fungal secondary metabolite 2,4-dihydroxy-3-methylacetophenone elicits a defined transcriptional response in N. crassa
We also evaluated the transcriptional response of N. crassa cells to 2,4-dihydroxy-3-methylacetophenone (termed ‘D1’ from now on) (Fig. 1), a secondary metabolite from the culture of Neosartorya siamensis KUFC 6349 collected from soil in Thailand [31]. A 1-hour treatment of N. crassa cells with 20 μM D1 resulted in the alteration of the expression levels of 3.5% of the genes (Fig. 2A, v, left panel), which is comparable with the TX34 profile. D1 caused a similar fraction of gene induction and repression (Fig. 2A, v, right panel). Functional enrichment among the genes that were either induced or repressed by D1 was evaluated using FunCat (Supplemental File 2 and Table 4). D1 induced genes involved in carbon compound and carbohydrate metabolism, fermentation, oxidative stress response and detoxification (Table 4), indicating an active response of the fungus in the presence of this compound. In fact, analysis of the genes included in the ‘detoxification’ category revealed that D1 led to the upregulation of genes within the ABC transporter family, namely NCU05591 (cdr4), NCU07546 and NCU09830 (atrb), which is a shared feature with some of the aforementioned xanthone derivatives. Among the repressed genes, there was an enrichment of genes involved in several categories related with cell metabolism, including metabolism of aspartate, nitrogen, sulfur, selenium, purines and tetrahydrofolate (Table 4). Interestingly, also like the tested (thio)xanthone derivatives, D1 led to the repression of NCU04907, NCU07235, NCU11307, NCU09428, NCU04910 and NCU11922 (Table 2), suggesting that D1 and (thio)xanthones activate common intracellular pathways and further emphasizes that the downregulation of these genes is related to a fungal stress response.
Table 4.
Functional enrichment analysis of genes whose expression was altered by D1 (P-value < 0.0001).
| FunCat ID | Category | P-value |
|---|---|---|
| D1 (induced genes) | ||
| 1 | Metabolism | 1.41E − 05 |
| 01.05 | C-compound and carbohydrate metabolism | 1.70E − 05 |
| 02.16 | Fermentation | 3.89E − 05 |
| 32.01.01 | Oxidative stress response | 2.48E − 05 |
| 32.07 | Detoxification | 9.99E − 08 |
| D1 (repressed genes) | ||
| 1 | Metabolism | 2.89E − 07 |
| 01.01 | Amino acid metabolism | 1.27E − 07 |
| 01.01.06 | Metabolism of the aspartate family | 2.69E − 06 |
| 01.02 | Nitrogen, sulfur and selenium metabolism | 3.97E − 05 |
| 01.03.01 | Purine nucleotide/nucleoside/nucleobase metabolism | 4.14E − 05 |
| 01.05.13 | Transfer of activated C-groups | 6.39E − 05 |
| 01.05.13.03 | Tetrahydrofolate-dependent C-transfer | 7.71E − 06 |
(Thio)xanthone derivatives and D1 inhibit the growth of N. crassa
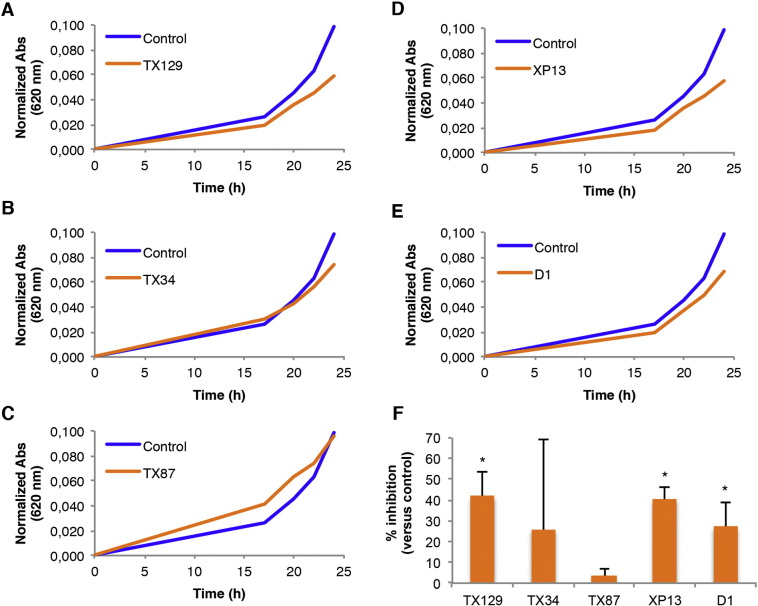
Previous reports have shown that xanthone derivatives possess anti-proliferative and pro-apoptotic activity [2], [11], [12], [13], [14]. Thus, we evaluated the growth of N. crassa cells in the presence of the (thio)xanthone derivatives and the acetophenone isolated from N. siamensis. TX129, XP13 and D1 markedly inhibited the growth of N. crassa while TX34 and TX87 did not have a significant impact on growth (see curves in Fig. 4A–E and quantification of the data for the 24 hour time point in Fig. 4F). Importantly, XP13 ranked first as the compound causing the most dynamic transcriptional response, altering the expression of approximately 16% of the N. crassa genes (Fig. 2A, iv). Thus, the aforementioned transcriptional alterations caused by the (thio)xanthone derivatives TX129 and XP13 and the acetophenone D1 are associated with an deleterious effect on fungal growth.
Fig. 4.
Growth of N. crassa cells in the presence of the (thio)xanthone derivatives and the N. siamensis-derived compound D1. (A-E) Growth was evaluated by following the absorbance at 620 nm of N. crassa cells in culture medium supplemented with TX129 (A), TX34 (B), TX87 (C), XP13 (D) and D1 (E). (F) The percentage of inhibition caused by each of the compounds at the 24 hour time point is presented. *, P-value < 0.05.
Conclusions
The availability of high-throughput methodologies to manipulate simple models like N. crassa constitutes a major advantage to study intracellular pathways that are targeted by exogenous compounds. Our results pointed out that ABC transporters and a group of mostly uncharacterized genes are targets of xanthone and acetophenone derivatives, suggesting that they correspond to common fungal stress response elements. In addition, our work provides several datasets that may orientate future studies on the mechanisms underlying the inhibitory effects on cell growth caused by xanthones.
Acknowledgments
A.P.G. was a recipient of a fellowship from Fundação Calouste Gulbenkian (104210) and a short-term fellowship from EMBO (329-2012). This work was supported by FCT Portugal (CEQUIMED-PEst-OE/SAU/UI4040/2014, PEst-C/SAU/LA0002/2013 and FCOMP-01-0124-FEDER-037277 to A.V.), the European POCI program of QCAIII co-participated by FEDER (NORTE-07-0124-FEDER-000003), a grant from the National Institutes of Health (NIH R24 GM081597 to N.L.G. with Drs. JW Taylor and RB Brem) and project MARBIOTECH (NORTE-07-0124-FEDER-000047) within the SR&TD Integrated Program MARVALOR—Building research and innovation capacity for improved management and valorization of marine resources, supported by the Programa Operacional Regional do Norte (ON.2—O Novo Norte) and by the European Regional Development Fund.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.gdata.2015.02.001.
Contributor Information
A. Pedro Gonçalves, Email: antoniopedrocardoso@gmail.com.
Arnaldo Videira, Email: avideira@ibmc.up.pt.
Appendix A. Supplementary data
Complete RNA-seq dataset obtained in the course of this work.
Complete functional enrichment analysis, using FunCat, of genes whose expression was altered by the (thio)xanthone derivatives or D1 in N. crassa cells.
References
- 1.Mazimba O., Nana F., Kuete V., Singh G.S. 11—Xanthones and anthranoids from the medicinal plants of Africa. In: Kuete V., editor. Medicinal Plant Research in Africa. Elsevier; Oxford: 2013. pp. 393–434. [Google Scholar]
- 2.Pinto M.M., Sousa M.E., Nascimento M.S. Xanthone derivatives: new insights in biological activities. Curr. Med. Chem. 2005;12:2517–2538. doi: 10.2174/092986705774370691. [DOI] [PubMed] [Google Scholar]
- 3.Pedro M., Cerqueira F., Sousa M.E., Nascimento M.S.J., Pinto M. Xanthones as inhibitors of growth of human cancer cell lines and their effects on the proliferation of human lymphocytes in vitro. Bioorg. Med. Chem. 2002;10:3725–3730. doi: 10.1016/s0968-0896(02)00379-6. [DOI] [PubMed] [Google Scholar]
- 4.Pinto M., Castanheiro R. Natural prenylated xanthones: chemistry and biological activities. In: Brahmachari G., editor. Natural Products: Chemistry, Biochemistry and Pharmacology. Alpha Science International; Oxford, UK: 2009. [Google Scholar]
- 5.Brahmachari G.E. Alpha Science International; Oxford, UK: 2009. Natural Products: Chemistry, Biochemistry and Pharmacology. [Google Scholar]
- 6.Kuete V.E. Elsevier; Oxford, UK: 2013. Medicinal Plant Research in Africa: Pharmacology and Chemistry. [Google Scholar]
- 7.Obolskiy D., Pischel I., Siriwatanametanon N., Heinrich M. Garcinia mangostana L.: a phytochemical and pharmacological review. Phytother. Res. 2009;23:1047–1065. doi: 10.1002/ptr.2730. [DOI] [PubMed] [Google Scholar]
- 8.Vieira L.M., Kijjoa A. Naturally-occurring xanthones: recent developments. Curr. Med. Chem. 2005;12:2413–2446. doi: 10.2174/092986705774370682. [DOI] [PubMed] [Google Scholar]
- 9.Azevedo C.M.G., Afonso C.M.M., Pinto M.M.M. Routes to xanthones: an update on the synthetic approaches. Curr. Org. Chem. 2012;16:2818–2867. [Google Scholar]
- 10.Lesch B., Brase S. A short, atom-economical entry to tetrahydroxanthenones. Angew. Chem. 2004;43:115–118. doi: 10.1002/anie.200352154. [DOI] [PubMed] [Google Scholar]
- 11.Palmeira A., Paiva A., Sousa E., Seca H., Almeida G.M., Lima R.T., Fernandes M.X., Pinto M., Vasconcelos M.H. Insights into the in vitro antitumor mechanism of action of a new pyranoxanthone. Chem. Biol. Drug Des. 2010;76:43–58. doi: 10.1111/j.1747-0285.2010.00978.x. [DOI] [PubMed] [Google Scholar]
- 12.Costa E., Sousa E., Nazareth N., Nascimento M.S.J., Pinto M.M.M. Synthesis of xanthones and benzophenones as inhibitors of tumor cell growth. Lett. Drug Des. Discovery. 2010;7:487–493. [Google Scholar]
- 13.Paiva A.M., Sousa M.E., Camoes A., Nascimento M.S.J., Pinto M.M.M. Prenylated xanthones: antiproliferative effects and enhancement of the growth inhibitory action of 4-hydroxytamoxifen in estrogen receptor-positive breast cancer cell line. Med. Chem. Res. 2012;21:552–558. [Google Scholar]
- 14.Sousa E., Paiva A., Nazareth N., Gales L., Damas A.M., Nascimento M.S.J., Pinto M. Bromoalkoxyxanthones as promising antitumor agents: synthesis, crystal structure and effect on human tumor cell lines. Eur. J. Med. Chem. 2009;44:3830–3835. doi: 10.1016/j.ejmech.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Palmeira A., Vasconcelos M.H., Paiva A., Fernandes M.X., Pinto M., Sousa E. Dual inhibitors of P-glycoprotein and tumor cell growth: (re)discovering thioxanthones. Biochem. Pharmacol. 2012;83:57–68. doi: 10.1016/j.bcp.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Leao M., Pereira C., Bisio A., Ciribilli Y., Paiva A.M., Machado N., Palmeira A., Fernandes M.X., Sousa E., Pinto M., Inga A., Saraiva L. Discovery of a new small-molecule inhibitor of p53–MDM2 interaction using a yeast-based approach. Biochem. Pharmacol. 2013;85:1234–1245. doi: 10.1016/j.bcp.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Palmeira A., Sousa E., Fernandes M.X., Pinto M.M., Vasconcelos M.H. Multidrug resistance reversal effects of aminated thioxanthones and interaction with cytochrome P450 3A4. J. Pharm. Pharm. Sci. 2012;15:31–45. doi: 10.18433/j3bg65. [DOI] [PubMed] [Google Scholar]
- 18.Davis R.H., de Serres F.J. Genetic and microbiological research techniques for Neurospora crassa. Meth Enzymol. 1970;17:79–143. [Google Scholar]
- 19.Vogel H.J. A convenient growth medium for Neurospora (Medium N) Microbial Genet. Bull. 1956;13:42–43. [Google Scholar]
- 20.Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., Pimentel H., Salzberg S.L., Rinn J.L., Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruepp A., Zollner A., Maier D., Albermann K., Hani J., Mokrejs M., Tetko I., Guldener U., Mannhaupt G., Munsterkotter M., Mewes H.W. The FunCat, a functional annotation scheme for systematic classification of proteins from whole genomes. Nucleic Acids Res. 2004;32:5539–5545. doi: 10.1093/nar/gkh894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveros J.C., VENNY An interactive tool for comparing lists with Venn Diagrams. 2007. http://bioinfogp.cnb.csic.es/tools/venny/index.html
- 23.Binder U., Chu M., Read N.D., Marx F. The antifungal activity of the Penicillium chrysogenum protein PAF disrupts calcium homeostasis in Neurospora crassa. Eukaryot. Cell. 2010;9:1374–1382. doi: 10.1128/EC.00050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goncalves A.P., Hall C., Kowbel D.J., Glass N.L., Videira A. CZT-1 is a novel transcription factor controlling cell death and natural drug resistance in Neurospora crassa. 2014;G3(4):1091–1102. doi: 10.1534/g3.114.011312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Videira A., Kasuga T., Tian C., Lemos C., Castro A., Glass N.L. Transcriptional analysis of the response of Neurospora crassa to phytosphingosine reveals links to mitochondrial function. Microbiology. 2009;155:3134–3141. doi: 10.1099/mic.0.029710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J., Yu X., Xie B., Gu X., Zhang Z., Li S. Transcriptomic profiling-based mutant screen reveals three new transcription factors mediating menadione resistance in Neurospora crassa. Fungal Biol. 2013;117:422–430. doi: 10.1016/j.funbio.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi M., Yamashita K., Shiozawa A., Ichiishi A., Fukumori F., Fujimura M. An AP-1-like transcription factor, NAP-1, regulates expression of the glutathione S-transferase and NADH:flavin oxidoreductase genes in Neurospora crassa. Biosci. Biotechnol. Biochem. 2010;74:746–752. doi: 10.1271/bbb.90790. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Zhang Z., Zhang X., Zhang H., Sun X., Hu C., Li S. CDR4 is the major contributor to azole resistance among four Pdr5p-like ABC transporters in Neurospora crassa. Fungal Biol. 2012;116:848–854. doi: 10.1016/j.funbio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes A.S., Castro A., Videira A. Reduced glutathione export during programmed cell death of Neurospora crassa. Apoptosis. 2013;18:940–948. doi: 10.1007/s10495-013-0858-y. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes A.S., Goncalves A.P., Castro A., Lopes T.A., Gardner R., Glass N.L., Videira A. Modulation of fungal sensitivity to staurosporine by targeting proteins identified by transcriptional profiling. Fungal Genet. Biol. 2011;48:1130–1138. doi: 10.1016/j.fgb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buttachon S., Chandrapatya A., Manoch L., Silva A., Gales L., Bruyere C., Kiss R., Kijjoa A. Sartorymensin, a new indole alkaloid, and new analogues of tryptoquivaline and fiscalins produced by Neosartorya siamensis (KUFC 6349) Tetrahedron. 2012;68:3253–3262. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete RNA-seq dataset obtained in the course of this work.
Complete functional enrichment analysis, using FunCat, of genes whose expression was altered by the (thio)xanthone derivatives or D1 in N. crassa cells.