Abstract
Neurocognitive deficits are evident both in established schizophrenia and bipolar disorder (BP). However, it has been suggested that schizophrenia, but not BP, is characterized by neurodevelopmental abnormalities that can lead to cognitive deficits at the earliest stages of the illness. The aim of this meta-analytic review was to compare neurocognitive deficits in first-episode BP (FEBP) with healthy controls and first-episode schizophrenia (FES) patients. The current meta-analysis included a total of 22 adult studies and involved comparisons of 533 FEBP patients with 1417 healthy controls and 605 FEBP and 822 FES patients. FEBP patients were significantly impaired in all cognitive domains (d = 0.26–0.80) and individual tasks (d = 0.22–0.66) investigated. FES patients significantly underperformed FEBP patients in most cognitive domains (d = 0.05–0.63) and on individual tasks (d = 0.13–0.77). Neuropsychological impairment, which is comparable to chronic BP, was evident in FEBP. Similar to chronic patients, cognitive functions in FEBP lie intermediate between FES and healthy controls. Neurodevelopmental factors are likely to play a significant role not only in schizophrenia but also in BP.
Key words: bipolar disorder, cognition/mania, psychosis, schizophrenia
Introduction
Cognitive dysfunction is a common and robust feature of schizophrenia.1–3 Bipolar disorder (BP) is also associated with cognitive deficits in a number of domains including executive functions, attention, and memory that persist in remission.4 In chronic samples, cognitive dysfunction is less severe in BP than schizophrenia but the magnitude of cognitive differences between schizophrenia and BP are modest.5,6
However, it is argued that cognitive deficits in BP and schizophrenia might have very different trajectories. In schizophrenia, there is general consensus that cognitive and intellectual deficits are evident early in neurodevelopment, including childhood, well before the onset of psychosis.7–10 It also seems that many of the cognitive deficits observed in chronic patients are also seen in first-episode schizophrenia (FES).11,12 Neurodevelopmental abnormalities play a major role in these deficits.13–16
In contrast to the findings in schizophrenia, a number of studies have suggested normal, at times superior, cognitive abilities, and school achievement in children and adolescents who develop adult BP.17,18 It has been suggested that developmental cognitive abnormalities might be specific to schizophrenia.9,19 It was also hypothesized that patients with BP only develop cognitive deficits during the course of illness as a result of neurodegenerative changes and that cognitive deficits would be absent or very modest in first-episode BP (FEBP)20,21; whereas in schizophrenia it is proposed that cognition is impaired before the onset of the illness and at first-episode which might be a key difference between BP and schizophrenia.
Recently, a number of neuropsychological studies comparing FEBP with healthy controls and FES were conducted. Some of these studies have supported the hypothesis of relative preservation of cognitive abilities in FEBP and specificity of such deficits to schizophrenia.22 On the other hand, many others have not supported the preserved cognition argument in FEBP casting doubt on the hypothesis that cognitive deficits in BP develop during the course of the illness. A recent meta-analysis found that cognitive function is significantly impaired in FEBP in comparison to healthy controls.23 However, it is not possible to know whether the severity of these findings are comparable to chronic BP as the meta-analysis of Lee et al23 reported effect sizes only for broad cognitive domains rather than for individual tasks. More importantly, it is not known if cognitive differences between FES and FEBP are large unlike modest differences in chronic patients. No previous meta-analysis has compared the cognitive functions of FEBP and FES. Therefore, a meta-analytic review of the literature is warranted in order to examine overall outcome of studies investigating cognitive deficits in FEBP in comparison to schizophrenia and healthy controls. Our aim was to conduct a meta-analysis of cognitive abilities in FEBP in comparison to healthy controls and FES. We hypothesized that cognitive deficits will also be evident in FEBP samples and differences between FEBP and FES, as in chronic samples, would be modest.
Methods
Study Selection
We followed MOOSE and PRISMA guidelines in conducting this meta-analysis.24,25 A literature search was conducted using the databases Pubmed, PsycINFO, ProQuest, and Scopus to identify relevant studies (January 1990 to July 2014). At the first step of the search, combinations of the following keywords were used: first-episode, recent onset, bipolar disorder, mania. All studies (over 500) identified by this strategy were screened for the use of neurocognitive assessments. Reference lists of published reports were also reviewed for additional studies. This step was followed for checking eligibility of the studies identified after screening. Inclusion criteria were studies that: (1) examined cognitive abilities after a FEBP (within a maximum of 2 years); (2) Compared FEBP with healthy controls and/or patients with FES spectrum disorder; (3) reported sufficient data to calculate the effect sizes and standard errors of the cognitive measures. We contacted the authors for unreported information when needed and additional information was received from three authors (see acknowledgments). Lopez-Jaramillo et al26 (which was included in Lee et al)23 was not included as the average duration of illness in this study was 13 years. In the case of multiple reports based on overlapping samples, the study with the largest sample size was selected for inclusion in the main meta-analysis. Data from additional reports were only used for calculation of effect sizes of individual tasks or cognitive domain data that were not included in the main study or calculation of effects size for the euthymic state.27–29 A total of 25 studies (21 main studies)22,27–50 were selected for the current meta-analysis (table 1) (see supplementary figure for a flow chart of the study selection process). However, 3 of these studies were based on pediatric samples that had very significant general intelligence deficits compared to adult samples (see below) and therefore were excluded from further analyses.
Table 1.
Characteristics of Adult Studies Included in Meta-analysis of First-Episode Bipolar Disorder in Comparison to FES and Healthy Controls
| Studies | Sample N | Male % | Age (y) | Education | Medications | Psychosis rating | BP state | Mania rating (BP) | Depression rating (BP) |
|---|---|---|---|---|---|---|---|---|---|
| Ayres et al 30 | 41 FEBP 98 FES 383 HC |
37% 57% 47% |
33.5 30.6 32.1 |
No difference | Most exposed to antipsychotics Many BP on mood stabilizers |
Not clear | |||
| Barrett et al 31 | 32 FEBP 46 FES 67 HC |
50% 70% 58% |
36.7 29.0 33.2 |
91 % of patients on antipsychotics | PANSS positive FEBP:17.4 FES:17.6 |
Subacute | PANSS excitement: 17.4 | BDI:10.0 | |
| Bucker et al 32 Torres et al 29 DeFreitas 33 | 74 FEBP 98 HC |
48% 40% |
23.0 22.5 |
No difference | Mild symptoms: Torres et al, euthymic | ||||
| 72 FES 64 FEBP |
50% 67% |
22.7 21.4 |
FES<BP | PANSS positive FES:20.5 |
Euthymic | YMRS:0 (median) | HDRS:3 (median) | ||
| Chan et al 34 | 40 FEBP 38 FES 37 HC |
45% 37% 27% |
22.4 21.8 22.4 |
FES=BP<HC | All but 3 on Antipsychotics 26 BP on mood stablizers | PANSS positive FEBP:8.2 FES:10.7 |
Euthymic | YMRS:2.4 | HDRS:1.1 |
| Daros et al 35 | 16 FEBP 24 FES 32 HC |
56% 79% 34% |
23.6 22.6 25.8 |
Matched for reading ability | No medications within 3 days of assessment >50% exposed to antipsychotics |
PANSS positive FEBP:24.5 FES:22.3 |
Symptomatic | YMRS:28.2 | HDRS:28.5 |
| Demmo et al 36 Hellvin et al 37 | 87 FEBP 87 FES 90 HC |
45% 43% 58% |
29.8 30.1 30.1 |
No difference | |||||
| 34 FEBP 110 HC |
44% 45% |
31.2 31.1 |
No difference | 28/34 on antipsychotics 18734 lithium or mood stabilizers |
PANSS positive FEBP:11.6 |
Some symptomatic | YMRS:2 (median) | IDS-C:14 (median) | |
| Dickerson et al 38 | 60 FEBP 56 FES 312 HC |
35% 82% 34% |
26.5 23.1 28.6 |
FES<BP<HC | 107/116 on antipsychotics 65% BP on lithium |
PANSS positive FEBP:19.9 FES:20.5 |
Symptomatic | CDS:7.6 | |
| Fleck et al 39 Lebowitzet al 28 Larson et al 27 | 21 FEBP 48 HC |
52% 42% |
25.7 28.2 |
No difference | Most on antipsychotics and mood stabilizers |
Manic or mixed but Larson et al: Euthymic | YMRS:21.7 | HDRS:16.0 | |
| Gruber et al 40 | 26 FEBP 20 HC |
73% 75% |
24.4 25.3 |
BP<HC | <2 weeks treatment | Not clear Within 2 weeks of manic admission |
|||
| Hill et al 41 | 22 FEBP 30 FES 41 HC |
59% 80% 59% |
22.7 23.0 24.9 |
No difference | Half antipsychotic naive, others brief exposure | PANSS positive FEBP:23.5 FES:23.6 |
Symptomatic | HDRS:29.1 | |
| Hirayisu et al 42 | 24 FEBP 20 FES 22 HC |
75% 80% 91% |
23.6 27.3 24.5 |
Most on antipsychotics Short duration |
Manic | ||||
| Minzenberg et al 44 | 26 FEBP 73 FES 54 HC |
62% 81% 52% |
20.6 21.6 20.1 |
FES<HC | 86% FES and FEBP 58% on antipsychotics and 57% mood stabilizer |
SAPS FEBP:7.9 FES:9.2 |
Euthymic or mildly symptomatic | ||
| Mojtabai et al 45 | 72 FEBP 102 FES |
50% 69% |
30.5 30.2 |
FES<BP | SAPS FEBP:0.3 FES:0.7 | Stable but mildly symptomatic | |||
| Nehra et al 46 | 16 FEBP 20 HC |
50% 60% |
28.4 38.7 |
BP<HC | 13/16 antipsychotics 7/16 mood stabilizers |
Euthymic | YMRS:1.4 | HDRS:1.4 | |
| Owoeye et al 47 | 73 FEBP 73 FES |
52% 74% |
30.9 32.6 |
PANSS positive FEBP:16.3 FES:17.4 |
Symptomatic | ||||
| Thomas et al 49 | 11 FEBP 38 FES 16 HC |
No difference | Acute manic | ||||||
| Zanelli et al 22 | 37FEBP 65 FES 177 HC |
41% 65% 44% |
28.1 26.5 37.2 |
FES<BP=HC | Symptomatic |
Note: FEBP, first-episode bipolar disorder; FES, first-episode schizophrenia; HC, healthy controls; PANSS, the positive and negative syndrome scale; YMRS, Young Mania rating scale; HDRS, Hamilton depression rating scale; SAPS, Scale for Assessment of Positive Symptoms.
Adult FEBP vs Healthy Controls
About 15 studies involving 533 FEBP patients and 1417 healthy controls were included. The groups did not differ in the percentage of females included (BP 51.3 %; healthy controls, 55.7 %), and did not differ significantly in age (d = 0.12, CI = −0.07 to 0.31, Z = 1.23, P = .22).
Adult FEBP vs FES
Fourteen studies involving 605 FEBP patients and 822 FES patients were included. The vast majority of FES patients had a diagnosis of schizophrenia but a small subset of these patients had diagnoses of schizoaffective or schizophreniform disorder. The percentage of females was significantly higher in the FEBP (50.5 %) than FES group (34.1 %), and FES patients were significantly younger than FEBP patients (d = 0.17, CI = 0.02–0.32, Z = 2.26, P = .02).
Statistical Analyses
Meta-analyses were conducted when at least 3 independent studies reported on a given measure. Individual cognitive tests were grouped into broader cognitive domains based on the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS), except for calculation of verbal fluency score separate from processing speed. Cognitive domains included were processing speed, verbal memory, visual memory, attention and vigilance (attention), reasoning and problem solving (reasoning), working memory, and verbal fluency. We classified cognitive tests that were not part of the MATRICS battery under the relevant cognitive domain based on agreement between the 2 authors and factor loadings as reviewed in Nuechterlein et al.51,52 A global cognitive index, “Global cognition,” for each study was calculated by averaging the effect sizes across different domains.
This step was undertaken because there were not sufficient studies to perform meta-analyses for all individual tasks. However, it was also possible to conduct individual task analyses for trail making A and B tests, letter and semantic fluency, list learning and delayed recall (based on similar list learning tasks, see supplementary table for more details), digits span forwards and backwards, letter number sequencing (LNS; only for FEBP vs controls), and Wisconsin Cart Sorting Test (WCST) perseverative errors (only for FEBP vs controls). Most working memory (WM) tests used in the studies included in the meta-analysis were verbal tasks. In addition to the WM domain, a separate effect size was also calculated for verbal WM.
Meta-analyses were performed using MIX software version 1.7 on a Windows platform.53 Effect sizes were weighted using the inverse variance method. A random effects model (DerSimonian-Laird estimate) was used, as the distributions of effect sizes were heterogeneous for the number of variables. Homogeneity of the distribution of weighted effect sizes was tested with the Q test, and degree of heterogeneity was quantified using the I 2 test. I 2 estimates the percentage of total variation across studies that is due to heterogeneity rather than chance.54 I 2 values between 0 and 0.25 suggest small magnitudes of heterogeneity, while I 2 values in the range 0.25 and 0.50 suggest medium magnitudes, and those >0.50 indicate large magnitudes. Tau squared (τ 2), an estimate of between study variance was used as measure of heterogeneity in the random-effects model. Publication bias was assessed by inspecting funnel plots and fail-safe N tests. The Fail Safe N test involves computing a combined P value for all studies included in the meta-analysis, and calculating how many additional studies with a zero effect (average Z of zero) would be necessary to create a nonsignificant P.55
Meta-regression analyses were conducted for age, gender (male ratio in FEBP and ratio of males in FES vs FEBP), in FEBP control and FEBP-FES comparisons whenever at least 6 studies reported these variables. Meta-regression analyses were also conducted for dichotomized variables of mood state (euthymic vs noneuthymic) and substance abuse (excluded vs not-excluded) and education (matched and not-matched) in FEBP-control comparisons. Meta-regression analyses (weighted generalized least squares regressions) were conducted using SPSS. Meta-regression analyses performed with a random effects model were conducted using the restricted information maximum likelihood method with a significance level set at P < .05.
Results
Cognitive Deficits in FEBP
The magnitude of IQ deficits in pediatric samples was large (d = 1.26) and the difference between adult and pediatric samples was highly significant (Q bet = 23.05, P < .001). Therefore, as mentioned above, the pediatric studies were excluded from further analyses.
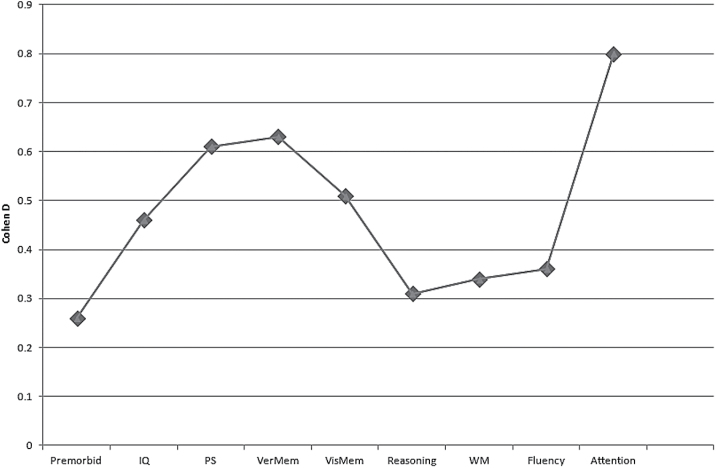
Healthy controls significantly outperformed euthymic adult FEBP patients in all cognitive domains (range of d = 0.31–0.80) as well as current (d = 0.45) and premorbid (d = 0.26) IQ (table 2; figure 1).
Table 2.
Mean Weighted Effect Sizes for Cognitive Differences Between Patients with First-Episode Bipolar Disorder and Healthy Controls in Adult Only Samples
| Test | Study N | FEBP | HC | D | 95 % CI | Z | P | Q test (P) | τ2 | I 2 | Fail safe N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Premorbid | 7 | 207 | 500 | 0.26 | 0.10–0.42 | 3.17 | .001 | 0.73 | 0 | 0 | 15 |
| IQ | 8 | 275 | 615 | 0.45 | 0.19–0.71 | 3.43 | <.001 | 0.007 | 0.09 | 63.6 | 58 |
| PS | 8 | 291 | 494 | 0.61 | 0.39–0.84 | 5.31 | <.001 | 0.07 | 0.05 | 47.2 | 107 |
| TMT A | 5 | 146 | 283 | 0.66 | 0.25–1.06 | 3.20 | .001 | ||||
| TMT B | 4 | 146 | 283 | 0.57 | 0.23–0.91 | 3.32 | <.001 | ||||
| Stroop | 3 | 156 | 135 | 0.47 | 0.23–0.72 | 3.83 | <.001 | ||||
| Fluency | 9 | 354 | 926 | 0.36 | 0.17–0.55 | 3.65 | <.001 | 0.06 | 0.04 | 47 | 54 |
| Letter | 8 | 280 | 828 | 0.30 | 0.05–0.55 | 2.35 | .02 | ||||
| Category | 5 | 181 | 358 | 0.39 | 0.–0.77 | 1.96 | .05 | ||||
| Verbal memory | 6 | 312 | 785 | 0.63 | 0.39–0.86 | 5.27 | <.001 | 0.02 | 0.05 | 61.9 | 111 |
| Learninga | 4 | 220 | 410 | 0.50 | 0.14–0.87 | 2.70 | .007 | ||||
| Delayeda | 4 | 220 | 406 | 0.56 | 0.27–0.84 | 3.78 | <.001 | ||||
| Reasoning | 7 | 268 | 785 | 0.31 | 0.05–0.56 | 2.36 | .02 | 0.01 | 0.07 | 62 | 23 |
| WCST per | 5 | 157 | 510 | 0.33 | −0.16 to 0.82 | 1.31 | .19 | ||||
| WM | 9 | 344 | 906 | 0.34 | 0.20–0.47 | 4.83 | <.001 | 0.42 | 0 | 0.6 | 43 |
| Verbal WM | 8 | 300 | 821 | 0.32 | 0.17–0.42 | 4.25 | <.001 | ||||
| Digit Span F | 5 | 205 | 603 | 0.22 | 0.05–0.40 | 2.47 | .01 | ||||
| Digit Span B | 6 | 217 | 615 | 0.49 | 0.31–0.66 | 5.54 | <.001 | ||||
| LNS | 3 | 116 | 312 | 0.35 | 0–0.70 | 1.94 | .05 | ||||
| Spatial WM | 3 | 114 | 157 | 0.38 | 0.06–0.71 | 2.32 | .02 | ||||
| Visual memory | 5 | 170 | 420 | 0.51 | 0.16–0.86 | 2.87 | .004 | 0.01 | 0.11 | 68.3 | 29 |
| Attention | 3 | 107 | 155 | 0.80 | 0.54–1.06 | 6.09 | <.001 | 0.96 | 0 | 0 | 22 |
| Global | 15 | 533 | 1417 | 0.54 | 0.41–0.66 | 8.42 | <.001 | 0.23 | 0.01 | 19.5 | 352 |
Note: D, Cohen’s D; FEBP, first-episode bipolar disorder; HC, healthy controls; PS, processing speed, TMT, trail making test; WCST, Wisconsin Card Sorting Test; WM, working memory.
aList learning task.
Fig. 1.
Cognitive deficits in FEBP in comparison to healthy controls.
The distribution of effect sizes was heterogeneous for IQ and verbal and visual memory, fluency, and reasoning domains (I 2 = 61–68.3 %). However, the magnitudes of this heterogeneity were small (range of τ 2 = 0–0.11) for all domains in the random effects model. Heterogeneity of IQ was driven by 2 studies with very large effect sizes. The IQ deficit in FEBP patients was smaller (d = 0.27, CI = 0.11–0.44, Z = 3.29, P = .001) and distribution of effect sizes were homogeneous (Q test = 2.33, P = .80) in a re-analysis of IQ differences without these 2 studies.
The funnel plots did not show evidence of publication bias in any of the cognitive domains and the trim and fill method did not suggest a different effect size for any of the variables. The fail-safe N number was between 15 and 352. Task-specific analyses indicated that healthy controls performed significantly better than FEBP patients on all cognitive tasks (d = 0.22–0.66).
In meta-regression analyses, gender, education, age, state (euthymic vs noneuthymic), and exclusion of drug use had no significant effects on global cognition.
Cognitive Deficits in FEBP and FES
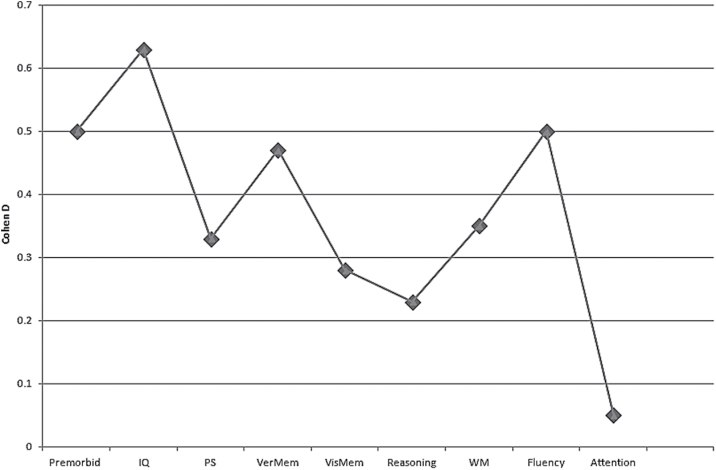
FEBP patients significantly outperformed FES patients in processing speed (d = 0.33), verbal fluency (d = 0.50), verbal memory (d = 0.47), and working memory (d = 0.35) but not reasoning, sustained attention, and visual memory. FEBP patients also outperformed FES patients in current (d = 0.63) and premorbid (d = 0.50) IQ (figure 2; table 3). The distribution of effect sizes was homogeneous except for IQ, processing speed, global cognition, and working memory (I 2 = 48.8–67.9 %). Magnitudes for heterogeneity were small (range of τ 2 = 0–0.07) in the random-effects model. The funnel plots did not show evidence of publication bias in any of the cognitive domains and the trim and fill method did not suggest a different effect size for any of the variables. The fail-safe N number was between 4 and 80 for the variables where significant between-group differences were found. In individual task analyses, FEBP patients significantly outperformed FES patients on all cognitive tasks other than digit span forwards (d = 0.18) and backwards (d = 0.13). The only significant finding in the meta-regression analyses was the relationship between age of FES patients and cognitive differences between FES and FEBP. Between-group differences in working memory (Z = 2.79, P = .005) were more significant in studies that included younger FES patients.
Fig. 2.
Cognitive deficits in FES in comparison to FEBP.
Table 3.
Mean Weighted Effect Sizes for Cognitive Differences Between Patients with First-Episode Bipolar Disorder and Schizophrenia
| Test | Study N | FEBP | HC | D | 95 % CI | Z | P | Q test (P) | τ 2 | I 2 | Fail safe N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Premorbid | 7 | 412 | 316 | 0.50 | 0.30–0.69 | 4.97 | <.001 | 0.15 | 0.03 | 36.8 | 61 |
| IQ | 6 | 318 | 215 | 0.63 | 0.36–0.91 | 4.49 | <.001 | 0.05 | 0.06 | 67.9 | 59 |
| PS | 6 | 413 | 266 | 0.33 | 0.08–0.59 | 2.59 | .009 | 0.03 | 0.06 | 58.9 | 21 |
| TMT A | 3 | 197 | 131 | 0.45 | 0.23–0.68 | 3.91 | <.001 | ||||
| TMT B | 3 | 197 | 131 | 0.47 | 0.14–0.80 | 2.76 | .006 | ||||
| Digit symbol | 3 | 254 | 196 | 0.71 | 0.36–1.06 | 4.01 | <.001 | ||||
| Fluency | 7 | 510 | 355 | 0.50 | 0.33–0.66 | 5.93 | <.001 | 0.26 | 0.01 | 22.0 | 80 |
| Letter | 5 | 300 | 242 | 0.42 | 0.24–0.60 | 4.66 | <.001 | ||||
| Category | 3 | 182 | 146 | 0.77 | 0.0–1.53 | 1.98 | .05 | ||||
| Verbal memory | 7 | 458 | 374 | 0.47 | 0.28–0.65 | 4.97 | <.001 | 0.13 | 0.02 | 39.5 | 69 |
| Learninga | 5 | 356 | 282 | 0.59 | 0.40–0.78 | 5.95 | <.001 | ||||
| Recalla | 5 | 356 | 282 | 0.38 | 0.20–0.55 | 4.30 | <.001 | ||||
| Reasoning | 2 | 121 | 97 | 0.23 | −0.09 to 0.56 | 1.42 | .16 | 0.24 | 0.01 | 26.3 | |
| WM | 8 | 456 | 318 | 0.35 | 0.11–0.59 | 2.84 | .005 | 0.02 | 0.07 | 59.2 | 35 |
| Verbal WM | 8 | 456 | 318 | 0.33 | 0.08–0.57 | 2.60 | .009 | ||||
| Digit span F | 4 | 251 | 184 | 0.18 | −0.03 to 0.38 | 1.70 | .09 | ||||
| Digit span B | 6 | 319 | 217 | 0.13 | −0.04 to 0.31 | 1.48 | .14 | ||||
| Visual memory | 4 | 243 | 163 | 0.28 | −0.05 to 0.60 | 1.68 | .09 | 0.05 | 0.06 | 66.2 | 4 |
| Attention | 2 | 68 | 33 | 0.05 | −0.38 to 0.47 | 0.56 | .83 | 0.62 | 0 | 0 | |
| Global | 14 | 822 | 605 | 0.28 | 0.12–0.44 | 3.54 | <.001 | 0.02 | 0.04 | 48.8 | 74 |
D, Cohen’s D, FEBP, first-episode bipolar disorder; HC, healthy controls, PS, processing speed; TMT, trail making test; WCST, Wisconsin Card Sorting Test; WM, working memory.
aList learning task.
Discussion
The current meta-analysis includes 22 main studies and involved comparisons of 545 FEBP patients with 1611 healthy controls and 644 FEBP with 878 FES patients. FEBP was associated with widespread impairment in cognitive functions. Cognitive impairment was generally more severe in FES in comparison to FEBP. However, cognitive differences between patient groups were modest.
In comparison with controls, FEBP patients were significantly impaired in all cognitive domains and individual tasks investigated. Medium effect sizes were noted for most of the cognitive variables. However, the effect size for sustained attention was large and the number of measures including premorbid IQ, reasoning, fluency and working memory had small effect sizes. These findings are consistent with the recent meta-analysis of Lee et al,23 however this meta-analysis, unlike Lee et al,23 also investigated individual cognitive tasks including trail making A and B tests, stroop interference, letter and category fluency, digit span backwards and forwards, LNS, and continuous performance test (CPT). The severity of cognitive impairment in FEBP was mostly comparable to findings in a previous meta-analysis of patients with established BP.4,56 Only a few measures had relatively small effect sizes in FEBP compared with chronic BP: WCST, trail making B, working memory, and fluency tasks had small to medium rather than medium to large effect sizes. However, such indirect comparisons have limited value, requiring longitudinal studies. Thus far, few studies have directly compared cognitive functions in multiepisode and first-episode (or single episode) patients with BP.26,28,37,46 These studies gave inconsistent findings and can be considered as providing evidence of no change or only limited progression of cognitive deficits as patients have recurring episodes. Moreover, such findings can reflect the sample differences rather than progression as chronic samples naturally include a higher percentage of patients with severe illness. The limited longitudinal neuropsychological studies in BP do not provide evidence of cognitive decline after onset of illness.57,58 Overall, these findings suggest that most cognitive deficits are already evident after the first manic episode. However, as indicated, the available studies are small and further work should explore evidence for progressive changes.
While most of the studies in the meta-analysis included symptomatic patients, similar findings in the euthymic samples suggest that cognitive deficits in FEBP persist in remission. This finding suggests that most of the cognitive impairment in FEBP, like in chronic samples, is not explainable by the effect of mood symptoms. Similar to Lee et al,23 younger age of illness onset in FEBP was not associated with more severe cognitive deficits in adult samples. However, the limited variance of mean age of onset in adult studies can mask a possible relationship between younger onset of illness and cognitive deficits in FEBP. In fact, findings of the few available pediatric FEBP studies clearly suggest that early onset of illness is associated with more severe cognitive deficits. This finding is not surprising as early onset in BP has been associated with poor prognosis.59 Such a finding might be interpreted as consistent with the notion that earlier age of onset may impact on maturational trajectories of certain abilities during critical developmental phases.60–62 Alternatively, severe premorbid cognitive abnormalities might be a susceptibility factor for early onset of BP.
Comparison of Cognitive Abilities in Schizophrenia and BP During the Early “Phases” of Illness
In chronic samples, previous meta-analyses compared cognitive abnormalities in schizophrenia and BP.5,6 Both meta-analyses found that cognitive impairment is significantly more severe in schizophrenia but between-group differences were modest, suggesting that there is a significant overlap between schizophrenia and BP. Our current meta-analysis suggested that this is also true for FEBP. Moreover, findings of this meta-analysis do not suggest that between-group differences (schizophrenia vs BP) for cognitive impairment are more pronounced in FE (d = 0.05–0.63) than in chronic samples (d = 0.26–0.67).6 Effect sizes for differences between FES and FEBP were small to moderate. Between-group differences for processing speed, working memory, verbal memory, and fluency deficits were significant but moderate suggesting that cognitive differences between schizophrenia and BP are quantitative rather than qualitative. Differences between FES and FEBP for IQ were relatively large (medium effect size). Also, in individual task analyses, effect sizes for a difference between FES and FEBP were relatively larger (medium effect size) for 2 variables (digit symbol and category fluency), while for all other measures the magnitude of between group differences were small. However, effect sizes for deficits in IQ, digit symbol and category fluency are among the very largest in chronic schizophrenia and differences between schizophrenia and BP for these tasks are relatively large in both FE and chronic samples. General intellectual abilities play a role in relative differences between schizophrenia and BP not only in established illness but also at the onset of the major psychoses.31 The current meta-analysis has not found evidence of significant change in the pattern of neuropsychological differences between schizophrenia and BP over time after first-episode. These findings are not supportive of the hypothesis that cognitive impairment at the beginning of illness is evident in schizophrenia but not in BP.
While FE studies fail to support specificity of early cognitive deficits to schizophrenia, it might be argued that cognitive deficits can develop during prodromal periods of BP before onset of the first-episode. Therefore, studies investigating cognitive deficits in at-risk subjects prior to the first-episode are important to test the hypothesis of specificity of early cognitive deficits to schizophrenia. To date, few studies have compared cognitive deficits in subjects at risk for BP. However, one study suggested that premorbid deficits in UHR subjects that develop schizophrenia and BP might be similar to each other.63 More studies investigating the cognitive differences between schizophrenia and BP before the first-episode would be very informative. These findings do not exclude the possibility that there might be subgroups of BP who have no neurodevelopmental cognitive deficits as premorbid studies suggest that not only impaired cognitive functioning but also above-average academic achievement predicts BP.18,64
This study has a number of limitations. Studies included in this meta-analysis were based on medicated patient samples. Therefore, it was not possible to explore the potential effects of medications on cognitive impairment in FEBP and cognitive differences between FEBP and FES. Also, we were not able to investigate the effects of BP subtypes on our findings (BP I vs BP II; Psychotic vs nonpsychotic BP) due to the lack of FE studies comparing such groups. This subject requires further investigation in future studies as BP I and history of psychosis have been associated with relatively more severe cognitive impairments in established BP.65–67 Another consideration in interpreting the findings of this analysis is the relationship between global cognition and between-group differences for other cognitive domains. For example, in Barrett et al,31 while there were significant differences between FES and FEBP, there were no significant cognitive differences between subgroups of FES and FEBP patients matched for IQ. In the current meta-analysis, many studies were not matched for IQ, with significant group differences apparent between FEBP, FES, and healthy controls. IQ differences may reflect true differences, or may represent potential problems in sample matching that would influence the findings in other cognitive domains. Thus, in neurodevelopmental conditions, like bipolar disorder and schizophrenia, matching for general intelligence on the one hand can mask true cognitive deficits that are strongly associated with IQ, while not matching can overestimate such deficits. Further, lack of comprehensive data across various domains in many studies, as well as limited studies investigating longitudinal changes, were further limitations of our findings.
Conclusion
As a conclusion, the evidence showing that cognitive deficits are already evident at FEBP suggests that neurodevelopmental factors play an important role in the development of cognitive deficits not only in schizophrenia but also in BP. Future studies investigating premorbid trajectories of cognitive functions in BP from childhood to first-episode are important to clarify potential differences and similarities of developmental trajectories of BP and schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
NHMRC Senior Principal Research Fellowship (628386 to C.P.) and a National Alliance for Research on Schizophrenia and Depression (NARSAD) Distinguished Investigator Award from the Brain and Behavior Research Foundation (USA; 18722 to C.P.)
Supplementary Material
Acknowledgments
We would like to thank to Dr Ingrid Melle, Dr Jolanta Zanelli, Dr Suzanne Barrett for providing additional data. The authors have declared that there are no conflicts of interest in relation to the subject of this study. Over the last 2 years, Christos Pantelis has participated on Advisory Boards for Janssen-Cilag and Lundbeck. He has received honoraria for talks presented at educational meetings organized by Janssen-Cilag, Astra Zenaca, and Lundbeck.
References
- 1. Gold JM, Harvey PD. Cognitive deficits in schizophrenia. Psychiatr Clin North Am. 1993;16:295–312. [PubMed] [Google Scholar]
- 2. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 3. Bora E, Yücel M, Pantelis C. Cognitive impairment in schizophrenia and affective psychoses: implications for DSM-V criteria and beyond. Schizophr Bull. 2010;36:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. [DOI] [PubMed] [Google Scholar]
- 5. Bora E, Yucel M, Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. Br J Psychiatry. 2009;195:475–482. [DOI] [PubMed] [Google Scholar]
- 6. Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80:137–149. [DOI] [PubMed] [Google Scholar]
- 7. Bora E. Developmental lag and course of cognitive deficits from the premorbid to postonset period in schizophrenia. Am J Psychiatry. 2014;171:369. [DOI] [PubMed] [Google Scholar]
- 8. Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159:1183–1189. [DOI] [PubMed] [Google Scholar]
- 9. Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. [DOI] [PubMed] [Google Scholar]
- 10. Reichenberg A, Caspi A, Harrington H, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bora E, Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophr Res. 2013;144:31–36. [DOI] [PubMed] [Google Scholar]
- 12. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. [DOI] [PubMed] [Google Scholar]
- 13. Bora E. Neurodevelopmental origin of cognitive impairment in schizophrenia. Psychol. Med. 2014b. In press. [DOI] [PubMed] [Google Scholar]
- 14. Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? BMJ(Clin Res Ed) 1987:681–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pantelis C, Yücel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. [DOI] [PubMed] [Google Scholar]
- 16. Weinberger DR. The pathogenesis of schizophrenia: a neurodevelopmental theory. In: Nasrallah RA, Weinberger DR, eds. The Neurology of Schizophrenia. Amsterdam, the Netherlands: Elsevier; 1986: 387–405. [Google Scholar]
- 17. Kumar CT, Frangou S. Clinical implications of cognitive function in bipolar disorder. Ther Adv Chronic Dis. 2010;1:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MacCabe JH, Lambe MP, Cnattingius S, et al. Excellent school performance at age 16 and risk of adult bipolar disorder: national cohort study. Br J Psychiatry. 2010;196:109–115. [DOI] [PubMed] [Google Scholar]
- 19. Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;71:405–416. [DOI] [PubMed] [Google Scholar]
- 20. Goodwin GM, Martinez-Aran A, Glahn DC, Vieta E. Cognitive impairment in bipolar disorder: neurodevelopment or neurodegeneration? An ECNP expert meeting report. Eur Neuropsychopharmacol. 2008;18:787–793. [DOI] [PubMed] [Google Scholar]
- 21. Demjaha A, MacCabe JH, Murray RM. How genes and environmental factors determine the different neurodevelopmental trajectories of schizophrenia and bipolar disorder. Schizophr Bull. 2012;38:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zanelli J, Reichenberg A, Morgan K, et al. Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am J Psychiatry. 2010;167:78–85. [DOI] [PubMed] [Google Scholar]
- 23. Lee RS, Hermens DF, Scott J, et al. A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. J Psychiatr Res. 2014;57:1–11. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 26. López-Jaramillo C, Lopera-Vásquez J, Gallo A, et al. Effects of recurrence on the cognitive performance of patients with bipolar I disorder: implications for relapse prevention and treatment adherence. Bipolar Disord. 2010;12:557–567. [DOI] [PubMed] [Google Scholar]
- 27. Larson ER, Shear PK, Krikorian R, Welge J, Strakowski SM. Working memory and inhibitory control among manic and euthymic patients with bipolar disorder. J Int Neuropsychol Soc. 2005;11:163–172. [DOI] [PubMed] [Google Scholar]
- 28. Lebowitz BK, Shear PK, Steed MA, Strakowski SM. Verbal fluency in mania: relationship to number of manic episodes. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:177–182. [PubMed] [Google Scholar]
- 29. Torres IJ, DeFreitas VG, DeFreitas CM, et al. Neurocognitive functioning in patients with bipolar I disorder recently recovered from a first manic episode. J Clin Psychiatry. 2010;71:1234–1242. [DOI] [PubMed] [Google Scholar]
- 30. Ayres AM, Busatto GF, Menezes PR, et al. Cognitive deficits in first-episode psychosis: a population-based study in São Paulo, Brazil. Schizophr Res. 2007;90:338–343. [DOI] [PubMed] [Google Scholar]
- 31. Barrett SL, Mulholland CC, Cooper SJ, Rushe TM. Patterns of neurocognitive impairment in first-episode bipolar disorder and schizophrenia. Br J Psychiatry. 2009;195:67–72. [DOI] [PubMed] [Google Scholar]
- 32. Bücker J, Popuri S, Muralidharan K, et al. Sex differences in cognitive functioning in patients with bipolar disorder who recently recovered from a first episode of mania: data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM). J Affect Disord. 2014;155:162–168. [DOI] [PubMed] [Google Scholar]
- 33. DeFreitas VG. The Nature and Specificity of Verbal Memory Interference in First Episode Schizophrenia. Simon Fraser University; 2014. (thesis) [Google Scholar]
- 34. Chan RC, Lui SS, Wang Y, et al. Patients with bipolar disorders share similar but attenuated prospective memory impairments with patients with schizophrenia. Psychol Med. 2013;43:1639–1649. [DOI] [PubMed] [Google Scholar]
- 35. Daros AR, Ruocco AC, Reilly JL, Harris MS, Sweeney JA. Facial emotion recognition in first-episode schizophrenia and bipolar disorder with psychosis. Schizophr Res. 2014;153:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Demmo C, Melle IM, Kvitland LK, Andreassen OAA, Vik Lagerberg TVL, Ueland TU. Neurocognitive performance in first episode bipolar i disorder compared to first episode schizophrenia and healthy controls. Bipolar Disord 2014;16:104. [Google Scholar]
- 37. Hellvin T, Sundet K, Simonsen C, et al. Neurocognitive functioning in patients recently diagnosed with bipolar disorder. Bipolar Disord. 2012;14:227–238. [DOI] [PubMed] [Google Scholar]
- 38. Dickerson F, Stallings C, Vaughan C, et al. Cognitive functioning in recent onset psychosis. J Nerv Ment Dis. 2011;199:367–371. [DOI] [PubMed] [Google Scholar]
- 39. Fleck DE, Shear PK, Madore M, Strakowski SM. Wisconsin Card Sorting Test performance in bipolar disorder: effects of mood state and early course. Bipolar Disord. 2008;10:539–545. [DOI] [PubMed] [Google Scholar]
- 40. Gruber SA, Rosso IM, Yurgelun-Todd D. Neuropsychological performance predicts clinical recovery in bipolar patients. J Affect Disord. 2008;105:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hill SK, Reilly JL, Harris MS, et al. A comparison of neuropsychological dysfunction in first-episode psychosis patients with unipolar depression, bipolar disorder, and schizophrenia. Schizophr Res. 2009;113:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hirayasu Y, McCarley RW, Salisbury DF, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Janssen J, Alemán-Gómez Y, Schnack H, et al. Cortical morphology of adolescents with bipolar disorder and with schizophrenia. Schizophr Res. 2014;158:91–99. [DOI] [PubMed] [Google Scholar]
- 44. Minzenberg MJ, Gomes GC, Yoon JH, Swaab TY, Carter CS. Disrupted action monitoring in recent-onset psychosis patients with schizophrenia and bipolar disorder. Psychiatry Res. 2014;221:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mojtabai R, Bromet EJ, Harvey PD, Carlson GA, Craig TJ, Fennig S. Neuropsychological differences between first-admission schizophrenia and psychotic affective disorders. Am J Psychiatry. 2000;157:1453–1460. [DOI] [PubMed] [Google Scholar]
- 46. Nehra R, Chakrabarti S, Pradhan BK, Khehra N. Comparison of cognitive functions between first- and multi-episode bipolar affective disorders. J Affect Disord. 2006;93:185–192. [DOI] [PubMed] [Google Scholar]
- 47. Owoeye O, Kingston T, Scully PJ, et al. Epidemiological and clinical characterization following a first psychotic episode in major depressive disorder: comparisons with schizophrenia and bipolar I disorder in the Cavan-Monaghan First Episode Psychosis Study (CAMFEPS). Schizophr Bull. 2013;39:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh MK, Chang KD, Chen MC, et al. Volumetric reductions in the subgenual anterior cingulate cortex in adolescents with bipolar I disorder. Bipolar Disord. 2012;14:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thomas P, Kearney G, Napier E, Ellis E, Leuder I, Johnson M. Speech and language in first onset psychosis differences between people with schizophrenia, mania, and controls. Br J Psychiatry. 1996;168:337–343. [DOI] [PubMed] [Google Scholar]
- 50. Zabala A, Rapado M, Arango C, et al. Neuropsychological functioning in early-onset first-episode psychosis: comparison of diagnostic subgroups. Eur Arch Psychiatry Clin Neurosci. 2010;260:225–233. [DOI] [PubMed] [Google Scholar]
- 51. Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. [DOI] [PubMed] [Google Scholar]
- 52. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 53. Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 55. Rosenthal R, Rosnow R. Essentials of Behavioral Research: Methods and Data Analysis. 1991; New York, NY: McGraw Hill. [Google Scholar]
- 56. Trotta A, Murray RM, MacCabe JH. Do premorbid and post-onset cognitive functioning differ between schizophrenia and bipolar disorder? A systematic review and meta-analysis. Psychol Med. 2014:1–14. [DOI] [PubMed] [Google Scholar]
- 57. Balanzá-Martínez V, Tabarés-Seisdedos R, Selva-Vera G, et al. Persistent cognitive dysfunctions in bipolar I disorder and schizophrenic patients: a 3-year follow-up study. Psychother Psychosom. 2005;74:113–119. [DOI] [PubMed] [Google Scholar]
- 58. Torres IJ, Kozicky J, Popuri S, et al. 12-month longitudinal cognitive functioning in patients recently diagnosed with bipolar disorder. Bipolar Disord. 2014;16:159–171. [DOI] [PubMed] [Google Scholar]
- 59. Tohen M, Zarate CA, Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160:2099–2107. [DOI] [PubMed] [Google Scholar]
- 60. Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull. 2011;37:504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pantelis C, Yücel M, Bora E, et al. Neurobiological markers of illness onset in psychosis and schizophrenia: The search for a moving target. Neuropsychol Rev. 2009;19:385–398. [DOI] [PubMed] [Google Scholar]
- 62. Testa R, Pantelis C. The role of executive functions in psychiatric disorders. In: Wood SJ, Allen N, Pantelis C, eds. The Neuropsychology of Mental Illness 2009:117–137. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 63. Olvet DM, Stearns WH, McLaughlin D, Auther AM, Correll CU, Cornblatt BA. Comparing clinical and neurocognitive features of the schizophrenia prodrome to the bipolar prodrome. Schizophr Res. 2010;123:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bora E. Developmental trajectory of cognitive impairment in bipolar disorder: comparison with schizophrenia. Eur Neuropsychopharmacol. 2014. doi:10.1016/j.euroneuro. 2014.09.007. [DOI] [PubMed] [Google Scholar]
- 65. Bora E, Yücel M, Pantelis C. Neurocognitive markers of psychosis in bipolar disorder: a meta-analytic study. J Affect Disord. 2010;127:1–9. [DOI] [PubMed] [Google Scholar]
- 66. Bora E, Yücel M, Pantelis C, Berk M. Meta-analytic review of neurocognition in bipolar II disorder. Acta Psychiatr Scand. 2011;123:165–174. [DOI] [PubMed] [Google Scholar]
- 67. Bora E, Berk M. Psychosis continuum and neurocognition in bipolar disorder. Rev Bras Psiquiatr. 2011;33:319–320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.