Abstract
Both cannabis use and the dopamine receptor (DRD2) gene have been associated with schizophrenia, psychosis-like experiences, and cognition. However, there are no published data investigating whether genetically determined variation in DRD2 dopaminergic signaling might play a role in individual susceptibility to cannabis-associated psychosis. We genotyped (1) a case-control study of 272 patients with their first episode of psychosis and 234 controls, and also from (2) a sample of 252 healthy subjects, for functional variation in DRD2, rs1076560. Data on history of cannabis use were collected on all the studied subjects by administering the Cannabis Experience Questionnaire. In the healthy subjects’ sample, we also collected data on schizotypy and cognitive performance using the Schizotypal Personality Questionnaire and the N-back working memory task. In the case-control study, we found a significant interaction between the rs1076560 DRD2 genotype and cannabis use in influencing the likelihood of a psychotic disorder. Among cannabis users, carriers of the DRD2, rs1076560, T allele showed a 3-fold increased probability to suffer a psychotic disorder compared with GG carriers (OR = 3.07; 95% confidence interval [CI]: 1.22–7.63). Among daily users, T carrying subjects showed a 5-fold increase in the odds of psychosis compared to GG carriers (OR = 4.82; 95% CI: 1.39–16.71). Among the healthy subjects, T carrying cannabis users had increased schizotypy compared with T carrying cannabis-naïve subjects, GG cannabis users, and GG cannabis-naïve subjects (all P ≤ .025). T carrying cannabis users had reduced working memory accuracy compared with the other groups (all P ≤ .008). Thus, variation of the DRD2, rs1076560, genotype may modulate the psychosis-inducing effect of cannabis use.
Key words: dopamine receptors type 2, G × E interaction, schizophrenia, schizotypy, working memory.
Introduction
Schizophrenia is a complex disorder in which both genetic variation and environmental factors play an etiological role.1–4 Findings from epidemiological studies suggest that cannabis use represents an environmental risk factor for schizophrenia and psychotic disorders.5,6 Meta-analyses indicate that psychosis risk rises with increasingly heavy cannabis exposure, and estimate that cannabis use may account for 8–14% of schizophrenia cases.7,8 Recent evidence from studies on healthy subjects suggests that cannabis use is associated with increased levels of schizotypy9 and can induce transient psychotic symptoms.10 However, the effect of cannabis use on cognition remains unclear.11 Inconsistent findings have been reported with cannabis use being associated with poor cognition in healthy subjects,12–15 but with better cognition among psychotic patients.16–20
The neurobiological mechanisms underlying how cannabis exposure may confer increased schizophrenia susceptibility are poorly understood.21–24 Increased dopamine synthesis capacity and release have been reported in psychotic patients as well as in the prodromal phase of psychosis.25,26 Drugs that increase dopamine release can induce or worsen psychosis27 and the effects of the main psychoactive ingredient of cannabis, delta-9-tetrahydrocannabinol (Δ9-THC),28 have also been related to dopamine dysfunction.29–31
Experimental data indicate that the dopamine receptor type 2 (D2) and schizophrenia are related.32–37 Two isoforms of the D2 receptor are known. The D2 long isoform (D2L) is mainly postsynaptic and is a target for haloperidol, and the D2 short (D2S) isoform is mainly presynaptic and serves as an autoreceptor regulating dopamine synthesis and release.38 These 2 isoforms are coded by D2 receptor gene (DRD2-11q23) with a mechanism of alternative splicing acting at exon 6. A previous study39 characterized a functional single-nucleotide polymorphism (SNP) within DRD2 at intron 6 (rs1076560—guanine > timine—G > T) associated with relative expression of the 2 isoforms in the frontal cortex. In particular, the T allele shifts splicing from D2S to D2L, decreasing the D2S/D2L ratio relative to the G allele and causing reduced prefrontal presynaptic D2 expression. The T allele has also been associated with putative greater levels of striatal dopamine.40
The DRD2 gene has been identified in a number of studies including a recent huge genome-wide association study35 as a risk gene for schizophrenia. However, to our knowledge, no research has been carried out to investigate the role of the DRD2 gene in the context of the association between cannabis use and psychosis and/or psychosis-related phenotypes. The aim of this study was 2-fold: first, to test if the functional genetic variation within DRD2 rs1076560 interacts with cannabis use to predict risk of psychosis in a case-control study; second, to test if such variation interacts with cannabis use to predict psychosis-related clinical and cognitive phenotypes in a study of healthy subjects. Given the relevance of DRD2 rs1076560 for dopaminergic signaling and psychosis-related phenotypes, including clinical symptoms and cognitive alteration, in both schizophrenia patients and controls,37–42 we hypothesized that rs1076560 T allele would interact with cannabis use to predict increased risk of psychosis in the case-control study as well as increased schizotypy and reduced cognitive performance in healthy subjects.
Methods
Case-Control Study
Participants.
Participants were recruited as part of the Genetic and Psychosis project (GAP),43 a case-control study, carried out at the Institute of Psychiatry, London. All patients presenting to the Adult services (18–65years) of the South London and Maudsley Mental Health NHS Foundation Trust, between December 2005 and October 2010, with their first episode of psychosis, were recruited into the study. Patient diagnosis of nonorganic psychosis (ICD 10: F20-F29 and F30-F33) was validated by administering the Schedules for Clinical Assessment in Neuropsychiatry (SCAN).44 Cases who met criteria for organic psychosis (F09) were excluded. About 23% of the patients approached refused to take part in the study; they were more likely to be of Black [χ2(3)=52.9; P < .01] and of male gender [χ2(1) = 75.3; P < .01] compared to those who consented. Therefore, ethnicity and gender were controlled for in all the analyses. Over the same time frame, from the area served by the mental health units, we recruited a sample of control subjects, aged 18–65 years, representing the local population in terms of ethnicity and other main sociodemographics according to the appropriate census data (www.statistics.gov.uk/census). Those willing to take part in the study were administered the Psychosis Screening Questionnaire (PSQ)45 and were excluded if they met criteria for a psychotic disorder or if they reported a previous diagnosis of psychotic illness. For a more detailed description of the GAP study methods, see Di Forti et al.46,47
The data presented here are based on the subset of the whole GAP sample (N cases = 272/461, 59%; N controls = 234/389, 60%) recruited up to 2008, when the DRD2 genotyping was carried out. On all subjects included in our analyses, we have complete data on sociodemographics, cannabis, and other drug use.
General Assessment.
Sociodemographic data (age, gender, and self-reported ethnicity) on patients and control subjects were collected using the Medical Research Council Social Scale (MRCSS).48
Validation of Self-Report of Ethnicity.
To confirm self-report of ethnicity (MRCSS),48 genetic ancestry was derived using a panel of 57 ancestry informative genetic markers, as done previously.46 These were genotyped using iPLEX technology developed for the MassArray platform (Sequenom Inc.). Ancestry scores were derived using the program Structure to implement a model-based (Markov Chain Monte Carlo) clustering algorithm. Having determined the best solution for K (the probable true number of underlying genetic groups) in initial analyses, individuals who scored between 96% and 100% for genetic cluster membership were used to create a 3-way ancestral axis based on Black African (n = 81), European Caucasian (n = 118), and Asian (n = 16) ancestry. These reference groups were used to index genetic ancestry for the remaining sample. Further information on the makeup of the marker panel as well as a figure reporting plots of 3-way ancestral axis based on Black African, European Caucasian and Other are available on request. Ninety percent (N = 453) of participants had information on both self-reported ethnicity and ancestry markers. The level of overall agreement between self-reported and genetic ethnicities (95%) was reassuringly high.
History of Cannabis and Other Drugs Use.
Participants were administered the the Cannabis Experience Questionnaire modified version (CEQmdv)43,46 to assess their lifetime use of cannabis, stimulants and other drugs use. They were also screened for nicotine dependence (Fagerström Test for Nicotine Dependence (FTND)49 and harmful drinking (HD) behavior.
Genotyping.
DNA was obtained from all participants recruited up to 2008 (N = 512). Seventy-five percent of DNA samples used originated from blood and 25% from cheek swabs. DNA extraction was performed using standard phenol-chloroform methods. Off the shelf Taqman assays for DRD2 rs1076560 are available as a kit, at http://www.appliedbiosystems.com.
Genotype calls, successful call rate 99% (N = 506), were discriminated based on algorithmic membership of 3 clusters representing homozygote G/G, heterozygote G/T, and homozygote T/T genotype classes. A comparison of genotype results for individuals with overlapping blood and cheek swab DNA revealed there was 100% concordance between blood- and cheek-derived genotype data.
Ethics.
The study was granted ethical approval by the South London and Maudsley and Institute of Psychiatry Local Research Ethics Committee. All cases and control subjects included gave informed written consent, signing the consent document, to the publication of data originating from the study.
Data Analysis.
For the statistical analyses, given the low number of subjects carrying 2 copies of the minor allele (TT subjects), we collapsed them with the GT subjects in group 1 = TT + GT (T carriers) to be compared to group 2 = GG carriers. This procedure is consistent with a series of earlier studies evaluating polymorphisms with low minor allele frequencies, especially when codominance of the alleles is not known.50 Additional sociodemographic and lifestyle variables (gender, age, ethnicity, nicotine dependence, harmful alcohol use, and other substance use) were modeled as potential confounders. Analysis of variance (ANOVAs) and χ2were used to evaluate the relation between genotype and demographics data as well as the relationship between lifetime cannabis use (yes/no) and demographics data, in both patients and control subjects. χ2 was used to evaluate the relationship between potential confounders and genotype/cannabis use as well as between potential confounders and presence of psychotic disorder. Further, χ2 were used to determine whether cannabis use was associated with DRD2 genotype in both patients and control subjects. Finally, a multivariable logistic regression, with history of lifetime cannabis use (yes/no) and DRD2 rs10764560 genotype (GG, T carrier) as independent variables, was used to evaluate the main effects as well as the interaction between cannabis use and rs1076560 on presence of a psychotic disorder after adjusting for the modeled potential confounders. The interaction model examined the probability of having a psychotic disorder among DRD2 rs1076560 T carrying cannabis users compared with GG subjects. Odds ratios (OR) of psychosis among T carrying cannabis-naïve subjects were also calculated from the estimates provided by the model. Also a multivariable logistic regression was performed, excluding from the analysis control subjects aged 30 (median) or less, in order to investigate the possible confounding effect of having included in the sample control subjects who had not passed the cases’ average age of psychosis onset (28.67±8.71 years). Further multivariable logistic regressions were performed for each ethnic group (White Caucasian, Black Caribbean, Black African, and Asian/other). A second multivariable logistic regression with lifetime frequency of cannabis use (daily/weekly or less) and DRD2 rs10764560 genotype (GG, T carrier) as independent variables, was used to evaluate the main effects as well as the interaction between frequency of cannabis use and rs1076560 on presence of a psychotic disorder, after adjusting for the modeled potential confounders. The statistical threshold was set at P < .05.
Healthy Subjects Study
Participants.
From a sample of 252 healthy subjects, 2 partially overlapped groups of respectively 170 (sample for schizotypal traits evaluation, SCZt) and 221 (sample for working memory performance evaluation, WMp) individuals entered the healthy subjects study. All participants were unrelated Caucasians from the region of Puglia in Italy. Protocols and procedures were approved by the local Institutional Review Board. After complete description of the study to the subjects, written informed consent was obtained. Subjects underwent the Structured Clinical Interview for DSM-IV to exclude any Axis I psychiatric disorder. Exclusion criteria were active cannabis use in the past 6 months, use of other stimulant drugs, head trauma with loss of consciousness, and any significant medical illness. Parental socioeconomic status (Hollingshead Scale), handedness (Edinburgh Inventory), and total intelligence quotient (IQ) (WAIS-R) were also measured.
Genotyping.
All subjects were genotyped for DRD2 rs1076560. This SNP was analyzed with allele-specific polymerase chain reaction primers as described previously.37,39
Substance Use Evaluation.
All participants were asked about their use of tobacco, alcohol, and cannabis. Users were interviewed using the Fagerström Test for Nicotine Dependence (FTND),49 the Tolerance, Worried, Eye-opener, Amnesia, and Cut down (TWEAK) alcohol screening test,51 and the CEQ,43 to determine lifetime nicotine dependence, HD behavior, and cannabis use, respectively.
Schizotypal Traits Evaluation.
Schizotypal traits were assessed using the Schizotypal Personality Questionnaire (SPQ).52 The SPQ is a self-report scale based on the DSM-III-R criteria for schizotypal personality disorder and it is widely used to screen for schizotypal traits in the general population. The SPQ has excellent internal consistency (0.91), good test-retest reliability (0.82), discriminant validity, and correlates highly with clinical schizotypy.52
Cognitive Performance Evaluation.
Subjects completed the N-back working memory task.40 Briefly, n back refers to how far back in the sequence of stimuli the subject has to recall. The stimuli consisted of numbers (1–4) shown in random sequence and displayed at the points of a diamond-shaped box. There was a visually paced motor task that also served as a nonmemory-guided control condition (0-back) that simply required subjects to identify the stimulus currently seen. In the working memory condition, the task required recollection of a stimulus seen 2 stimuli previously (2-back) while continuing to encode additionally incoming stimuli.
Data Analysis.
As in the case-control study, we collapsed the subjects according to their genotype into group 1 = TT + GT and group 2 = GG. Gender, age, HD behavior, and nicotine dependence were modeled as potential confounders. ANOVAs and χ2were used to evaluate the relation between genotype and demographics data as well as the relationship between cannabis use (yes/no) and demographics data. χ2 was used to evaluate the relationship between potential confounders and genotype/cannabis use. Finally, factorial ANOVAs, with cannabis use (yes/no) and DRD2 rs1076560 genotype (GG, T carrier) as independent variables were used to evaluate the main effects as well as the interaction between cannabis use and rs1076560 on schizotypy and working memory performance. Furthermore, factorial analysis of covariance (ANCOVAs), with cannabis use (yes/no) and DRD2 rs1076560 genotype (GG, T carrier) as independent variables, and the potential confounders as covariates, were used to evaluate the main effects as well as the interaction between cannabis use and rs1076560 on schizotypy and working memory performance respectively. Fisher’s test was used for post hoc analyses. The statistical threshold was set at P < .05.
Results
Case-Control Study
Demographic Measures and Allelic Distribution of the DRD2 rs1076560 Genotype.
First-episode psychosis (FEP) patients and control subjects differed significantly for some demographic characteristics. FEP patients tended to be significantly younger (F = 3.71, P = .055) and were also more likely to belong to the Black African/Caribbean ethnic group (χ2 = 41.53, P < .001); the 2 groups did not differ for gender (P > .1; table 1). On average, FEP patients’ psychosis onset occurred before age 30 years (table 1). Table 1 describes the allelic distribution of the rs1076560 genotype in the 272 FEP and in the 234 control subjects. After genotype determination, the groups (GG, T carriers) displayed Hardy-Weinberg equilibrium (P > .1). DRD2 genotype was not associated with any demographic measure or with diagnosis status (all P > .1). Among FEP patients DRD2 genotype was not associated with age of psychosis onset (P > .1; table 1).
Table 1.
Demographic Measures of First-Episode Psychosis Patients and Control Subjects
| FEP Patients n = 272 (181 Males) | Control Subjects n = 234 (141 Males) | Statistical Comparisons | |
|---|---|---|---|
| ANOVA | |||
| Age (M ± SD) | 29.64±8.72 | 31.23±9.84 | F = 3.71, P = .055 |
| Range | 18–55 | 18–61 | |
| Distribution | n (%) | n (%) | X 2 |
| 18–29 | 126 (46.3) | 101 (43.2) | P > .1 |
| 30–39 | 109 (40.1) | 75 (32) | |
| 40–49 | 31 (11.4) | 46 (19.7) | |
| 50–59 | 6 (2.2) | 11 (4.7) | |
| 60–65 | — | 1 (0.4) | |
| ANOVA | |||
| Age of psychosis onset (M ± SD) | 28.67±8.71 | ||
| GG (M ± SD) | 28.51±8.92 | P > .1 | |
| T car (M ± SD) | 27.62±9.1 | ||
| n (%) | n (%) | X 2 | |
| Self-reported ethnicity | χ2 = 41.53, P < .001 | ||
| White Caucasian | 86 (31.6) | 136 (58.1) | |
| Black Caribbean | 81 (29.8) | 38 (16.2) | |
| Black African | 75 (27.6) | 32 (13.7) | |
| Asian/other | 30 (11) | 28 (12) | |
| DRD2 rs1076560 allelic frequency | P > .1 | ||
| GG | 205 (75.4) | 174 (74.4) | |
| GT | 62 (22.8) | 55 (23.5) | |
| TT | 5 (1.8) | 5 (2.1) | |
| Tobacco use | χ2 = 27.14, P < .001 | ||
| Nicotine dependence | 191 (70.2) | 111 (47.4) | |
| Not nicotine dependence | 81 (29.8) | 123 (52.6) | |
| Stimulants use | χ2 = 4.42, P = .036 | ||
| Yes | 113 (41.5) | 76 (32.5) | |
| Never | 159 (58.5) | 158 (67.5) | |
| Alcohol use | χ2 = 17.37, P < .001 | ||
| Harmful drinking behavior | 196 (72.1) | 204 (87.2) | |
| Not harmful drinking behavior | 76 (27.9) | 30 (12.8) | |
| Cannabis use | P > .1 | ||
| Yes | 184 (67.6) | 142 (60.7) | |
| GG | 143 (77.7) | 100 (70.4) | |
| T car | 41 (22.3) | 42 (29.6) | |
| Never | 88 (32.4) | 92 (39.3) | |
| GG | 62 (70.4) | 74 (80.4) | |
| T car | 26 (29.6) | 18 (19.6) | |
| Frequency of use | χ2 = 14.30, P < .001 | ||
| Daily a | 96 (63.6) | 43 (39.8) | |
| GG | 75 (78.1) | 30 (69.8) | |
| T car | 21 (21.9) | 13 (30.2) | |
| Weekly or less a | 55 (36.4) | 65 (60.2) | |
| GG | 42 (76.4) | 46 (70.8) | |
| T car | 13 (23.6) | 19 (29.2) | |
| No detailsa | 33 | 34 | |
| Age of first use (M ± SD)a | 16.18±4.82 | 16.71±2.91 | P > .1 |
| GG (M ± SD)a | 16.24±5.02 | 16.69±2.96 | |
| T car (M ± SD)a | 15.97±4.13 | 16.76±2.87 |
Note: FEP, first-episode psychosis.
aIn those who had ever used cannabis.
Pattern of Substance Use.
More than two-thirds of the FEP patients (70.2%) reported nicotine dependence compared with 47.4% of the control subjects (χ2 = 27.14, P < .001). Similarly, FEP patients reported more frequently other illicit drug use (41.5%) compared to control subjects (32.5%; χ2 = 4.42, P = .036) but control subjects reported more frequently HD behavior (87.2%) compared to FEP patients (72.1%; χ2=17.37, P < .001). 67.6% of the FEP patients and 60.7% of the control subjects reported ever having used cannabis. Among those who had a history of lifetime cannabis use, FEP patients were more likely than control subjects to report daily use (63.6% vs 39.8%; χ2 = 14.30, P < .001). FEP patients and control subjects did not differ in their age of first use of cannabis (P > .1; table 1). On average, psychosis onset among FEP patients occurred 12.19±9.38 years after the first use of cannabis (table 1). No patient involved in this study started to use cannabis for the first time after the psychosis onset.
Case-Control G × E Analyses.
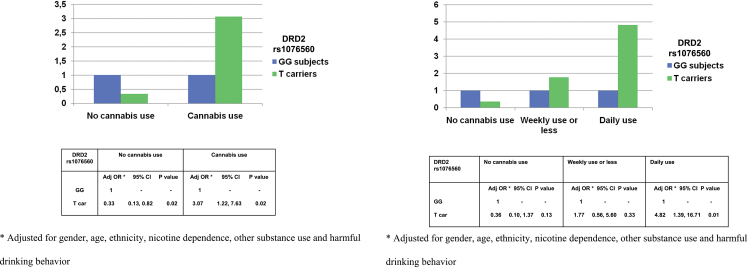
The 2 genotype groups (TT+GT vs GG) did not differ in their pattern of substance use (prevalence, frequency or age of first use of cannabis) in both FEP patients and control subjects (all P > .1; table 1). A multivariable logistic regression adjusting for gender, age, ethnicity, tobacco use (nicotine dependence), stimulants use, and alcohol use (HD behavior) showed a significant interaction between lifetime cannabis use and DRD2 rs1076560 on probability of suffering from a psychotic disorder (N = 506, likelihood ratio test =5.75; P = .017). Among subjects having a lifetime history of cannabis use, T carrying subjects showed a 3-` increased odds of having psychotic disorder (OR = 3.07; 95% confidence interval [CI]: 1.22,7.63) when compared with GG subjects. On the contrary, among those who had never used cannabis, the presence of the T allele was associated with lower odds of suffering a psychotic disorder (OR = 0.33; 95% CI: 0.13,0.82) compared with GG genotype (figure 1a). A further multivariable logistic regression on psychosis risk, adjusting for the modeled potential confounders and excluding from the analysis the younger half of the control subjects’ sample (age < 30), indicated that the presence in the main analysis of control subjects who have not passed the cases’ average age of psychosis onset is not likely to affect the results significantly. Further multivariable logistic regressions on psychosis risk performed separately for each ethnic group adjusting for the modeled potential confounders indicated that ethnicity did not affect the results significantly. A second multivariable logistic regression adjusting for the modeled potential confounders showed a significant interaction between lifetime frequency of cannabis use and DRD2 rs1076560 on risk of psychosis. The analysis showed an increasing probability of suffering from a psychotic disorder in T carrying cannabis users depending on frequency of use (N = 439, likelihood ratio test =6.50; P = .039). Among both occasional and daily cannabis users, T carrying subjects showed increased odds of having psychotic disorder when compared with GG subjects, but only among daily cannabis users did the increased odds of psychosis shown by T carrying subjects reach significance (OR = 4.82; 95% CI: 1.39,16.71). On the contrary, among those who had never used cannabis, the presence of the T allele was associated with lower odds of suffering a psychotic disorder (OR = 0.36; 95% CI: 0.10, 1.37) compared with GG genotype, even if the OR failed to reach significance (figure 1b).
Fig. 1.
(a) Interaction between DRD2 rs1076560 and lifetime cannabis use on psychosis risk. (b) Interaction between DRD2 rs1076560 and lifetime frequency of cannabis use on psychosis risk.
Healthy Subjects Study
Demographic Measures and Allelic Distribution of the DRD2 rs1076560 Genotype.
Tables 2 and 3 describe demographic measures and allelic distribution of the rs1076560 genotype of the 2 samples for the evaluation of schizotypal traits and cognitive performance respectively. After genotype determination, both groups (GG, T carriers) displayed Hardy-Weinberg equilibrium (all P > .1). Furthermore, SCZt and WMp genotype groups did not differ for any demographic measure (sex, age, parental socioeconomical status, handedness and total IQ, all P > .1). Moreover, there were no significant differences in demographics between cannabis users and cannabis naïve subjects (all P > .1; tables 2 and 3).
Table 2.
Description of the Sample for the Schizotypal Traits Evaluation
| Sample n = 170 (82 Males) | DRD2 rs1076560 GG Subjects n = 116 (57 Males) | DRD2 rs1076560 T Carriers n = 54 (1 TT) (25 Males) | Cannabis-Naïve Subjects n = 103 (50 Males) | Cannabis Users n = 67 (32 Males) | |
|---|---|---|---|---|---|
| Age (M ± SD) | 28.36 (8.23) | 28.41 (8.29) | 28.26 (8.18) | 28.63 (8.64) | 27.94 (7.60) |
| Range | 19–51 | 19–51 | 19–49 | 19–51 | 19–50 |
| Distribution | n (%) | n (%) | n (%) | n (%) | n (%) |
| 18–29 | 134 (78.8) | 91 (78.5) | 43 (79.6) | 82 (79.6) | 52 (77.6) |
| 30–39 | 24 (14.1) | 16 (13.8) | 8 (14.8) | 14 (13.6) | 10 (14.9) |
| 40–49 | 10 (5.9) | 7 (6) | 3 (5.6) | 6 (5.8) | 4 (6) |
| 50–59 | 2 (1.2) | 2 (1.7) | 0 | 1 (1) | 1 (1.5) |
| 60–65 | — | — | — | — | — |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| PSES | 42.65 (16.97) | 43.01 (16.94) | 41.67 (17.21) | 42.80 (17.40) | 42.42 (16.40) |
| Handedness | 0.84 (0.26) | 0.84 (0.26) | 0.83 (0.27) | 0.84 (0.27) | 0.83 (0.27) |
| IQ | 107.52 (11.78) | 107.74 (11.41) | 107.09 (12.72) | 107.50 (11.32) | 107.55 (12.72) |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Tobacco use | |||||
| ND yes | 52 (41.9) | 34 (40.5) | 18 (45) | 27 (36.5) | 25 (50) |
| ND no | 72 (58.1) | 50 (59.5) | 22 (55) | 47 (63.5) | 25 (50) |
| No details | 46 | 32 | 14 | 29 | 17 |
| Alcohol use | |||||
| HD yes | 57 (46) | 38 (45.2) | 19 (47.5) | 33 (44.6) | 24 (48) |
| HD no | 67 (54) | 46 (54.8) | 21 (52.5) | 41 (55.4) | 26 (52) |
| No details | 46 | 32 | 14 | 29 | 17 |
| Cannabis use | |||||
| Yes | 67 (39.4) | 46 (39.7) | 21 (38.9) | ||
| Never | 103 (60.6) | 70 (60.3) | 33 (61.1) | ||
| Dailya | 8 (11.9) | 5 (10.9) | 3 (14.3) | ||
| Weekly or lessa | 59 (88.1) | 41 (89.1) | 18 (85.7) | ||
Note: HD, harmful drinking; IQ, Intelligence Quotient; ND, nicotine dependence; PSES, parental socioeconomical status.
aIn those who had ever used cannabis; there were no significant differences between GG subjects and T carrying subjects as well as between cannabis users and cannabis-naïve subjects among demographic measures and substance use (all P > .1).
Table 3.
Description of the Sample for the Cognitive Performance Evaluation
| Sample n = 221 (120 Males) |
DRD2 rs1076560 GG Subjects n =173 (93 Males) | DRD2 rs1076560 T Carriers n = 48 (3 TT) (27 Males) | Cannabis-Naïve Subjects n = 126 (67 Males) | Cannabis Users n = 95 (53 Males) | |
|---|---|---|---|---|---|
| Age (M ± SD) | 27.40 (7.69) | 27.31 (7.70) | 27.75 (7.71) | 27.23 (7.46) | 27.63 (8.02) |
| Range | 18–63 | 18–63 | 18–58 | 18–58 | 18–63 |
| Distribution | n (%) | n (%) | n (%) | n (%) | n (%) |
| 18–29 | 173 (78.3) | 137 (79.2) | 36 (75) | 99 (78.6) | 74 (77.9) |
| 30–39 | 29 (13.1) | 21 (12.1) | 8 (16.7) | 17 (13.5) | 12 (12.6) |
| 40–49 | 14 (6.3) | 11 (6.4) | 3 (6.2) | 7 (5.5) | 7 (7.5) |
| 50–59 | 4 (1.8) | 3 (1.7) | 1 (2.1) | 3 (2.4) | 1 (1) |
| 60–65 | 1 (0.5) | 1 (0.6) | — | — | 1 (1) |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| PSES | 43.13 (16.53) | 43.45 (16.67) | 41.99 (16.14) | 44.54 (16.51) | 41.26 (16.45) |
| Handedness | 0.82 (0.31) | 0.81 (0.32) | 0.85 (0.29) | 0.80 (0.36) | 0.85 (0.29) |
| IQ | 107.05 (11.71) | 107.55 (11.96) | 105.25 (10.71) | 107.48 (11.74) | 106.47 (11.72) |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Tobacco use | |||||
| ND yes | 68 (43) | 53 (42.4) | 15 (45.5) | 37 (42) | 31 (44.3) |
| ND no | 90 (57) | 72 (57.6) | 18 (54.5) | 51 (58) | 39 (55.7) |
| No details | 63 | 48 | 15 | 38 | 25 |
| Alcohol use | |||||
| HD yes | 76 (48.1) | 58 (46.4) | 18 (54.5) | 42 (47.7) | 34 (48.6) |
| HD no | 82 (51.9) | 67 (53.6) | 15 (45.5) | 46 (52.3) | 36 (51.4) |
| No details | 63 | 48 | 15 | 38 | 25 |
| Cannabis use | |||||
| Yes | 95 (43) | 77 (44.5) | 18 (37.5) | ||
| No | 126 (57) | 96 (55.5) | 30 (62.5) | ||
| Dailya | 10 (10.5) | 8 (10.4) | 2 (11.1) | ||
| Weekly or lessa | 85 (89.5) | 69 (89.6) | 16 (88.9) | ||
Note: HD, harmful drinking; IQ, Intelligence Quotient; ND, nicotine dependence; PSES, parental socioeconomical status.
aIn those who had ever used cannabis; there were no significant differences between GG subjects and T carrying subjects as well as between cannabis users and cannabis-naïve subjects among demographic measures and substance use (all P > .1).
Pattern of Substance Use.
Patterns of tobacco use (nicotine dependence), alcohol use (HD behavior), and cannabis use (lifetime exposure, frequency of use) are described in tables 2 and 3. SCzt and WMp genotype groups did not differ in their pattern of substance use; in addition, cannabis users and cannabis naïve subjects did not differ in their pattern of tobacco or alcohol use, in either the SCZt or WMp samples (all P > .1; tables 2 and 3).
Schizotypal Traits.
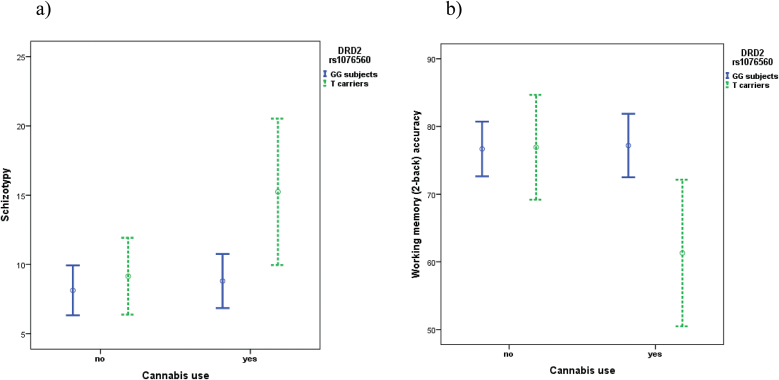
Factorial ANOVA on SPQ score data revealed: a main effect of DRD2 rs1076560, with T carriers having increased schizotypy compared with GG subjects (F 1,169 = 5.47; P = .020); a trend toward a main effect of cannabis use approaching significance, with cannabis users tending to have increased schizotypy compared to cannabis naïve subjects (F 1,169 = 3.42; P = .066). More importantly, there was an interaction between genotype and cannabis use (F1,166 = 4.05; P = .046). Fisher’s post hoc analysis revealed that, while cannabis use did not significantly affect schizotypy in the context of GG genotype, T carrying cannabis users had increased schizotypy compared with T carrying cannabis naïve subjects (P = .025), GG cannabis users (P = .001), and GG cannabis naïve subjects (P = .005) (figure 2a). Further ANCOVA on schizotypy in 124 out of 170 individuals using gender, age, nicotine dependence and HD behavior as covariates, indicated that these potentially confounding variables did not affect the results significantly.
Fig. 2.
Interaction between DRD2 rs1076560 and lifetime cannabis use on healthy subjects’ Schizotypy (SPQ questionnaire) and Working Memory Test (2-back) accuracy.
Cognitive Performance.
Factorial ANOVA on working memory accuracy data demonstrated: a trend towards a main effect of DRD2 rs1076560 approaching significance, with T carriers tending to have reduced accuracy compared with GG subjects (F 1,220 = 3.04; P = .083); and a significant interaction between genotype and cannabis use (F 1,217 = 6.49; P = .012). Fisher’s post hoc analysis revealed that, while cannabis use did not significantly affect working memory performance in the context of GG genotype, T carrying cannabis users performed less accurately than T carrying cannabis naïve subjects (P = .008), GG cannabis users (P = .003), and GG cannabis naïve subjects (P = .003) (figure 2b). No significant main effect or interaction of genotype and cannabis use was found on working memory reaction time. Further ANCOVA on accuracy and reaction time in 158 out of 221 individuals using gender, age, nicotine dependence, and HD behavior as covariates indicated that these potentially confounding variables do not affect the results.
Discussion
The present results indicate that rs1076560 interacts with cannabis use in modulating the risk of having a psychotic disorder, as well as schizotypal traits and cognitive performance in healthy subjects. These results provide another instance of interaction between cannabis exposure and genetic variation on risk of develop a psychotic disorder. Moreover, they shed new light on the potential for cannabis use to alter cognition and induce psychosis-like symptoms in healthy people of specific genetic backgrounds.
Studies on gene by cannabis use interaction (G × E) on psychosis risk and psychosis-related endophenotypes have mainly included genes involved in the regulation of dopaminergic system.7,53–56 Caspi et al53 reported that variation in COMT, which encodes the enzyme catechol-O-methyltransferase (COMT) that plays an important role in the degradation of dopamine in brain, modulated the risk of cannabis users developing psychosis; however, this report has not been consistently replicated.57–59 On the other hand both published reports examining AKT1, which encodes the serine/treonine kinase AKT1, an integral part of the dopamine receptor 2 (DRD2) signaling cascade in the striatum, reported an interaction with cannabis use on the risk of psychosis.46,54 Therefore, other genes that regulate signaling pathways and impact on dopamine transmission may be plausible candidates for such G × E interaction.
Our candidate for a mediating role in the association between cannabis use and psychosis risk as well as between cannabis use and psychosis-related endophenotypes was the DRD2 gene. D2 receptors are privileged targets of antipsychotic drugs, which antagonize their activity.32 Previous reports have suggested association between psychosis and relatively greater D2 density in striatum,33 and an aberrant D2 signaling has been associated with clinical symptoms.34 Also DRD2 rs1076560 has been associated with putatively greater levels of striatal dopamine.40 Importantly, a recent study from the Schizophrenia Working Group of the Psychiatric Genomics Consortium has indicated that DRD2, rs2514218, is also associated with diagnosis of schizophrenia,35 and this polymorphism is in linkage disequilibrium with rs1076560 (r 2 = 0.3, D’ = 0.54), albeit not strongly. In addition, the primary central effects of the psychostimulant drugs are enhancement of the dopamine concentration in the synaptic cleft and activation of D2-like dopamine receptors in the nucleus accumbens.60–62
Given the evidence that dopaminergic signaling is altered in schizophrenia patients and cannabis users31,63 and that DRD2 variation seems to be relevant for both psychotic symptoms and cognitive alteration,36,37,40,41 we investigated if DRD2 variation concurs to the psychosis-inducing effects of cannabis use. Our results indicate that the effect of lifetime cannabis use on the risk of suffering from a psychotic disorder as well as on the likelihood of presenting psychosis-like symptoms and altered cognition differ according to DRD2 rs1076560 genotype.
In the case-control study, we found an interaction between cannabis use and rs1076560 on psychosis risk, T carrying subjects showing an increased risk of having a psychotic disorder in the context of cannabis use compared to GG subjects. Our results suggest that specific DRD2 variation could represent a genetic vulnerability to the cannabis-induced increased risk of psychosis. A previous study indicated that the risk of psychosis depends, in part, on how frequently people use cannabis.43 Consistent with that study, a second analysis showed that in the context of occasional cannabis use, T carrying cannabis users have a relatively small increase in their psychosis risk which failed to reach significance. However, T carrying subjects who reported daily cannabis use showed the greatest risk of psychosis.
Our findings support previous reports suggesting that genetic variation within genes that regulate signaling pathways and impact on dopamine transmission influences the risk of developing a psychotic disorder in cannabis users depending on the frequency of use.46,54 Our results indicate a model of interaction known as “qualitative G × E interaction” with a cross over pattern: carriers of the DRD2 rs1076560 T allele, compared to GG subjects, have a lower probability of psychotic disorder if they never used cannabis but a higher probability if they have a history of cannabis use, especially of daily use. Our findings are in line with previous results in the field,15,46,53 and indicate that specific minor alleles may prevent or promote the risk for psychosis depending on the presence of a history of cannabis use. Such findings require validation in experimental designs and animal studies where both changes in the exposure and in the genotype can be modelled.
In the healthy subjects study, cannabis users had higher schizotypal symptoms than cannabis naïve subjects, in line with previous reports on a relation between cannabis use and greater schizotypy.9 In addition, we found an interaction between cannabis use and rs1076560 on schizotypy. In particular, in the context of cannabis use, carriers of the rs1076560 T allele had greater schizotypal symptoms compared to the other groups while cannabis use did not significantly increase schizotypal symptoms in the context of GG genotype. This result extends previous findings64 and suggests a moderating effect of variation in DRD2 on the relationship between cannabis use and schizotypy. Moreover, as elevated schizotypy and cannabis use have been associated with increased risk for psychosis,8,65 our result implicates the T allele of rs1076560 in genetic vulnerability to cannabis-induced increased psychosis-proneness in healthy subjects.
Finally, we find an interaction between cannabis use and rs1076560 on cognitive performance. Consistently with other results from this study, carriers of the rs1076560 T allele performed less accurately on a working memory task compared to other groups. Again, cannabis use did not significantly affect working memory performance in the context of GG genotype. This finding extends previous reports on association between cannabis use and cognitive processing in healthy subjects,12–15 suggesting that discrepancies in these studies may also be because of genetic variation having an impact on cognitive phenotypes.66 This result is also consistent with previous findings indicating that T carrying subjects show less efficient prefronto-striatal activity and reduced performance during working memory.37 Our result, together with this latter report, suggest that rs1076560 T allele, which per se seems to be related to less efficient cognitive performance,37,41 may represent a genetic vulnerability to cannabis-induced deleterious effects on cognition.
The lack of a T allele “protective” effect in cannabis-naïve healthy subjects’ schizotypy levels and cognitive performance could be due to several factors. Even if an allele conferred protection against a disease, it is not obvious that it would give advantage in terms of cognitive performance or in reducing schizotypal traits among healthy individuals. Several studies indicate that brain-imaging techniques have the potential to address a number of cognitive psychology questions with a higher degree of inferential and statistical power than the more traditional measures of cognitive psychology.67 Thus, a previous study reported that behavioral analyses alone were not able to detect a “protective” effect of CNR1 genetic variation on working memory performance in cannabis-naïve healthy subjects (ie better performance), while related imaging analyses revealed a more efficient prefrontal connectivity.15 Finally, the fact that the T allele in the absence of cannabis use is protective in the context of a disease does not necessarily translate to a population of healthy subjects in whom schizotypy does not translate into a full blown disease, probably because their genetics are more protective by definition. Also, the interaction between frequency of cannabis use and rs1076560 on risk of psychosis fails to reach significance for the T allele “protective” effect in cannabis-naïve subjects, possibly due to reduced statistical power.
The rs1076560 T allele has been previously associated with putatively greater levels of striatal dopamine,40 thus providing a possible neurobiological mechanism for the results of this study. More specifically, our results together with this previous report suggest that genetically determined increased dopamine levels in the striatum40 may modulate the psychosis-inducing effect of cannabis use. This interpretation is consistent with the classical hypothesis of a high release of striatal dopamine in schizophrenia32 and with the evidence that drugs increasing dopamine release can induce or worsen psychosis.27 In other words, as Δ9-THC can alter dopaminergic signaling,28–31 the interaction between DRD2 rs1076560 and cannabis use elicits the T allele related genetic vulnerability, due to an alteration in striatal dopamine. However, these findings need to be replicated. Even if gene by environment (G × E) research has received widespread attention, G × E findings remain controversial, suggesting that direct replications deserve more attention that novel findings or indirect replications.68 Moreover, longitudinal studies are needed to express conclusively on the direction of the association between DRD2 rs1076560 and cannabis-related psychosis. Even if our findings together with the previous literature43 suggest that cannabis users could develop psychosis based on a specific genetic background, results do not exclude the possibility that some patients use cannabis to ameliorate certain internal feelings or symptoms of psychosis. Cannabis use as self-medication for psychosis could coexist with cannabis-induced psychosis via genetics. Further studies are also needed to assess possible differences in cannabis-related psychosis risk among T carrying individuals due to ethnicity. Even if our analyses revealed that ethnicity is not likely to strongly affect the results of this study, the data are too limited to come to strong conclusions concerning ethnicity. Finally, the presence of only a limited number of subjects with daily cannabis use in the healthy subjects’ study is a limitation which needs to be taken into account. We could not test for an interaction between frequency of cannabis use and DRD2 genotype on healthy subjects’ levels of schizotypy and working memory performance because of the very limited statistical power.
These results support the theory that some individuals carry genetic vulnerability to the psychotogenic effect of cannabis. The character of this vulnerability is likely to be a polygenic gene-environment interaction. Identifying those genes and organizing them into genetic pathways will facilitate understanding the mechanisms that modulate the relationship between cannabis exposure and psychosis.
Funding
UK National Institute of Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health, South London and Maudsley (SLAM) and the Institute of Psychiatry at King’s College London; The Psychiatry Research Trust, the Maudsley Charity Research Fund; European Community’s Seventh Framework Program (HEALTHF2-2009–241909; Project EU-GEI).
Acknowledgments
This study has been carried out with the support of (1) The Genetic and Psychotic Disorders (GAP) Study, Institute of Psychiatry, King’s College London; (2) The South London and Maudsley (SLAM) Mental Health NHS Foundation Trust; (3) The Group of Psychiatric Neuroscience at the University of Bari. Prof. Alessandro Bertolino is a full time employee of Hoffman-La Roche Ltd. Conflict of interest statement: All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1. Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis”. J Psychiatr Res. 1999;33:543–548. [DOI] [PubMed] [Google Scholar]
- 2. van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 4. Tost H, Meyer-Lindenberg A. Puzzling over schizophrenia: schizophrenia, social environment and the brain. Nat Med. 2012;18:211–213. [DOI] [PubMed] [Google Scholar]
- 5. Di Forti M, Murray RM. Cannabis consumption and risk of developing schizophrenia: myth or reality? Epidemiol Psichiatr Soc. 2005;14:184–187. [DOI] [PubMed] [Google Scholar]
- 6. Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. [DOI] [PubMed] [Google Scholar]
- 7. Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. [DOI] [PubMed] [Google Scholar]
- 8. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. [DOI] [PubMed] [Google Scholar]
- 9. Fridberg DJ, Vollmer JM, O’Donnell BF, Skosnik PD. Cannabis users differ from non-users on measures of personality and schizotypy. Psychiatry Res. 2011;186:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. [DOI] [PubMed] [Google Scholar]
- 11. Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57:688–691. [DOI] [PubMed] [Google Scholar]
- 12. Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. [DOI] [PubMed] [Google Scholar]
- 13. Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. [DOI] [PubMed] [Google Scholar]
- 14. Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. [DOI] [PubMed] [Google Scholar]
- 15. Colizzi M, Fazio L, Ferranti L, et al. Functional genetic variation of the cannabinoid receptor 1 and cannabis use interact on prefrontal connectivity and related behavior. Neuropsychopharmacology. 2015;40:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jockers-Scherübl MC, Wolf T, Radzei N, et al. Cannabis induces different cognitive changes in schizophrenic patients and in healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1054–1063. [DOI] [PubMed] [Google Scholar]
- 17. Sevy S, Burdick KE, Visweswaraiah H, et al. Iowa gambling task in schizophrenia: a review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr Res. 2007;92:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mata I, Rodríguez-Sánchez JM, Pelayo-Terán JM, et al. Cannabis abuse is associated with decision-making impairment among first-episode patients with schizophrenia-spectrum psychosis. Psychol Med. 2008;38:1257–1266. [DOI] [PubMed] [Google Scholar]
- 19. Schnell T, Koethe D, Daumann J, Gouzoulis-Mayfrank E. The role of cannabis in cognitive functioning of patients with schizophrenia. Psychopharmacology (Berl). 2009;205:45–52. [DOI] [PubMed] [Google Scholar]
- 20. Ferraro L, Russo M, O’Connor J, et al. Cannabis users have higher premorbid IQ than other patients with first onset psychosis. Schizophr Res. 2013;150:129–135. [DOI] [PubMed] [Google Scholar]
- 21. D’Souza DC, Sewell RA, Ranganathan M. Cannabis and psychosis/schizophrenia: human studies. Eur Arch Psychiatry Clin Neurosci. 2009;259:413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeLisi LE. The effect of cannabis on the brain: can it cause brain anomalies that lead to increased risk for schizophrenia? Curr Opin Psychiatry. 2008;21:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumra S. Schizophrenia and cannabis use. Minn Med. 2007;90:36–38. [PubMed] [Google Scholar]
- 24. Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. Int Rev Psychiatry. 2009;21:152–162. [DOI] [PubMed] [Google Scholar]
- 25. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 27. Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl). 2002;161:331–339. [DOI] [PubMed] [Google Scholar]
- 29. French ED. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226:159–162. [DOI] [PubMed] [Google Scholar]
- 30. Egerton A, Chaddock CA, Winton-Brown TT, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74:106–112. [DOI] [PubMed] [Google Scholar]
- 31. Murray RM, Mehta M, Di Forti M. Different dopaminergic abnormalities underlie cannabis dependence and cannabis-induced psychosis. Biol Psychiatry. 2014;75:430–431. [DOI] [PubMed] [Google Scholar]
- 32. Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. [DOI] [PubMed] [Google Scholar]
- 33. Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- 34. Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9:696–709. [DOI] [PubMed] [Google Scholar]
- 35. Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Serretti A, Lattuada E, Lorenzi C, Lilli R, Smeraldi E. Dopamine receptor D2 Ser/Cys 311 variant is associated with delusion and disorganization symptomatology in major psychoses. Mol Psychiatry. 2000;5:270–274. [DOI] [PubMed] [Google Scholar]
- 37. Bertolino A, Fazio L, Caforio G, et al. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain. 2009;132:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Usiello A, Baik JH, Rougé-Pont F, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y, Bertolino A, Fazio L, et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA. 2007;104:20552–20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bertolino A, Taurisano P, Pisciotta NM, et al. Genetically determined measures of striatal D2 signaling predict prefrontal activity during working memory performance. PLoS One. 2010;5:e9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blasi G, Lo Bianco L, Taurisano P, et al. Functional variation of the dopamine D2 receptor gene is associated with emotional control as well as brain activity and connectivity during emotion processing in humans. J Neurosci. 2009;29:14812–14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blasi G, Napolitano F, Ursini G, et al. DRD2/AKT1 interaction on D2 c-AMP independent signaling, attentional processing, and response to olanzapine treatment in schizophrenia. Proc Natl Acad Sci USA. 2011;108:1158–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Di Forti M, Morgan C, Dazzan P, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195:488–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. World Health Organization. Schedules for Clinical Assessment in Neuropsychiatry (SCAN). Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 45. Bebbington PE, Nayani T. The Psychosis Screening Questionnaire. Int J Methods Psychiatr Res. 1995;5:11–19. [Google Scholar]
- 46. Di Forti M, Iyegbe C, Sallis H, et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry. 2012;72:811–816. [DOI] [PubMed] [Google Scholar]
- 47. Di Forti M, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mallett R, Leff J, Bhugra D, Pang D, Zhao JH. Social environment, ethnicity and schizophrenia. A case-control study. Soc Psychiatry Psychiatr Epidemiol. 2002;37:329–335. [DOI] [PubMed] [Google Scholar]
- 49. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 50. Blasi G, De Virgilio C, Papazacharias A, et al. Converging evidence for the association of functional genetic variation in the serotonin receptor 2a gene with prefrontal function and olanzapine treatment. JAMA Psychiatry. 2013;70:921–930. [DOI] [PubMed] [Google Scholar]
- 51. Chan AW, Pristach EA, Welte JW, Russell M. Use of the TWEAK test in screening for alcoholism/heavy drinking in three populations. Alcohol Clin Exp Res. 1993;17:1188–1192. [DOI] [PubMed] [Google Scholar]
- 52. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 53. Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. [DOI] [PubMed] [Google Scholar]
- 54. van Winkel R; Genetic Risk and Outcome of Psychosis (GROUP) Investigators. Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry. 2011;68:148–157. [DOI] [PubMed] [Google Scholar]
- 55. van Winkel R, van Beveren NJ, Simons C; Genetic Risk and Outcome of Psychosis (GROUP) Investigators. AKT1 moderation of cannabis-induced cognitive alterations in psychotic disorder. Neuropsychopharmacology. 2011;36:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Verdejo-García A, Fagundo AB, Cuenca A, et al. COMT val158met and 5-HTTLPR genetic polymorphisms moderate executive control in cannabis users. Neuropsychopharmacology. 2013;38:1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Henquet C, Rosa A, Krabbendam L, et al. An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology. 2006;31:2748–2757. [DOI] [PubMed] [Google Scholar]
- 58. Costas J, Sanjuán J, Ramos-Ríos R, et al. Interaction between COMT haplotypes and cannabis in schizophrenia: a case-only study in two samples from Spain. Schizophr Res. 2011;127:22–27. [DOI] [PubMed] [Google Scholar]
- 59. Zammit S, Owen MJ, Evans J, Heron J, Lewis G. Cannabis, COMT and psychotic experiences. Br J Psychiatry. 2011;199:380–385. [DOI] [PubMed] [Google Scholar]
- 60. Drago J, Padungchaichot P, Accili D, Fuchs S. Dopamine receptors and dopamine transporter in brain function and addictive behaviors: insights from targeted mouse mutants. Dev Neurosci. 1998;20:188–203. [DOI] [PubMed] [Google Scholar]
- 61. Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. [DOI] [PubMed] [Google Scholar]
- 62. Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. [DOI] [PubMed] [Google Scholar]
- 63. Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry. 2014;75:470–478. [DOI] [PubMed] [Google Scholar]
- 64. Taurisano P, Romano R, Mancini M, et al. Prefronto-striatal physiology is associated with schizotypy and is modulated by a functional variant of DRD2. Front Behav Neurosci. 2014;8:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. J Abnorm Psychol. 1994;103:171–183. [DOI] [PubMed] [Google Scholar]
- 66. Barnes JJ, Dean AJ, Nandam LS, O’Connell RG, Bellgrove MA. The molecular genetics of executive function: role of monoamine system genes. Biol Psychiatry. 2011;69:e127–e143. [DOI] [PubMed] [Google Scholar]
- 67. D’Esposito M, Zarahn E, Aguirre GK. Event-related functional MRI: implications for cognitive psychology. Psychol Bull. 1999;125:155–164. [DOI] [PubMed] [Google Scholar]
- 68. Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]