Abstract
It has been proposed that both positive and negative symptoms in schizophrenia (SZ) may derive, at least in part, from a disrupted ability to accurately and flexibly represent the value of stimuli and actions. To assess relationships between dimensions of psychopathology in SZ, and the tendency to devalue food stimuli, on which subjects were fed to satiety, we administered a sensory-specific satiety (SSS) paradigm to 42 SZ patients and 44 controls. In each of 2 sessions, subjects received 16 0.7-ml squirts of each of 2 rewarding foods and 32 squirts of a control solution, using syringes. In between the 2 sessions, each subject was instructed to drink one of the foods until he/she felt “full, but not uncomfortable.” At 10 regular intervals, interspersed throughout the 2 sessions, subjects rated each liquid for pleasantness, using a Likert-type scale. Mann-Whitney U-tests revealed group differences in SSS effects. Within-group tests revealed that, while controls showed an effect of satiety that was sensory specific, patients showed an effect of satiety that was not, devaluing the sated and unsated foods similarly. In SZ patients, we observed correlations between the magnitude of SSS effects and measures of both positive and negative symptoms. We argue that the ability to flexibly and rapidly update representations of the value of stimuli and actions figures critically in the ability of patients with psychotic illness to process salient events and adaptively engage in goal-directed behavior.
Key words: schizophrenia, satiety, value, anhedonia, avolition
Introduction
Schizophrenia (SZ) is a devastating mental illness, affecting approximately 0.5%–1% of the adult population worldwide.1 Along with positive (psychotic) symptoms, SZ is characterized by negative symptoms, which are among the most enduring and debilitating aspects of the illness.2,3 A reduced ability to initiate goal-oriented behavior (avolition)4 and a reduced ability to experience pleasure (anhedonia)5 are 2 aspects of negative symptomatology characteristic of many patients with SZ.
One possible explanation for avolition in SZ is a diminished hedonic response to rewarding stimuli—if individuals do not experience a stimulus as pleasurable, they will not want it, or work to get it. The idea that anhedonia in SZ patients is characterized by diminished consummatory pleasure is not supported by the literature, however, as most studies indicate that SZ patients do not differ from controls in their self-reported experience of pleasure.6 That is, despite a large and growing literature pointing to deficits in reward-sensitivity and reinforcement learning,7 as well as associated neural signals,8 in SZ patients (especially those with moderate-to-severe negative symptoms), patients have generally given normative ratings for reinforcer valence/pleasurability,9,10 when prompted directly. Reduced reward-seeking behavior could, however, persist in the presence of seemingly intact consummatory pleasure in SZ if patients and controls assign affective value using dissimilar internal “scales.” That is potential differences in the ranges of hedonic experience might not be captured by cross-sectional studies prompting patients and controls to rate their hedonic experiences on Likert-type scales. An additional possibility is that patients do such ratings, not based on actual experiences, but rather based on a sense of what is likely normative and socially desirable.
One way to shed light on this issue is to examine approach or avoidance behavior, with regard to stimuli that have been variably been associated with appetitive and aversive feedback. Studies using such paradigms, however, typically bear on the ability of patients to learn about the value of stimuli, based on the experience of rewards and punishments. The purpose of the current study was to investigate whether the experience of appetitive stimuli in SZ is genuinely normative, by examining changes in the valuation of stimuli, not as a consequence of learning, but as a consequence of satiation. In order to investigate this issue, we adapted a paradigm from Kringelbach and colleagues,11 which uses a sensory-specific satiety (SSS) design to assess subjective evaluations of rewards, before and after satiation, and to investigate the extent to which neural responses in reward-sensitive brain regions track, or fail to track, reported experience. This paradigm allowed us to directly vary the value of a reinforcer under experimental control. Importantly, the SSS effect depends on differential devaluation of food stimuli with different sensory properties, based on the fact that one is consumed to satiety, and the other not.12 Based on the idea that motivational deficits in SZ are driven, at least in part, by a reduced ability to flexibly and precisely update representations of value, we hypothesized that patients with SZ would show a reduced SSS effect, relative to control participants. Furthermore, we predicted that the magnitude of the SSS effect would correlate significantly with clinical ratings of avolition and anhedonia.
Methods
Participants
Our initial sample consisted of 49 patients meeting DSM-IV-TR criteria for schizophrenia or schizoaffective disorder, as determined by the Structured Interview for the DSM-IV (SCID-I),13 and 48 healthy controls. All individuals volunteered to participate in the study and provided written informed consent, and all subjects were compensated for study participation. All patients were recruited from the Maryland Psychiatric Research Center (MPRC) and were clinically stable (as determined by their treating physician) and stably medicated (no changes in medication type or dose within 4 weeks of study; details of antipsychotic drugs (APDs) are given in supplementary table 1). Healthy volunteers were recruited from the community via random phone number dialing and advertisements and were screened for Axis I and II disorders using the SCID-I.13 All control participants were free of any significant personal psychiatric and medical history, had no history of severe mental illness in first-degree relatives, and did not meet criteria for current substance abuse or dependence.
General Procedures
All participants fasted (no food or drink, except for water and necessary medications) for at least 3 hours prior to the 2 experimental sessions described below. The total time of the 2 experimental sessions was 75–90 minutes. Study participants completed standard cognitive assessments including the MATRICS battery,14 Wechsler Abbreviated Scale of Intelligence (WASI),15 Wide Ranging Achievement Test Four (WRAT-4),16 and Wechsler Test of Adult Reading (WTAR).17 Patients and controls were also administered the Chapman Scales for Physical and Social Anhedonia.18 Overall symptom severity in patients was characterized using the Brief Psychiatric Ratings Scale (BPRS),19 and negative symptom severity was quantified using the Scale for the Assessment of Negative Symptoms (SANS)20 and the Brief Negative Symptom Scale (BNSS).21 These assessments were generally administered within a week of the experimental sessions (the median interval was 5 days). Patients in the study exhibited moderate degrees of negative and overall symptoms (mean SANS item score = 1.5; SD = 0.7; mean BPRS item score = 1.8; SD = 0.3).
SSS Task
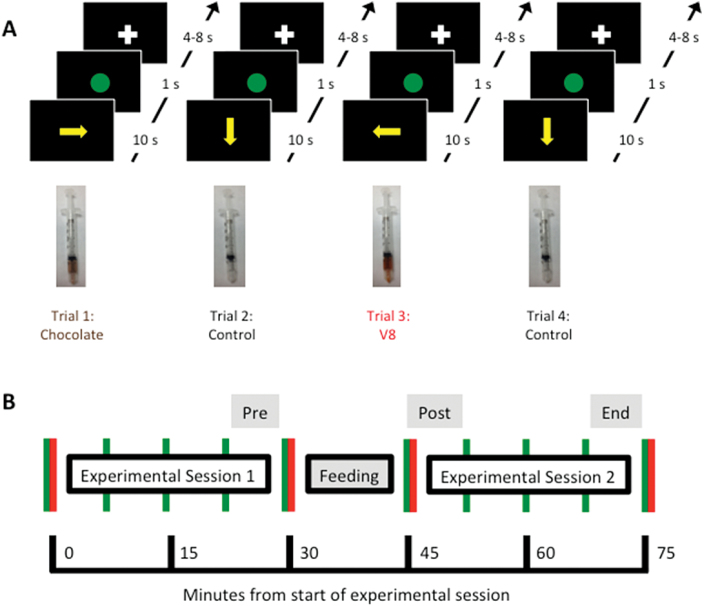
Food stimuli (V8 100% Vegetable Juice, from the Campbell Soup Company, and All Natural Chocolate Hazelnut Non-dairy Beverage, from Pacific Natural Foods) were presented in 2 sessions (pre- and postfeeding), each consisting of 16 four-trial epochs (figure 1A). Each epoch consisted of the delivery of one of the liquid foods, followed by a tasteless solution (consisting of the ionic components of saliva, KCl, and NaHCO3, dissolved in sterile water), then by the other liquid food, then again by the tasteless solution. At the beginning of each trial, 0.7ml of one of the gustatory stimuli was delivered. The subject was instructed to roll the stimulus around on the tongue and to swallow the liquid after 10 seconds (following a visual cue). Immediately after sampling each of the food stimuli 16 times in the first experimental session, and immediately before sampling each of the food stimuli 16 times in the second experimental session, subjects were fed to satiety on one of the foods (see supplementary methods for details). As illustrated in figure 1B, 10 pleasantness ratings were taken, at regular intervals (prior to each session and after every 4 epochs of each session). Ratings were entered on a visual analog scale, using a wheel manipulandum, with anchors of “Not at all” (far left; cursor position 0) and “Extremely” (far right; cursor position 800). A value corresponding to the final position of the cursor was scaled to range from 0 to 100.
Fig. 1.
Illustration of the experimental paradigm. (A) In each epoch of 4 trials, participants received 1 squirt of each food and 2 squirts of the control solution. (B) Time course of ratings across the sessions. Subjects were asked to manually rate each liquid for pleasantness, using a wheel manipulandum (green vertical lines). Subjects also rated each food for intensity, before and after each session, and also gave ratings of hunger and thirst at these times (red vertical lines).
Seven patients and 4 controls were removed from the analysis, because they gave low initial pleasure ratings to one of the food stimuli. The remaining sample of 42 patients and 44 controls did not differ in demographic variables such as age, gender, race, parental educational attainment (as a proxy for socioeconomic status), and proportions of smokers (table 1). In 26 patients and 26 controls, behavioral data were collected along with the acquisition of functional magnetic resonance imaging (fMRI) data; results of analyses of fMRI data will be reported elsewhere.
Table 1.
Subject Characteristics
| Patients (N = 42) |
Controls (N = 44) |
P of Group Difference | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Demographics | |||
| Age | 37.9 (11.2) | 38.6 (11.7) | .783 |
| Gender | 10 F, 32 M | 17 F, 27 M | .167 |
| Race | 27W, 15 NW | 26W, 18 NW | .662 |
| Smokers | 14 Y, 28 N | 8 Y, 36 N | .140 |
| Subject education (y) | 13.0 (2.0) | 15.2 (2.0) | <.001 |
| Parental education (y) | 13.8 (3.5) | 14.5 (2.8) | .335 |
| Neuropsychological testing/ questionnaires | |||
| IQ (from WASI 2-subtest) | 102.3 (12.8) | 118.1 (9.6) | <.001 |
| WTAR Scaled Score | 100.5 (16.5) | 112.0 (10.7) | <.001 |
| MATRICS Composite Score | 34.5 (13.5) | 54.7 (9.3) | <.001 |
| Chapman – Phys. Anhed. | 14.7 (7.2) | 8.0 (4.3) | <.001 |
| Chapman – Soc. Anhed. | 11.9 (7.5) | 7.4 (6.6) | .005 |
Note: F, Female; M, Male; W, White; NW, Non-White; WASI, Wechsler Abbreviated Scale of Intelligence; WTAR, Wechsler Test of Adult Reading; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; Phys., Physical; Anhed., Anhedonia; Soc., Social.
Statistical Analyses
In order to quantify the acute SSS/devaluation effect, we computed, for both the sated and unsated foods, the differences in average ratings between the 2 time points immediately prior to feeding and the 2 immediately after feeding (Postfeeding Pleasantness − Prefeeding Pleasantness; see figure 1B). The main SSS score we used was the difference in the pleasantness change scores for the sated and unsated foods from immediately prefeeding to immediately postfeeding:
[(Sated-Post − Sated-Pre) − (Unsated-Post − Unsated-Pre)]
According to this formula, a negative change score represents devaluation, from pre- to postfeeding, and a negative SSS score represents greater devaluation of the sated food, relative to the unsated food. We used initial and postfeeding ratings for sated and unsated foods, change scores for sated and unsated foods, and SSS scores in correlation analyses with clinical variables, including symptom ratings and antipsychotic drug dose (converted to oral-haloperidol-equivalent units; see supplementary materials for details).
In order to quantify the extent to which the SSS/devaluation effect endured (after Rolls et al12), we also computed, for both the sated and unsated foods, the differences in average ratings between the 2 time points immediately prior to feeding and the last 2 time points of the experiment (End-of-Session Pleasantness − Prefeeding Pleasantness). From these change scores, we computed a second SSS measure. Details of these analyses are reported in the supplementary materials.
Results
Tests of Group Differences in SSS: All Subjects
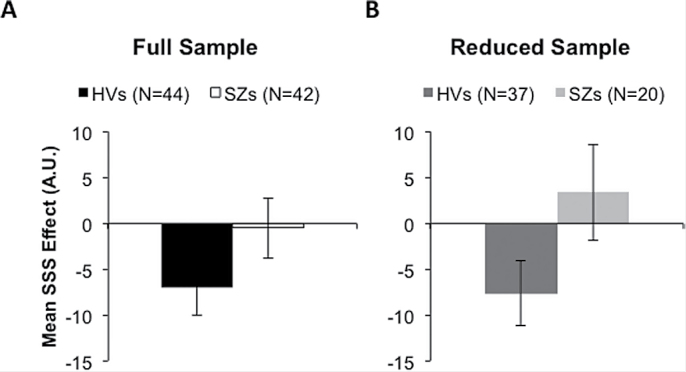
As shown in figure 2A, patients and controls showed a strong trend toward a group difference in mean SSS scores, from prefeeding to time points immediately following feeding (Z of Mann-Whitney U = 1.845, P = .065). This was true, despite similar amounts of liquid consumed during the feeding interval (see supplementary results) and similar initial pleasantness ratings for the 2 foods across groups (supplementary table 2). Patients showed an effect of satiety on pleasantness ratings that was not sensory specific. Unlike controls, who showed a greater decrement in pleasantness ratings for the fed food than the unfed food (Z of Wilcoxon Signed Rank Test = 2.305, P = .021), patients devalued both of the food stimuli equally, following the consumption of only one of the foods to satiety (Z of Wilcoxon Signed Rank Test = 0.518, P = .604). That is, for patients, the experience of consuming one food to the point of satiety led to a generalization to the other food—an effect not seen in controls.
Fig. 2.
Sensory-specific satiety effects in (A) the full sample of patients with schizophrenia (SZs; N = 42) and healthy volunteers (HVs; N = 44; left set of bars) and (B) in the subsample of SZs (N = 20) and HVs (N = 37) showing reduced hunger ratings, postfeeding (right set of bars). Sets of bars show differences in valuation change, for the sated and unsated foods, from prefeeding to immediately postfeeding.
Tests of Group Differences in SSS: Subjects Reporting Changes in Hunger
Because patients reported less of a reduction in the overall feeling of satiety, relative to controls (see supplementary materials), we did additional analyses, comparing only the 20 patients and 37 controls who reported a reduction in hunger (prefeeding hunger rating > postfeeding hunger rating; characterizing information reported in supplementary table 3). These subsamples of patients and controls did not differ in their initial ratings of hunger, thirst, the intensity of either food stimulus, or the pleasantness of either food stimulus (supplementary table 4). They also did not differ in the mean changes in their ratings of the intensity of either food stimulus, or in mean changes in hunger and thirst ratings, from immediately prefeeding to immediately postfeeding. As shown in figure 2, these subsamples of patients and controls showing reduced hunger differed significantly in mean SSS scores (Z of Mann-Whitney U = 2.157, P = .031). As with the entire sample of patients, patients reporting hunger reductions exhibited an effect of satiety on pleasantness ratings that was not sensory specific (supplementary table 4). Unlike controls reporting hunger reductions, who showed greater devaluation of the fed food than the unfed food (Z of Wilcoxon Signed Rank Test = 2.142, P = .032), the subsample of patients devalued both of the food stimuli similarly, following the consumption of only one of the foods to satiety (Z of Wilcoxon Signed Rank Test = 1.045, P = .296).
Correlation Analyses
In the entire sample of patients, the magnitudes of SSS effects correlated significantly with BPRS measures of psychosis severity (table 2). In SZ patients showing changes in hunger from immediately pre- to immediately postfeeding, the magnitude of the SSS effect correlated significantly with measures of anhedonia and avolition from both questionnaires (the Chapman scales) and clinical ratings (the SANS; table 2). All of these results indicated that patients with higher levels of anhedonia/avolition showed devaluation that was less specific to the food on which they were sated (supplementary figure 1).
Table 2.
Spearman Correlations Between Sensory-Specific Satiety Scores and Clinical Variables, in the Full Sample, and in the Reduced Sample, of Patients
| Full Sample (N = 42) |
Reduced Sample (N = 20) |
|||
|---|---|---|---|---|
| ρ | P | ρ | P | |
| BPRS Item Average | 0.281 | .071 | 0.224 | .343 |
| BPRS Psychosis Average | 0.343 | .026* | 0.293 | .211 |
| SANS Item Average | 0.034 | .831 | 0.306 | .190 |
| SANS Avolition Item Average | 0.268 | .086 | 0.525 | .017* |
| SANS Avolition/Anhedonia Item Average | 0.186 | .238 | 0.538 | .014* |
| Chapman – Physical Anhedonia Score | 0.141 | .374 | 0.547 | .013* |
| Chapman – Social Anhedonia Score | 0.250 | .120 | 0.709 | .001* |
| MATRICS Composite Score | −0.308 | .047* | −0.421 | .064 |
Note: Sensory-specific satiety scores explained in text. BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms.
Perhaps of note, average baseline pleasantness ratings for the 2 foods showed strong correlations with BPRS ratings of psychotic symptoms (ρ = −0.353, P = .022), in the full sample of patients (supplementary table 5), and with both psychotic (ρ = −0.714, P < .001) and general (ρ = −0.561, P = .010) symptoms, in the reduced sample of patients (supplementary table 6). However, given the lack of a correlation between average baseline pleasantness ratings and SSS scores, in both the full (ρ = −0.196, P = .213) and reduced (ρ = −0.260, P = .268) samples of patients, it is unlikely that the correlation between average baseline pleasantness ratings and ratings of psychotic symptoms can account for the observed correlation between SSS scores and ratings of psychotic symptoms.
We also observed that average baseline pleasantness ratings for the 2 foods showed weak trends toward correlations with self-reports of both physical (ρ = −0.382, P = .096) and social (ρ = −0.386, P = .103) anhedonia, from the Chapman scales, in the reduced sample of patients (supplementary table 6). However, these relationships were not observed in the reduced sample of controls, or in either group, in the full sample (all P’s >> .1). Furthermore, average baseline pleasantness ratings for the 2 foods did not correlate at all with clinical ratings from the SANS, arguing against the idea that correlations between SSS scores and measures of anhedonia and avolition are attributable to correlations between baseline pleasantness ratings and measures of anhedonia and avolition.
Discussion
Our analyses of reports of subjective hedonic experience in SZ patients and controls produced 3 main findings. First, patients with SZ showed a reduced SSS effect, such that they tended to devalue both the sated and the unsated food stimulus. Second, the magnitude of the SSS effect in patients with SZ, as well as with the degree of devaluation of the sated stimulus, correlated with clinical ratings for avolition/anhedonia; as expected, patients with lower ratings for avolition/anhedonia showed devaluation that was more specific to the sated food stimulus. Finally, SSS scores in SZ patients also correlated with clinical ratings for positive symptoms.
The current results have important implications for our understanding of negative symptoms in SZ. In particular, these findings suggest a need conceptualize the nature of anhedonia in SZ, not only in terms of feelings of in-the-moment pleasure at given time points, but also in terms of the modification of these feelings over time. It has been established that environmental stimuli can vary in their incentive value/ability to motivate behavior, depending on the organism’s current status or internal state.22,23 As illustrated by the SSS effect,12,24 food stimuli, in particular, vary in value based on an individual’s level of satiety for a certain class of food. The ability to precisely update and represent the value of stimuli and actions in context is a key aspect of adaptive behavior, and a reduced ability to do this could account for some of the motivational deficits observed in psychiatric illness.
It is noteworthy that attenuated SSS effects were not just characteristic of SZ patients with the most severe negative symptoms; we also found correlations between severity of positive symptoms and impaired SSS. One might see this relationship as indicating a different kind of aberrant salience than is typically discussed in the literature25,26: the devaluation of one food transferred to a different food, an inappropriate generalization of negative valence. It is not hard to imagine how a similar process could underlie impaired social functioning if even appropriate discomfort with a specific person generalized more broadly.
SSS is a phenomenon that reflects a reduced experience of pleasure with regard to a specific food based on satiety on that food, thus relying on a high degree of sensitivity to interoceptive signals and neural representations of the value and magnitude of rewards and punishments, in which orbitofrontal cortex (OFC) and associated structures have been observed to play a critical role.27–31 Neuroimaging research has linked the rapid updating the subjective value of food stimuli to activity in OFC.11 This finding, of a direct correspondence between OFC activity and self-reports of pleasure, ties the psychological experience of taste hedonics to a brain region that is known to play a more general role in the active maintenance of value representations. Importantly, additional research has provided evidence of OFC abnormalities in SZ,32–35 which could factor in the reduced ability to learn and precisely represent the value of environmental stimuli36–38 and underlie motivational deficits.
The fact that SZ patients (especially more symptomatic patients) tend to devalue sated and unsated foods similarly, in the context of a SSS paradigm, suggests a lack of precision in value updating, supporting the idea that OFC is dysfunctional in SZ. One potential explanation for the observation that SZ patients often show normative ratings for consummatory hedonics in cross-sectional studies6 is that these studies, depending on single-instance ratings of in-the-moment pleasure, do not genuinely require participants to report hedonic experience in the way a SSS paradigm does. While a devaluation paradigm, like SSS, prompts subjects to report fine-grained interoceptive feelings at multiple instances over time, cross-sectional studies may allow subjects to report feelings based on a sense of what is likely normative and socially desirable, rather than directly on the basis of value signals from OFC.
A recent study by Morris et al39 examining the impact of food devaluation on goal-directed action in SZ39 suggests an additional role for the caudate in value-driven behavior. These authors also found that higher avolition scores were related to reduced devaluation-related changes in caudate activity in SZ. It should be noted, however, that while Morris et al39 used a Pavlovian-Instrumental Transfer (PIT) paradigm, showing that SZ patients failed to adjust their approach/avoidance behavior, with regard to devalued food stimuli, the current study used a simpler paradigm, not dependent on any form of associative learning, revealing that SZ patients failed to selectively adjust their hedonic assessment of food stimuli as a consequence of the devaluation of one of the stimuli.
The interpretations of the results of this study are constrained by several limitations. First, SZ patients in our study were taking antipsychotic drugs (supplementary table 1), which may have had an impact on feelings of pleasure or satiety. Although an effect of APD type and/or dose can only be ruled out by random assignment to drug in the context of a clinical trial, our analyses (supplementary table 7) revealed no systematic relationships between drug dose (converted to haloperidol equivalent units) and SSS effects. Thus, we had no reason to believe that antipsychotic dose accounted for changes in feelings of either sensory-specific or overall feelings of satiety.
Second, it is possible that methodological issues may have affected the robustness of the satiety effects observed in this study, relative those observed in the study after which it was modeled.11 These differences included the length of time that subjects were required to fast (6 h in the original study), the time of day at which subjects typically performed the study (usually late morning in our study, later afternoon in the original study), and the inclusion of females and older subjects in our study (the mean age of participants in the Kringelbach et al11 study was 28.5).
Perhaps as a consequence of some of these methodological considerations, it was apparent that a much lower proportion of patients reported a change in hunger, following the consumption of only one of the foods to satiety, relative to controls. Despite the consumption of similar amounts of each drink, roughly half of patients did not report a reduction in hunger. The reduced tendency of patients with SZ to report changes in hunger, following the consumption of foods, may be consistent with reports that SZ patients show a reduced experience of pain,40,41 possibly reflecting a more general abnormality in the physiology underlying interoception.42 It is worth noting that evidence points to involvement of the insula in multiple aspects of interoception,43 including feelings of hunger and satiety,44 and that there have been numerous reports of insula abnormalities in SZ.45–47
In order to assess whether the 20 patients reporting reductions in hunger differed from the 22 who did not, we compared the 2 groups on a number of standard clinical, cognitive, and experimental measures (supplementary tables 8 and 9). In addition to changes in hunger and thirst ratings, these comparisons revealed group differences in the average of SANS Avolition items and devaluation of the unsated stimulus, as well as mean devaluation of both food stimuli. Thus, patients who felt less full, also devalued the food stimuli less. When we performed further analyses on the 20 patients reporting changes in hunger from pre- to postfeeding sessions (excluding the 22 who did not), we observed a close relationship, in this group, between the extent to which the feeling of satiety was sensory specific, and the severity of hedonic and motivational impairments in patients. The reporting of SSS requires a sensitivity to internal state that is even more fine-grained than the ability to report overall changes in satiety. It is likely that systematic relationships between clinical symptoms and the magnitude of the SSS effect were observed in only a subset of SZ patients, because the ability to detect and report SSS is contingent on the ability to detect and report feelings of satiety, in general.
In sum, the results of our study validate the use of a SSS paradigm as a probe of stimulus-specific devaluation, specifically in conjunction with neuroimaging. Furthermore, similar paradigms could be used to test whether specific physiological abnormalities related to the psychological experience of rewards, and the translation of this experience into motivation, have a genetic basis and are present in unaffected first-degree relatives of SZ patients.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work supported by National Institutes of Health (NIH) grants R21 MH086739, R01 MH080066, K12 RR023250 and by the National Institute on Drug Abuse - Intramural Research Program (NIDA-IRP).
Supplementary Material
Acknowledgments
Clinical staff at the MPRC and NIDA-IRP performed screening and evaluation of research participants. Sharon August and Leeka Hubzin assisted with the assessment of research participants. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. [DOI] [PubMed] [Google Scholar]
- 2. Tamminga CA, Buchanan RW, Gold JM. The role of negative symptoms and cognitive dysfunction in schizophrenia outcome. Int Clin Psychopharmacol. 1998;13(suppl 3):S21–S26. [DOI] [PubMed] [Google Scholar]
- 3. Fenton WS, McGlashan TH. Natural history of schizophrenia subtypes. II. Positive and negative symptoms and long-term course. Arch Gen Psychiatry. 1991;48:978–986. [DOI] [PubMed] [Google Scholar]
- 4. Foussias G, Remington G. Negativesymptoms inschizophrenia:avolition and Occam’srazor. Schizophr Bull. 2010;36:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf DH. Anhedonia in schizophrenia. Curr Psychiatry Rep. 2006;8:322–328. [DOI] [PubMed] [Google Scholar]
- 6. Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gold JM, Waltz JA, Kasanova Z, et al. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch Gen Psychiatry. 2012;69:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deserno L, Boehme R, Heinz A, Schlagenhauf F. Reinforcement learning and dopamine in schizophrenia: dimensions of symptoms or specific features of a disease group? Front Psychiatry. 2013;4:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waltz JA, Schweitzer JB, Gold JM, et al. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. 2009;34:1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dowd EC, Barch DM. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry. 2010;67:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kringelbach ML, O’, Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. [DOI] [PubMed] [Google Scholar]
- 12. Rolls BJ, Rolls ET, Rowe EA, Sweeney K. Sensory specific satiety in man. Physiol Behav. 1981;27:137–142. [DOI] [PubMed] [Google Scholar]
- 13. First MB, Spitzer RL, Gibbon M, Williams JBW.Structured Clinical Interview for DSM-IV- Axis I Disorders (SCID-I). Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 14. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. [DOI] [PubMed] [Google Scholar]
- 15. Wechsler D.Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 16. Wilkinson GS, Robertson GJ.Wide Range Achievement Test 4: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2006. [Google Scholar]
- 17. Wechsler D.Wechsler Test of Adult Reading (WTAR). San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 18. Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. [DOI] [PubMed] [Google Scholar]
- 19. Overall JE, Gorman DR. TheBrief Psychiatric Rating Scale. Psychol Reports. 1962;10:799–812. [Google Scholar]
- 20. Andreasen NC.The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 21. Kirkpatrick B, Strauss GP, Nguyen L, et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull. 2011;37:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Killgore WD, Yurgelun-Todd DA. Affect modulates appetite-related brain activity to images of food. Int J Eat Disord. 2006;39:357–363. [DOI] [PubMed] [Google Scholar]
- 23. Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. [DOI] [PubMed] [Google Scholar]
- 24. Rolls ET, Rolls BJ, Rowe EA. Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol Behav. 1983;30:185–192. [DOI] [PubMed] [Google Scholar]
- 25. Roiser JP, Stephan KE, den Ouden HE, Barnes TR, Friston KJ, Joyce EM. Do patients with schizophrenia exhibit aberrant salience? Psychol Med. 2009;39:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. [DOI] [PubMed] [Google Scholar]
- 27. O’, Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. [DOI] [PubMed] [Google Scholar]
- 28. Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. [DOI] [PubMed] [Google Scholar]
- 29. Rolls ET, Kringelbach ML, de Araujo IE. Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci. 2003;18:695–703. [DOI] [PubMed] [Google Scholar]
- 30. Tremblay L, Hollerman JR, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate striatum. J Neurophysiol. 1998;80:964–977. [DOI] [PubMed] [Google Scholar]
- 31. Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex. 2000;10:263–271. [DOI] [PubMed] [Google Scholar]
- 32. Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex. Focal abnormalities in orbitofrontal cortex in schizophrenia. Arch Gen Psychiatry. 1997;54:1089–1095. [DOI] [PubMed] [Google Scholar]
- 33. Crespo-Facorro B, Kim J, Andreasen NC, O’, Leary DS, Magnotta V. Regional frontal abnormalities in schizophrenia: a quantitative gray matter volume and cortical surface size study. Biol Psychiatry. 2000;48:110–119. [DOI] [PubMed] [Google Scholar]
- 34. Goldstein JM, Goodman JM, Seidman LJ, et al. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. [DOI] [PubMed] [Google Scholar]
- 35. Bertollo DN, Cowen MA, Levy AV. Hypometabolism in olfactory cortical projection areas of male patients with schizophrenia: an initial positron emission tomography study. Psychiatry Res. 1996;60:113–116. [DOI] [PubMed] [Google Scholar]
- 36. Brown JK, Waltz JA, Strauss GP, McMahon RP, Frank MJ, Gold JM. Hypothetical decision making in schizophrenia: the role of expected value computation and “irrational” biases. Psychiatry Res. 2013;209:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strauss GP, Robinson BM, Waltz JA, et al. Patients with schizophrenia demonstrate inconsistent preference judgments for affective and nonaffective stimuli. Schizophr Bull. 2011;37:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brambilla P, Perlini C, Bellani M, et al. Increased salience of gains versus decreased associative learning differentiate bipolar disorder from schizophrenia during incentive decision making. Psychol Med. 2013;43:571–580. [DOI] [PubMed] [Google Scholar]
- 39. Morris RW, Quail S, Griffiths KR, Green MJ, Balleine BW. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biol Psychiatry. 2015;77:187–195. [DOI] [PubMed] [Google Scholar]
- 40. Bonnot O, Anderson GM, Cohen D, Willer JC, Tordjman S. Are patients with schizophrenia insensitive to pain? A reconsideration of the question. Clin J Pain. 2009;25:244–252. [DOI] [PubMed] [Google Scholar]
- 41. Potvin S, Marchand S. Hypoalgesia in schizophrenia is independent of antipsychotic drugs: a systematic quantitative review of experimental studies. Pain. 2008;138:70–78. [DOI] [PubMed] [Google Scholar]
- 42. Linnman C, Coombs G, III, Goff DC, Holt DJ. Lack of insula reactivity to aversive stimuli in schizophrenia. Schizophr Res. 2013;143:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. [DOI] [PubMed] [Google Scholar]
- 44. Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123:105–115. [DOI] [PubMed] [Google Scholar]
- 46. Shepherd AM, Matheson SL, Laurens KR, Carr VJ, Green MJ. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72:775–784. [DOI] [PubMed] [Google Scholar]
- 47. Moran LV, Tagamets MA, Sampath H, et al. Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biol Psychiatry. 2013;74:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.