Abstract
Hypofunctioning of the N-methyl-D-aspartate receptor (NMDA-R) has been prominently implicated in the pathophysiology of schizophrenia (ScZ). The current study tested the effects of ketamine, a dissociative anesthetic and NMDA-R antagonist, on resting-state activity recorded with magnetoencephalography (MEG) in healthy volunteers. In a single-blind cross-over design, each participant (n = 12) received, on 2 different sessions, a subanesthetic dose of S-ketamine (0.006mg/Kg) and saline injection. MEG-data were analyzed at sensor- and source-level in the beta (13–30 Hz) and gamma (30–90 Hz) frequency ranges. In addition, connectivity analysis at source-level was performed using transfer entropy (TE). Ketamine increased gamma-power while beta-band activity was decreased. Specifically, elevated 30–90 Hz activity was pronounced in subcortical (thalamus and hippocampus) and cortical (frontal and temporal cortex) regions, whilst reductions in beta-band power were localized to the precuneus, cerebellum, anterior cingulate, temporal and visual cortex. TE analysis demonstrated increased information transfer in a thalamo-cortical network after ketamine administration. The findings are consistent with the pronounced dysregulation of high-frequency oscillations following the inhibition of NMDA-R in animal models of ScZ as well as with evidence from electroencephalogram-data in ScZ-patients and increased functional connectivity during early illness stages. Moreover, our data highlight the potential contribution of thalamo-cortical connectivity patterns towards ketamine-induced neuronal dysregulation, which may be relevant for the understanding of ScZ as a disorder of disinhibition of neural circuits.
Key words: NMDA-receptor, ketamine, GABA, schizophrenia, MEG, gamma-band oscillations
Introduction
Schizophrenia (ScZ) is a debilitating psychiatric condition characterized by positive (eg, hallucinations and delusions) and negative symptoms (eg, flat affect), as well as cognitive deficits. Recent evidence suggests that a deficit in excitation/inhibition (E/I) balance parameters may constitute a pathophysiological mechanism that could underlie impairments in cognition and certain clinical symptoms.1 This is because during normal brain functioning, the generation of coherently organized large-scale networks is critically dependent upon the activity of gamma-aminobutyric acid (GABA) inhibitory interneurons expressing the calcium (Ca2+) binding protein parvalbumin (PV)2 and glutamatergic activation of PV interneurons,3 leading to rhythmic fluctuations of neuronal excitability at low and high-frequency ranges.4
Specifically, N-methyl-D-aspartate receptors (NMDA-Rs) number and functionality have been critically implicated in the pathophysiology of ScZ5,6 and abnormalities in glutamatergic transmission are a candidate mechanism for disturbed high frequency oscillations in the disorder. In vivo and in vitro electrophysiological studies using NMDA-R antagonists have revealed an increase of spontaneous power at both low (30–60 Hz) and high (60–130 Hz) gamma-band ranges as well as at ripple frequencies (130–200 Hz).7 In contrast, oscillations at lower-frequencies, such as in the theta-band, are reduced.7 Only a small number of studies have reported no effects8 or a decrease.9 Different gamma-band frequencies, however, are not always equally modulated by ketamine and regional differences have been reported in some studies.8
In the current study, we investigated the impact of ketamine on resting-state activity in magnetoencephalography (MEG) recordings in healthy volunteers to establish links between preclinical research and findings from EEG/MEG-recordings in ScZ-patients. Recent evidence from spontaneous electroencephalogram (EEG) recordings in ScZ-patients has reported increased spontaneous high-frequency activity in ScZ10,11 although this has not been confirmed in all studies.10 In addition, several studies with functional magnetic resonance imaging (fMRI) indicated increased connectivity in large-scale networks following ketamine administration12 which parallels findings in participants at-risk for psychosis and patients with first-episode ScZ.13
Together with findings of elevated glutamate levels in early-stage ScZ,14 these findings raise the possibility that NMDA-hypofunctioning may underlie certain neuronal signatures of the disorder, highlighting the need to investigate the effects of Ketamine on gamma-band oscillations in healthy volunteers. However, in humans, only preliminary evidence exists on increased gamma-band power at rest following ketamine-administration at subanesthetic dosages.15
Methods and Materials
Participants
Twelve participants (2 females) with a mean age of 29.6 years (range: 27–39) were recruited and the structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (SCID-II)16 was administered. If criteria were met for a past or present Axis I or II diagnosis or endorsed a family history of psychosis, the participant was excluded from the study. The medical screening consisted of a physical examination along with regular ECG, vital signs, blood tests, drug screening, and psychological testing (see supplementary methods for a list of the tests adopted). The study was carried out according to the Declaration of Helsinki and approved by the ethical committees of the Goethe University Frankfurt. After complete description of the study to the participants, written informed consent was obtained.
Experimental Procedure
This study follows a single-blind, randomized, placebo-controlled, crossover design. At the beginning of the experimental session, a bolus of 10mg S-ketamine (drug condition) or 10ml of NaCl 0.9% (placebo condition) was injected. This was followed by continuous intravenous infusion at 0.006mg S-ketamine per Kg body weight per min or NaCl 0.9%, respectively. We recorded 8min (4min with eyes open and 4min with eyes closed) of resting-state activity during the continuous drug (ketamine or placebo) infusion. Only the eyes closed condition will be reported. Resting state activity was recorded circa 45min after bolus injection, time in which participants performed a visual task (data not reported here). Following the MEG-recording, participants were examined using the Positive and Negative Syndrome Scale (PANSS)17 with the addition of the “disorganization” factor.18 For each subject the placebo and ketamine conditions were completed between 2 and 4 weeks apart.
Anatomical MRI Data Acquisition
Prior to the MEG-measurement, a high-resolution anatomical MRI scan was acquired for each participant using a 3D magnetization-prepared rapid-acquisition gradient echo sequence (160 slices; voxel size: 1×1 × 1mm; field of view: 256mm; repetition time [TR]: 2300ms; transfer entropy [TE]: 3.93ms). Scanning was performed using a 3-Tesla Siemens Trio scanner.
MEG-Data Acquisition
MEG data were acquired using a 275-sensors whole-head system (Omega 2005, VSM MedTech Ltd, BC, Canada) at a sampling rate of 600 Hz in a synthetic third order axial gradiometer configuration. Data were band-passed filtered offline between 1–150 Hz, and participants’ head movements were monitored before and after each recording using coils placed on the nasion and 1cm anterior of the tragus of the left and right ear. Head movements were monitored before and after the recording. Recordings with movements larger than 5mm were excluded from the analysis.
MEG Data Processing and Analysis
Preprocessing and analysis of the MEG data was performed with the open source Matlab toolbox “FieldTrip.”19 The continuous recording was divided in segments of 2 s, each constituting a trial. Trials containing eye blinks or artifacts due to muscle activity or sensors (SQUIDs) jumps were discarded using automatic artifact rejection routines. Data were processed and statistically analyzed both at the sensor- and at the source-level. In addition, to investigate the effects of ketamine on the interactions between “drug-reactive” sources, we quantified changes in information transfer by measuring TE,20 as implemented in the TRENTOOL toolbox.21,22
Sensor- and Source-Level Analysis.
Sensor-level beta (13–30 Hz) and gamma (30–90 Hz) frequency activity was estimated using Morlet-wavelet convolution (5 cycles per wavelet). A non-parametric dependent samples t-test based on a permutation approach (1500 permutations)23 was used to test differences between the placebo and ketamine conditions on all MEG sensors. To minimize the influence of differences in the distance between MEG-sensors and head position on amplitude fluctuations, data was normalized both for high and low frequency analysis by dividing the amplitude of each frequency by the sum of the amplitudes of all frequencies.
Power-spectra were source-localized using a Dynamical Imaging of Coherent Sources (DICS) frequency beamformer.24 Single-subject source power estimates were normalized to the template brain of the Montreal Neurological Institute (MNI) using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Source data were statistically analyzed using cluster-based permutations (1500 permutations).
Transfer Entropy.
Source-level functional connectivity between regions showing spectral changes after ketamine administration (see Results section below) was estimated using TE.25,26 TE estimates the amount of information communicated from a source to a target process. This is achieved by quantifying how much information in the future of the target process is only predictable when knowing the past states of the source process. In this sense, TE can be seen as a more general, information theoretic version of Wiener–Granger causality (see supplementary information for details on TE analysis). In the first step of our analysis, we extracted the time-course (ie, virtual channels) of all sources showing ketamine-reactivity in the beta and gamma bands. We then assessed the global, drug-driven, change in TE between all sources through averaging TE values across all links per participant and conditions. To localize these changes across the links post hoc, a 1-sided permutation test for each link was performed and the alpha level was corrected for multiple comparisons using Bonferroni correction. Given the number of sources (n = 16) and the number of potential interactions for each source (n = 15), P was set to .05 / 240 (2.08×10−4). In addition, we investigated TE-changes separately for interactions between sources in the beta- and gamma-frequency ranges as well as between sources that were active in 2 different spectral bands (for this additional analysis, if no connectivity survived the Bonferroni-corrected threshold of 2.08×10−4, we adopted a more liberal threshold of P < .0005 uncorrected).
Results
PANSS-Data
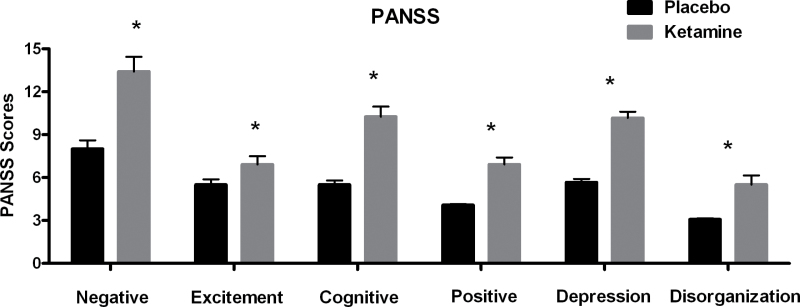
Ketamine lead to a statistically significant increase in all of the 6 PANSS subscales (see figure 1 and supplementary table S1).
Fig. 1.
Average scores on the 6 different Positive and Negative Syndrome Scale subscales for the placebo (black) and ketamine (gray) conditions. Error bars indicate the SEM (*P < .05).
Sensors- and Source-Level MEG Results
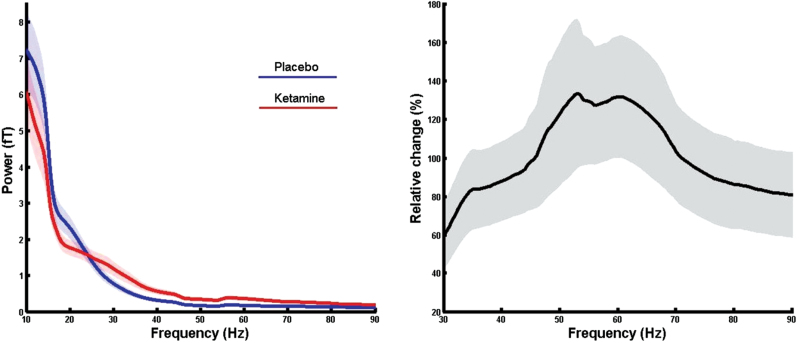
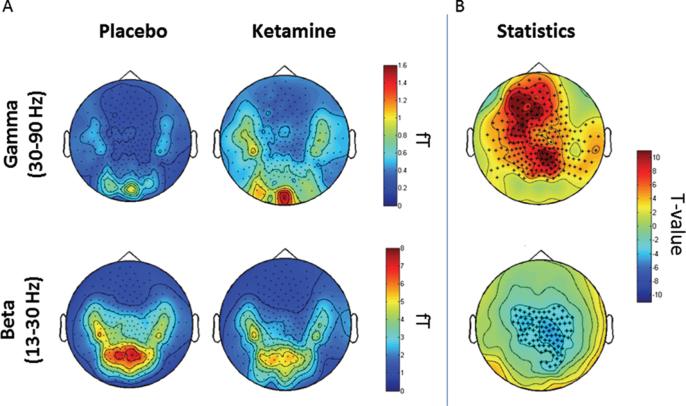
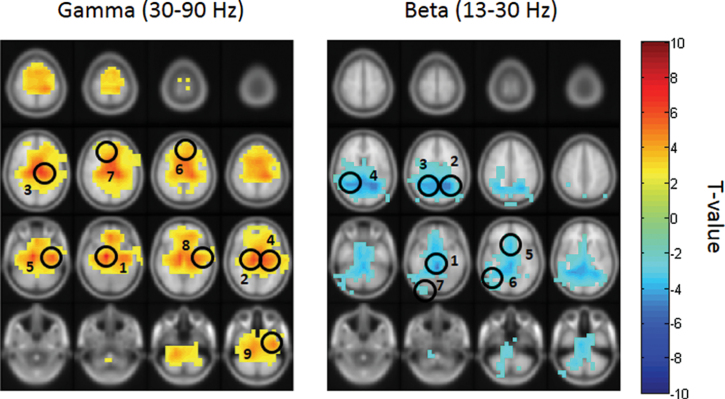
After artifact rejection, 77 trials (SD = 21) remained in the placebo and 84 trials (SD = 12) in the ketamine condition. Ketamine administration caused an increase in gamma-band (30–90 Hz) power (figure 2) over frontal, parietal and temporal MEG sensors (figure 3). Similar to the sensor-level results, source-analysis demonstrated an increase in the gamma-band frequency range following ketamine administration in a number of cortical and subcortical regions. 30–90 Hz power was most prominently increased in the right hippocampus and right/left thalami, followed by cortical structures, such as the left fusiform gyrus, right medio frontal cortex, left frontal pole, left superior frontal gyrus, left superior temporal gyrus, and left middle temporal gyrus (MTG-L; see figure 4 and supplementary table S2).
Fig. 2.
Power spectra analysis. (Left) Placebo and ketamine power-spectra as averaged across all subjects and calculated considering all MEG sensors (shades indicated the SEM). (Right) Relative change (ie, [[Ketamine power−Placebo power] / Placebo power] × 100) of the ketamine power with respect to the placebo power in the gamma-band range.
Fig. 4.
Sensor-level analysis. (A) Topoplots representing the average power spectra (fT) of gamma (top) and beta (bottom) frequency ranges in the placebo (left) and ketamine (right) conditions. (B) Results of the nonparametric cluster-based statistic highlighting sensors showing a statistically significant effect for gamma (top) and beta (bottom) frequencies (red: ketamine > placebo; blue: colors placebo > ketamine) (*P < .001).
In contrast to gamma-band power, beta-band (13–30 Hz) activity was reduced after ketamine administration in particular over central MEG sensors. At the source-level, beta-band decreases were localized figure 3 to the cerebellum, left/right precunei, right middle temporal gyrus, left anterior cingulate cortex, right inferior temporal gyrus, and visual cortex (see figure 4 and supplementary table S3).
Fig. 3.
Source-level analysis. Cluster-based nonparametric statistic highlights statistically significant differences between the placebo and ketamine condition across the gamma (left) and beta (right) frequency bands (red: ketamine > placebo; blue: placebo > ketamine). Gamma-band (30–90 Hz): (1) R-hippocampus [10 −10 −20], (2) R-thalamus [10 −10 10], (3) L-thalamus [−10 −20 10], (4) L-fusiform gyrus [−40 −10 −30], (5) R-medial frontal cortex [0 40 −20], (6) L-frontal pole [−20 40 −10], (7) L-superior frontal gyrus [−20 40 40], (8) L-superior temporal gyrus [−70 −20 0], and (9) L-middle temporal gyrus [−60 0 −30]. Beta-band (13–30 Hz): (1) cerebellum [0 −40 −20], (2) L-precuneus [−20 −50 20], (3) R-precuneus [30 −50 10], (4) R-middle temporal gyrus [60 −30 −10], (5) L-anterior cingulate [0 20 −10], (6) R-inferior temporal gyrus [50 −60 −20], and (7) R-visual cortex [30 −90 −10].
Correlation Between MEG-Source Activity and PANSS
Source-power in 2 anatomical regions showing the strongest ketamine effect in the gamma-band range (right hippocampus and right thalamus) were correlated to the PANSS subscales using a nonparametrical Spearman correlation. Results showed a negative correlation between right hippocampus power after ketamine injection and PANSS Positive scale (rs12 = −0.70, P = .011).
TE Results
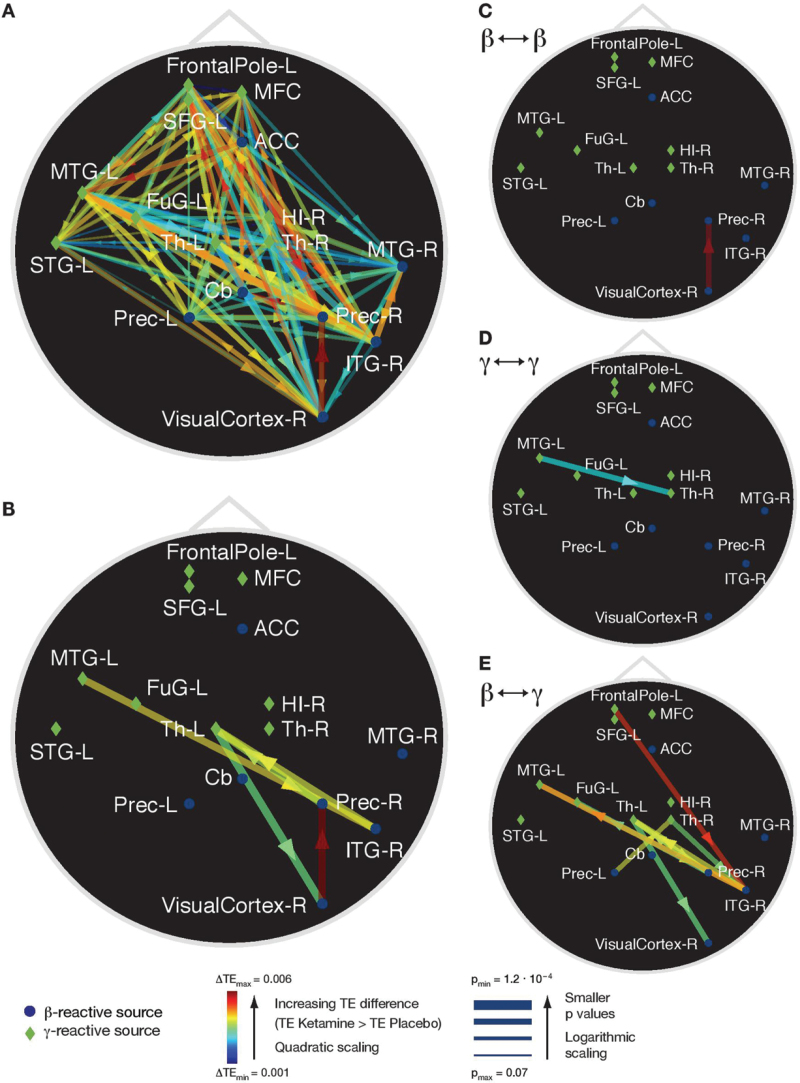
Ketamine administration caused an increase in the average TE-values (see figure 5A for uncorrected effects). Connections that survived multiple comparisons correction were localized to links from MTG-L to right inferior temporal gyrus (ITG-R); ITG-R to the left Thalamus (Th-L,); Th-L to right visual cortex (Visual Cortex-R); Visual Cortex-R to right precuneus (Prec-R); Prec-R to Th-L (figure 5B). Connections with significant increases in information transfer were found for source pairs comprising 3 of 4 possible types: between sources with ketamine-induced changes in the beta-band, between sources with changes in the gamma band, and from sources with changes in the gamma band to sources with changes in the beta band. Changes in TE values for the remaining type, from sources with changes in the beta band to sources with changes in the gamma band were found at slightly less conservative thresholds of P < .0005 (figure 5D). For the purpose of comparison we also present the other connection types at this threshold (figures 5C–E).
Fig. 5.
Transfer entropy (TE) analysis. TE differences between ketamine and placebo conditions. Green diamonds indicate MEG sources reactive to ketamine in the gamma band, blue circles indicate sources reactive in the beta band (figure 3). Arrow colors indicate strength of the difference. (A) Uncorrected differences in TE. (B) Statistically significant differences (Bonferroni corrected: P < 2.08×10−4). For illustration purposes we also provide the TE differences at a significance threshold of P < .0005 uncorrected, for the TE between (C) sources reactive in the beta frequency band, (D) in the gamma frequency band, and (E) between beta- and gamma-sources. Legend: FrontalPole-L = left frontal pole, MFC = medial frontal cortex, SFG-L = left superior frontal gyrus, ACC = anterior cingulate cortex, MTG-L = left middle temporal gyrus, FuG-L = left fusiform gyrus, Th-L= left thalamus, Cb = cerebellum, Prec-L = left precuneus, HI-R = right hippocampus, Th-R = right thalamus, MTG-R = right medial temporal gyrus, Prec-R = right precuneus, ITG-R = right inferior temporal gyrus, VisualCortex-R = right visual cortex.
Discussion
There is increasing evidence suggesting that core features of ScZ may be the consequence of hypofunctioning of NMDA-Rs.5 In the current study, we investigated the effects of ketamine, a noncompetitive antagonist of the NMDA-R, on resting-state MEG-activity in healthy volunteers, to establish whether changes in amplitude fluctuations and connectivity patterns following ketamine administration allow links to the pathophysiology of ScZ. Currently, only preliminary evidence exists on the effects of ketamine in humans on spontaneous beta/gamma- band activity.15 Our findings of pronounced increases in gamma-band power and increased functional connectivity are in agreement with extensive preclinical data on the effects of ketamine on neural oscillations.27–32 Moreover, there are similarities with evidence from EEG and fMRI-recordings in ScZ,13,33 which together have potentially important implications for the understanding of ScZ as a disorder involving fundamentally a disinhibition of cortico–subcortical circuits.
Ketamine Effects on High-frequency Activity: Potential Neurophysiological Mechanisms
The spectra during resting-state activity corresponded to the usual 1/f distribution both in the placebo and in the ketamine condition. The contrast between ketamine-induced spectral activity and the placebo condition, however, suggests an upregulation of high-frequency activity with a peak ~60 Hz (figure 2). The frequency as well as the magnitude of this effect are comparable to visually-induced activity that has been described in recent MEG-studies,34 suggesting that NMDA-R hypofunctioning could be associated with an oscillatory process in cortical and subcortical regions. In contrast to 30–90 Hz power, beta-band activity was strongly reduced, which is in agreement with recent data.35
Source-reconstruction of resting-state MEG-activity allowed us to determine the neural generators in different frequency ranges. In the 30–90 Hz frequency band, ketamine caused an upregulation in subcortical and cortical areas. The largest increases of gamma-band activity were observed in the right hippocampus and right/left thalami, followed by parietal, temporal and frontal structures (figure 4). This is in agreement with previous in vivo studies in rodents,9,27,36–38 which consistently demonstrated a ketamine-induced increase in spontaneous gamma-band activity in cortical and subcortical areas. Decreases in beta-band were localized to brain regions that were overall distinct from gamma-band generators. Maximal reductions in 13–30 Hz were localized to the cerebellum, temporal and visual cortex.
Potential mechanisms for the ketamine-driven upregulation of gamma-band activity are increased excitability of pyramidal cells due to reduced activation of GABAergic interneurons and a shift in the relative contribution of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA-R mediated excitatory post synaptic potentials (EPSPs) to the drive of interneurons.39 Previous studies have shown that NMDA-R hypofunction leads to an increase in firing rate of pyramidal neurons.40 In addition, AMPA receptor mediated excitation becomes relatively more preponderant when NMDA-Rs are deficient as AMPA-R mediated EPSPs have much faster kinetics than NMDA-R mediated EPSPs and are more numerous than NMDA-Rs on PV+-interneurons.41 Accordingly, reducing the NMDA-R mediated excitatory input on GABAergic interneurons increases the ratio of fast over slow EPSPs39 and this, together with increased activity of pyramidal cells, provides favorable conditions for fast oscillations with important implications for information processing and network-interactions.
Gamma-band oscillations are particularly prominent in superficial layers (layers 2/3),42 the main origin of feedforward projections, and are dependent upon fast, transient excitation of fast-spiking interneurons.43 In contrast, beta oscillations are largely found in infragranular layers and can be independent of excitatory or inhibitory synaptic transmission.44 Current theories suggest that beta-band oscillations are therefore involved in the mediation of feedback to lower sensory areas and important for predictive coding processes.45 Accordingly, 1 effect of the NMDA-R hypofunctioning is a possible shift towards feed-forward mediated information transmission and/or increase in background activity which could interfere with incoming sensory information. As a result, a decrease in signal-to-noise in neural circuits occurs,46,47 which could impact upon the ability to differentiate between relevant and irrelevant information, a symptom commonly observed in the early stages of ScZ.48
This hypothesis is supported by our result of increase functional connectivity (TE) between sources, which included the thalamus, hippocampus, parietal and temporal cortices. Increased TE-values have to be interpreted as information in one source closely following information available in another source.26 Thus, at an information theoretical level, our data suggests that MEG-derived generators follow more readily the input they receive, which could further amplify the shift towards a higher-frequency regime and thus contribute to a breakdown of filtering capabilities with respect to a source’s input.
Interestingly, elevated hippocampal gamma-band activity after ketamine administration correlated negatively with positive symptoms as measured with PANSS, which contrasts with findings in ScZ-patients indicating an opposite relationship.49 One possibility is that elevated hippocampal gamma-band activity during acute ketamine administration indexes an initial mechanism to compensate for dysfunctional thalamo-cortical sensory transmission through enhanced gating of neuronal responses, a process that is least in part mediated by hippocampal interneurons,50 and thus reduce the occurrence of psychotic symptoms.
NMDA-R, Gamma-Band Activity, Network Organization, and ScZ
An important question concerns the similarities between the changes in rhythmic activity and connectivity patterns resulting from NMDA-R hypofunction in the current study and the evidence on abnormal large-scale network-activity in ScZ-patients. As pointed above, ketamine and associated NMDA-R hypofunction lead to an upregulation of high-frequency activity and increased interactions between nodes of the network.
Currently, the large majority of studies in patients with ScZ have reported decreases of task related gamma band power51 and connectivity.52,53 However, a recent study33 showed that background gamma activity is increased during auditory steady-state stimulation in SZ which was interpreted as a disruption in E/I-balance parameters. This is furthermore supported by preliminary evidence for increased gamma-band in medication-naïve first-episode ScZ-patients at-rest11,54 which has not, however, been confirmed in other studies.10,51,55
Additional evidence supporting the relationship between the role of glutamatergic abnormalities and neurophysiological dysfunctions in ScZ comes from several studies that have examined functional connectivity in early-stage ScZ with resting-state fMRI. Consistent with our finding of increased connectivity in thalamo-cortical circuits, individuals at high risk for ScZ and patients with early-course ScZ were characterized by increased connectivity which was not present in chronic ScZ-patients.13
The possibility of NMDA-R mediated disinhibition in ScZ at illness-onset is supported by our recent data in unmedicated first-episode-ScZ patients which suggests an excessive spreading of neural activity as indexed by event-related fields during sensory processing in MEG-data,56 as well as by findings suggesting elevated glutamate levels during early illness stages which decrease progressively with illness duration.14 Accordingly, these findings highlight the possibility of a stage-specific elevation of network-activity and organization, which is compatible with a large-scale disinhibition of neural circuits in ScZ.
Our results of MEG-informed source-localization furthermore are consistent with recent data that have highlighted the importance of thalamo-cortical interactions and hippocampal circuits in the pathophysiology of ScZ. Several resting-state fMRI studies reported increased functional connectivity between thalamus and cortical regions,57–59 albeit some report mixed findings.59 In addition, the increase in gamma-band activity in hippocampal sources, a brain region with a large number of NMDA-R sites,60 is consistent with findings highlighting the possible contribution of elevated metabolism as a result of NMDA-R hypofunctioning in the early stages of ScZ.61
Issues for Further Research and Limitations
It should be noted that the interpretation of the physiological effects of ketamine is complicated by the fact that, in addition to blocking NMDA-Rs, ketamine also increases the systemic levels of dopamine, acetylcholine, and norepinephrine.62 However, more recent evidence suggests that the increase in gamma-band activity is mainly due to a specific blockade of NMDA-Rs containing the NR2A subunit.63
In addition, several brain regions with significant modulation at beta/gamma-band frequencies were localized to subcortical areas (thalamus and hippocampus). Albeit MEG subcortical source localization remains challenging due to the rapid decay of the neuromagnetic field, recent studies have however reported robust signals obtained from thalamic and hippocampal sources,64–66 suggesting the potential suitability of MEG to detect rhythmic activity from deeper brain regions.
The important role of the thalamus in the dysregulation of high-frequency oscillations in our MEG-data is supported by previous findings showing that the thalamus is centrally involved in the regulation of synchronous cortical activity and in the gating of sensory information.67 Specifically, there is a large body of evidence showing that gamma-band oscillations are robust signature of thalamic activity as indicated by pacemaker function of cells in the thalamic reticular nucleus (RT),68 and pronounced 30–90 Hz oscillations in the lateral geniculate nucleus.69 Moreover, data from animal model indicate that ketamine reduces extracellular GABA levels by acting on NMDA-R PV interneurons.70 In particular, GABA release reduction from the RT to other thalamic nuclei, due to inactivation of NMDA-R on RT-neurons, would lead to increase firing rate of thalamic relay neurons and pathological activation of thalamo-cortical circuits, which could trigger a widespread shift in excitability levels.71
This evidence is consistent with current findings of thalamic-driven dysregulation of connectivity patterns following acute ketamine administration.72 Together, these findings highlight an important convergence between preclinical findings and MEG-reconstructed resting-state networks that identify the thalamus as a core region of ketamine-induced network changes. Moreover, NMDA-R blockade by ketamine increases global-based connectivity12 and increased functional inputs to regions such as the thalamus, frontal lobe, and occipital cortex, in human fMRI resting-state recordings.
Summary
The findings of the current study support previous data from invasive electrophysiological investigations, which have demonstrated a profound effect of NMDA-hypofunction on coordinated high-frequency activity.7,29 Because some evidence suggests that spontaneous gamma-band power may be increased in ScZ-patients, especially at illness-onset,73 and because gamma-band activity is constitutive for cognition and normal brain functions,1 it is important to further identify the mechanisms through which ketamine leads to the upregulation of gamma-band activity. This will require an integration of both targeted pharmacological studies in in vitro and in vivo preparations as well as further investigations of the connectivity and dynamics of large-scale neuronal networks. Further research into the mechanisms underlying the effects of NMDA-R on high-frequency activity promises insights into the role of beta/gamma-band oscillations for normal brain functions, the pathophysiology of ScZ, but also of affective disorders, such as depression, because ketamine has recently been demonstrated to act rapidly as an antidepressant.74
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
LOEWE grant Neuronale Koordination Forschungsschwerpunkt Frankfurt (NeFF) to D.R.
Supplementary Material
Acknowledgments
We wish to thank Alla Brodski and Patricia Wollstadt for their support in the data processing and analysis. In addition, we would like to acknowledge the helpful suggestions of Prof. Boris Quednow in regards to the design of the study. P.J.U. has received research support from Lilly.
References
- 1. Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. [DOI] [PubMed] [Google Scholar]
- 2. Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. [DOI] [PubMed] [Google Scholar]
- 4. Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirov G, Pocklington AJ, Holmans P, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunt MJ, Kasicki S. A systematic review of the effects of NMDA receptor antagonists on oscillatory activity recorded in vivo. J Psychopharmacol. 2013;27:972–986. [DOI] [PubMed] [Google Scholar]
- 8. Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci. 2012;32:3022–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hunt MJ, Falinska M, Łeski S, Wojcik DK, Kasicki S. Differential effects produced by ketamine on oscillatory activity recorded in the rat hippocampus, dorsal striatum and nucleus accumbens. J Psychopharmacol. 2011;25:808–821. [DOI] [PubMed] [Google Scholar]
- 10. Rutter L, Carver FW, Holroyd T, et al. Magnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition. Hum Brain Mapp. 2009;30:3254–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kikuchi M, Hashimoto T, Nagasawa T, et al. Frontal areas contribute to reduced global coordination of resting-state gamma activities in drug-naïve patients with schizophrenia. Schizophr Res. 2011;130:187–194. [DOI] [PubMed] [Google Scholar]
- 12. Driesen NR, McCarthy G, Bhagwagar Z, et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18:1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anticevic A, Corlett PR, Cole MW, et al. N-methyl-d-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry. 2015;77:569–580. [DOI] [PubMed] [Google Scholar]
- 14. Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies. Schizophr Bull. 2013;39:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwartz MS, Virden S, Scott DF. Effects of ketamine on the electroencephalograph. Anaesthesia. 1974;29:135–140. [DOI] [PubMed] [Google Scholar]
- 16. First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II). Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 17. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 18. Cuesta MJ, Peralta V. Cognitive disorders in the positive, negative, and disorganization syndromes of schizophrenia. Psychiatry Res. 1995;58:227–235. [DOI] [PubMed] [Google Scholar]
- 19. Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schreiber T. Measuring information transfer. Phys Rev Lett. 2000;85:461–464. [DOI] [PubMed] [Google Scholar]
- 21. Lindner M, Vicente R, Priesemann V, Wibral M. TRENTOOL: a Matlab open source toolbox to analyse information flow in time series data with transfer entropy. BMC Neurosci. 2011;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wollstadt P, Martinez-Zarzuela M, Vicente R, Diaz-Pernas FJ, Wibral M. Efficient transfer entropy analysis of non-stationary neural time series. PLoS One. 2014;9:e102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. [DOI] [PubMed] [Google Scholar]
- 24. Gross J. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci. 2001;98:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wibral M, Vicente R, Lindner M. Transfer Entropy in Neuroscience. In: Springer, ed. Directed Information Measures in Neuroscience. Berlin, Germany: Springer; 2014. [Google Scholar]
- 26. Wibral M, Pampu N, Priesemann V, et al. Measuring information-transfer delays. PLoS One. 2013;8:e55809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. [DOI] [PubMed] [Google Scholar]
- 28. Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct Funct. 2012;217:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kocsis B, Brown RE, McCarley RW, Hajos M. Impact of ketamine on neuronal network dynamics: translational modeling of schizophrenia-relevant deficits. CNS Neurosci Ther. 2013;19:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Yoshida T, Katz DB, Lisman JE. NMDAR antagonist action in thalamus imposes δ oscillations on the hippocampus. J Neurophysiol. 2012;107:3181–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hakami T, Jones NC, Tolmacheva EA, et al. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4:e6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caixeta FV, Cornelio AM, Scheffer-Teixeira R, Ribeiro S, Tort AB. Ketamine alters oscillatory coupling in the hippocampus. Sci Rep. 2013;3:2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, Spencer KM. Spontaneous Gamma Activity in Schizophrenia. JAMA Psychiatry. 2015. doi:10.1001/jamapsychiatry.2014.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM, Fries P. Localizing human visual gamma-band activity in frequency, time and space. Neuroimage. 2006;29:764–773. [DOI] [PubMed] [Google Scholar]
- 35. Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–1464. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Yoshida T, Katz DB, Lisman JE. NMDAR antagonist action in thalamus imposes oscillations on the hippocampus. J Neurophysiol. 2012;107:3181–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ehrlichman RS, Gandal MJ, Maxwell CR, et al. N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience. 2009;158:705–712. [DOI] [PubMed] [Google Scholar]
- 38. Phillips KG, Cotel MC, McCarthy AP, et al. Differential effects of NMDA antagonists on high frequency and gamma EEG oscillations in a neurodevelopmental model of schizophrenia. Neuropharmacology. 2012;62:1359–1370. [DOI] [PubMed] [Google Scholar]
- 39. Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci U S A. 2011;108:11262–11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. [DOI] [PubMed] [Google Scholar]
- 44. Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arnal LH, Giraud AL. Cortical oscillations and sensory predictions. Trends Cogn Sci. 2012;16:390–398. [DOI] [PubMed] [Google Scholar]
- 46. Kulikova SP, Tolmacheva EA, Anderson P, et al. Opposite effects of ketamine and deep brain stimulation on rat thalamocortical information processing. Eur J Neurosci. 2012;36:3407–3419. [DOI] [PubMed] [Google Scholar]
- 47. Saunders JA, Gandal MJ, Siegel SJ. NMDA antagonists recreate signal-to-noise ratio and timing perturbations present in schizophrenia. Neurobiol Dis. 2012;46:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. [DOI] [PubMed] [Google Scholar]
- 49. Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–230. [DOI] [PubMed] [Google Scholar]
- 50. Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience. 1995;69:371–381. [DOI] [PubMed] [Google Scholar]
- 51. Grutzner C, Wibral M, Sun L, et al. Deficits in high- (>60 Hz) gamma-band oscillations during visual processing in schizophrenia. Front Hum Neurosci. 2013;7:88. doi:10.3389/fnhum.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Uhlhaas PJ, Linden DE, Singer W, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun L, Castellanos N, Grützner C, et al. Evidence for dysregulated high-frequency oscillations during sensory processing in medication-naïve, first episode schizophrenia. Schizophr Res. 2013;150:519–525. [DOI] [PubMed] [Google Scholar]
- 56. Rivolta D, Castellanos NP, Stawowsky C, et al. Source-reconstruction of event-related fields reveals hyperfunction and hypofunction of cortical circuits in antipsychotic-naive, first-episode schizophrenia patients during Mooney face processing. J Neurosci. 2014;34:5909–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klingner CM, Langbein K, Dietzek M, et al. Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014;264:111–119. [DOI] [PubMed] [Google Scholar]
- 58. Anticevic A, Cole MW, Repovs G, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24:3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Monaghan DT, Cotman CW. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985;5:2909–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schobel SA, Chaudhury NH, Khan UA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gunduz-Bruce H. The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev. 2009;60:279–286. [DOI] [PubMed] [Google Scholar]
- 63. Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry. 2012;71:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ribary U, Ioannides AA, Singh KD, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci U S A. 1991;88:11037–11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Poch C, Fuentemilla L, Barnes GR, Duzel E. Hippocampal theta-phase modulation of replay correlates with configural-relational short-term memory performance. J Neurosci. 2011;31:7038–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roux F, Wibral M, Singer W, Aru J, Uhlhaas PJ. The phase of thalamic alpha activity modulates cortical gamma-band activity: evidence from resting-state MEG recordings. J Neurosci. 2013;33:17827–17835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Saalmann YB, Kastner S. Cognitive and perceptual functions of the visual thalamus. Neuron. 2011;71:209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pinault D, Deschenes M. Control of 40-Hz firing of reticular thalamic cells by neurotransmitters. Neuroscience. 1992;51:259–268. [DOI] [PubMed] [Google Scholar]
- 69. Ghose GM, Freeman RD. Oscillatory discharge in the visual system: does it have a functional role? J Neurophysiol. 1992;68:1558–1574. [DOI] [PubMed] [Google Scholar]
- 70. Grasshoff C, Gillessen T, Wagner E, Thiermann H, Szinicz L. Ketamine reduces cholinergic modulated GABA release from rat striatal slices. Toxicol Lett. 2005;156:361–367. [DOI] [PubMed] [Google Scholar]
- 71. Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dawson N, McDonald M, Higham DJ, Morris BJ, Pratt JA. Subanesthetic ketamine treatment promotes abnormal interactions between neural subsystems and alters the properties of functional brain networks. Neuropsychopharmacology. 2014;39:1786–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim SY, Lee H, Kim HJ, et al. In vivo and ex vivo evidence for ketamine-induced hyperglutamatergic activity in the cerebral cortex of the rat: potential relevance to schizophrenia. NMR Biomed. 2011;24:1235–1242. [DOI] [PubMed] [Google Scholar]
- 74. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.