Abstract
In an effort to increase applicability of preclinical research to both men and women, the National Institute of Health is anticipated to put forth guidelines for the incorporation of sex as a variable in animal studies. Common myths and perceived barriers to the inclusion of females in neuroscience research are discussed and a roadmap for implementation provided. Accounting for sex as a variable does not require studying sex differences and is easier to implement than generally assumed.
Key words: sex differences, hormones, behavior
Introduction
In 1993, the United States Congress passed the National Institutes of Health Revitalization Act which mandated equal representation of women and minorities in clinical research funded by that agency. While the positive impact of this act on the health of women and minorities continues to be debated, it is unambiguously clear that the mandate did not trickle down to preclinical research involving animal models and cell lines.1,2 Surveys as recently as 2009 across a wide range of biological disciplines reveal overwhelming reliance on exclusively male animals or that subject sex was undefined. The discipline of neuroscience is one of the most heavily skewed with a ratio of 5.5:1 male to female subjects. The deleterious consequences of the under representation of females in preclinical studies is evident in the 8 out of 10 drugs withdrawn from the market in the past 10 years being due to unexpected adverse events in women, some of them life threatening (see3). The “failure to replicate” phenomenon that has alarmed the large funding agencies has been attributed to multiple variables, including a lack of consideration of sex differences.4 The needle began to move towards the middle in recent years as more and more journals began to require the sex of the animals be reported, sometimes even in the title.5
The research landscape now has the potential for a seismic shift after the announcement by the NIH in Spring of 2014 that they would begin requiring equal representation of both sexes in preclinical research except for a few “narrowly defined” exceptions.6 Reactions were swift and heated, both those that were celebratory7 and those that decried the move as poorly conceived, unnecessary, and potentially disastrous.8 Both sides can be accused of over reacting given there have been no clear guidelines, mandates or requirements established to-date. But there has been a great deal of discussion,9 and these have revealed the many factors that have to be considered, including when to include both sexes and how, the cost, the necessity, where to find the appropriate grant and manuscript review expertize and defining the success metrics that demonstrate it has all been worth the effort.
For animal model studies of neuropsychiatric disorders, the consideration of sex as a variable would seem self-evident given the strong gender bias in diagnostic frequency of so many conditions, yet in most preclinical models the comparison of males and females remains relatively rare, with the exception of those few who are explicitly mining for sex differences. The goal of this article is thus 2-fold. First is to convince readers of this journal that it is to their great advantage to begin incorporation of both sexes in their preclinical research. Second is to lay out a road map that provides guidance and suggestions for how to relatively painlessly include males and females in preclinical studies, as well as allay commonly repeated myths about what is required to do so. It is not the goal of this review to comment on, substitute for or in any way usurp the National Institute of Health (NIH) guidelines for research that incorporates both sexes. At this writing those guidelines have not been clearly articulated but there has been much thoughtful discussion both within and between the NIH and the extramural research community.3,9 Announcement of the guidelines is anticipated in Fall of 2015 for the FY16 round of grant applications, thus now is the time to be begin best practices for incorporating the influences of sex in preclinical research.
Why Should I Study Both Males and Females?
Not surprisingly, the earliest reports of sex differences in the brain were related to reproductive functions, either the sexual and parenting behavior of the animals, or the neural control of the anterior pituitary and the release of gonadotropins essential for ovulation in females but not males. All of these end-points are controlled by neuroanatomical and physiological parameters that are robustly different in males and females.10 Moreover, these reproduction-related sex differences are almost exclusively localized to the hypothalamus and closely associated preoptic area, regions far from the more intensely studied cerebral cortex and hippocampus. Early attention on sex differences was understandably focused in these brain regions and while it was perfectly acceptable to note that the brains of males and females are different when it comes to reproduction, it was a brave man who would say this was also true for brain regions relevant to cognition or emotionality. This began to change with the work coming from the McEwen lab in the 1990s convincingly demonstrating that the gonadal hormone, estradiol, exerted potent effects on the plasticity of hippocampal pyramidal neurons.11 Nonetheless, for many investigators the notion still persists that there is no difference in male and female brains outside of reproduction, yet ironically, most investigators limit themselves to studying only one sex, overwhelmingly males. But the burden of proof has shifted as we now know that the potential for a sex difference exists for every endpoint one studies in the brain.12 This includes neurogenesis, glialgenesis, migration, myelination, dendritic branching, synaptogenesis, synapse elimination, apoptosis, astrocyte and microglia morphology, neurocircuitry, neurochemistry, and even fundamental parameters such as basal calcium level and resting membrane potential (see for review13). If we consider the behavioral assays so commonly used as endophenotypes of neuropsychiatric disorders, all of these also have inherent sex differences depending on timing and paradigm. These include open field, elevated plus maze, sucrose preference, forced swim test, tail suspension, and more. Exposure to stress prenatally or postnatally has different effects on males and females and in opposite directions depending on the timing and nature of the stress.14–16 The list goes on but suffice it to say it should never be assumed there is not a sex difference in a particular endpoint, in fact the assumption should be the opposite until proven otherwise.17,18
And by doing so, one may discover the heuristic value inherent in comparing and contrasting males and females. Novel mechanisms related to pain,19 cell death,20 synaptic physiology21, and synapse formation22 are just a few examples of biological processes that would have remained undiscovered if not for the active comparison of males and females.
Common Myths That Create Barriers to Studying Females
There are lots of reasons offered for why not to study females, but as scientists the burden is on us to ask are they legitimate reasons? Below are just a few that I think my colleagues would agree are the most commonly heard.
“If I include females I will have to control for the phase of the estrus cycle and thereby quadruple my N, which I cannot afford”.
For many years, it was a de facto truth that estrus cycle had to be controlled for when studying females, but was it really necessary? The answer was empirically answered by a meta-analyses of 293 neuroscience focused articles which measured 9932 traits in 30 broad categories.23 The variability in the response of cycling females was no greater than that for males, and in some cases was even less. What could be causing variability between males? Housing. Males that are group housed form dominance hierarchies, producing a range of affective states which are directed by varying levels of hormonal and other changes. Isolating males can be highly stressful, introducing an unanticipated variable. The bottom line is, you don’t need to control for estrus cycle but you should be alert to the possibility that it is contributing to variability in your data, just as you should also be alert to a similar possibility if your animals are group vs singly housed.
“You can’t tell males from females until they go through puberty”
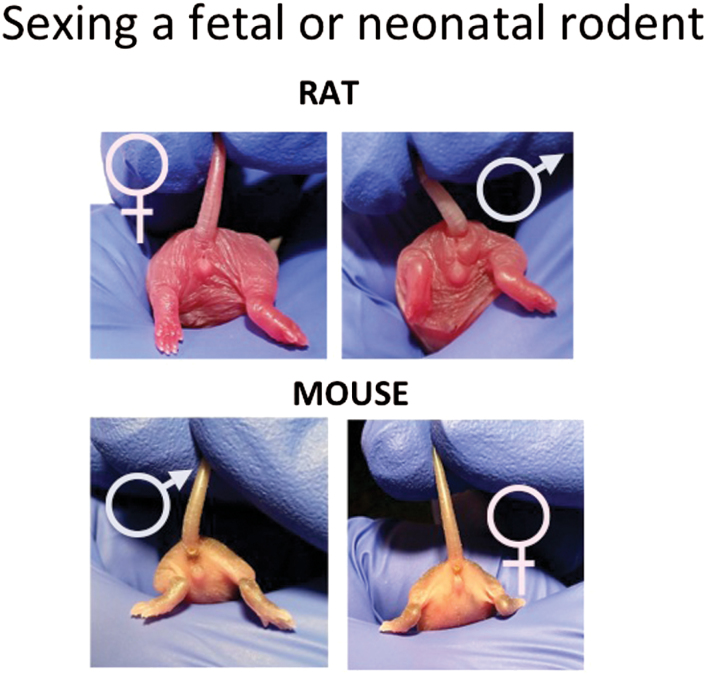
The sex of rat and mouse pups can be easily determined on the day of birth by visual inspection of the genitalia. The anogenital distance is greater in males and usually has a slight pigmentation (figure 1). Visual inspection also works in late gestation fetuses and can be further confirmed by dissection of the abdomen and localization of the testis which have not yet descended. Earlier gestation fetuses can be sexed genetically for Sry or by an even simpler recently developed PCR using a single primer pair that amplifies different sized fragment of the X and Y chromosome.24 Often researchers need to pool samples from fetuses and this is done without consideration of sex. This is fine as it meets the mandate of including both sexes and should simply be reported as mixed sex.
Fig. 1.
Sexing neonatal rats and mice. Examination of the anogenital region in animals as young as a few hours reveals a clear difference in males and females, with males having a longer anogenital distance, some scrotal swelling, and slight pigmentation.
“There are no differences in the brain prior to puberty”
Sex differences in the brain begin to be established in utero and an early organizational period is complete by the first week of life in mammals. The increase in gonadal hormones at puberty then acts on a brain that has been permanently impacted by developmental processes that are different in males and females. Even the experience of male and female pups can be different in unappreciated ways. For example, male pups receive far more anogenital grooming from the dam25 and are preferentially retrieved to the nest if dislocated.26 Both of these have the potential to alter brain development.
“Other variables such as age are more important to me than sex”.
There are many biological variables that are important to consider but the one that doesn’t change, is most easily identified, is most potently acted upon by evolutionary pressures and accounts for half of the population at any give time, is sex. The impact of sex certainly changes across the life span and its influence may wax or wan depending on the endpoint, but its centrality in biological processes cannot be denied.
“I am only studying cells in a dish and so it doesn’t matter”
Even cells have a sex,27 each one is an XX or XY and again a systematic comparison across studies found that any number of parameters varied across both primary cultures and those cell lines in which sex could be identified (sex is lost in many cell lines due to chromosomal aneuplodies and deletions).28 This is also true for primary neuronal culture where factors as fundamental as resting calcium can vary in male and female hippocampal neurons.29
“I prefer to study gender as opposed to sex”
Often investigators using animal models are uncomfortable with the word sex and use gender to refer to males vs females. But gender is a purely human construct that incorporates both persons perception of their sex and societies views and attitudes towards that person based on the perception of sex. Because we cannot know if animals have a perception of their sex, the term gender cannot be applied.
Roadmap
The incorporation of sex as a variable does not mean that one has to study sex differences. This is an important distinction in experimental design. Studying sex differences requires sufficient subjects that even small differences can be detected with a high probability, ie sufficient power. However, just incorporating sex as a variable provides the ability to detect a large difference if it exists. This takes much less power and can be tolerated at a lower probability level. Researchers often confuse the power analyses used to calculate the n for their primary endpoint as the same power needed to detect a sex difference in that endpoint. They are not necessarily the same as the parameters that go into a power analyses, ie variance, magnitude effect, confidence etc., will not be the same. It is not unlikely that the power required to detect a sex difference is greater than that to detect an effect of a particular manipulation.
Step One: Keep Doing What You Are Already Doing But Change Half the Animals in Your Study to Female
This simple step will now incorporate sex as a variable into your design. Whether sex is contributing to your variability is determined statistically. So if you were previously comparing 2 groups by t-test, now conduct a 2-way ANOVA with sex as a factor. My recommendation would be if your habit is a group size of 6, increase it to 8 and use 4 males and 4 females as a way of slightly hedging your bets in favor of confirming or denying a sex difference. If there is no statistical effect of sex, you can go back to your t-test. If you have multiple groups, still conduct a 2-way ANOVA but with sex as a factor and so on. Once sex is eliminated as a contributor to your effect, it is legitimate to no longer incorporate it into your statistical analyses (at least in this authors opinion). However, it does not mean you should stop using females. Indeed you now have the added benefit of using all of the animals at your disposal. If there is no effect of sex then there is no justification for excluding females. This can be particularly valuable in the use of genetically modified mouse strains in which the females are often discarded.
While statistics are valuable in telling you if there is a main effect of sex, you should not be a slave to a P value. This would be a time when a “trend” shouldn’t be ignored. Examine your data for signs of increased variability or a bimodal distribution when females are incorporated. These would both be signs that females are responding differently than males either at baseline or in response to your manipulation.
Step Two: Embrace the Difference and Exploit it for Discovery, or, Report and Move On
If your data is telling you there might be a sex difference in responsiveness this can be a great tool for further mechanistic exploration. There are 2 ways to address this. One is to ask, what is the source of the sex difference, the other is to ask what does the sex difference tell you about your phenomena. Sex differences arise from 3 biological sources; (1) hormones, either exposure early in life or in adulthood, (2) genes on the X or Y chromosome, and (3) experience. In our rodent animal models experience can generally be considered last in the list of potential sources and hormones generally the first. Determining if a sex difference is due to the unique hormonal profiles of adult males and females is relatively easy, but is often a direction most investigators don’t want to go. Moreover, many adult sex differences are due to the imprinting effects of hormones in development and here things get a lot more complicated, but are still manageable. See Becker et al30 and McCarthy et al31 for “how to” guides on studying hormonal effects. If hormones are not involved the next best guess is chromosome compliment and here things are even trickier, involving genetically engineered mice in which the Sry gene has been translocated to an autosome, thereby separating genetic sex from gonadal sex.32
But one doesn’t need to know the source of a sex difference to use it to advantage. Instead one can simply ask what is different in males and females that is altering the response? Here is an imaginary example. An investigator is using optogenetics to stimulate opening of a calcium channel in the basolateral amygdala to induce an antianxiety effect. It is working well in males, but when the investigator tries it in females, no effect. So what is different about females? Detection of early gene activation (ie, cfos) indicates that optogenetic stimulation does not excite the female neurons as it does the males but pharmacological manipulation to open the calcium channels works equally well in males and females. Injection of retrograde tracers into the amygdala indicates a robust projection to the nucleus accumbens present in males is lacking in females. Thus both males and females have the calcium channel, but females have a different circuitry. This provides the investigator a clear tool for elucidating the neural circuit mediating their antianxiety effect and would provide grounds for examining public databases on the human connectome to see if a similar variability existed in men vs women or some other population of interest, such as those with anxiety disorders. This is an entirely imaginary example but a parallel and real example of how preclinical work has unexpectedly informed clinical research on neuropsychiatric disorders in the context of sex differences can be found in33.
In summary, incorporating sex as a variable into preclinical research is not as daunting as it might first appear. There is no need to double sample sizes, monitor the estrus cycle, perform gonadectomies etc if one is not specifically pursuing the origins of a sex difference. But including females in each experiment allows for any findings to be generalized to the entire population, not just half. And, if one is lucky, including both sexes might provide unexpected insights into the origins of a phenomenon or even disorder, a prospect that is becoming a reality in difficult and gender-biased conditions such as schizophrenia34 and autism spectrum disorder.35
Funding
National Institutes of Health of the United States (R01MH52716, R01NS050525, and R01MH091424 to M.M.M.)
Acknowledgments
The author has declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2010;35:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zucker I, Beery AK. Males still dominate animal studies. Nature 2010;465:690. [DOI] [PubMed] [Google Scholar]
- 3. Sandberg K, Umans JG, the Georgetown Consensus Conference Work G. Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research. FASEB J. 2015;29:1646–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature 2014;505:612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blaustein JD. Animals have a sex, and so should titles and methods sections of articles in Endocrinology. Endocrinology 2012;153:2539–2540. [DOI] [PubMed] [Google Scholar]
- 6. Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014;509:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCullough LD, McCarthy MM, de Vries GJ. NIH policy: status quo is also costly. Nature 2014;510:340. [DOI] [PubMed] [Google Scholar]
- 8. Fields RD. NIH policy: mandate goes too far. Nature 2014;510:340. [DOI] [PubMed] [Google Scholar]
- 9. McCullough LD, de Vries GJ, Miller VM, Becker JB, Sandberg K, McCarthy MM. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol Sex Differ. 2014;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCarthy M, De Vries G, Forger N. Sexual differentiation of the brain: mode, mechanisms and meaning. In: Pfaff D Arnold AP Etgen AM Fahrbach SE and Rubin RT, eds. Hormones, Brain and Behavior. San Diego, CA: Academic Press; 2009:1707–1744. [Google Scholar]
- 11. Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cahill L. Sex influences on brain and emotional memory: the burden of proof has shifted. Progress Brain Res. 2010;186:29–40. [DOI] [PubMed] [Google Scholar]
- 13. McCarthy MM. Estradiol and the developing brain. Physiol. Rev. 2008;88:91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci. 2004;19:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howerton CL, Bale TL. Targeted placental deletion of OGT recapitulates the prenatal stress phenotype including hypothalamic mitochondrial dysfunction. Proc Natl Acad Sci USA 2014;111:9639–9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. [DOI] [PubMed] [Google Scholar]
- 18. Jazin E, Cahill L. Sex differences in molecular neuroscience: from fruit flies to humans. Nat Rev Neurosci. 2010;11:9–17. [DOI] [PubMed] [Google Scholar]
- 19. Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Progress Brain Res. 2010;186:141–157. [DOI] [PubMed] [Google Scholar]
- 20. Hill CA, Fitch RH. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurol Res Int. 2012;2012:867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 2012;74:801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. [DOI] [PubMed] [Google Scholar]
- 23. Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. [DOI] [PubMed] [Google Scholar]
- 24. McFarlane L, Truong V, Palmer JS, Wilhelm D. Novel PCR assay for determining the genetic sex of mice. Sex Dev. 2013;7:207–211. [DOI] [PubMed] [Google Scholar]
- 25. Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev Psychobiol. 1984;17:347–356. [DOI] [PubMed] [Google Scholar]
- 26. Bowers JM, Perez Pouchoulen M, Edwards NS, McCarthy MM. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J Neurosci. 2012;33:2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15:6–11. [DOI] [PubMed] [Google Scholar]
- 28. Shah K, McCormack CE, Bradbury NA. Do you know the sex of your cells? Am J Physiol Cell Physiol. 2014;306:C3–C18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nunez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing GABA and possible role of local estradiol synthesis in the developing male and female hippocampus. Neuroscience 2009;158:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Becker JB, Arnold AP, Berkley KJ, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 2005;146:1650–1673. [DOI] [PubMed] [Google Scholar]
- 31. McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: The not so inconvenient truth. J Neurosci. 2012;32:2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dao DT, Mahon PB, Cai X, et al. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry 2010;68:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry 2010;22:417–428. [DOI] [PubMed] [Google Scholar]
- 35. Werling DM, Lowe JK, Luo R, Cantor RM, Geschwind DH. Replication of linkage at chromosome 20p13 and identification of suggestive sex-differential risk loci for autism spectrum disorder. Mol Autism. 2014;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]