Abstract
Amotivation and reward-processing deficits have long been described in patients with schizophrenia and considered large contributors to patients’ inability to integrate well in society. No effective treatments exist for these symptoms, partly because the neuromechanisms mediating such symptoms are poorly understood. Here, we propose a translational neuroscientific approach that can be used to assess reward/motivational deficits related to the negative symptoms of schizophrenia using behavioral paradigms that can also be conducted in experimental animals. By designing and using objective laboratory behavioral tools that are parallel in their parameters in rodents and humans, the neuromechanisms underlying behaviors with relevance to these symptoms of schizophrenia can be investigated. We describe tasks that measure the motivation of rodents to expend physical and cognitive effort to gain rewards, as well as probabilistic learning tasks that assess both reward learning and feedback-based decision making. The latter tasks are relevant because of demonstrated links of performance deficits correlating with negative symptoms in patients with schizophrenia. These tasks utilize operant techniques in order to investigate neural circuits targeting a specific domain across species. These tasks therefore enable the development of insights into altered mechanisms leading to negative symptom-relevant behaviors in patients with schizophrenia. Such findings will then enable the development of targeted treatments for these altered neuromechanisms and behaviors seen in schizophrenia.
Introduction
Schizophrenia is a lifelong debilitating neurodevelopmental disorder affecting approximately 1% of the population.1 Patients with schizophrenia exhibit myriad of symptoms, with treatments for positive symptoms (eg, hallucinations, delusions), being available since the 1950s. In the late 1990s, it was recognized that cognitive symptoms (eg, inattention, poor working memory, and executive dysfunction) predicted functional outcome in patients. Since then, several National Institute of Mental Health (NIMH) initiatives were undertaken to improve our understanding of neuromechanisms underlying cognitive deficits (eg, Measurement And Treatment Research to Improve Cognition in Schizophrenia2; Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia [CNTRICS3]; and parallel efforts in the United Kingdom, Cambridge Neuropsychological Test Automated Battery [CANTAB4,5]). These initiatives led to the Food and Drug Administration (FDA) recognizing that treatments for cognitive dysfunction could be approved. Most relevant to the present review, are the CNTRICS and CANTAB efforts that focused on developing cross-species tests to investigate specific domains. These efforts highlighted the fact that cross-species tests are required to improve our understanding of the neurosubstrates of schizophrenia symptoms that are not adequately treated today. Despite the recognition that cognitive symptoms require such efforts, 1 major symptom group characterizing schizophrenia that has not seen the same efforts are negative symptoms.
A NIMH-initiated consensus conference held on negative symptoms clarified that negative symptoms are distinct from cognitive deficits.6–8 This conference confirmed that negative symptoms of schizophrenia include affective flattening (diminished emotional expression), anhedonia (diminished ability to experience pleasure), avolition (amotivation), alogia (impoverished speech), and asociality9 but can be divided into 2 main classes: (1) reduced expression of observable verbal and nonverbal communication (eg, reduced facial expression or voice tone) and (2) reduced motivation (ie, avolition).6,10,11 This consensus meeting resulted in FDA-endorsement of negative symptoms as a drug target but it was not designed to develop/recommend objective laboratory-based tests for these symptoms. Hence, clinical rating scales remain the primary measure of negative symptoms, creating difficulty in identifying neural mechanisms underlying these symptoms given the lack of objective quantification of behaviors.
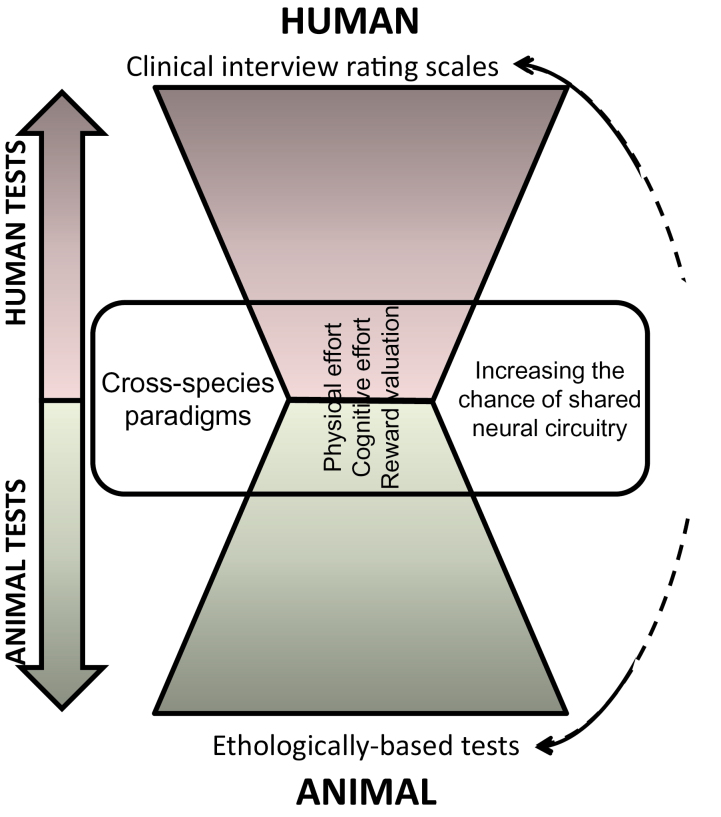
More germane to this review and our current understanding is that motivational factors of negative symptoms have been linked with poor functional outcome in patients.12–14 To-date, rodent studies investigating the putative neural mechanisms underlying negative symptom-related behaviors have relied largely on nonoperant based tests thought to relate to negative symptoms of schizophrenia.15,16 For example, the sweet-preference test in animals measures preference for a sweet vs a nonsweet solution, thought to reflect anhedonia in schizophrenia. Despite the large number of studies investigating this phenomenon in rodents claiming this relation,17–20 when patients with schizophrenia were finally tested, they exhibited normal preference for juice (ie, sweet solution) despite their high negative symptom scores.21 Considering the difficulty of linking interview-based rating scales to laboratory behavioral tasks in experimental animals (figure 1), “the present review describes procedures that quantify motivation and reward valuation in rodents in a similar parametric fashion to tests that can be conducted in humans and briefly describes what is known of the neuromechanisms underlying these behaviors.” Such cross-species tasks have high translational value in terms of both identifying neuromechanisms mediating negative symptoms, as well as targets for medication development.
Fig. 1.
A schematic of the translational approach described here towards the development of cross-species applicable procedures to quantify behaviors with relevance to the negative symptoms of schizophrenia. Traditional measures of negative symptoms in patients with schizophrenia utilize interview-based rating scales. On the other end of the spectrum, animal paradigms have largely utilized behaviors that are relevant to the animal’s natural behavior (ethological) in the hope that these measures will provide similarities to the human rating scales. The approach proposed here is to identify paradigms that can be conducted across species, providing quantifiable measures that are consistent across species. By utilizing consistent paradigms across species, it is hoped that there will be greater consistency in the neural circuitry between species. Thus, any mechanistic findings in animals will have an increased chance of representing the same circuitry in humans.
The approach taken here is consistent with the Research Domain Criteria (RDoC) initiative of the NIMH,22 designed to bypass diagnostic categories and focus instead on classifying psychopathology based on dimensions of functioning in patients present across diagnostic categories.23–25 Several behavioral procedures discussed below are listed in the Positive Valence System domain of the RDoC as tasks assessing constructs of approach motivation. Specifically, the subconstructs of expectancy/reward prediction-error action selection/preference-based decision making, effort valuation/willingness to work, reward valuation, and the construct of reward learning are some of the constructs assessed by the procedures described below.
There have been several recent attempts to use effort-based decision-making paradigms that involve reward delivery upon emission of correct responses (borrowed from cognitive neuroscience) to quantify effortful choice-preference in schizophrenia patients (Green et al,26 this issue). Some effort-based decision-making tasks have been developed for use in rodents and can be readily adapted for humans, eg, motivation to exert physical or cognitive effort.27 Other tasks include quantifying the value placed on a reward, integrating feedback from reward/loss, and translating this feedback into reward-seeking action. Tasks that can quantify each of these aspects of reward processing will be discussed below (table 1).
Table 1.
Paradigmatic Differences Between Described Tasks
| Paradigm | Physical Effort | Cognitive Effort | Punishment Risk | Choosing Between Stimuli |
|---|---|---|---|---|
| Progressive Ratio Breakpoint Paradigm | Increase over time | Constant low | None | Choice is only when to stop |
| Effort-based discounting task | Choice of high/low | Constant low | Lack of reward | Choice only in difficulty level of trial |
| Cognitive/perceptive effort task | Constant low | Choice of high/low | Lack of reward | Choice only in difficulty level of trial |
| Probabilistic learning task (explicit reward feedback association) | Constant low | Constant high | Lack of reward | Choice between 2 stimuli with varied reward probabilities |
| Iowa Gambling Task (feedback-based decision making under risk) | Constant low | Constant high | Varied punishment levels/probabilities | Choice between 4 stimuli with varied reward/punishment probabilities |
| Response Bias Probabilistic reward task (reward responsiveness) | Constant low | Constant high | Lack of reward | Accuracy between 2 choices irrelevant: Reward probability biases selection toward 1 option |
Reward Valuation From Physical and/or Cognitive Effort Tasks
A classic test for measuring the motivation to exert physical effort to gain rewards is the Progressive Ratio Breakpoint (PRBP) procedure that can be used in almost every species. The PRBP involves progressively increased effort requirements (eg, pressing a lever for rodents or pressing a key on a keyboard for humans) to obtain the same amount of reward. In this procedure, the willingness of an animal to work for a reward is quantified by assessing the breaking point, that is the point where the subject ceases to respond because “the juice is no longer worth the squeeze”.28 In 2000, Ellenbroek and Cools15 suggested using the PRBP to quantify negative symptom-relevant behavior in animal models of schizophrenia, a premise supported by others in the field.16,29
In 2014, Wolf and colleagues30 conducted a PRBP-based study in patients with schizophrenia. Patients were given information on the reward level and number of trials required to obtain a reward. The task was simple and required that subjects identify the highest of 2 numbers). The number of trials to be completed progressively increased with the subjects terminating the testing session at any point of their choosing, enabling the determination of their breaking point. Patients with schizophrenia exhibited significantly lower PRBP scores than healthy control participants, and breakpoints correlated with amotivation levels in the patients assessed via negative symptom rating scales.30 This task involves both a physical and cognitive effort, although the cognitive requirement is kept purposefully very low due to cognitive difficulties of patients (discussed below). The cognitive load therefore does not increase over the session. Likewise in rodents, the difficulty is raised by increasing number of responses to earn a reward and the response itself is simple. Thus, the PRBP may provide a viable methodology for investigating neurocircuitry underlying the motivation to exert physical and cognitive effort to gain rewards across species with relevance specifically to the lack of motivation exhibited by schizophrenia patients.
Additional operant-based tasks exist for rats to quantify choice-based effortful motivation. Floresco and colleagues developed a physical effort-based discounting/decision-making task (EDT) that presents the rat with hard- or easy-effort-based choice levers. Whenever the easy choice lever was chosen, the rats received 1 reward. When selecting the hard choice the rats received 4 rewards, but only if they could make enough responses in a short space of time with the number of responses required increasing as the session continued.31 Thus, the breakpoint, defined as the effort requirement when the rat stopped selecting the high-reward lever, indicates at what point the reward was insufficient for its cost in physical effort. Other techniques to measure rodent motivation involve maze-based tasks.32–36 These maze tasks are not discussed here due to the limited number of trials that can be completed in a single session, thus introducing possible consolidation-related confounds. PRBP demands can be physical, as in most of the procedures described above, while others may be mentally taxing requiring “cognitive” effort. This cognitive effort aspect of motivation also requires study in the context of the fact that schizophrenia patients exhibit cognitive deficits that may contribute to amotivation and vice versa.37–43
A task for rodents that varies cognitive load requiring rats to choose between cognitive effort levels has been developed recently. Winstanley and colleagues44 designed this task, requiring rats to select between performing easy or hard trials (involving short and long visual stimulus durations respectively). After their choice, they are presented with a single light in 1 of 5 holes requiring a response in that hole within the stimulus duration to receive a reward. Detection of a light that is presented briefly is more difficult than detection of a long duration light but rats receive twice the reward for correct responses when choosing difficult trials. Although there is trial-by-trial variation in choices for individual rats, individuals exhibited a natural inclination to select easy or hard trials, determining them as “slackers” or “workers”. Although it is labor intensive to train rats in this task, the inherent trait was stable in rats and has been manipulated in various studies.
In its inaugural study, it was revealed that amphetamine and caffeine treatment lowered the preference for hard choices in “workers”, while only amphetamine raised the preference for hard choices in “slackers”.44 Importantly, both treatments increased responding prior to stimulus cue onset (premature responding) irrespective of the rat’s preference for easy or hard trials, indicating separable mechanisms underlying selection of hard vs easy choices and premature responses. Furthermore, inactivation of specific brain regions induced effects that were baseline preference dependent. For example, inactivation of the basolateral amygdala increased preference for hard trials in “slackers”, while reducing such preference in “workers”. Inactivation of the anterior cingulate cortices45 and prefrontal cortices46 reduced the preference for hard trials (cognitive effort) in all rats irrespective of baseline preference. Importantly, unlike the physical effort task (EDT) described above, dopamine receptor antagonists did not reduce the preference of rats to select hard trials.46 These data support the separation of mechanisms underlying choices of different quantitative levels of physical vs cognitive effort. Specifically, it appears that mesocorticolimbic dopamine is critically involved in physical effort valuation while cortical areas, such as the prefrontal and cingulate cortex are involved in cognitive effort valuation. This cognitive effort task has been reverse translated for use in humans and is described in detail as a perceptual effort task elsewhere in this issue.47,48 Although largely consistent with the rodent task, the difficulty level for patients was titrated based on the patients’ baseline abilities. Future studies will be required to demonstrate the cross-species validity of findings for this task.
Integrating Reward Feedback for Decision Making
Quantifying the motivation to expend physical or cognitive effort required for a straightforward reward enables the investigation of circuitry related to negative symptoms. It is also important to study how rewarding feedback is processed for future decisions and actions, providing insights into the value a subject places on that feedback (reward or no reward). These procedures, quantify learning in an environment that is more relevant to the “real-world” than the reward-for-effort tasks described above. Deficits in such reward-associative learning correlate with negative symptoms in schizophrenia.49 Numerous tasks exist that quantify reward-associative learning that are available for use in both rodents and humans. Although these tasks evaluate associative learning (a cognitive process), the subject must process reward feedback (ie, reward responsiveness), which will influence future choices and actions in the pursuit of rewards. It is for these reasons that it is hypothesized that quantifying of such reward-related decision making is related to negative symptoms in patients with schizophrenia.
A classic method of testing such feedback-based decision making in humans is the probabilistic learning task.50–52 In this task, subjects are required to select the target from 2 options. While ordinarily rewarded for selecting the target, feedback can be misleading as the selection of a specific target will not always result in a reward. Likewise, selecting the nontarget would occasionally be rewarded. Hence, the subjects’ choices result in unreliable feedback. This ratio of reward/nonreward can vary but classically starts at an 80/20 ratio, with variations at 70/30 or 60/40 etc. for different stimuli. Furthermore, some human studies utilized reversal stages where the contingencies were switched, or extra stages whereby stimuli were mixed with previously presented stimuli. Negative symptoms correlated with learning deficits in this task when multiple ratio contingencies were presented.49 Importantly, patients exhibited poor learning in these tasks driven by poor reward-associative learning, while exhibiting normal learning in response to punishments.49,53–55 This pattern of results suggests mostly intact associative learning but decreased sensitivity to reward, an effect that could be conceptualized as amotivation. This reduced associative learning from rewards can be quantified by examining post-hoc choices after receiving a reward, referred to as the win-stay ratio. Changes to punishment-associative learning can be quantified similarly using the lose-shift ratio.56–59 Thus, utilizing feedback-based decision-making tasks could illuminate reward processing capabilities in patients that may underlie negative symptoms in patients. These paradigmatic challenges to learning can readily be tested in animals.
Some possible confounds using probabilistic learning tasks should be addressed however. Although it is recognized that probabilistic learning tasks primarily assess contingency learning—possibly more relevant to cognitive deficits in schizophrenia—such learning involves processing of reward feedback with deficits associated with negative symptoms.49 When using a probabilistic learning task to quantify reward-association behaviors in rodents, certain confounds should be considered. The probabilistic learning task has a strong learning component—conclusions about selectivity of deficits to reward valuation (and hence negative symptoms) would receive greater support if differences in lose-shift behavior were absent. Further support for selectivity of reward valuation/integration effects in rodents would be provided if no deficits in learning tasks were seen that do not involve a strong positive reinforcement, eg, aversively motivated tasks such as the Morris water maze or Barnes maze. That is, converging operations need to be used to determine whether it is learning or reward valuation that drive potential deficits in this task. A further complication stems from the finding that performance in these tasks was linked to working memory performance in humans.60 This working memory link likely derives from presenting stimuli pairs simultaneously and not sequentially. In other words, while 1 stimulus pair is presented in trial 1 a different pair would be presented in trial 2, a third pair would be presented in trial 3, with each pair at different probabilistic reward levels. Presenting stimuli pairs sequentially (eg, the first stimuli pair is shown until all trials for that pair are completed then the next stimuli pair is presented) may provide a more succinct quantification of reward-associative learning not complicated by working memory confounds. Such confounds are best avoided if the neural circuitry underlying a specific behavior are to be clearly investigated.
Additional concerns stems from the cognitive underpinnings required to complete a probabilistic learning task. The cognitive deficits experienced by patients with schizophrenia could affect performance in tasks requiring subjects to choose between options. Although conceptually the cognitive load is kept low in tasks intended to assess reward and motivational processes, patients with schizophrenia do exhibit numerous cognitive deficits relative to healthy subjects. The concern about cognitive load in patient studies is discussed in the theoretical approach article on humans (Green et al,26 this issue). For animal studies, the concern derives from whether the manipulation has effects beyond the primary outcome measure. Such concerns can be examined by investigating effects on additional behavioral measures (eg, water maze as described above) and tasks that assess different processes relevant to negative symptoms in schizophrenia.
Ultimately, numerous procedures use probabilistic rewards and punishments in response to choices that could also prove useful to understanding neuromechanisms underlying reward-based decision making.31,56,61–66 The optimal tasks will be those where direct comparisons can be made across species. One example that measures decision making under risk originally developed for humans is the Iowa Gambling Task (IGT) which presents choices of stimuli and varies reward, punishment levels, and probabilities for reward and punishment.67 Similarly to probabilistic learning, poor reward-associative learning measured in the IGT correlated with negative symptoms in patients with schizophrenia.55 Hence, the win-stay behavior of patients in the IGT could help identify the underlying mechanism(s) of this reward-associative learning. This task has since been adapted for use in both rats68,69 and mice.65 Unlike the probabilistic learning task however, the IGT utilizes more punishment and risk during task learning and so incorporates even more aspects of cognition that could confound behavioral results. Hence, making choices in this task based on feedback may provide a window into reward anticipation that links to motivation in patients with schizophrenia.
Another task that can measure response to feedback is the Response-Bias Probabilistic Reward Task (RBPRT). This task was originally developed by Pizzagalli and colleagues70 as a computer-based laboratory task for humans to quantitatively and objectively assess how and whether reward feedback alters future behavior and choices. In the RBPRT, subjects are presented with a cartoon face on the computer screen that lacks a mouth. Then during discrete trials, a long or a short mouth is added to the cartoon face for a brief period, and the subject has to press 1 key if she/he saw a long mouth and another key in response to a short mouth. The target (ie, mouth) is presented for a very brief of time so that discrimination of short vs long is very difficult. Further, both stimuli are only partially and differentially reinforced with 1 reinforced 60% (ie, rich stimulus) and the other reinforced 20% (ie, lean stimulus) of the time. By providing ambiguous stimuli that are rarely rewarded, the task enables a response bias to develop towards the more frequently reinforced stimulus, irrespective of a subjects ability to discriminate between the stimuli. Indeed, healthy subjects gradually develop a response bias for the richer stimulus, accompanied by decreased accuracy, indicating that reinforcement history affected their subsequent behavioral choices. Importantly, depressed patients or college students with high depression scores do not develop this response bias yet maintain their discrimination accuracy demonstrating a failure to incorporate reinforcement history to affect their future pursuit of rewards.70,71 Two published studies indicated no differences between controls and schizophrenia patients in developing a response bias in the RBPRT.72,73 A third study indicated slowed response bias development in schizophrenia patients (Erican Duncan, personal communication). The original negative results may be related to the patients smoking status or negative symptom levels. In support, of this hypothesis data in healthy humans74 and rats75 demonstrate that nicotine increases response bias while nicotine withdrawal, characterized by anhedonia, reduced response bias development in rats75
Most relevant to this review article is the fact that Markou and colleagues translated the RBPRT that was originally developed in humans to rats.76 Short and long duration tones are used as the stimuli that the rats have to discriminate while performing in this task. All parameters of the rat version of the task, including percentage of responses that are reinforced and ratio of reinforcement for the 2 target stimuli, are identical in the human and rat versions. Thus, the neurosubstrates enabling positive reinforcement to influence future pursuit of rewards may be studied using this task.
Despite the apparent similarities in the traditional probabilistic learning tasks and the RBPRT, there are important differences. The former provides explicit feedback for subjects chosen stimuli (providing measures of learning driven by accuracy) while the latter provides rich vs lean feedback unrelated to their choices, and thus leads to stimulus bias irrespective of accuracy). Therefore, these 2 tasks may provide evidence for distinct aspects of reward-association driven feedback learning.
Neuromechanisms
Each of the behaviors discussed above was chosen because of the opportunity to use them to assess negative symptom-related behaviors in both humans and rodents. These tasks measure conceptually different aspects of reward/motivation-relevant behaviors, covering physical and cognitive effortful motivation, as well as reward-related feedback decision making. The differences in the constructs assessed by these tasks are reflected in evidence from rodent studies showing that distinct neuromechanisms underlie these behaviors, known as discriminant validity. While distinct mechanisms may subserve these behaviors, there is also evidence for overlap with interactive mechanistic effects at the receptor levels. Although limited evidence on neuromechanisms have been generated to date, evidence for their divergence and interaction will be described below.
Several studies highlight mechanistic interaction at the receptor level. For example, it is clear that systemic treatment with stimulants that increase catecholaminergic release (eg, amphetamine) increase physical effort as measured in the PRBP and in the EDT. Similarly, such stimulants also increased cognitive effort in rats, although only in rats identified as exerting low cognitive effort.44 Furthermore, systemic amphetamine improved reward-associative learning in mice,77 and increased reward responsiveness in rats.78 Additionally, reducing Sp4 expression in mice from birth reduced physical effort and lowered reward-associative learning,77 although the precise mechanism(s) underlying these deficits as adults have yet to be determined. Evidence from other effort-based tasks provide additional insights about striatal involvement in effort-based decision making in rat mazes79,80 and reward-associative probabilistic learning in rats81,82 and humans.52 Humans with focal orbitofrontal cortex (OFC) lesions exhibit impaired probabilistic learning both in terms of initial and reversal learning.83 This effect has not yet been replicated in rodents but the OFC likely plays an important updating stimulus-association reward values.84 Hence, a catecholaminergic-driven frontostriatal network likely underlies numerous aspects of motivation whether it is willingness to exert physical or cognitive effort, or to process reward feedback utilizing either explicit or ambiguous stimuli.
The differences between these tasks may provide greater insight into targeting treatments toward patients with specific deficits quantified by human versions of these tasks. For example, while inactivation of the basolateral amygdala in rats reduced physical effortful responding85 and high cognitive effort preference in rats,45 such a lesion increased cognitive effort preference in low effort rats.45 The dopaminergic system may be less important for cognitive than physical effort given that dopamine receptor antagonists reduced effort in the latter46,86 but not the former.46 Additionally, mice without alpha7 nAChRs exhibited normal physical effort motivation but impaired reward-associative learning,87 despite such mice exhibiting normal learning in aversively motivated environments.88 Other differences include the need of the dorsal striatum in rats performing an effort-based decision-making task89,90 but not in the PRBP.91 Future studies using specific cross-species tests and manipulations may solidify the neuromechanisms mediating these specific behaviors in both humans and rodents for as yet there remain too few studies for a complete understanding. Once greater knowledge is acquired however, targeted treatments could then be developed for the domain affected specifically in 1 patient group vs another.
Most studies reported to-date that investigate the neural mechanisms of motivation focus on the role of the dopaminergic system. This dopaminergic focus could stem from a desire to increase the likelihood of observing an experimental effect (since several effects have already been reported), or due to publication bias where negative studies do not get reported as often. The battery of tasks described here provide an opportunity to investigate whether other neurotransmitter systems implicated in reward, eg, cholinergic interaction with the dopaminergic system to mediate these behaviors. For example, activation of the alpha7 nAChR increases striatal dopamine release,92 which preferentially activates dopamine D1 receptors93 as this effect is absent in mice lacking alpha7 nAChRs.94 This mechanistic interaction may provide the explanation of how alpha7 nAChR agonists could improve negative symptoms in patients with schizophrenia in early trials because they result in the reward-associated dopamine D1 receptor pathway.95–97 Future studies are required, however, to corroborate striatal dopamine D1 receptors and alpha7 nAChRs interacting with a functional significance on behavior.
Conclusion
Here, we provided a translational approach identifying tasks that can be used in both humans and animals to enhance the investigation of neural mechanisms underlying physical, cognitive, and reward-related effort-based decision making (table 2). Cross-species tasks have been devised with increasing recognition for the need of biomarkers that relate to specific cognitive domains.98–100 Each task described above can be conducted in both humans and rodents because the tasks developed for humans do not involve verbal responses or rating scales. Instead, human subjects are required to emit responses upon presentation of discrete stimuli, providing performance-based quantified data. This review has not covered behavioral tasks that may link between species. For example, recreating measurements of facial expression or vocal tone communication in rodents has proven problematic in the past, although future studies may utilize rodent ultrasonic vocalizations (UV).101 Since positive UV are emitted in expectation of positive reinforcement, they may link to anticipatory as opposed to consummatory behavior. Future effort is required to confirm such links. Similarly, a rodent social recognition/preference task exists which may be relevant to asociality in schizophrenia. CNTRICS suggested however, that this task is more closely linked to quantifying social cognition.102 While it is interesting to investigate the neural mechanisms underlying these behaviors for their own sake, their investigation will be more relevant when they are linked to proven deficits in patients with schizophrenia using the cross-species tasks described here (Horan et al,47 this issue).
Table 2.
Summary of Tasks and Findings With Putative Cross-Species Relevance to Negative Symptoms of Patients With Schizophrenia
| Domain | Paradigm | Rodent-based Manipulation | Reference |
|---|---|---|---|
| Physical Effort | Progressive Ratio Breakpoint Paradigm | Reduced Sp4 expression reduced breakpoint. Psychostimulants that elevate catecholamine levels (eg, amphetamine/modafinil) increased breakpoint. Ventral striatal lesion increased breakpoint. Dopamine D2 receptor over-expression reduced breakpoint Withdrawal from amphetamine reduced breakpoint. | 77 , 78 , 87 103–105 |
| Effort-based discounting task | Amphetamine and ketamine increased breakpoint at lower doses, reducing effort at higher. Basolateral amygdala inactivation increased breakpoint. Dopamine receptor antagonists blunted effort. | 46 , 85 , 86 | |
| Cognitive Effort | Cognitive effort task | Psychostimulants (eg, amphetamine) increased effort in “slackers”, while dopamine receptor antagonists did not affect effortful choices. | 44 , 46 |
| Reward Valuation | Probabilistic learning task (explicit reward feedback association) | Psychostimulants (eg, amphetamine) increased breakpoint. Citalopram increases reversals. Reduced Sp4 expression impairs learning. Isolation rearing impaired between-, but not within-session learning. Ventral striatal inactivation reduced win-stay in rats. | 44 , 59 , 77 , 81 |
| Iowa Gambling Task (feedback-based decision making under risk) | Amphetamine treatment increases low-reward choices while selectively reduced DAT function increases high-reward options. | 65 | |
| Response Bias Probabilistic reward task (reward responsiveness) | The psychostimulant amphetamine enhanced bias learning; presynaptic doses of dopamine D2/3 receptor agonist or nicotine withdrawal blunted bias learning. | 75,76 |
Finally, 1 must always be cognizant of the species-specific behavioral divide when using this cross-species translational approach. Despite attempting to make the tasks as equitable across species as possible, verified cross-species validity is required. Differences in effects could arise from the differences in the way humans vs animal subjects were tested—dome differences remain unavoidable as species differences are inevitable.106 In human tests, for example, it is easy to instruct patients on the task and provide some practice prior to testing. In animal procedures, the rodents must be trained to perform the tasks prior to testing. That is, training in animals (sometimes quite labor intensive) is in lieu of the instructions given to human subjects. Some techniques can be used to ensure as equivalent procedures between humans and rodents. For example, humans can be provided explicit instructions on the value of discrete stimuli. For rodents, they can be made to sample every possibility prior to testing (forced-choice trials), hence they obtain a better more explicit understanding of the reinforcing contingencies irrespective of their overall choices. Once these tests have been refined—and with corroborative evidence that patients with schizophrenia exhibit impaired performance—biomarkers linked to impaired performance can be more readily generated. Future rodent studies could then be conducted that involve experimental manipulations that are relevant to the genetic and/or environmental factors shown to lead to the development of schizophrenia. The effects of these targeted manipulations could be assessed in a battery of tests relevant to negative symptoms and the effects of putative treatments could also be investigated in experimental animals. Theoretically, because the treatments will have biomarker-based findings in tasks linked closely to the neural mechanism in both human and animals, the likelihood of a positive treatment crossing the species divide would be greatly increased.
The limited data available from these tasks indicate overlap of some neuromechanisms but also dissociations that indicate divergent validity (ie, the tasks assess different aspects of reward processing and motivation). Dopamine D1 receptors on the direct frontostriatal pathway likely underlie reward associative learning, while indirect manipulation of this pathway (eg, via alpha7 nAChR activation) could alter such reward associative learning. Direct dopamine activation can increase physical but not cognitive effort. The OFC likely underlies reward valuation particularly during periods of uncertainty. When testing these neuromechanisms, dissociations are also required to determine possible separable effects on motivation vs cognition in these tasks. While further validation is required, we propose that by developing these tasks across species the neuromechanisms can be investigated in detail in animals and the resulting deficits in patients more readily explained. Hence, quantifying different types of deficits in reward and motivational processes of patients with schizophrenia would promote the development of improved rodent procedures that assess the same construct and eventually the discovery of targeted treatments for the underlying altered neuromechanisms.
Funding
National Institutes of Health (R01-087989 to A.M., R01-MH104344 to J.Y., R21-MH101579 to J.Y.); Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center.
Acknowledgments
We thank Drs Samuel Barnes, Andre Der-Avakian, Mark Geyer, Michael Green, William Horan, and Steve Marder for their support. In the past 3 years Dr Young’s work has been funded by NIDA and NIMH, as well as the US. Veteran’s Administration VISN 22 Mental Illness, Research, Education, and Clinical Center, Cerca Insights, Lundbeck Ltd, and Omeros, and has received consulting compensation for Amgen, and honorarium from Arena Pharmaceuticals. In the past 3 years, Dr Markou’s work has been funded by NIDA, NIMH, and NIAAA. In addition, Dr Markou has received contract research support from Astra-Zeneca and Forest Laboratories and hononaria from AbbVie, Germany.
References
- 1. Cannon M, Jones P. Schizophrenia. J Neurol Neurosurg Psychiatry. 1996;60:604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9. [DOI] [PubMed] [Google Scholar]
- 3. Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sahakian BJ, Owen AM, Morant NJ, et al. Further analysis of the cognitive effects of tetrahydroaminoacridine (THA) in Alzheimer’s disease: assessment of attentional and mnemonic function using CANTAB. Psychopharmacology (Berl). 1993;110:395–401. [DOI] [PubMed] [Google Scholar]
- 5. Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. [DOI] [PubMed] [Google Scholar]
- 6. Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marder SR, Daniel DG, Alphs L, Awad AG, Keefe RS. Methodological issues in negative symptom trials. Schizophr Bull. 2011;37:250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laughren T, Levin R. Food and Drug Administration commentary on methodological issues in negative symptom trials. Schizophr Bull. 2011;37:255–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 10. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirkpatrick B, Fischer B. Subdomains within the negative symptoms of schizophrenia: commentary. Schizophr Bull. 2006;32:246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF. Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med. 2011;41:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32:259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellenbroek BA, Cools AR. Animal models for the negative symptoms of schizophrenia. Behav Pharmacol. 2000;11:223–233. [DOI] [PubMed] [Google Scholar]
- 16. Young JW, Zhou X, Geyer MA. Animal models of schizophrenia. In: Swerdlow NR, ed. Behavioral Neurobiology of Schiozphrenia and Its Treatment. Berlin: Springer; 2010:391–433. [DOI] [PubMed] [Google Scholar]
- 17. Distler MG, Opal MD, Dulawa SC, Palmer AA. Assessment of behaviors modeling aspects of schizophrenia in Csmd1 mutant mice. PLoS One. 2012;7:e51235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barkus C, Feyder M, Graybeal C, et al. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2012;62:1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM. MK-801 produces a deficit in sucrose preference that is reversed by clozapine, D-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: relevance to negative symptoms associated with schizophrenia? Pharmacol Biochem Behav. 2010;95:223–229. [DOI] [PubMed] [Google Scholar]
- 20. Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. [DOI] [PubMed] [Google Scholar]
- 22. Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–397. [DOI] [PubMed] [Google Scholar]
- 26. Green MF, Horan WP, Barch DM, Gold JM. Effort-based decision making: A novel apprach for assessing motivation in schizophrenia. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–988. [DOI] [PubMed] [Google Scholar]
- 28. HODOS W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. [DOI] [PubMed] [Google Scholar]
- 29. Barnes SA, Der-Avakian A, Markou A. Anhedonia, avolition, and anticipatory deficits: assessments in animals with relevance to the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 2014;24:744–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolf DH, Satterthwaite TD, Kantrowitz JJ, Katchmar N, Vandekar L, Elliott MA, Ruparel K. Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull. 2014;40:1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31:8625–8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. [DOI] [PubMed] [Google Scholar]
- 33. Markou A, Salamone JD, Bussey TJ, et al. Measuring reinforcement learning and motivation constructs in experimental animals: relevance to the negative symptoms of schizophrenia. Neurosci Biobehav Rev. 2013;37:2149–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. [DOI] [PubMed] [Google Scholar]
- 35. Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl). 2007;191:461–482. [DOI] [PubMed] [Google Scholar]
- 36. Izquierdo A, Belcher AM. Rodent models of adaptive decision making. Methods Mol Biol. 2012;829:85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barch DM, Carter CS; CNTRICS Executive Committee. Measurement issues in the use of cognitive neuroscience tasks in drug development for impaired cognition in schizophrenia: a report of the second consensus building conference of the CNTRICS initiative. Schizophr Bull. 2008;34:613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carter CS, Barch DM, Bullmore E, et al. Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia II: developing imaging biomarkers to enhance treatment development for schizophrenia and related disorders. Biol Psychiatry. 2011;70:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gold JM, Hahn B, Zhang WW, et al. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Arch Gen Psychiatry. 2010;67:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 41. Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67(Suppl 9:3–8; discussion 36–42. [PubMed] [Google Scholar]
- 42. Marder SR. The NIMH-MATRICS project for developing cognition-enhancing agents for schizophrenia. Dialogues Clin Neurosci. 2006;8:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Young JW, Geyer MA, Rissling AJ, et al. Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Transl Psychiatry. 2013;3:e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cocker PJ, Hosking JG, Benoit J, Winstanley CA. Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacology. 2012;37:1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hosking JG, Cocker PJ, Winstanley CA. Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacology. 2014;39:1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hosking JG, Floresco SB, Winstanley CA. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology. 2015;40:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Horan WP, Reddy LF, Barch DM, et al. Effort-based decision making paradigms for clinical trials in schizophrenia: Part 2 - external validity and correlates. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reddy LF, Horan WP, Barch DM, et al. Effort-based decision making paradigms for clinical trials in schizophrenia: Part 1 - psychometric characteristics of five paradigms. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bari A, Theobald DE, Caprioli D, et al. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chamberlain SR, Müller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frank MJ, Seeberger LC, O’reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. [DOI] [PubMed] [Google Scholar]
- 53. Waltz JA, Frank MJ, Wiecki TV, Gold JM. Altered probabilistic learning and response biases in schizophrenia: behavioral evidence and neurocomputational modeling. Neuropsychology. 2011;25:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nestor PG, Choate V, Niznikiewicz M, Levitt JJ, Shenton ME, McCarley RW. Neuropsychology of reward learning and negative symptoms in schizophrenia. Schizophr Res. 2014;159:506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. [DOI] [PubMed] [Google Scholar]
- 57. Stopper CM, Floresco SB. What’s better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat Neurosci. 2014;17:33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. St Onge JR, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 2010;20:1816–1828. [DOI] [PubMed] [Google Scholar]
- 59. Amitai N, Young JW, Higa K, Sharp RF, Geyer MA, Powell SB. Isolation rearing effects on probabilistic learning and cognitive flexibility in rats. Cogn Affect Behav Neurosci. 2014;14:388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Collins AG, Brown JK, Gold JM, Waltz JA, Frank MJ. Working memory contributions to reinforcement learning impairments in schizophrenia. J Neurosci. 2014;34:13747–13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van den Bos R, Jolles J, van der Knaap L, Baars A, de Visser L. Male and female Wistar rats differ in decision-making performance in a rodent version of the Iowa Gambling Task. Behav Brain Res. 2012;234:375–379. [DOI] [PubMed] [Google Scholar]
- 62. Worthy DA, Hawthorne MJ, Otto AR. Heterogeneity of strategy use in the Iowa gambling task: a comparison of win-stay/lose-shift and reinforcement learning models. Psychon Bull Rev. 2013;20:364–371. [DOI] [PubMed] [Google Scholar]
- 63. Stopper CM, Floresco SB. Contributions of the nucleus accumbens and its subregions to different aspects of risk-based decision making. Cogn Affect Behav Neurosci. 2011;11:97–112. [DOI] [PubMed] [Google Scholar]
- 64. van Enkhuizen J, Geyer MA, Young JW. Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task: relevance to mania. Psychopharmacology (Berl). 2013;225:661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van Enkhuizen J, Henry BL, Minassian A, et al. Reduced dopamine transporter functioning induces high-reward risk-preference consistent with bipolar disorder. Neuropsychopharmacology. 2014;39:3112–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Young J, van Enkhuizen J, Geyer M. Dopamine transporter knockdown mice exhibit poorer within-session risk learning in a mouse Iowa Gambling Tasl consistent with bipolar mania patients. American College of Neuropsychopharmacology. 2011; Oral Presentation. [Google Scholar]
- 67. Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. [DOI] [PubMed] [Google Scholar]
- 68. Rivalan M, Ahmed SH, Dellu-Hagedorn F. Risk-prone individuals prefer the wrong options on a rat version of the Iowa Gambling Task. Biol Psychiatry. 2009;66:743–749. [DOI] [PubMed] [Google Scholar]
- 69. Rivalan M, Coutureau E, Fitoussi A, Dellu-Hagedorn F. Inter-individual decision-making differences in the effects of cingulate, orbitofrontal, and prelimbic cortex lesions in a rat gambling task. Front Behav Neurosci. 2011;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol Sci. 2005;16:805–813. [DOI] [PubMed] [Google Scholar]
- 71. Vrieze E, Pizzagalli DA, Demyttenaere K, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 2008;64:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ahnallen CG, Liverant GI, Gregor KL, et al. The relationship between reward-based learning and nicotine dependence in smokers with schizophrenia. Psychiatry Res. 2012;196:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008;63:1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pergadia ML, Der-Avakian A, D’Souza MS, et al. Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA Psychiatry. 2014;71:1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Der-Avakian A, D’Souza MS, Pizzagalli DA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry. 2013;3:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Young J, Kamenski M, Higa K, Light G, Geyer M, Zhou X. GlyT-1 inhibition attenuates the attentional but not learning or motivational deficits of the Sp4 hypomorphic mouse model relevant to psychiatric disorders [published online ahead of print April 24, 2015]. Neuropsychopharmacology. doi: 10.1038/npp.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170:1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Salamone JD, Correa M, Nunes EJ, Randall PA, Pardo M. The behavioral pharmacology of effort-related choice behavior: dopamine, adenosine and beyond. J Exp Anal Behav. 2012;97:125–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex. 2007;17:251–260. [DOI] [PubMed] [Google Scholar]
- 81. Dalton GL, Phillips AG, Floresco SB. Preferential involvement by nucleus accumbens shell in mediating probabilistic learning and reversal shifts. J Neurosci. 2014;34:4618–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Atallah HE, Rudy JW, O’Reilly RC. The role of the dorsal striatum and dorsal hippocampus in probabilistic and deterministic odor discrimination tasks. Learn Mem. 2008;15:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Res. 2006;1105:93–101. [DOI] [PubMed] [Google Scholar]
- 84. Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: insights from animal models [published online ahead of print June 11, 2015]. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci. 2009;29:5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. [DOI] [PubMed] [Google Scholar]
- 87. Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA. Delayed procedural learning in α7-nicotinic acetylcholine receptor knockout mice. Genes Brain Behav. 2011;10:720–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. Alpha7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learn Mem. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- 89. Kurniawan IT, Seymour B, Talmi D, Yoshida W, Chater N, Dolan RJ. Choosing to make an effort: the role of striatum in signaling physical effort of a chosen action. J Neurophysiol. 2010;104:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ostlund SB, Maidment NT. Dopamine receptor blockade attenuates the general incentive motivational effects of noncontingently delivered rewards and reward-paired cues without affecting their ability to bias action selection. Neuropsychopharmacology. 2012;37:508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Eagle DM, Humby T, Dunnett SB, Robbins TW. Effects of regional striatal lesions on motor, motivational, and executive aspects of progressive-ratio performance in rats. Behav Neurosci. 1999;113:718–731. [DOI] [PubMed] [Google Scholar]
- 92. Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. J Mol Neurosci. 2006;30:137–140. [DOI] [PubMed] [Google Scholar]
- 93. Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A. Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J Neurochem. 2004;90:1094–1103. [DOI] [PubMed] [Google Scholar]
- 94. Quarta D, Naylor CG, Barik J, Fernandes C, Wonnacott S, Stolerman IP. Drug discrimination and neurochemical studies in alpha7 null mutant mice: tests for the role of nicotinic alpha7 receptors in dopamine release. Psychopharmacology (Berl). 2009;203:399–410. [DOI] [PubMed] [Google Scholar]
- 95. Umbricht D, Keefe RS, Murray S, et al. A randomized, placebo-controlled study investigating the nicotinic α7 agonist, RG3487, for cognitive deficits in schizophrenia. Neuropsychopharmacology. 2014;39:1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an α-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol. 2013;86:1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Barch DM, Moore H, Nee DE, Manoach DS, Luck SJ. CNTRICS imaging biomarkers selection: Working memory. Schizophr Bull. 2012;38:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Luck SJ, Ford JM, Sarter M, Lustig C. CNTRICS final biomarker selection: Control of attention. Schizophr Bull. 2012;38:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Young JW, Geyer MA. Developing treatments for cognitive deficits in schizophrenia: the challenge of translation. J Psychopharmacol. 2015;29:178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–283. [DOI] [PubMed] [Google Scholar]
- 102. Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci Biobehav Rev. 2013;37:2166–2180. [DOI] [PubMed] [Google Scholar]
- 103. Li YC, Kellendonk C, Simpson EH, Kandel ER, Gao WJ. D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:12107–12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Barr AM, Fiorino DF, Phillips AG. Effects of withdrawal from an escalating dose schedule of d-amphetamine on sexual behavior in the male rat. Pharmacol Biochem Behav. 1999;64:597–604. [DOI] [PubMed] [Google Scholar]
- 105. Bensadoun JC, Brooks SP, Dunnett SB. Free operant and discrete trial performance of mice in the nine-hole box apparatus: validation using amphetamine and scopolamine. Psychopharmacology (Berl). 2004;174:396–405. [DOI] [PubMed] [Google Scholar]
- 106. Young JW, Jentsch JD, Bussey TJ, Wallace TL, Hutcheson DM. Consideration of species differences in developing novel molecules as cognition enhancers. Neurosci Biobehav Rev. 2012;37:2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]