Abstract
We present the biochemical and molecular diagnosis of dihydrolipoamide dehydrogenase (DLD) deficiency (also known as E3 deficiency) and Leigh syndrome in a 14 year-old girl with previous history of learning disability and episodic encephalopathy and ketoacidosis. The diagnosis was suggested by biochemical laboratory values from plasma amino acids and urine organic acids, which were obtained during an acute episode of encephalopathy, lactic ketoacidosis and liver failure all precipitated by infectious mononucleosis. DLD deficiency was confirmed via enzymatic and molecular analyses. E3 activity from cultured skin fibroblasts ranged between 9% and 29% of the mean. Molecular analysis showed compound heterozygosity for novel and previously reported pathogenic mutations; p.I353T and p.G136del, respectively. The patient was managed using a combination of dietary augmentation as well as continuous renal replacement therapy given her severe and persistent lactic acidosis. Her acute decompensation resulted in brain MRI changes involving the posterior aspect of the putamina, lateral and medial thalami, substantia nigra, lateral geniculate bodies and splenium of the corpus callosum. Additional affected regions included the cortex and subcortical white matter of the right and left occipital lobes and the peri-rolandic region. We review the literature of molecularly confirmed patients with DLD deficiency and note that Leigh syndrome is common in reported patients. This case provides further evidence of the heterogeneous presentation of DLD deficiency as our patient presented with her most severe decompensation at an age much more advanced than in previously reported patients.
Keywords: Dihydrolipoamide dehydrogenase deficiency, E3 deficiency, Maple Syrup Urine Disease type III, Leigh syndrome, metabolic
INTRODUCTION
Dihydrolipoamide dehydrogenase (DLD) functions as the E3 subunit of three mitochondrial multienzyme complexes: pyruvate dehydrogenase (PDH), alpha-ketoglutarate dehydrogenase (α-KGDH) and branched chain 2-oxoacid dehydrogenase (BCKDH). In these complexes, DLD functions as a homodimer of ~50 kDa subunits which reoxidize the reduced lipoyl moiety of E2. DLD deficiency (also known as E3 deficiency or maple syrup urine disease, type III) is a rare autosomal recessive disorder that presents with variable phenotypes ranging from a severe neonatal presentation consisting of ketoacidosis, hypoglycemia and encephalopathy to a milder presentation consisting of variable hepatic dysfunction and/or neurological findings. The majority of patients develop symptoms within the first 1 to 2 years of life with presentation in the neonatal period associated with a worse prognosis. While a large percentage of early-onset patients die in the neonatal period, multiple patients have been reported surviving into their second and third decade of life [1–3].
Here we present the biochemical and molecular diagnosis of DLD deficiency associated with Leigh syndrome in a 14 year-old girl with learning disability and a history of episodic encephalopathy and ketoacidosis. We identify a novel pathogenic DLD mutation in this report. Furthermore, this case provides further evidence of the heterogeneous presentation of DLD deficiency as our patient presented with her most severe decompensation following primary mononucleosis infection at an age much more advanced than in previously reported patients. We also review molecularly confirmed cases of DLD deficiency previously published and note that Leigh syndrome is common in patients with this disorder.
CASE REPORT
Our female patient was the second child of non-consanguineous parents of mixed European ancestry. She was born at term via vaginal delivery following an uncomplicated pregnancy. Following delivery, she did well and was discharged from the hospital without incident. Throughout the first year of life, her development was apparently normal and she achieved all developmental milestones on time.
At about 1 year of age, she began experiencing episodes of recurrent emesis and encephalopathy with laboratory evaluations showing lactic acidosis and hypoglycemia (as low as 9 mg/dL). The episodes typically lasted between 2 and 10 days with return to baseline following each episode. She was admitted to a community hospital for these episodes and supported with intravenous (IV) dextrose. Laboratory evaluations performed during these episodes showed lactic acidosis and hypoglycemia and at age 5 also included transaminase elevations (of unknown degree). At school, she exhibited evidence of some learning disability requiring special education in all grades. At 12 years of age, she was functioning at a 1st- to 2nd-grade level. She had experienced 1 seizure that occurred at less than 5 years of age and was associated with an episode of hypoglycemia, which required a rapid infusion of dextrose-containing parenteral fluids. Over time, she developed an avoidance of high protein and high fat foods, maintaining a diet primarily composed of carbohydrates.
A biochemical workup was initiated at 12 years of age. Her plasma amino acids demonstrated small amounts of alloisoleucine (~8 μM) on two occasions, with normal or low concentrations of branched chain amino acids. Urinary organic acid never displayed ketoacids. A fibroblast culture was assayed at Emory University, GA for BCKDH activity showing 24.3% of normal leucine decarboxylation.
At 14 years of age the patient was admitted to a community hospital following three days of recurrent emesis and intermittent fever. Initial laboratory evaluation showed an anion gap (32) metabolic acidosis, transaminitis with aspartate aminotransferase (AST) of 496 IU/L (normal 8–30 IU/L), alanine aminotransferase (ALT) of 279 IU/L (normal 7–35), and glucose of 75 mg/dL. Following 3 days of increasing encephalopathy including the onset of aphasia, incontinence, inability to ambulate, and eventually unresponsiveness, she was transferred to our pediatric intensive care unit for further management. Upon admission she was encephalopathic, moaning but not responding to commands. She withdrew from pain but had no other purposeful movements. Her optic disc margins were sharp. Deep tendon reflexes were diminished. Her lactate was 5.6 mmol/L (normal 0.5–2.0 mmol/L), AST of 821 IU/L, ALT of 692, an international normalized ratio of 2.3, partial thromboplastin time of 39.7 seconds (normal 22.0–32.0 seconds), ammonia of 154 μmol/L (normal 11–60 μmol/L), normal anion gap of 11, venous pH of 7.39, and normal blood glucose of 88 mg/dL. She was placed on hemodialysis and later transitioned to continuous renal replacement therapy for 6 days given the persistently elevated lactate with a maximum of 15 mmol/L on intensive care unit day 2. Throughout this time, her liver function continued to worsen with AST and ALT reaching a maximum of 2,810 IU/L and 2,181 IU/L, respectively on intensive care unit day 2. Evaluation for viral hepatitis, cytomegalovirus, varicella, herpes simplex virus and toxoplasmosis were all negative. Ebstein-Barr virus serologies showed positive EBV viral capsid antigen IgM consistent with primary mononucleosis infection.
Plasma amino acids at the time of admission to the intensive care unit showed a valine of 406 μmol/L (normal 155–343 μmol/L), isoleucine of 183 μmol/L (normal 34–106 μmol/L), leucine of 428 μmol/L (normal 86–206 μmol/L), alloisoleucine of 26 μmol/L (normal 0 μmol/L), glutamine of 2,594 μmol/L (normal 457–857 μmol/L), citrulline of 165 μmol/L (normal 19–52 μmol/L) and arginine of 148 μmol/L (1–81 μmol/L). Urine organic acids showed a massive lactic peak, small 2-hydroxybutyric, 2-hydroxyisovaleric, glutaric, adipic, and 2-oxoglutaric peaks, and moderate 2-hydroxyglutaric and 4-hydroxyphenyllactic peaks. Maximum ammonia level during this hospital course was 172 μmol/L. Acylcarnitine profile was within normal limits. Brain MRI performed on day 3 of the patient’s illness is shown in Figure 1, and showed multiple bilateral and symmetric areas of impeded diffusion as well as increased T2 signal involving the posterior aspect of the putamina, lateral and medial thalami, substantia nigra, lateral geniculate bodies and splenium of the corpus callosum. There was also less symmetric areas on increased T2 signal in the cortex and subcortical white matter of the right and left occipital lobes and in the peri-rolandic region. An echocardiogram performed during this admission showed normal cardiac anatomy and function.
Figure 1.
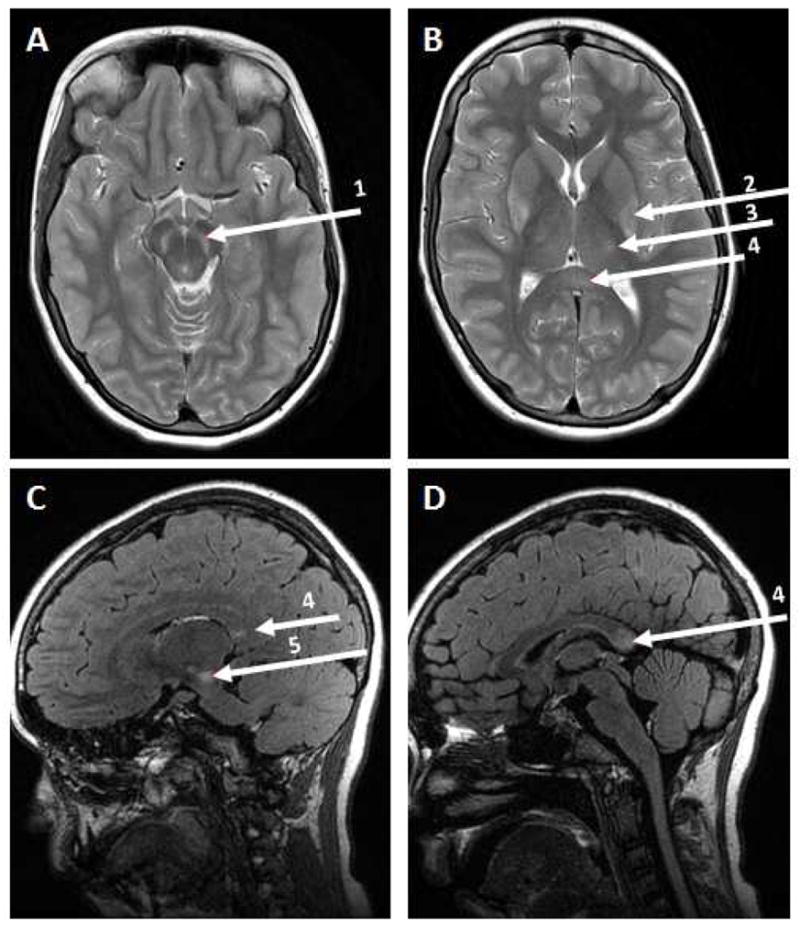
MRI performed 3 days after the onset of the patient’s acute illness, showing symmetric areas of increased signal. A, B are axial T2 images and C, D sagittal FLAIR images. 1, midbrain; 2 basal ganglia; 3, thalamus; 4, posterior corpus callosum; and 5, upper midbrain.
With the combination of dialysis and stringent nutritional monitoring the patient’s mental status and laboratory values continued to improve. After 2 weeks of inpatient medical care she was transferred to a long-term rehabilitation facility, where she remained for 6 weeks. Throughout her hospital stay the patient was maintained on a high carbohydrate diet (70–75% of daily calories) with protein intake kept at recommended daily allowance. She continued this diet following hospital discharge.
Throughout the later part of her hospitalization, with normalized biochemical profiles, she exhibited slurred speech, dysmetria by finger-nose-finger test, hypotonia, and an ataxic gait. On return visit to our genetics and neurology clinic following 6 weeks of inpatient rehabilitation, she continued to have slurred speech and an ataxic gait and required a walker to ambulate long distances, and tired quickly. Biochemical evaluation at 9 weeks post-illness showed a normal urine organic acid profile, normal lactic acid, leucine, isoleucine, low valine, and absent alloisoleucine. In addition, an MRI done about 4 months after discharge showed cystic changes of the posterior basal ganglia, with resolution of the other abnormalities.
RESULTS
Initially, our patient was suspected of having a variant of Maple Syrup Urine Disease. After the current metabolic decompensation an enzyme assay of cultured skin fibroblasts (Center for Inherited Disorders of Energy Metabolism, Case Medical Center, OH) showed pyruvate dehydrogenase complex activity of 80% of mean, within reference range. Pyruvate dehydrogenase E3 activity was decreased at 29% of the mean. Alpha-ketoglutarate dehydrogenase complex activity was normal at 107% of the mean but with a decreased E3 component at 9% of the mean. Molecular testing following her decompensation showed compound heterozygosity for two DLD mutations, a previously reported pathogenic variant (c.405_407delAGG; p.G136del) and a novel mutation (c.1058T>C; p.I353T) presumed pathogenic by multiple functional prediction software programs including PolyPhen-2. Both variants were confirmed to be inherited in trans by parental testing. Other tests included negative molecular analysis of BCKDHA, BCKDHB, and DBT.
DISCUSSION
Neonatal-onset DLD deficiency typically presents with encephalopathy, recurrent emesis, hypotonia, ketoacidosis, and hypoglycemia as shown in Table 1. Other findings can include liver failure, cardiomyopathy, seizures and failure to thrive (Table 1). The typical biochemical profile of individuals with DLD deficiency consists of elevated branched-chain amino acids, elevated glutamine and the presence of allo-isoleucine in plasma, as well as detection of alpha-ketoglutarate, branched-chain ketoacids, ketones, and lactate in urine [4,5]. Patients who present after the neonatal period typically have a milder phenotype with morbidity and mortality significantly lower than the neonatal presentation. Given the lack of phenotypic information in the literature, the degree of morbidity that surviving patients with DLD deficiency experience is difficult to determine. Our patient experienced a major metabolic decompensation at 14 years of age after contracting infectious mononucleosis. Biochemical and molecular work-up confirmed DLD deficiency via enzyme assay and DLD gene analysis, which showed compound heterozygosity for a novel mutation (I353T) and a previously reported pathogenic mutation (G136del).
Table 1.
Genotype-Phenotype Relationship in our patient and those reported with DLD deficiency
| Case | Age | Genotype | Phenotype | Ref | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LD | FTT | DD | Enceph | Hypotonia | Seizure | Hepato | Liver dysfunction | Lactic acidosis | Hypoglycemia | Leigh-like MRI | Other | ||||
| 1 | 1 day | R482G/R482G | + | + | N | Limb spasticity, ptosis, hypertrophic cardiomyopathy | 14 | ||||||||
| 2 | 1 day | R482G/R482G | + | + | + | N | Splenomegaly, limb hypertonia | 14 | |||||||
| 3 | 1 day | R482G/R482G | + | + | + | 14 | |||||||||
| 4 | 2 days | Y35X/G229C | + | + | Normal IQ | 1 | |||||||||
| 5 | 3 days | D479V/D479V | + | + | + | + | Microcephaly | 16 | |||||||
| 6 | 3 days | Y35X/G229C | + | + | N | Recurrent emesis, normal IQ | 1 | ||||||||
| 7 | 3 days | G136del/E375K | + | + | + | N | Optic atrophy | 13 | |||||||
| 8 | 1–2 mos | G229C/G229C | Repetitive hypoglycemic episodes | 6 | |||||||||||
| 9 | Infancy | G229C/G229C | Episodic emesis | 6 | |||||||||||
| 10 | Infancy | G229C/G229C | + | Recurrent emesis | 6 | ||||||||||
| 11 | 7 mos | I480M/I480M | N | Ptosis, photophobia, weakness | 3 | ||||||||||
| 12 | 8 mos | G229C/G229C | + | + | + | + | 15 | ||||||||
| 13 | 8 mos | G229C/G229C | + | 6 | |||||||||||
| 14 | 14 mos | I47T/E375K | + | N | Microcephaly, ptosis | 12 | |||||||||
| 15 | 2 yrs | G229C/G229C | + | Recurrent emesis | 1 | ||||||||||
| 16 | 2–3 yrs | G229C/G229C | + | Recurrent emesis, exertional fatigue | 1 | ||||||||||
| 17 | 3 yrs | G229C/G229C | Sepsis with multiorgan failure | 1 | |||||||||||
| 18 | 3 yrs | G229C/G229C | Exertional fatigue, porphyria | 1 | |||||||||||
| 19 | 20 yrs | G229C/G229C | + | Recurrent emesis, myoglobinuria, weakness | 1 | ||||||||||
| 20 | 2 days | I47T/G229C | + | + | + | + | Y | Myocardial dysfunction | 12 | ||||||
| 21 | 2 days | M361V/E375K | + | Y | Poor feeding, | 10 | |||||||||
| 22 | 6 mos | K72E/P488L | + | + | Y | Spastic quadriplegia, dystonia | 8 | ||||||||
| 23 | 6 mos | Y35Xins/R495G | + | + | + | Y | 9 | ||||||||
| 24* | 12 mos | G137del/I353T | + | + | + | + | + | Y | |||||||
| 25 | 2 yrs | IVS+1G>A/I393T | + | + | Y | Ataxia, microcephaly, stroke-like episode, tetraspasticity | 11 | ||||||||
LD, learning disability; FTT, failure to thrive; DD, developmental delay; Enceph, encephalopathy; Hepato, hepatomegaly; and *, our patient (in bold).
Table 1 shows the genotypes of molecularly confirmed patients with DLD deficiency and an overview of phenotypic information available. Genotype-phenotype correlations remain difficult given the variable phenotype and age of presentation. However, patients with the homozygous G229C mutation have been reported to have milder phenotypes with some patients presenting in their second decade of life [1]. However, this is not absolute, as Hong et al. reported a patient homozygous for the G229C mutation who died as an infant during a metabolic decompensation [6].
Leigh syndrome (subacute nectrotizing encephalomyelopathy) is a neurodevelopmental disorder that consists of characteristic clinical findings and brain pathology. Diagnostic criteria for Leigh syndrome includes (1) progressive neurological disease with motor and intellectual developmental delay; (2) signs and symptoms of brainstem and/or basal ganglia disease; (3) elevated lactate levels in blood and/or cerebrospinal fluid; and (4) one or more of the following: (a) characteristic features of Leigh syndrome on neuro-radioimaging (symmetrical hypodensities in the basal ganglia on CT or hyperintense lesions on T2-weighted MRI), (b) typical neuropathological changes at postmortem exam, or (c) typical neuropathology in a similarly affected sibling [7]. Our patient fulfills criteria for Leigh syndrome based on her developmental delay, which worsened recently, basal ganglia disease as evidenced by her ataxic gait and dysmetria, lactic acidosis and classic brain MRI findings. As previously reported, Leigh syndrome is observed in patients with DLD deficiency [5]. This development is likely attributable to deficiency of pyruvate dehydrogenase complex activity. Review of the literature (Tables 1 and 2) shows that at least five molecularly confirmed patients and one enzymatically confirmed patient had brain imaging findings consistent with Leigh syndrome [5,8–12]. Though pyruvate dehydrogenase may be involved in the development of Leigh syndrome, review of Tables 1 and 2, including our patient’s data, suggest that enzyme activity or genotype are not useful predictors for risk of changes on brain MRI.
Table 2.
Biochemical analysis of our patient and those reported with DLD deficiency
| Case | Enzyme Activity (%)
|
Biochemical Tests
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDH | BCKDH | αKGDH | DLD | Tissue Type | Plasma Amino Acids
|
Urine Organic Acids
|
||||||
| BCAA | Glutamine | Alloisoleucine | Alanine | Lactate | αKG | Other | ||||||
| 1 | + | |||||||||||
|
| ||||||||||||
| 2 | 63 | 56 | 0 | 20 | F | + | Succinic, fumaric, malic | |||||
|
| ||||||||||||
| 3 | 0 | 20 | F | Fumaric | ||||||||
|
| ||||||||||||
| 4 | 20 | M | + | Ketones | ||||||||
| 18 | L | |||||||||||
|
| ||||||||||||
| 5 | 0 | 15 | 2 | M | Branched chain keto acids | |||||||
|
| ||||||||||||
| 6 | 8 | M | + | + | + | Ketones, α-ketoadipic | ||||||
| 9 | L | |||||||||||
|
| ||||||||||||
| 7 | 12 | 6 | 3 | F | + | + | + | + | Succinic, hydroxyisovaleric, isocaproic | |||
| 14 | 1 | 11 | M | |||||||||
| 13 | 9 | L | ||||||||||
|
| ||||||||||||
| 8 | 8 | F | ||||||||||
|
| ||||||||||||
| 9 | 16 | F | ||||||||||
|
| ||||||||||||
| 10 | 30 | F | ||||||||||
|
| ||||||||||||
| 11 | 13–97 | 0 | F | + | + | |||||||
|
| ||||||||||||
| 12 | + | + | + | + | Ketones, branched chain keto acids | |||||||
|
| ||||||||||||
| 13 | 33 | F | ||||||||||
|
| ||||||||||||
| 14 | 59 | 62 | 25 | 9 | F | + | + | + | Ketones | |||
|
| ||||||||||||
| 15 | 12 | L | ||||||||||
|
| ||||||||||||
| 16 | 9 | M | + | + | + | Ketones, α-aminoadipic, α-ketoadipic, α-hydroxyadipic | ||||||
| 13–31 | L | |||||||||||
|
| ||||||||||||
| 17 | 8 | M | ||||||||||
| 17 | L | |||||||||||
|
| ||||||||||||
| 18 | 19 | L | ||||||||||
|
| ||||||||||||
| 19 | 21 | M | + | + | + | Ketones | ||||||
| 18 | L | |||||||||||
|
| ||||||||||||
| 20 | 69 | 58 | 44 | 10 | F | + | + | + | Ketones, DCAs | |||
|
| ||||||||||||
| 21 | 5–44 | 4–14 | F | + | + | Branched chain acids | ||||||
| 3–14 | 3–6 | M | ||||||||||
| 5–38 | 3–12 | L | ||||||||||
|
| ||||||||||||
| 22 | 6 | F | + | + | + | Branched chain keto acids | ||||||
|
| ||||||||||||
| 23 | 11 | 20 | 14 | F | + | + | + | |||||
| 26 | 1.5 | L | ||||||||||
|
| ||||||||||||
| 24* | 80 | 24 | 107 | 9–29 | F | + | + | + | + | + | Hydroxyglutaric, ketones, hydroxyisovaleric, succinic | |
|
| ||||||||||||
| 25 | 100 | F | + | + | + | + | Branched chain oxoacids, ketones, 2-hydroxyglutaric acid | |||||
| 14 | 29 | M | ||||||||||
, our patient (in bold); F, fibroblasts; M, muscle; and L, lymphocytes. Please see Table 1 for references corresponding to each case. PDH, pyruvate dehydrogenase; BCKDH, branched chain ketoacid dehydrogenase; αKGDH, alpha-ketoglutrate dehydrogenase; DLD, dihydrolipoamide dehydrogenase; BCAA, branched chain amino acids; Gln, glutamine; αKG, alpha-ketoglutarate; DCA, dicarboxylic acids
This case provides further evidence of the variable phenotype of DLD deficiency and identifies a novel disease-causing mutation. It also indicates the difficulty of recognizing subtle signs and symptoms of a rare biochemical disorder which sometimes results in severe metabolic decompensation.
Acknowledgments
JKB was in part supported by NIH K12 HD028820 and an award from the Elizabeth E. Kennedy (Children’s Research) Fund through the Department of Pediatrics, the University of Michigan. We thank Deborah Weigel for technical help with the urine organic acids and plasma amino acids analyses and Dr. Shiao-Pei Weathers for support with patient care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaag A, Saada A, Berger I, Mandel H, Joseph A, Feigenbaum A, Elpeleg ON. Molecular basis of lipoamide dehydrogenase deficiency in Ashkenazi Jews. Am J Med Genet. 1999;82:177–82. [PubMed] [Google Scholar]
- 2.Barak N, Huminer D, Segal T, Ben Ari Z, Halevy J, Tur-Kaspa R. Lipoamide dehydrogenase deficiency: a newly discovered cause of acute hepatitis in adults. J Hepatol. 1998;29:482–4. doi: 10.1016/s0168-8278(98)80069-x. [DOI] [PubMed] [Google Scholar]
- 3.Quintana E, Pineda M, Font A, Vilaseca MA, Tort F, Ribes A, Briones P. Dihydrolipoamide dehydrogenase (DLD) deficiency in a Spanish patient with myopathic presentation due to a new mutation in the interface domain. J Inherit Metab Dis. 2010 doi: 10.1007/s10545-010-9169-4. [DOI] [PubMed] [Google Scholar]
- 4.Robinson BH, Taylor J, Kahler SG, Kirkman HN. Lactic Acidemia, Neurologic deterioration and carbohydrate dependence in a girl with dihydrolipoyl dehydrogenase deficiency. Eur J Pediatr. 1981;136:35–9. doi: 10.1007/BF00441708. [DOI] [PubMed] [Google Scholar]
- 5.Craigen WJ. Leigh disease with deficiency of lipoamide dehydrogenase: treatment failure with dichloroacetate. Pediatr Neurol. 1996;14:69–71. doi: 10.1016/0887-8994(96)00005-7. [DOI] [PubMed] [Google Scholar]
- 6.Hong YS, Korman SH, Lee J, Ghoshal P, Wu Q, Barash V, Kang S, Oh S, Kwon M, Gutman A, Rachmel A, Patel MS. Identification of a common mutation (Gly194Cys) in both Arab Moslem and Ashkenazi Jewish patients with dihydrolipoamide dehydrogenase (E3) deficiency: possible beneficial effect of vitamin therapy. J Inherit Metab Dis. 2003;26:816–8. doi: 10.1023/b:boli.0000010004.12053.5b. [DOI] [PubMed] [Google Scholar]
- 7.Rahman S, Blok RB, Dahl HH, Danks DM, Kirby DM, Chow CW, Christodoulou J, Thorburn DR. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann Neurol. 1996;39:343–51. doi: 10.1002/ana.410390311. [DOI] [PubMed] [Google Scholar]
- 8.Liu TC, Kim H, Arizmendi C, Kitano A, Patel MS. Identification of two missense mutations in a dihydrolipoamide dehydrogenase-deficient patient. Proc Ntal Acad Sci U S A. 1993;90:5186–90. doi: 10.1073/pnas.90.11.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong YS, Kerr DS, Craigen WJ, Tan J, Pan Y, Lusk M, Patel MS. Identification of two mutations in a compound heterozygous child with dihydrolipoamide dehydrogenase deficiency. Hum Mol Genet. 1996;5:1925–30. doi: 10.1093/hmg/5.12.1925. [DOI] [PubMed] [Google Scholar]
- 10.Cerna L, Wenchich L, Hansikova H, Kmoch S, Peskova K, Chrastina P, Brynda J, Zeman J. Novel mutations in a boy with dihydrolipoamide dehydrogenase deficiency. Med Sci Monit. 2001;7:1319–25. [PubMed] [Google Scholar]
- 11.Grafokou O, Oexle K, van den Huevel L, Smeets R, Trijbels F, Goebel HH, Bosshard N, Superti-Furga A, Steinmann B, Smeitink J. Leigh sydrome due to compound heterozygosity of dihydrolipoamide dehydrogenase gene mutations. Description of the first E3 slice site mutation. Eur J Pediatr. 2003;162:714–18. doi: 10.1007/s00431-003-1282-z. [DOI] [PubMed] [Google Scholar]
- 12.Cameron JM, Leyandoyskiy V, Mackay N, Raiman J, Renaud DL, Clarke JT, Feigenbaum A, Elpeleg O, Robinson BH. Novel mutations in dihydrolipoamide dehydrogenase deficiency in two cousins with borderline-normal PDH complex activity. Am J Med Genet A. 2006;140:1542–52. doi: 10.1002/ajmg.a.31313. [DOI] [PubMed] [Google Scholar]
- 13.Hong YS, Kerr DS, Liu TC, Lusk M, Powell BR, Patel MS. Deficiency of dihydrolipoamide dehydrogenase due to two mutant alleles (E340K and G101del) Analysis of a family and prenatal testing. Biochim Biophys Acta. 1997;1362:160–8. doi: 10.1016/s0925-4439(97)00073-2. [DOI] [PubMed] [Google Scholar]
- 14.Odievre MH, Chretien D, Munnich A, Robinson BH, Dumoulin R, Masmoudi S, Kadhom N, Rotig A, Rustin P, Bonnefont JP. A novel mutation in the dihydrolipoamide dehydrogenase E3 subunit gene (DLD) resulting in an atypical form of α-ketoglutarate dehydrogenase deficiency. Hum Mutat. 2005;25:323–4. doi: 10.1002/humu.9319. [DOI] [PubMed] [Google Scholar]
- 15.Sansaricq C, Pardo S, Balwani M, Grace M, Raymond K. Biochemical and molecular diagnosis of lipoamide dehydrogenase deficiency in a North American Ashkenazi Jewish family. J Inherit Metab Dis. 2006;29:203–4. doi: 10.1007/s10545-006-0175-5. [DOI] [PubMed] [Google Scholar]
- 16.Shany E, Saada A, Landau D, Shaag A, Hershkovitz E, Elpeleg ON. Lipoamide dehydrogenase deficiency due to a novel mutation in the interface domain. Biochem Biophys Res Commun. 1999;262:163–6. doi: 10.1006/bbrc.1999.1133. [DOI] [PubMed] [Google Scholar]


