Abstract
Oral cancer is a substantial, often unrecognized issue globally, with close to 300,000 new cases reported annually. It is a management conundrum: a cancer site that is easily examined; yet more than 40% of oral cancers are diagnosed at a late stage when prognosis is poor and treatment can be devastating. Opportunistic screening within the dental office could lead to earlier diagnosis and intervention with improved survival.
Objectives
To describe how clinicians make decisions about referral based on the risk classification of the lesion.
Methods
18 dentists from 15 dental offices participated in a 1-day workshop on oral cancer screening. Participants then screened patients (medical history, conventional oral exam, fluorescent visualization exam) in-office for 11 months, triaging patients by apparent clinical risk: low-risk (common benign conditions, geographic tongue, candidiasis, trauma), intermediate-risk (lichenoid lesions) and high-risk (white or red lesions or ulcers without apparent cause). Clinicians made the decision on which lesions to reassess in 3 weeks based on risk assessment and clinical judgment. Lesions of concern were seen by a community facilitator or referred to an oral medicine specialist.
Results
2542 patients were screened and 389 lesions were identified (15% of patients). 350 were determined to be low-risk (90%), 19 IR (5%) and 20 HR (5%). One hundred and sixty-six (43%) patients were recalled for 3-week reassessment: 90% of HR lesions, 63% of IR lesions (63%) and 39% of low-risk lesions. Compliance to recall was high (92% of cases). Reassessment eliminated the referral of 99/166 (60%) of lesions that had resolved. 6 lesions were biopsied with 3 low-grade dysplasias identified.
Conclusions
Three key decision points were tested: risk assessment, need for reassessment and need for referral. A 3-week reassessment appointment was invaluable to prevent the unnecessary referral due to confounders. There is a need for a well-defined triage pathway to facilitate oral cancer screening and a methodical and consistent approach to opportunistic screening in the dental office.
Keywords: Oral cancer screening, precancerous conditions, diagnosis, mouth neoplasms, prevention & control, early detection of cancer, methods, awareness, questionnaires, early diagnosis, health professionals, education, referral and consultation
Introduction
A primary reason for oral cancer’s poor prognosis is late diagnosis: 5-year relative survival rates are 83% and 28% for early- (Stage 1) and late- (Stage 4) stage oral disease, respectively. (1) Proportions of oral cancer diagnosed with late stage disease vary by geographic region; in the United States (US), 67% are diagnosed after the disease has metastasized regionally or distantly (2); in India over 75% are diagnosed at a late stage. (2)
The potential impact of screening on disease outcome has been demonstrated in the only oral cancer screening randomized controlled trial (RCT), which involved 190,000 individuals in India. Associations were found between screening by community health workers and early stage diagnosis, decreased morbidity, and a 24% reduction in mortality in people with high-risk habits. (3) Of interest, this paper highlighted the benefit of repeated screenings with reductions noted in oral cancer incidence and mortality in high-risk patients who took part in all four rounds of screening.(3)
Given the low prevalence of oral cancer in developed countries as defined by ICD-10 codes (lip (C00), tongue (C02), gum (C03), floor of mouth (C04), palate (C05), buccal mucosa, retromolar, vestibule (C06) and tonsillar fossa and pillar (C09)), opportunistic screening in general dental practitioner clinics is recommended by numerous sources as part of regular clinic activity including the American Cancer Society (4), the Canadian Task Force on the Periodic Health Examination(5), the American Dental Association(6), the Cochrane Collaboration(7), the British Dental Association(8) and the UK working group on screening for oral cancer and precancer(9), and may provide the most cost-effective approach. (10). Dental practices are also promising venues for intervention. Within the US, 69% of Americans over age 18 visit a dental office annually; similar numbers are found in Canada. (11, 12) Many dental practitioners already self-report as conducting oral cancer exams: in a study of US dentists, 81% reported screening all patients over age 40 at the first appointment and 78% screened at recall appointments (13); in Canada, these proportions were 71% and 51%, respectively. (14) To date, however, efforts to standardize such behaviour and its integration into day-to-day practice have been limited.
As a first step in this direction in British Columbia (BC), the College of Dental Surgeons of BC released a set of guidelines (15) and protocols developed by the BC Oral Cancer Prevention Program in March 2008. (16–18) These guidelines recommended a systematic approach to evaluating the head and neck and oral regions, including the methodical collection of background information and a step-by-step clinical examination. This methodical process is important given the many mucosal conditions that have similar appearance; we believe “quick checks” or cursory looks provide insufficient information that could result in misdiagnosis.
This paper will present findings regarding the screening decisions and referral practices in 15 community dental practices in BC who followed such a step-by-step process. The dental practitioners had attended a training workshop on screening protocols with a hands-on demonstration and their screening activities were followed for 11 months. We sought to describe how well a triage pathway based on this step-by-step screening protocol as described in the BC guidelines assisted practitioners in differentiating between high-, intermediate- and low-risk lesions and in making decisions for appropriate and timely follow-up.
Materials and Methods
Study participants
This study was approved by the BC Cancer Agency and Simon Fraser University research ethics boards. Dental practitioners were selected from responders to a notice in a local dental association publication; interest was limited with 25 dentists responding to the ad. Eligibility was restricted to practitioners from established practices in the Greater Vancouver area who agreed to attend a one-day workshop and to follow the study protocol. Of the 25 responders, 4 did not want to participate once the protocol was explained, 1 did not show up to the workshop, 1 worked outside the Greater Vancouver area and 1 had to withdraw due to a death in the family. A total of 18 dentists participated from 15 offices (3 offices with 2 dentists), each dentist signing a consent form. The mean age of the dentists was 47 ± 8 years, 59% were male, with an average of 21 ± 9 years in practice.
Data collection
The study included 3 components: 1) a one-day workshop to orient dental participants to the study protocols; 2) subsequent follow-up of screening activities in each dental office, with facilitation and referral to dysplasia clinics for patients requiring further assessment; and 3) a final evening meeting of the participants to present the study results and gather further information through focus group discussions on their experiences with screening during the follow-up period. The workshop was held in November, 2007 and the follow-up period extended forward to September 2008.
Description of Workshop
Participants were encouraged to bring a staff member or an associate to the workshop in order to facilitate the integration of the study protocol into their dental practices. Thirty people attended the workshop: 18 dentists, 8 registered dental hygienists (RDH) and 4 certified dental assistants (CDA).
The workshop was comprised of three parts. Firstly, before the start of the workshop, two short self-administered questionnaires were completed to assess knowledge of oral cancer risk factors and to collect personal demographics on the participating dentists and information on their current screening activities. This questionnaire was adapted from Yellowitz et al (19) and Horowitz et al (13) and included multiple-choice questions related to oral cancer screening clinical risk factors, common terms, incidence and identification of known risk factors from amongst a variety of alleged risk factors. Demographic information included age, gender, years of practice, the number of hours worked weekly, study club participation, self-perceived adequacy of oral cancer screening training and counselling for tobacco or alcohol cessation. Questions also addressed the characteristics of the dental practice: type (general or specialty practice), staff (e.g., whether they employed RDHs), estimated number of patients seen weekly and estimated number of oral cancer exams among new and recall patients per week. 17 of the 18 dentists completed the questionnaire.
Secondly, a presentation was given, including a short review of oral cancer statistics, etiological factors, clinical risk factors and oral histopathology. An introduction to fluorescence visualization (FV), as a complementary approach to lesion examination (20, 21) was also given followed by a presentation on the step-by-step protocol for clinical assessment of patients, including extraoral and intraoral examination as described in Williams et al (16). Finally, the referral pathway for suspicious lesions and follow-up procedures were described.
Thirdly, the workshop concluded with a hands-on clinical session where each participant observed and performed an oral cancer screening examination of patients with active disease under both white light and FV conditions. Suggestions were also offered on how to talk to patients about screening.
Assessment of oral cancer screening activities during follow-up of dental practices
After completion of the workshop, participants were asked to screen all patients over age 21 for the period from November 2007 to September 2008. The study community facilitator (DML) then contacted each dental practice monthly for data acquisition and to address any questions on the study protocol. Participating offices were contacted regularly via email for the duration of the study to inform them of any issues that required clarification by their peers. If the dentists had any questions regarding a patient with a lesion, the community facilitator was available to view the lesion. Each patient screened was given a unique identifier which was also used at the follow-up clinic and on further diagnostic reports, if required.
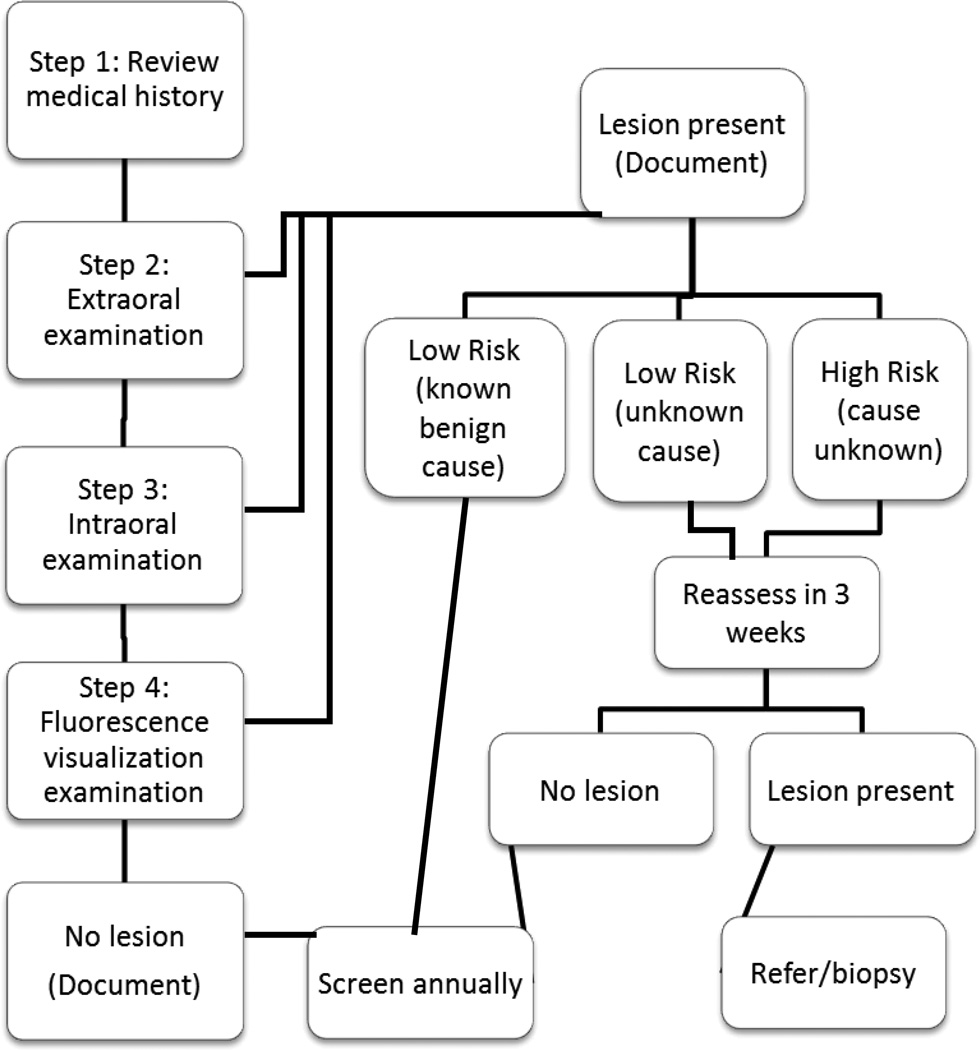
The dental offices were asked to complete the screening at each new patient or recall examination. The study protocol (Figure 1) included:
Figure 1.
Flow chart of study screening pathway.
Step 1. Patient History
This step involved recording the patient’s age, gender, personal and family history of oral cancer, tobacco use and alcohol consumption.
Step 2. Visual Screening Examination
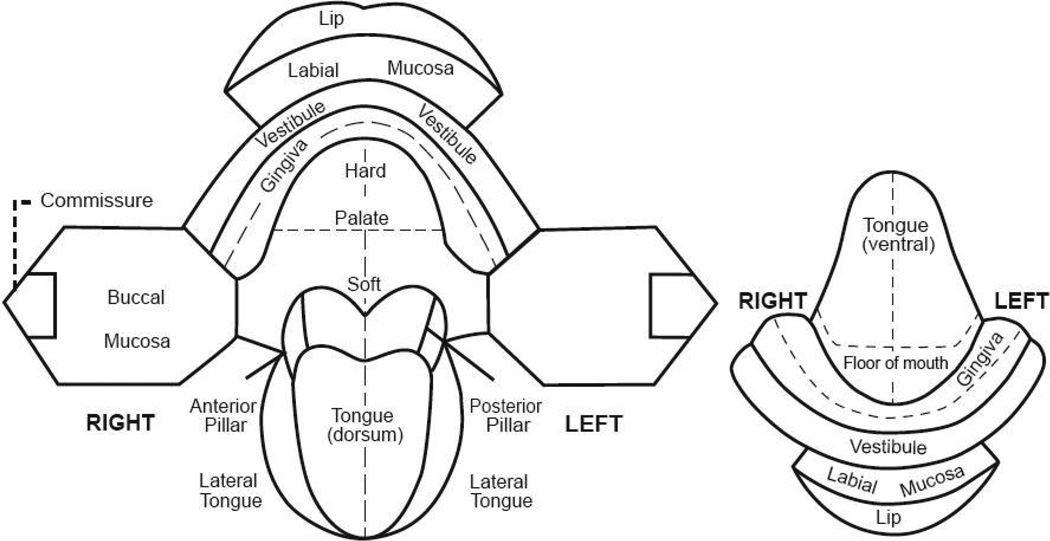
This step involved both an extraoral and intraoral examination. The extraoral examination included inspection and palpation of the head and neck region, focusing on asymmetry and swelling or tenderness. Participants were asked to refer to a medical doctor any patient with fixed, firm or unexplained lymph nodes or asymmetries. An intraoral exam under incandescent (white light) conditions was then undertaken. If an anomaly was present, the site, colour, texture and appearance of the lesion was documented by checking off the appropriate boxes on a screening form and drawing the anomaly’s location on an oral cavity diagram (Figure 2). Benign common mucosal changes not to be recorded included amalgam tattoos, Fordyce’s granules, vascularities and pigmentation due to skin colour.
Figure 2.
Map of the oral cavity.
Oral cancer sites included in this study are those sites visible to the clinician directly and are defined as the International Classification of Diseases (ICD)-10 and ICD-O3 site codes, C00 (lip), C02 (tongue), C03 (gum), C04 (floor of mouth), C05 (hard and soft palate), C06 (buccal mucosa, vestibules, and retromolar area), and C09 (tonsillar fossa and pillar).
Step 3. Lesion Assessment
This step involved assessing the risk of an anomaly. Low-risk lesions (LR) included obvious trauma, aphthous lesions, melanotic macules, candidiasis (including median rhomboid glossitis) and geographic tongue. Anomalies without apparent cause, non-healing ulcers, red or white patches and lichenoid lesions were considered high-risk lesions (HR). Lichenoid lesions were later reclassified as intermediate-risk lesions (IR) because lichenoid lesions have a variation in clinical presentation from faint white striae to red and erosive and some may have increased cancer risk. Lesions in this latter group require further follow-up for clinical management.
Step 4. Direct FV
The use of FV in this project was exploratory in nature, to see how dental professionals within the general dental community would use the device as all evidence to date has been primarily completed within referral clinics with experienced clinicians. All patients were screened using an FV imaging device and their FV status was recorded on the screening form. The FV examination followed the same methodical examination of all oral mucosa tissue as the conventional exam; however, this was done under reduced room lighting and with a handheld autofluorescence imaging device, marketed as the Velscope™, (LED Dental, Inc., White Rock, British Columbia, Canada). Lesions that retained the normal green autofluorescence under FV were classified as FV−. Tissue that showed a reduction in the normal pale green and appearing as dark patches were categorized as FV+. In cases where the examiner was unsure of FV loss, lesions were categorized as FV equivocal (FVE). (22) Detailed results of FV use on decision making will be published separately.
Lesion follow-up
Patients with LR lesions that were not obviously due to trauma or another benign condition were asked to return for reassessment in 3 weeks, allowing time for benign reactive or inflammatory lesions to resolve. If the lesion was still present, the dental practice was requested to notify the study community facilitator (DML) who reassessed the patient’s lesion within their dental office and referred any suspicious lesions to an Oral Mucosal Disease (OMD) referral clinic. In some cases, the dental offices directly referred patients to this clinic. HR lesions still present at 3 weeks were either reassessed by DML or referred directly by the dentist to the OMD clinic. Oral medicine specialists at OMD determined if a biopsy or further follow-up was warranted.
Statistical analysis
Descriptive statistics were used to summarize the data collected at the workshop from the study dentists on demographics, knowledge and baseline screening behaviour. Patient screening forms were imaged and uploaded directly into a Microsoft Excel study database using Teleform (version 10.1, 2006, Vista, California). Data analysis was performed with SPSS software, version 16.0 for Windows, 2007 (SPSS Inc., Chicago, Illinois).
Results
Knowledge of oral cancer risk factors at study entry
Participants were questioned on the adequacy of their training for the oral cancer screening exam (adapted from Horowitz et al)(13). When asked about intraoral and extraoral exams, most agreed or strongly agreed that they had received adequate training, including the palpation of lymph nodes; however, few felt adequately prepared to offer tobacco and alcohol cessation counselling. Less than half felt that their scholastic training weighted oral cancer screening similar to their other clinical training. When asked about current screening behaviour, the participants self-reported performing fewer extraoral exams than intraoral exams, with more exams in those over the age of 40. Only three clinicians reported collecting information about alcohol use.
All study participants’ knowledge of oral cancer risk factors and screening awareness were assessed at study entry. Respondents knew screening information such as the meaning of leukoplakia, the increased risk of nonhomogeneous appearing lesions and that early detection of oral cancer is the most significant factor in the long-term survival of oral cancer, and understood the histology underlying risk of premalignant lesions of different and increasing degrees of dysplasia. Most respondents were able to correctly identify squamous cell carcinoma (SCC) as the most common form of oral cancer and the dorsal tongue as the least common site for oral cancer. In contrast, only a few clinicians (18%) could identify the steps of an extraoral examination, or the most common lesion site in a patient with no apparent risk factors.
Screening activity during the study period
A total of 2631 screening examinations were completed over the study period. However, 48 had incomplete documentation of clinical data, 33 patients were under age 21 and hence not eligible, and 8 patients had a past history of oral cancer. These cases were removed, resulting in 2542 screening examinations for further analysis.
Overall, the average number of exams per practice over the study period was 169 ± 98, with a wide variation between offices (ranging from 17 – 367).
Step 1. Patient History
The first step of the oral cancer screening exam involved collection of patient history, including a medical history, patient characteristics and risk habits; the results are shown in Table 1. The majority of patients screened were 40 years of age and older, considered to be a key indicator to screen according to the BC guidelines for early detection of oral cancer. (15) Only 11% of patients were current smokers and 70% of former smokers had quit 10 or more years prior to their screening exam. Almost 60% of patients had a past history of drinking alcohol for one year or more.
Table 1.
Description of study patients and their risk habits.
| All (%) |
Lesion (%) |
No lesion (%) |
P value |
RR | |
|---|---|---|---|---|---|
| All | 2542 | 389 (15%) | 2153 (85%) | ||
| Age (N=2432) | |||||
| ≤40 | 531 (22%) | 61 (12%) | 470 (89%) | 0.004 | 1 |
| >40 | 1901 (78%) | 315 (17%) | 1586 (83%) | 1.5 (95%CI:1.1–2.1) | |
| Gender (N=2528) | |||||
| Male | 1069 (42%) | 175 (16%) | 894 (84%) | 0.242 | |
| Female | 1459 (58%) | 214 (15%) | 1245 (85%) | ||
| Family history of mouth cancer (N=2477) | |||||
| No | 2411 (97%) | 360 (15%) | 2051 (85%) | 0.023 | 1 |
| Yes | 66 (3%) | 17 (26%) | 49 (75%) | 2.0 (95%CI:1.1–3.5) | |
| History of smoking (N=2477) | |||||
| No | 1475 (60%) | 197 (13%) | 1278 (87%) | 0.001 | 1 |
| Yes | 1002 (41%) | 186 (19%) | 816 (81%) | 1.5 (95%CI:1.2–1.8) | |
| Smoking status (N=2477) | |||||
| Non-smoker | 1475 (60%) | 197 (13%) | 1278 (87%) | <0.001 | 1 |
| Former smoker | 733 (30%) | 138 (19%) | 595 (81%) | 1.4 (95%CI:1.0–2.0) | |
| Current smoker | 269 (11%) | 48 (18%) | 221 (82%) | 1.5 (95%CI:1.2–1.9) | |
| Tobacco amount (packs/day) (N=2360) | |||||
| 0 | 1475 (63%) | 197 (13%) | 1278 (87%) | 0.006 | 1 |
| <1/2 | 388 (16%) | 68 (18%) | 320 (83%) | 1.4 (95%CI:1.0–1.9) | |
| 1/2-1 | 313 (13%) | 58 (19%) | 255 (82%) | 1.5 (95%CI:1.1–2.0) | |
| >1 | 184 (8%) | 38 (21%) | 146 (79%) | 1.7 (95%CI:1.1–2.5) | |
| Tobacco duration (years) (N=2405) | |||||
| 0 | 1475 (61%) | 197 (13%) | 1278 (87%) | <0.001 | 1 |
| ≤10 | 343 (14%) | 57 (17%) | 286 (83%) | 0.8 (95%CI:0.5–1.3) | |
| 11–20 | 308 (13%) | 44 (14%) | 264 (86%) | 1.7 (95%CI:1.1–2.5) | |
| ≥21 | 279 (12%) | 70 (25%) | 209 (75%) | 0.8 (95%CI:0.6–1.1) | |
| Chewing tobacco (N=1926) | |||||
| No | 1884 (98%) | 305 (16%) | 1579 (84%) | 0.396 | |
| Yes | 42 (2%) | 9 (21%) | 33 (79%) | ||
| History of alcohol use (N=2488) | |||||
| No | 1019 (41%) | 126 (12%) | 893 (88%) | 0.001 | 1 |
| Yes1 | 1469 (59%) | 253 (17%) | 1216 (83%) | 1.5 (95%CI:1.2–1.9) | |
| Alcohol amount | |||||
| Beer (8 oz. drinks/week) (N=1851) | |||||
| 0 | 1019 (55%) | 126 (12%) | 893 (88%) | 0.016 | 1 |
| ≤14 | 787 (43%) | 139 (18%) | 648 (82%) | 1.7 (95%CI:1.2–2.0) | |
| 15–20 | 26 (1%) | 5 (19%) | 21 (81%) | 1.7 (95%CI:0.6–4.6) | |
| ≥21 | 19 (1%) | 3 (16%) | 16 (84%) | 1.3 (95%CI:0.4–4.6) | |
| Wine (4oz. drinks/week) (N=2150) | |||||
| 0 | 1019 (47%) | 126 (12%) | 893 (88%) | 0.024 | 1 |
| ≤14 | 1085 (51%) | 168 (16%) | 917 (85%) | 1.3 (95%CI:1.0–1.7) | |
| 15–20 | 31 (1%) | 6 (19%) | 25 (81%) | 1.7 (95%CI:0.7–4.2) | |
| ≥21 | 15 (1%) | 5 (33%) | 10 (67%) | 3.5 (95%CI:1.2–10.5) | |
| Spirits (1oz. drinks/week) (N=1715) | |||||
| 0 | 1019 (59%) | 126 (12%) | 893 (88%) | <0.001 | 1 |
| ≤14 | 668 (39%) | 117 (18%) | 551 (83%) | 1.5 (95%CI:1.1–2.0) | |
| 15–20 | 20 (1%) | 8 (40%) | 12 (60%) | 4.7 (95%CI:1.9–11.8) | |
| ≥21 | 8 (<1%) | 2 (25%) | 6 (75%) | 2.4 (95%CI:0.5–11.8) | |
| Alcohol duration (years) (N=2306) | |||||
| 0 | 1019 (44%) | 126 (12%) | 893 (88%) | 0.001 | 1 |
| ≤10 | 273 (12%) | 40 (15%) | 233 (85%) | 1.2 (95%CI:0.8–1.8) | |
| 11–20 | 369 (16%) | 58 (16%) | 311 (848%) | 1.3 (95%CI:0.9–1.9) | |
| ≥21 | 644 (28%) | 126 (20%) | 518 (80%) | 1.7 (95%CI:1.3–2.3) | |
| History of tobacco and alcohol (N=2440) | |||||
| NS + ND2 | 746 (31%) | 76 (10%) | 670 (90%) | <0.001 | 1 |
| NS + D | 703 (29%) | 116 (17%) | 587 (84%) | 1.7 (95%CI:1.3‒2.4) | |
| S + ND | 247 (10%) | 44 (19%) | 199 (81%) | 2.1 (95%CI:1.4–3.2) | |
| S + D | 744 (31%) | 135 (18%) | 609 (82%) | 2.0 (95%CI:1.4–2.6) | |
N = the number of respondents with applicable data
Alcohol use – consumed more than 2 drinks per week for more than 1 year
NS=non-smoker; ND=non-drinker; S=smoker; D=drinker
Step 2. Visual screening examination
The second step was the visual screening exam and, of the 2542 intraoral screenings completed, 2354 (93%) also had an extraoral exam. Of these, 134 (6%) patients had palpable lymph nodes, 2 of which were referred to their medical doctor. Also noted was a patient with an enlarged thyroid awaiting thyroid surgery and a patient with a history of chronic lymphocytic leukemia. Comments for positive lymph nodes included recent illness, infection, mobile and soft nodes, and those which had had medical follow-up; the remaining 77 had no explanation for positive nodes.
Intraoral examination resulted in the identification of 389 lesions, present as single lesions in 15% of patients. Table 2 is a graphic display of the flow of patients through the critical steps of this risk assessment strategy.
Table 2.
The triage of all patients with lesions from detection through to biopsy.
| N = 389 | ALL | Low-risk | Intermediate- risk | High-risk |
|---|---|---|---|---|
| No. of lesions | 389 (100%) | 350 (90%) | 19 (5%) | 20 (5%) |
| Recalled | 166 (43%) | 136 (39%) | 12 (63%) | 18 (90%) |
| Complied | 153 (92%) | 124 (91%) | 12 (100%) | 17 (94%) |
| Referred | 54 (35%) | 36 (29%) | 9 (75%) | 9 (53%) |
| Biopsied | 6 (11%) | 3 (8%) | 1 (11%) | 2 (22%) |
| Dysplasia | 3 (50%) | 1 (3%) | 0 | 2 (100%) |
Step 3. Lesion assessment
Of the 389 lesions identified, 350 (90% of lesions) were classified as low risk and 39 (10%) as high risk. Nineteen (5%) of the HR lesions were lichenoid and reclassified as IR leaving 20 (5%) as HR lesions.
LR lesions were categorized as trauma and/or nonspecific ulcer (N = 246, 70% of LR lesions), geographic tongue (N = 34, 14%) and ‘candidiasis and other’ (melanotic macule, scar, fistula, nevi, papilloma, pigmentation and mucocele) (N = 70, 28%). The participating clinicians further categorized these lesions as to need for follow-up. Of the 350 LR lesions, 214 (61%) had a known cause and were felt to not require reassessment (e.g., trauma such as burns, linea alba and other cheek biting, aphthous ulcers, herpetic lesions, denture sores, pigmentation, mucoceles, flossing trauma, varicosities, fistulas, candidiasis and geographic tongue).
Step 4. Direct FV
The fourth step, determining the FV status of these lesions, is the topic of a companion paper to be published separately.
Lesion follow-up
One hundred and thirty-six (39%) patients were asked to return in 3 weeks for reassessment and 124 (91%) complied. At reassessment, 88 (71%) lesions had resolved, leaving 36 lesions to be evaluated by the community facilitator (DML) to identify lesions requiring referral to oral medicine specialists at the Oral Mucosal Disease Program (OMDP). Four of these lesions required further assessment by an oral medicine specialist: 3 were biopsied and one is in follow-up. Of these biopsies, 1 showed mild dysplasia, 1 melanotic macule and 1 focal mucositis with melanin incontentia (the latter biopsy was done at the request of the patient out of concern over a family history of oral cancer and not due to a concern by the clinician of the presence of cancer risk).
Of 19 IR lesions with lichenoid characteristics, 12 were asked to return for reassessment in 3 weeks and all complied. Nine (75%) were then rescheduled for review by the study community facilitator (DML) or at the OMDP. Three lesions required further assessment, resulting in 1 biopsy (diagnosed as lichenoid mucositis); the other 2 patients are in follow-up and being monitored at OMDP.
Of the 20 HR lesions, 18 (90%) were asked to return for reassessment at 3 weeks and 17 (94%) complied. Nine lesions were still present at reassessment and all were seen by the study community facilitator (DML) or referred directly to the OMDP. Four (44%) patients’ required further follow-up and 2 have since been biopsied (1 mild and 1 moderate dysplasia).
Discussion
Few papers have examined the process by which dental practitioners make decisions on clinical anomalies. In our study, participating dentists were presented with a fixed protocol, a triage pathway and follow-up plan for at-risk lesions which extended to referral to oral specialists and biopsy, where necessary. We identified the key decision points and where the process may need improvement. This information will provide a framework for planning and development of oral screening activity in the general dental community.
One of the key questions addressed was whether a triage protocol facilitated the screening process. We describe what decisions were made at each step in this process, with respect to collection of patient history information, detection of lesions at screening, risk classification and triage of lesions.
The smoking prevalence amongst those screened was similar to that reported for the general British Columbian population (15% ever smokers, 14% aged 45 years and older) (23). Sixty percent of those screened consumed alcohol regularly with only 2% reporting drinking 21 or more drinks of alcohol per week. (24) All participants reported collecting current tobacco use yet only approximately half collected data about the amount and duration of the habit. The collection of alcohol habit information was poor with less than 20% collecting current use and none of the participants reported collecting amount and duration of the alcohol habit. Since risk of oral cancer and dysplasia is dose dependent for both alcohol and tobacco this is valuable information for ascertaining patient risk that is being missed by clinicians. (25, 26)
It is encouraging that most patients received an extraoral exam along with the intraoral exam. Of the 134 patients with palpable lymph nodes, two were referred to their medical doctor for further workup and 59 had an associated illness or medical history. However, there was no explanation for the remaining 77 individuals, which is a concern. The authors were unable to collect information about the patients referred to their medical doctors for possible lymph nodes. Further work is required to better guide clinicians in this step. The intraoral examinations resulted in the identification of 389 lesions, representing 15% of cases. This percentage is similar to that found in 18 dental practices in the UK; 2265 patients were examined in that study with 14.1% showing an anomaly. (24) It can be difficult to compare oral screening studies as lesion classification can vary; however, lesion groupings between the UK study (24), which used a similar lesion classification (ICD-9), and the current study were similar.
Risk classification represented a key decision point. Of interest, the vast majority of lesions (90%) were classified by the dentists into the LR category, 19 lesions (5%) into the IR category and 20 lesions (5%) into the HR category for oral cancer. In total, 10% of the lesions were categorized as IR or HR at the initial appointment and later reduced to 7.7% at the 3 week reassessment appointment. This is higher than the UK study where 4.2% of lesions were thought to be malignant or potentially malignant (included lichen planus). This is of interest as our study had fewer smokers than the UK study (11% as compared to 29%). A second key decision point involved identifying LR lesions with known cause. Generally, the dentists were able to rule out most of these lesions due to trauma (such as burns and cheek biting), linea alba, aphthous and herpetic ulcers, denture sores, and amalgam tattoos, of which they are familiar.
The request for a 3- week reassessment was another critical point. Only 39% of patients with LR lesions were asked back for a 3-week reassessment by the dentist, as compared to 63% with IR and 90% with HR lesions. Altogether, the 3-week reassessment eliminated the apparent unnecessary referral of 99 of 166 (60%) lesions for assessment of lesions, as these lesions resolved (88 LR, 3 IR, and 8 HR). Of interest, compliance for reassessment was high, with only 13 (8%) of 166 patients failing to return for the 3-week reassessment appointment.
A final key decision involved the request from dentists for assessment of lesions by the study community facilitator. This step reduced the patients’ time and anxiety for further unnecessary assessments and also reduced by 73% the number of patients referred to the OMDP clinic. Of the 8 facilitator-referred patients, 3 were biopsied (1 dysplasia, 2 lichenoid mucositis), 2 patients are in continued follow-up and 1 patient had hyperplastic lymphoid tissue on the tonsil (patient requested referral). An added value of the community facilitator was that dentists could observe the interaction between the facilitator and the patient. The discussion of referrals and biopsies with patients had been raised as a difficulty and a barrier to screening by clinicians in previous studies. (27)
We identified several components of the process that require change in screening practice. Reinforcing the use of a methodical, consistent approach for each patient is critical, with an emphasis that short-cuts can lead to misdiagnosis. The rationale and importance of each step needs to be emphasized, both for the screening exam and for the medical history. Results of the study entry questionnaire for the participating dentists showed similar patterns to earlier studies on the knowledge of risk factors (14, 19, 28) and screening practices. (13, 29) It was a particular concern that information on alcohol consumption was infrequently collected, despite the growing concern in the literature with respect to oral cancer risk. (30–32) Documentation of risk habits was often poor as has been previously reported in earlier studies. (13, 29) The importance of collecting and documenting risk habit information is reinforced by the increased proportion of lesions in this study, found in patients with a history of alcohol use, tobacco use and older age. The lack of documentation of the oral cancer screening itself is also a concern. (33–35) Only 65% of our dentists reported documenting these exams regularly; some noted documenting only when abnormal findings were discovered.
There were several limitations for this study. Firstly, the sample size was small and relied on volunteers for participation. The results may not reflect the response of the general dental profession. Out of approximately 1800 eligible general dentist in Greater Vancouver, very few responded to the advertisement and only 18 dentists (1%) participated. Limited interest in this study by general practice dentists could be associated with lack of interest or perceived need of the new technology for screening, or concerns on how participation in a study about oral cancer may affect their patients. However, there was considerable variation amongst the dentists in this study with respect to age, years of practice, screening behavior and knowledge base, a finding which suggests that data may reflect the screening practices of a fairly broad range of dental professionals. The numbers of participating dentists were too small to look for further relationships between clinician characteristics and screening behaviour and knowledge. Secondly, all oral lesions were not reviewed by the study facilitator. Some patients were not asked to return for reassessment, based upon the judgement of the participating dentist. Other patients were referred directly to the OMD clinic without first seeing the study facilitator. However, a review of all lesions by the study facilitator was not required in order to meet the objective of this study which was to gain information on screening decisions and how they are made in community practices. Thirdly, not all lesions referred to the OMD clinic were biopsied as it was felt not to be ethical to biopsy obviously benign lesions. Some patients with lichenoid lesions remained in follow-up and may be biopsied at a later date. Finally, data was not obtained from all patients seen in the participating dental offices, as this data was voluntarily collected from the dentists. However, attempts were made to get as complete data as possible with regular contact between the study facilitator (DML) and the participating dentists.
The data presented in this paper supports the need for well-defined triage pathway to facilitate the implementation and evaluation of oral cancer screening programs. Guidelines can only be viewed as a starting point towards standardizing such activity. There is also the need for hands-on educational processes. Moving such activities into study group settings in the community might be an attractive way to accomplish this and should be explored. There is also the need to provide the results of such activity back to the community in order to further guide the development of highquality screenings. Key decision points that need strengthening are: the evaluation of the extraoral exam; classification of lesions by degree of risk for oral cancer; and the importance of reassessment at 3 weeks for suspicious lesions. Of interest, out of 2542 patients screened, 3 dysplasias were identified (1 in ~850 patients). This is one of the few indications of the potential frequency of premalignant lesions in community practices in North America. Such information is valuable when planning screening and intervention strategies.
In conclusion, it is important to emphasize that fine-tuning and tailoring of education strategies for community practitioners is only a first step. There is also the need to develop well-defined referral pathways, efficient means to relay information back to community practitioners, and appropriate infrastructure and guidelines for the further assessment and treatment of cases identified in the screening process. A streamlined linkage of screening, referral and treatment is critical.
Table 3.
Low-risk lesion pathway
| N = 325 | Low risk | FV+ | FVE | FV− |
|---|---|---|---|---|
| No. of lesions | 325 (100%) | 175 (54%) | 24 (7%) | 126 (38%) |
| Recalled | 121 (37%) | 90 (51%) | 12 (50%) | 19 (15%) |
| Complied1 | 113 (93%) | 84 (93%) | 10 (83%) | 19 (100%) |
| Referred2 | 17 (15%) | 16 (19%) | 0 | 1 (5%) |
| Biopsied | 2 (12%) | 2 (13%) | 0 | 0 |
| Dysplasia | 0 | 0 | 0 | 0 |
At reassessment 18 FV+ lesions remained FV+, all the FVE lesions were FV− and the 1 of the FV− lesions was FV+.
5 FV+ lesions were referred directly without a recall visit; 18 lesions were seen by the facilitator (9 FV+, 5 FVE and 4 FV−) 2 FV+ lesions were referred by the facilitator.
Table 4.
Intermediate-risk lesion pathway
| N = 16 | Intermediate risk |
FV+ | FVE | FV− |
|---|---|---|---|---|
| No. of lesions | 16 (100%) | 8 (50%) | 1 (6%) | 7 (44%) |
| Recalled | 10 (63%) | 6 (75%) | 1 (100%) | 3 (43%) |
| Complied1 | 10 (100%) | 6 (100%) | 1 (100%) | 3 (100%) |
| Referred2 | 5 (15%) | 3 (19%) | 1 | 1 (5%) |
| Biopsied | 1 (12%) | 0 | 0 | 1 |
| Dysplasia | 0 | 0 | 0 | 0 |
At reassessment 4 of the 6 FV+ lesions remained FV+ and 1 FV− lesion was FV+.
7 lesions were seen by the facilitator (4 FV+ and 3 FV−) 2 FV+ and 1 FV− lesions were referred by the facilitator.
Table 5.
High-risk lesion pathway
| N = 16 | High risk | FV+ | FVE | FV− |
|---|---|---|---|---|
| No. of lesions | 16 (100%) | 9 (56%) | 1 (6%) | 6 (38%) |
| Recalled | 10 (63%) | 8 (89%)1 | 1 (100%) | 4 (67%) |
| Complied2 | 10 (100%) | 8 (100%) | 1 (100%) | 4 (100%) |
| Referred3 | 5 (50%) | 4 (50%) | 0 | 1 (25%) |
| Biopsied | 2 (40%) | 2 (50%) | 0 | 1 |
| Dysplasia | 2 (100%) | 0 | 0 | 0 |
One of the 9 high risk FV+ lesion was referred directly without reassessment.
At reassessment 5 of the 8 FV+ lesions remained FV+ and 1 FV− lesion was FV+.
3 lesions were seen by the facilitator (1 FV+, 1 FVE and 1 FV−) 1 FV+ and 1 FV− lesions were referred by the facilitator.
Acknowledgments
Financial support: This research was funded by the National Institutes of Health and the National Institute of Dental and Craniofacial Research (R01DE13124 and R01DE17013) and a Senior Graduate Studentship from the Michael Smith Foundation for Health Research/BC Cancer Foundation (DML).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Levin B. World Cancer Report. 2008 [Google Scholar]
- 3.Sankaranarayanan R, Ramadas K, Thara S, Muwonge R, Thomas G, Anju G, et al. Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncol. 2013;49(4):314–321. doi: 10.1016/j.oraloncology.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. American Cancer Society Guidelines for the Early Detection of Cancer, 2006. CA Cancer J Clin. 2006;56:11–25. doi: 10.3322/canjclin.56.1.11. [DOI] [PubMed] [Google Scholar]
- 5.Canadian Task Force on the Periodic Health Examination. The Canadian Guide to Clinical Preventive Health Care. Ottawa: Ministry of Supply and Services Canada; 1994. p. 1009. [Google Scholar]
- 6.Rethman MP, Carpenter W, Cohen EEW, Epstein J, Evans CA, Flaitz CM, et al. Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. J Am Dent Assoc. 2010;141(5):509–520. doi: 10.14219/jada.archive.2010.0223. [DOI] [PubMed] [Google Scholar]
- 7.Brocklehurst P, Kujan O, O'Malley LA, Ogden G, Shepherd S, Glenny AM. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev. 2013;11:CD004150. doi: 10.1002/14651858.CD004150.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.British Dental Association. Opportunistic Oral Cancer Screening. BDA News. 2000;(6):1–36. [Google Scholar]
- 9.Speight PM, Downer MC, Zakrzewska JMe. UK working group on screening for oral cancer and precancer: conclusions and recommendations. Community Dent Health. 1993;10(Suppl 1):87–89. [PubMed] [Google Scholar]
- 10.Speight P, Palmer S, Moles D, Downer M, Smith D, Henriksson M, et al. The cost-effectiveness of screening for oral cancer in primary care. Health Technol Assess. 2006;10(14):1–144. doi: 10.3310/hta10140. [DOI] [PubMed] [Google Scholar]
- 11.Statistics Canada. Table 105–0460 - Contact with dental professionals in the past 12 months, by age group and sex, household population aged 12 and over, Canada, provinces, territories, health regions (June 2005 boundaries) and peer groups, every 2 years, CANSIM (database) [updated August 27, 2009];2005 Available from: http://cansim2.statcan.gc.ca/cgi-win/cnsmcgi.exe?Lang=E&CNSM-Fi=CII/CII_1-eng.htm. [Google Scholar]
- 12.National Center for Chronic Disease Prevention and Health Promotion. National Oral Health Surveillance System Dental Visit: Center for Disease Control and Prevention. [updated 2009 11 06];2004 Available from: http://apps.nccd.cdc.gov/nohss/ListV.asp?qkey=5&DataSet=2.
- 13.Horowitz AM, Drury TF, Goodman HS, Yellowitz JA. Oral pharyngeal cancer prevention and early detection. Dentists' opinions and practices. J Am Dent Assoc. 2000;131(4):453–462. doi: 10.14219/jada.archive.2000.0201. [DOI] [PubMed] [Google Scholar]
- 14.Clovis JB, Horowitz AM, Poel DH. Oral and pharyngeal cancer: knowledge and opinions of dentists in British Columbia and Nova Scotia. J Can Dent Assoc. 2002;68(7):415–420. [PubMed] [Google Scholar]
- 15.Early Detection of Oral Cancer Working Group. Guideline for the Early Detection of Oral Cancer in British Columbia 2008. J Can Dent Assoc. 2008;74(3):245–252. [PubMed] [Google Scholar]
- 16.Williams PM, Poh CF, Hovan AJ, Ng S, Rosin MP. Evaluation of a suspicious oral mucosal lesion. J Can Dent Assoc. 2008;74(3):275–280. [PubMed] [Google Scholar]
- 17.Laronde DM, Hislop TG, Elwood JM, Rosin MP. Oral cancer: Just the facts. J Can Dent Assoc. 2008;74(3):269–272. [PubMed] [Google Scholar]
- 18.Currie BL, Williams PM, Poh CF. Is the message clear? Talking with you patient about oral cancer screening. J Can Dent Assoc. 2008;74(3):255–256. [PubMed] [Google Scholar]
- 19.Yellowitz JA, Horowitz AM, Drury TF, Goodman HS. Survey of US dentists' knowledge and opinions about oral pharyngeal cancer. J Am Dent Assoc. 2000;131(5):653–661. doi: 10.14219/jada.archive.2000.0239. [DOI] [PubMed] [Google Scholar]
- 20.Lane PM, Gilhuly T, Whitehead P, Zeng H, Poh CF, Ng S, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. Journal of Biomedical Optics. 2006;11(2):024006. doi: 10.1117/1.2193157. [DOI] [PubMed] [Google Scholar]
- 21.Poh CF, Ng SP, Williams PM, Zhang L, Laronde DM, Lane P, et al. Direct fluorescence visualization of clinically occult high-risk oral premalignant disease using a simple hand-held device. Head Neck. 2007;29(1):71–76. doi: 10.1002/hed.20468. [DOI] [PubMed] [Google Scholar]
- 22.Poh CF, MacAulay CE, Laronde DM, Williams PM, Zhang L, Rosin MP. Squamous cell carcinoma and precursor lesions: diagnosis and screening in a technical era. Periodontol 2000. 2011;57(1):73–88. doi: 10.1111/j.1600-0757.2011.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Health Canada. Canadian Tobacco Use Monitoring Survey (CTUMS) [updated 2009 0813];2009 [cited 2009]. Available from: www.hc-sc.gc.ca. [Google Scholar]
- 24.Lim K, Moles DR, Downer MC, Speight PM. Opportunistic screening for oral cancer and precancer in general dental practice: results of a demonstration study. Br Dent J. 2003;194(9):497–502. doi: 10.1038/sj.bdj.4810069. [DOI] [PubMed] [Google Scholar]
- 25.Bagnardi V, Blangiardo M, La Vecchia C, Corroa G. Alcohol Consumption and the Risk of Cancer. Alcohol Res Health. 2001;26(4):263–270. [PMC free article] [PubMed] [Google Scholar]
- 26.Hashibe M, Brennan P, Chuang S, Boccia S, Castellsague X, Chen C, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laronde DM, Bottorff JL, Hislop TG, Poh CY, Currie B, Williams PM, et al. Voices from the community--experiences from the dental office: initiating oral cancer screening. J Can Dent Assoc. 2008;74(3):239–241. [PubMed] [Google Scholar]
- 28.Canto M, Drury T, Horowitz A. Maryland Dentists' knowledge of oral cancer risk factors and diagnostic procedures. Health Promot Pract. 2001;2(3):255–262. [Google Scholar]
- 29.Clovis JB, Horowitz AM, Poel DH. Oral and pharyngeal cancer: practices and opinions of dentists in British Columbia and Nova Scotia. J Can Dent Assoc. 2002;68(7):421–425. [PubMed] [Google Scholar]
- 30.Allen E, Beral V, Casabonne D, Wan Kan S, Reeves G, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:298–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 31.Purdue MP, Hashibe M, Berthiller J, La Vecchia C, Dal Maso L, Herrero R, et al. Type of alcoholic beverage and risk of head and neck cancer--a pooled analysis within the INHANCE Consortium. Am J Epidemiol. 2009;169(2):132–142. doi: 10.1093/aje/kwn306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado M, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 33.Mignogna M, Fedele S, Lo Russo L, Ruoppo E, Lo Muzio L. Oral and pharyngeal cancer: Lack of prevention and early detection by health care providers. Eur J of Cancer Prev. 2001;10:381–383. doi: 10.1097/00008469-200108000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Alexander RE, Wright JM, Thiebaud S. Evaluating, documenting and following up oral pathological conditions. A suggested protocol. J Am Dent Assoc. 2001;132(3):329–335. doi: 10.14219/jada.archive.2001.0175. [DOI] [PubMed] [Google Scholar]
- 35.Hapcock CP. Risk management considerations for oral cancer. J Am Dent Assoc. 2005;136:1566–1567. doi: 10.14219/jada.archive.2005.0089. [DOI] [PubMed] [Google Scholar]