Abstract
Vertebrate reservoirs of arboviruses are often infected with microfilariae (MF). Laboratory studies have shown that MF can enhance the infectivity of arboviruses to mosquitoes. Soon after being ingested, MF penetrate the mosquito midgut. If the host blood also contains virus (i.e., vertebrate is dually infected), penetrating MF may introduce virus into the hemocoel. This can transform otherwise virus-incompetent mosquito species into virus-competent species and simultaneously accelerate viral development, allowing mosquitoes to transmit virus sooner than normal. This phenomenon is termed microfilarial enhancement of arboviral transmission. The prevalence of MF is very high in many passerine populations in North America. Therefore, we investigated if microfilarial enhancement could have facilitated the establishment and rapid spread of West Nile virus (WNV) across the mid-western United States. Our investigations revealed that mosquitoes, WNV, and passerine MF do interact in nature because; 1) 17% of 54 common grackles (Quiscalus quiscula L.), 8% of 26 American robins (Turdus migratorius L.), and 33% of three eastern kingbirds (Tyrannus tyrannus L.) were concurrently microfilaremic and seropositive to WNV; 2) feeding activities of mosquitoes overlapped temporally with the appearance of MF in the blood of common grackles; 3) mosquitoes fed on common grackles and American robins in nature; and 4) mosquito ingestion of two taxonomically distant species of passerine MF (i.e., Chandlerella quiscali and Eufilaria spp.) resulted in penetration of mosquito midguts. To estimate the theoretical effect that MF enhancement could have on WNV transmission in areas of high MF prevalence, vectorial capacity values were calculated for Culex mosquitoes feeding on common grackles, whereby MF enhancement was either invoked or ignored. For Cx. pipiens, vectorial capacity increased over three-fold when potential effects of MF were included in the calculations. For Cx. tarsalis, the effect was less (i.e., 1.4-fold increase). Closer attention should be paid to the potential of MF to enhance mosquito transmission of arboviruses.
Keywords: microfilarial enhancement of arboviral transmission, microfilariae, West Nile virus, mosquito, Culex
Defining vector competence is fundamental to understanding arboviral transmission cycles. Not every arthropod species can transmit every arboviral species, meaning there can be barriers to the infection process. The most important is the “midgut barrier” (Chamberlain and Sudia 1961). Ingested virus may either be unable to enter the arthropod midgut cells because of a receptor-ligand incompatibility (=midgut infection barrier) or, once inside, virus may be unable to disseminate from the midgut to the hemocoel (=midgut escape barrier) (Kramer et al., 1981, Hardy et al., 1983). In many cases, once the midgut barrier is overcome, viral infection of the salivary glands and subsequent transmission ensues. Thus, any mechanism that effectively bypasses midgut barriers will greatly increase the potential transmission of arboviruses by vectors.
The standard way to identify midgut barriers is to feed suspected vectors on viremic blood. Individuals are then sampled at various time intervals and their extremities (e.g., legs) are excised and assayed for virus to determine if ingested virus disseminated from the gut into the hemocoel. Vectors are fed using in vitro methods (e.g., membrane feeder) or by allowing them to feed on anesthetized or restrained vertebrates purposefully infected with the virus in question. Almost always, the vertebrates used are “clean,” laboratory-raised species (e.g., rodents, poultry). However, neither method accounts for the fact that the actual vertebrate reservoirs involved in arboviral transmission cycles are often infected with other blood-borne parasites. Particularly important are the filarioid nematodes.
Filarioid nematodes produce chronic infections among a wide variety of amphibians, reptiles, birds, and mammals, including humans. The prevalence of filarioid infections among wildlife can be very high (Higby 1943, Pung et al. 1966, Sousa et al. 1974, Weinmann et al. 1973). Indeed, blood surveys often underestimate the prevalence of filarioid infections (Homstad et al. 2003) because many filarioid species are nocturnally periodic, that is, the transmissible larval form (=microfilariae) only appear in the peripheral blood at night when field biologists are unlikely to be collecting blood samples. Many hematophagous arthropods (e.g., mosquitoes) feed at night and are likely to ingest nocturnally periodic microfilariae (MF). Laboratory studies have demonstrated that concurrent ingestion of MF and arboviruses by mosquitoes and biting midges can result in significantly greater infectivity of the arbovirus than when the same dose of arbovirus is ingested alone (Mellor and Boorman 1980; Turell et al. 1984, 1987; Zytoon et al. 1993; Vaughan and Turell 1996; Vaughan et al. 1999). As part of their developmental cycle, MF may penetrate the arthropod midgut after being ingested. If the bloodmeal also contains infectious virions, some of the virions can enter the hemocoel directly, circumventing the initial developmental events required for normal arboviral infection of arthropod vectors (i.e., viral attachment, invasion, replication, and release from within the mosquito midgut epithelium). This phenomenon, termed microfilarial enhancement of arboviral transmission, can simultaneously enhance two important transmission parameters. First, virus-incompetent species that are normally refractory to infection because of midgut barriers, may now develop infections. Increasing the susceptibility of otherwise refractory vectors can increase the number of secondary vector species involved in an arboviral transmission cycle. Second, immediate dissemination of virus by MF can accelerate viral development within the vector, shortening the time required for infected vectors to become infectious vectors (=extrinsic incubation period [EIP]). Because EIP affects transmission in an exponential fashion, small reductions in EIP can lead to large increases in vectorial capacity (MacDonald 1952).
Theoretically, MF enhancement could apply to any hematophagous arthropod species (e.g., sand flies, ticks) as long as the following requirements are met. First, the vertebrate must be concurrently viremic and microfilaremic (i.e., dually infected). Second, the arthropod must ingest both virus and MF. Third, the MF must penetrate the arthropod midgut. Fourth, upon MF penetration sufficient virus must pass from the bloodmeal into the hemocoel to establish a disseminated viral infection. And finally, the arthropod must be able to transmit the virus by bite (i.e., there must be no salivary gland barriers to viral infection or secretion).
The phenomenon of MF enhancement was described over 30 yr ago and has been demonstrated by three different research groups for at least nine different vector-arbovirus-MF model systems (Mellor and Boorman 1980; Turell et al. 1984, 1987; Zytoon et al. 1993; Vaughan and Turell 1996; Vaughan et al. 1999). However, its true impact on arboviral transmission cycles remains uncertain because many of the model systems used to demonstrate the concept were artificial and do not occur in nature. To understand the likelihood of MF enhancement occurring in nature, we investigated the interaction of mosquitoes with co-indigenous songbirds and their filarioid parasites during the time when West Nile virus (WNV) was becoming established in the Red River Valley of eastern North Dakota. Specifically, we determined the applicability of requirements one (=dual infections) and three (=MF penetration of the midgut) as they related to the possible role of MF enhancement of WNV transmission. We then used our findings, together with reported, regional-wide prevalences of MF infections in common grackles, to compare vectorial capacities whereby we either invoked or did not invoke the scenario of MF enhancement into our calculations. In this way, we quantified the theoretical potential that MF enhancement, at least in common grackles, could have had in facilitating the rapid spread of WNV across the mid-western United States.
Materials and Methods
Mosquitoes
To determine the circadian habits of host-seeking mosquitoes, a Mosquito Magnet trap was operated in a residential neighborhood in south Grand Forks, ND (47° 52′36″ N, 97° 01′51″ W) from 11 July through 24 July 2005. Collections were made at sunrise (5–6:30 a.m.) and at sunset (9–10:00 p.m.) over nine 24 h cycles. Mosquitoes were sorted by species and counted. To investigate the feeding preferences of local mosquitoes, engorged mosquitoes were collected from natural resting places (e.g., inside barns, culverts, etc.) and from Mosquito Magnet traps placed in and around Grand Forks, ND, and in Steele Co., ND. Engorged mosquitoes were identified to species, abdomens were removed, and DNA was extracted with guanidine thiocyanate (Tkach and Pawlowski 1999). Most of the mosquito species used in feeding trials (see below) were wild mosquitoes collected in eastern North Dakota using Mosquito Magnet traps. The exception was a recently colonized strain of Culex pipiens (L.) that originated from Larimer Co., CO (kind gift of K. Kobylinski and B. Foy, Colorado State University).
Birds and MF Parasites
Songbirds were collected in eastern North Dakota and western Minnesota under the authority of U.S. Fish & Wildlife scientific collection permit (MB072162), state collecting permits (North Dakota GNF02136294 and Minnesota 13083), and University of North Dakota IACUC protocol (0605–1). Because many MF species of songbirds are nocturnally periodic (Anderson 2000), two methods were used to examine birds for MF. For general surveys on MF prevalence and serology, birds were collected by gunshot and necropsied. Lungs were dabbed/smeared onto a microscope slide and the resultant “lung blood” was examined (40–100×) for live MF. Additional blood was collected in capillary tubes or on filter paper for detection of WNV antibodies (see below). For feeding trials requiring live birds, birds were captured by baited live-trap or by mist net and transported to the Biology Department where they were maintained in outdoor aviaries. At night, blood was collected from the brachial vein in heparinized capillary tubes. Tubes were centrifuged and motile MF were easily visualized (100×) at the interface of plasma and cell pack (Collins 1971). To determine the pattern of MF periodicity, seven grackles displaying varying intensities of MF infections were bled from the wing vein (≈60 μl) every 26 h, beginning at 8:00 p.m. and ending at 6:00 a.m. This regime allowed birds to recuperate between bleeds. Blood was immediately expelled into a tube containing 10 μl of liquid K3EDTA and thoroughly mixed to prevent clotting. Total volumes were measured and the contents were then placed on a clean microscope slide, covered with a glass coverslip and the total number of MF were counted. All counts were expressed as MF per 20 μl.
Immunoassays
Bird sera and blood were assayed for the presence of antibodies against WNV using the methods of Blitvich et al. (2003). If blood was collected by capillary tube, the tubes were centrifuged and the sera stored at –80°C until assayed. If blood was collected on filter paper, a small punch was taken and blood eluted into ≈250 μl phosphate buffered saline (PBS). Standard flat-bottomed 96-well plates (Costar, Fisher, Pittsburg, PA) were incubated overnight at 4°C with 100 μl WNV antigen and diluted in casein blocking buffer (Wirtz et al. 1993). Plates were emptied and 200μl of blocking buffer was added to each well. Plates were incubated for 1 h at 37°C, then washed four times with PBS + 0.1% Tween 20. Test samples (50μl diluted 1:10 in casein blocking buffer) were added to appropriate wells and incubated at 37°C for 2 h. Plates were washed four times and 50μl of dilute anti-WNV monoclonal antibodies (Mab 3.1112G = 1:2,000, Mab 6B6C-1 = 1:9,000) were added to appropriate wells and incubated for 37°C for 1 h. Plate were washed four times and 50 μl of dilute (1:2,000) horseradish peroxidase (HPR)-labeled rabbit antimouse IgG (KPL, Gaithersburg, MD) were added to appropriate wells and incubated for 37°C for 1 h. Plates were washed four times and 75 μl of ABTS substrate (KPL) were added to appropriate wells. Optical densities (OD) were read at regular intervals until the average OD for the control serum exceeded 0.3 (usually 10–20 min). The percentage of inhibition value of the test serum was calculated as follows: % inhibition = 100 – [100*(TS-B/CS-B)]; where TS = OD of test serum, CS = OD of control serum, and B = background OD. Samples displaying a percent inhibition of 45% or more were interpreted as positive for anti-WNV antibodies.
Mosquito Bloodmeal Identification
The DNA samples extracted from bloodmeals of field-collected mosquitoes were coded and stored at −80°C until analyzed. Vertebrate cytochrome B gene sequences present in the extracted DNA were amplified by polymerase chain reaction (PCR) using vertebrate specific primers, as previously described (Hassan et al. 2003). The resulting amplicons were sequenced and aligned with published sequences to identify the vertebrate species composition of the bloodmeals.
MF Penetration of Mosquito Midguts
Feeding trials were conducted with different species of MF parasitizing the common grackle, American robin, and darkeyed junco. Microfilaremic blood was administered to mosquitoes using either membrane feeders or live birds. For membrane feeding, blood from nonmicrofilaremic grackles were collected into citrated saline, centrifuged, and the erythrocytes were stored in sterile Alsevere's solution. Microfilaremic birds were euthanized and the lungs were removed, minced, suspended in buffered saline, vortexed, filtered through ultra-fine nylon mesh, and centrifuged. The MF densities of lung filtrates were determined by counting the MF in a small (≈10 μl) aliquot. Measured amounts of MF were then added to a 1:1 mixture of uninfected grackle erythrocytes and domestic goose sera. Two dilutions were prepared; one that approximated the normal MF density found in infected birds and one that was ≈10-fold higher than a normal microfilaremia. Microfilaremic blood (≈2 ml) was placed in water-jacketed membrane feeders maintained at 38°C via a circulating water bath and fitted with de-salted sausage casing (=pig intestine). Feeders were placed on the screen tops of cages containing mosquitoes for ≈1 h in darkness. For live bird feedings, microfilaremic birds were anesthetized with a mixture of ketamine (20 mg/kg intramuscular [IM]) and xylazine (4 mg/kg IM) injected with a 27 gauge needle into the pectoral muscles. Our preliminary studies indicated that ketamine-xylazine anesthesia did not alter the nocturnal periodicity of grackle MF, as has been reported for Brugia malayi (Brug) MF in gerbils (Beerntsen et al. 1996). Birds were anesthetized at night (≈2:00 a.m.) and placed on their backs inside a screen cage containing mosquitoes. Mosquitoes were allowed to feed in complete darkness for ≈1 h after which the bird was removed and a blood sample taken from the wing vein to obtain an estimate of microfilaremia at the time of mosquito feeding. Most mosquitoes fed on the face and feet of anesthetized birds. Mosquitoes were maintained at 24°C until morning (≈4 h after feeding), whereupon engorged mosquitoes were sorted by species and dissected. Replete midguts were carefully excised, opened, and the contents were placed on a slide with coverslip and examined for MF. Likewise, eviscerated carcasses were carefully teased apart, compressed with a coverslip and examined microscopically for MF. MF found in mosquito carcasses were interpreted as having penetrated the midgut. The numbers of MF in a bloodmeal and in the corresponding carcass represented the total number of MF ingested by a mosquito.
Vectorial Capacity
To assess the magnitude by which MF enhancement could theoretically have contributed to the rapid spread of WNV across the midwestern United States, we calculated vectorial capacities for Cx. pipiens and Cx. tarsalis mosquitoes feeding on common grackle, whereby MF enhancement was either invoked (VCYES) or not invoked (VCNO). Vectorial capacity (VC) is defined as the daily rate at which infective mosquito bites arise from a single infective host within an otherwise uninfected population (MacDonald 1952). It can be expressed by the formula
where:
ma = mosquito biting rate, expressed as number of mosquito bites per bird per night.
a = “avian host preference” or the probability of a mosquito feeding on a bird during 1 d. This can be calculated by dividing the bird blood index (i.e., proportion of the vector population feeding on birds) divided by the daily biting frequency of a mosquito (i.e., the gonotrophic period).
b = infectiousness of birds to the vectors (=vector competence).
P = daily probability of survival for the vector.
T = extrinsic incubation period of the virus within the vector.
Data from the literature, as well as results reported here, provided the parameter estimates used to calculate vectorial capacities (Tables 1 and 2). Certain parameters were held constant. For example, the intensity and selective behavior of mosquito feeding on birds (i.e., `ma' and `a') were held constant because we know of no empirical studies or compelling reason to suggest that these parameters would be affected by the MF status of viremic birds. In addition, we showed that daily mosquito survival (`P') was unaffected by mosquito ingestion of Ch. quiscali MF (see below). However, co-ingestion of virus and MF has been shown experimentally to alter both vector competence (b) and extrinsic incubation period (T). Thus, these were the two parameters that differed between calculations for VCYES and VCNO. For VCNO, estimates of `b' were based on transmission rates of mosquitoes that were orally exposed to WNV whereas for VCYES, estimates of `b' were based on transmission rates of mosquitoes that were intrathoracically inoculated and/or had disseminated infections of WNV. The VCYES, estimates of `T' were calculated as 42.4% reductions in the normal T reported in the literature, as determined experimentally for the dengue virus/Brugia MF/Aedes aegypti model system by Vaughan et al. (2009).
Table 1.
Parameter estimates used in calculations of vectorial capacity for WNV in Culex pipiens mosquitoes feeding on common grackles whereby MF enhancement is invoked (VCYES) or not invoked (VCNO)
| Scenario | Biting rate (ma)a | Avian host preference (a)b | Vector competence (b)c | Daily Survival (P)d | EIP (T)e |
|---|---|---|---|---|---|
| VCYES | 1.53 | 0.186 | 0.87 | 0.90 | 5 d |
| VCNO | 1.53 | 0.186 | 0.17 | 0.90 | 12 d |
Biting rate (no. bites/bird/night)—based on bird-baited mosquito traps (Darbro and Harrington 2006).
Avian host preference—based on bird blood index of 0.93 (Molaei et al. 2006) divided by gonotrophic value of 5 d (Reisen et al. 1992).
Vector competence—based on experimental transmission rates of an unpassed isolate of WNV (Crow 397–99 strain) obtained from the brain of a crow that died in the epicenter (i.e., New York, NY) during the initial year of the WNV outbreak (i.e., 1999) and using Cx. pipiens reared from larvae collected 20 km away from the epicenter. Values for vector competence represent viral transmission rates of mosquitoes that were either orally exposed to WNV (VCNO; i.e., 31/178) or were intrathoracially inoculated and/or had disseminated infections (VCYES; i.e., 21/24) (data are combined from Turell et al. 2000, Turell et al. 2001).
Daily survival—based on mark-release-recapture with Cx. pipiens in Washington, DC (Jones et al. 2012). Mortality of Cx. pipiens because of ingestion of Ch. quiscali MF was assumed to be negligible (≈4%; see text).
Extrinsic incubation period (EIP)—the `normal' EIP (i.e., VCNO) of 12 d is based on orally exposed Cx. pipiens held at 26°C where dissemination rate exceeded 15% (Dohm et al. 2002, Anderson et al. 2008). The MF-enhanced EIP (i.e., VCYES) of 5 d was calculated as 42.4% of normal EIP, based on the avg reductions of EIP observed with dengue 1 virus development within Ae. aegypti fed blood that was either dually infected (virus + Brugia malayi MF) or singly infected (virus only) (Vaughan et al. 2009).
Table 2.
Parameter estimates used in calculations of vectorial capacity for WNV in Culex tarsalis mosquitoes feeding on common grackles whereby MF enhancement is invoked (VCYES) or not invoked (VCNO)
| Scenario | Biting rate (ma)a | Avian host preference (a)b | Vector competence (b)c | Daily survival (P)d | EIP (T)e |
|---|---|---|---|---|---|
| VCYES | 14.1 | 0.176 | 1.00 | 0.80 | 5 d |
| VCNO | 14.1 | 0.176 | 0.82 | 0.80 | 12 d |
Biting rate (no. bites/bird/night)—based on the avg no. of engorged Culex tarsalis captured over 25 trap-nights by net traps baited with four species of wild birds (Anderson and Brust 1995, 1997; Lura et al. 2012).
Avian host preference—based a bird blood index of 0.88 during the summer months in California (Thiemann et al. 2011) divided by a gonotrophic value of 5 d (McHugh 1990).
Vector competence—based on experimental transmission rates of WNV (Crow 397–99 strain) for orally exposed Cx. tarsalis (VCNO; i.e., 58/71) and for disseminated infections of Cx. tarsalis (VCYES; i.e., 6/6) (Turell et al. 2002).
Daily survival—based on parity rates of wild-caught Cx. tarsalis in Imperial and Coachella Valleys, CA, during the summer months (Reisen et al. 1995).
Extrinsic incubation period (EIP)—the `normal' EIP (i.e., VCNO) of 12 d is based on orally exposed Cx. tarsalis held at 26°C where dissemination rate exceeded 50% (Anderson et al. 2012). The MF-enhanced EIP (i.e., VCYES) of 5 d was calculated as 42.4% of normal EIP, based on the avg reductions of EIP observed with dengue 1 virus development within Ae. aegypti fed blood that was either dually infected (virus + Brugia malayi MF) or singly infected (virus only) (Vaughan et al. 2009).
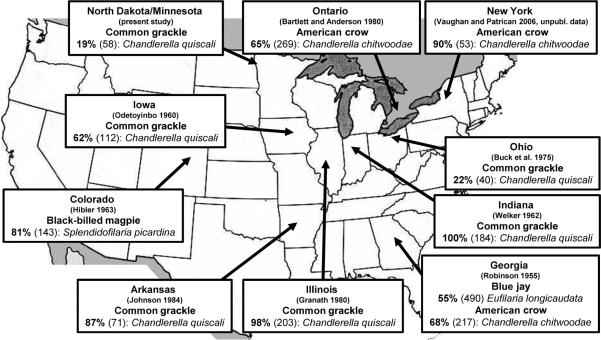
Because the point of this exercise was to obtain a quantitative estimate on how MF enhancement could affect the spread of WNV within naïve host populations, parameter values for vector competence, and extrinsic incubation periods were based on studies using the original progenitor strain of WNV in North America, NY99. The common grackle was identified as the most appropriate avian species for these calculations because 1) grackles are synanthropic and very common throughout the Midwest, 2) grackles produce high viremias when infected with WNV (Komar et al. 2003), 3) grackles are known to be fed on by Culex mosquitoes (Savage et al. 2007), and 4) unlike most other passerine reservoirs of WNV (e.g., American robin, house sparrow, etc.), there are several robust estimates of MF prevalence for grackles that span a large geographic area (see Fig. 1).
Fig. 1.
Prevalence (number birds examined) of microfilarial infections in North American populations of American crow, blue jay, black-billed magpie, and common grackle. Parasite species are in italics and references are in parentheses.
Once a value for VCYES was obtained, the value was further adjusted to reflect a realistic estimate of the proportion of mosquitoes that might be expected to experience midgut penetration in nature. This reflects the fact that not all hosts in a population are microfilaremic and not all mosquitoes feeding on microfilaremic hosts may have their midguts penetrated by ingested MF. For example, if 50% of the hosts in a particular area were microfilaremic and 30% of the mosquitoes that ingested MF had their midguts penetrated, then the scaling factor would be 0.5 × 0.3 or 0.15. In such a scenario, only 15% of the mosquitoes feeding on grackles would be involved in MF enhancement (VCYES) whereas the remaining 85% would not (VCNO). Thus:
These calculations were performed for each different region in the United States where estimates of MF prevalence exist (Fig. 1). The data for MF penetration were obtained during this study (see below).
Results
Passerine MF
In total, 144 songbirds were examined in 2005, comprised mostly of common grackle (n = 58), American robin (n = 26), and red-winged blackbird (Agelaius phoeniceus L.) (n = 26). Five of the 10 bird species examined had MF infections including; common grackle (11/58), American robin (4/26), brown-headed cowbird (Molothrus ater Boddaert) (1/6), blue jay (Cyanocitta cristata L.) (1/3), and eastern kingbird (1/3). Three types of MF were observed. Common grackle harbored sheathed MF with blunt tails and moved in an undulating, snake-like fashion (Fig. 2a). Adult worms were recovered from the ventricles of the brains of microfilaremic grackles and identified morphologically as Chandlerella quiscali (von Linstow), the filarioid most commonly found in common grackle (Anderson 2000). The four microfilaremic robins and the cowbird, blue jay, and kingbird harbored smaller (≈100 μm), unsheathed MF with pointed tails that moved in an erratic, often jerky fashion (Fig. 2b). Six additional robins collected in 2011 and 2012 harbored a third MF type that were long (>250 μm), unsheathed, with tapering pointed tails and moved in coiling/uncoiling fashion. The small MF were morphologically identical to taxonomic descriptions given for MF of the genus Eufilaria Seurat (Anderson 2000, Bartlett and Anderson 1980, Bartlett 2008) and the large MF were morphologically identical to taxonomic descriptions given for MF in the genus Cardiofilaria Strom (Bartlett 2008). Efforts to identify these filarioids to species by recovering adult worms from microfilaremic birds were unsuccessful. Two grackles and one of the robins each harbored more than one type of MF, indicative of multiple infections.
Fig. 2.
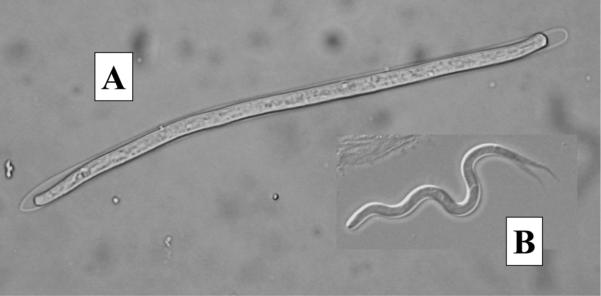
(A) Sheathed Chanderella quiscali microfilaria (150 μm long) from common grackle; (B) Unsheathed Eufilaria sp. microfilaria (100 μm long) from American robin.
Dual Exposure of Birds to MF and WNV
All bird species examined had antibody to WNV. Seroprevalences ranged from 17% in brown-headed cowbird (n = 6) to 100% in eastern kingbird (n = 3). Evidence of dual exposure (i.e., birds having both MF and antibodies to WNV) was detected in common grackle, American robin, and eastern kingbird (Table 3). The prevalence of dual exposure in these bird species did not differ from what would be predicted based on the combined prevalences of MF and sero-positivity (Fisher exact tests; P > 0.05). Grackles and robins are common songbirds in Grand Forks, ND, during the summer (J.A. Vaughan, personal observation) and both produce high viremias when infected with WNV (Komar et al. 2003), making them important local amplifying hosts for WNV. A significantly higher percentage of grackles had antibodies to WNV (67%) than did robins (38%) (Fisher's exact test; P < 0.03), suggesting that local grackles had been more frequently exposed to the bites of infected vectors than were robins.
Table 3.
Prevalence of common grackles, American robins, and eastern kingbirds having microfilarial infections, antibodies to West Nile virus (WNV), and concurrently microfilaremic and sero-positive to WNV (=dually exposed)
| Species | Microfilaremic (%) | WNV seropositive (%) | Dually exposed (%) |
|---|---|---|---|
| Common grackle | 11/58 (19%) | 36/54 (67%) | 9/54 (17%) |
| American robin | 4/26 (15%) | 10/26 (33%) | 2/26 (8%) |
| Eastern kingbird | 1/3 (33%) | 3/3 (100%) | 1/3 (33%) |
Red River Valley; northeast North Dakota and northwest Minnesota, summer 2005.
Nocturnal Periodicity of Grackle MF (Chandlerella quiscali)
Peak microfilaremias among grackles varied by as much as 45-fold, ranging from four MF (Bird 307) to 185 MF (Bird 302) per 20 μl blood (Fig. 3). Even so, the patterns of periodicity were similar. Microfilaremias were nil at 8:00 p.m., but rose steadily throughout the night, reaching a peak at 2:00 to 4:00 a.m. Microfilaremias rapidly subsided to almost nothing by sunrise (6:00 a.m.) (Fig. 3). These patterns were similar to those reported previously for Ch. quiscali MF (Odetoyinbo 1960). Thus, nocturnal periodicity of Ch. quiscali MF limits the phenomenon of MF enhancement in common grackle to those vector species that feed at night.
Fig. 3.
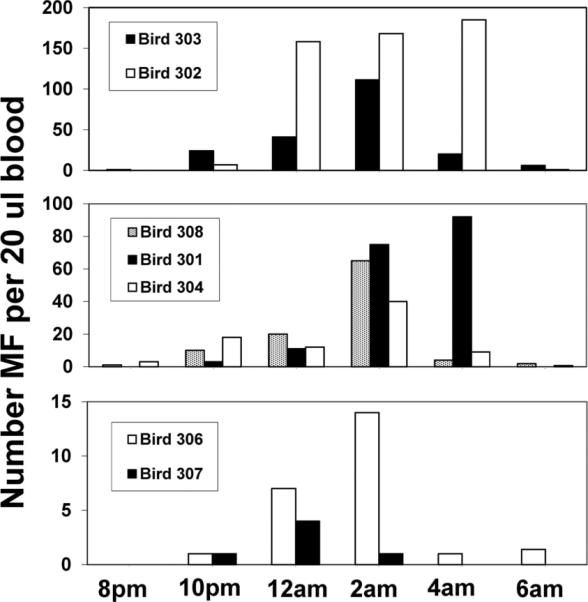
Nocturnal periodicity of Chandlerella quiscali MF in common grackles.
Likelihood of Mosquitoes Feeding on Birds at Night
The main mosquito species collected in Mosquito Magnets were Aedes vexans (Meigen), Ae. dorsalis (Meigen), and Cx. tarsalis Coquillett. Mean abundances of host-seeking Cx. tarsalis were significantly higher at night than during the day (paired t-test on log10 transformed data; t = 5.3; df = 8; P = 0.0007), indicating that Cx. tarsalis is primarily a “night-biting” species (Table 4). There were no significant differences between the mean numbers of host-seeking Ae. vexans or A. dorsalis caught during the day versus the night (paired t-tests; P > 0.10), indicating that these floodwater species hunt both day and night. Sixty-eight of 79 (86%) bloodmeals extracted from field-collected mosquitoes provided sufficient quality of host DNA to make accurate determinations (Table 5). Although most Ae. vexans (68%) collected had fed on mammals, a substantial proportion (26%) had fed on common grackle and one had fed on American Robin. Although sample sizes were small, it was clear that other Aedes species and Cx. pipiens also fed on common grackle. The combined results of MF periodicity studies (Fig. 3), day versus night sampling (Table 4), and bloodmeal analysis (Table 5) indicate that local mosquitoes, even mammophilic floodwater species such as Ae. vexans, and Ae. dorsalis, fed on grackles (and possibly robins) and hunted at night when MF are circulating in peripheral blood of the birds.
Table 4.
Geometric mean (95% CL) of host-seeking mosquitoes collected in a Mosquito Magnet trap over nine 24 h sampling intervals
| Mosquito species | Sunrise to sunset (day) | Sunset to sunrise (night) |
|---|---|---|
| Aedes vexans | 9 (4, 22) | 12 (4, 33) |
| Aedes dorsalis | 7 (4, 14) | 13 (5, 32) |
| Culex tarsalis | 4 (1, 10) | 67 (29, 156) |
Collections were taken at sunrise and at sunset. Grand Forks, ND, July, 2005.
Table 5.
Number of bloodmeals identified to host species for engorged mosquitoes collected in Grand Forks County, ND, summer 2005 and Steele County, ND, summer 2010 (Culex pipiens)
| Host species | Aedes vexans | Aedes dorsalis | Aedes triseriatus | Culiseta inornata | Culex tarsalis | Culex pipiens | Total |
|---|---|---|---|---|---|---|---|
| Common grackle | 10 | 2 | 1 | 3 | 16 | ||
| American robin | 1 | 1 | |||||
| Chipping sparrow | 1 | 1 | |||||
| Pigeon | 1 | 5 | 6 | ||||
| Chicken | 1 | 1 | 2 | 4 | |||
| Deer | 21 | 5 | 1 | 27 | |||
| Cow | 3 | 1 | 1 | 5 | |||
| Human | 2 | 1 | 1 | 3 | 1 | 8 | |
| Total | 38 | 4 | 2 | 11 | 10 | 3 | 68 |
MF Penetration of Mosquito Midguts
All five of the wild-caught mosquito species tested were susceptible to midgut penetration by co-indigenous Ch. quiscali MF (Table 6). However, the prevalence of penetration differed among mosquito species, densities of MF ingested, and in some cases, between the two feeding techniques. Ae. dorsalis and Ae. vexans experienced higher rates of midgut penetration when Ch. quiscali MF were ingested from a membrane feeder than from a live bird. Perhaps the lack of blood coagulation in the membrane feeders facilitated MF motility and penetration through the midgut whereas normal coagulation within bloodmeals taken from a live host hindered MF motility and penetration. Whatever the reason, the different outcomes suggest that live bird feedings should be the method of choice to assess MF penetration in nature. When fed on a live bird, the rates of MF penetration in Ae. vexans, Ae. dorsalis, Ae. flavescens (Muller), and Cx. tarsalis were all <20%.
Table 6.
Penetration and movement across the mosquito midgut by Chandlerella quiscali microfilariae (MF) when ingested by co-indigenous, wild-caught mosquitoes
| Mosquito species | Type of feeding | MF ingested per mosquito (range) | MF penetrating midgut (range) | Prevalence of MF penetration (n) |
|---|---|---|---|---|
| Aedes vexans | Membrane-high density | 3211 (420–48,880) | 6 (1–176) | 42% (40) |
| Membrane-low density | 24 (1–350) | 2 (1–4) | 9% (34) | |
| Live bird | 16 (6–68) | 0 | 0% (14) | |
| Aedes dorsalis | Membrane-high density | 1073 (396–5205) | 65 (3–670) | 67% (12) |
| Membrane-low density | 52 (1–292) | 5 (1–113) | 43% (14) | |
| Live bird | 26 (3–88) | 1 (1–2) | 16% (25) | |
| Aedes fiavescens | Live bird | 70 (22–136) | 2 | 17% (6) |
| Aedes triseriatits | Membrane-high density | 1406 (607–4380) | 4 (1–7) | 50% (4) |
| Membrane-low density | 142 (48–375) | 1 | 17% (6) | |
| Culex tarsalis | Membrane-high density | 2060 (500–12,125) | 3 (1–9) | 15% (52) |
| Membrane-low density | 145 (1–432) | 1 (1–3) | 12% (33) | |
| Live bird | 21 (1–156) | 1 | 12% (40) |
Mosquitoes were either fed MF harvested from bird lungs, mixed with bird blood, and placed into prewarmed membrane feeders (=membrane) or were allowed to feed directly on anesthetized microfilaremic birds at night (=live bird).
Because of the importance of Cx. pipiens as a major vector of WNV in the eastern United States, additional feeding trials were conducted with a recently colonized strain of Cx. pipiens, comparing the abilities of three different genera of passerine MF to penetrate Cx. pipiens midguts (Table 7). When mosquitoes fed on microfilaremic birds at night, the proportion of midguts penetrated by Chandlerella (37%) and Eufilaria (60%) MF did not differ significantly from one another (χ2 = 2.55; P = 0.11). In contrast, when Cx. pipiens fed on a microfilaremic robin harboring Car diofilaria MF, none of the ingested MF penetrated the midguts. This may have been a function of the extremely low intensity of Cardiofilaria microfilaremia (2 MF per 20 μl) within the robin. Microfilaremias within the Chandlerella-infected grackle (693 MF per 20 μl) and the Eufilaria-infected junco (938 MF per 20 μl) were much higher. Indeed when data for all three MF species were pooled, there was a significant relationship between densities of MF ingested and numbers penetrating the midgut (F = 13.00; df = 1, 82; P = 0.0005). Thus, the intensity of a host's microfilaremia may be important in determining its relative potential to contribute to MF enhancement. Nevertheless, these results demonstrated that at least two species of passerine MF possess the ability to penetrate the midgut of Cx. pipiens when ingested from a live host. The grackle MF, Ch. quiscali, can penetrate the midgut of Cx. tarsalis, albeit to a lesser extent.
Table 7.
Penetration and movement across the mosquito midgut by three different genera of passerine microfilariae (MF) when ingested by laboratory-reared Culex pipiens mosquitoes
| MF species | Bird host | Type of feeding | MF ingested per mosquito (range) | MF penetrating midgut (range) | Prevalence of MF penetration (n) |
|---|---|---|---|---|---|
| Chandlerella quiscali | Grackle | Membrane high density | 1028 (587–1487) | 4 (1–13) | 100% (9) |
| Membrane-low density | 37 (17–62) | 1 (1–2) | 25% (16) | ||
| Live bird | 29 (1–347) | 2 (1–12) | 37% (49) | ||
| Cardiofilaria spp. | Robin | Membrane-low density | 5 (2–9) | 1 | 11% (9) |
| Live bird | 3 (1–5) | 0 | 0% (19) | ||
| Eufilaria spp. | Junco | Live bird | 71 (48–125) | 1 (1–2) | 60% (15) |
Mosquitoes were either fed MF harvested from bird lungs, mixed with bird blood, and placed into prewarmed membrane feeders (=membrane) or were allowed to feed directly on anesthetized microfilaremic birds at night (=live bird).
Effect of Ch. quiscali MF on Cx. pipiens Mortality
To determine if Ch. quiscali MF penetration of the midgut causes excessive mortality in Cx. pipiens, survivorship of the same cohort of mosquitoes used in the midgut penetration studies described above was monitored for 5 d after blood feeding. Of 354 engorged Cx. pipiens mosquitoes, only 15 (4%) died, all within 32 h after feeding. The remaining mosquitoes survived and oviposited normally. Fourteen of the 15 dead mosquitoes were “red mosquitoes,” indicating that midgut integrity had been disrupted and that blood had leaked into the hemocoel. The dead mosquitoes were carefully dissected and found to contain significantly more MF in their hemocoels (geometric mean = 15; range = 3–71) than their seemingly healthy counterparts dissected the day before (geometric mean = 2; range = 1–12) (Wilcoxon Rank Sum Test; P < 0.001). These results indicate that ingested Ch. quiscali MF had negligible effect on the overall mortality of Cx. pipiens but in those few cases where mortality did occur, the amount of host blood leaking into the hemocoel was so great that it was plainly visible and probably toxic to the mosquito.
Vectorial Capacity
The five parameters necessary to calculate vectorial capacity were available from literature sources for Cx. pipiens and Cx. tarsalis (Tables 1 and 2). For each mosquito species, separate calculations were performed for each of six regions in the mid-west United States where estimates on Ch. quiscali MF prevalence in common grackles were available (Fig. 1). For both Cx. pipiens and Cx. tarsalis, the inclusion of potential MF effects yielded significantly higher values of vectorial capacity than when MF effects were excluded from the calculations (one-sample t-tests; P values <0.008) (Table 8). When MF effects were included, the average vectorial capacity for Cx. pipiens (VCYES = 0.43 ± 0.17) increased over three time that of the baseline vectorial capacity value (VCNO = 0.13). For Cx. tarsalis, the difference between the average VCYES value and the VCNO value was not as divergent (i.e., 1.4-fold difference). One reason for this relates to the lower efficiency of Ch. quiscali MF in penetrating the midguts of Cx. tarsalis (i.e., 12%; Table 6) compared with that in Cx. pipiens (i.e., 37%; Table 7). In addition, the baseline vector competence of Cx. tarsalis for the NY-99 strain of WNV is already so high (VCNO; b = 0.82) that MF have little net effect on increasing vector competence (VCYES; b = 1.00). Thus, the effects of MF enhancement in Cx. tarsalis (at least as pertains to Ch. quiscali MF and grackles) would seem to be confined to decreasing the extrinsic incubation period in a small proportion of mosquitoes. However, the theoretical effects of MF enhancement in Cx. pipiens are much greater and may help explain why WNV was able to spread so rapidly across the eastern half of North America where Cx. pipiens is the predominate vector.
Table 8.
Theoretical vectorial capacity values computed tor Culex pipiens and Cx. tarsalis mosquitoes feeding on West Nile virus-infected common grackles whereby the potential effect of concurrent Chandlerella quiscali microfilaremias (MF) on increasing the vector competence and simultaneously reducing the extrinsic incubation of the virus has either been invoked (VCYES) or has been ignored (VCNO) in the calculations
| State | Reported MF |
Culex pipiens
|
Culex tarsalis
|
||
|---|---|---|---|---|---|
| prevalence (n) | VCYES | VCNO | VCYES | VCNO | |
| Ohio | 22% (40) | 0.23 | 0.13 | 0.71 | 0.63 |
| Indiana | 100% (184) | 0.59 | 0.13 | 0.99 | 0.63 |
| Illinois | 98% (203) | 0.58 | 0.13 | 0.98 | 0.63 |
| Iowa | 62% (112) | 0.42 | 0.13 | 0.85 | 0.63 |
| Arkansas | 87% (71) | 0.54 | 0.13 | 0.95 | 0.63 |
| North Dakota | 19% (58) | 0.22 | 0.13 | 0.70 | 0.63 |
| Average (±SD) | 80% (668) | 0.43 ± 0.17 | 0.13 | 0.86 ± 0.13 | 0.63 |
Discussion
Laboratory studies have demonstrated that MF enhancement of arboviral transmission can increase the susceptibility of marginally susceptible mosquito species to infection with arboviruses, and at the same time, can accelerate the infectious process within refractory and fully susceptible mosquitoes alike. Central to the concept's validity is the existence in nature of vertebrates that are infected concurrently with MF and arbovirus during the time when vectors are feeding. Throughout the world, examples of multiple parasitism are common (Petney and Andrews 1998) and there is enormous geographic overlap between many arboviral and filarioid infections. Filarioid infections are chronic, often life-long infections. Once individuals within a vertebrate population become microfi-laremic, they have the potential to serve as dually infected hosts should they later acquire arboviral infections.
This is exactly the scenario that played out for many songbird populations when WNV expanded outward from its point of introduction in New York City during the early to mid-2000s. Parasitological surveys of American crow, blue jay, black-billed magpie, and common grackle conducted before the arrival of WNV indicate that there were extremely high prevalences of pre-existing MF infection among these WNV-susceptible species over a large expanse of North America (Fig. 1). In areas where nonimmune bird populations had MF prevalences approaching 100% (e.g., Indiana, Illinois), it is probable that dually infected birds constituted a major part of the infectious reservoir for WNV for those bird species.
However, the existence of dual infections by itself does not satisfy all the requirements needed for MF enhancement to occur. An important aim of this study was to determine the degree of interaction occurring between mosquitoes and the MF of songbirds in nature. We found that the common grackle and American robin were naturally exposed to both MF and WNV infections (Table 3) and that local mosquitoes fed on these bird species (Table 5) and fed during the night (Table 4) when nocturnally periodic MF were present in the peripheral blood (Fig. 3). Importantly, we observed that mosquito ingestion of two taxonomically distant genera of passerine MF (i.e., Chandler-ella [subfamily: Splendidofilariinae] and Eufilaria [subfamily: Lemdaninae]) resulted in penetration of mosquito midguts (Tables 6 and 7); even though mosquitoes are not the vectors for Chandlerella or Eufilaria and do not support their development. These findings underscore the notion that MF species not normally associated with mosquitoes can, nevertheless, penetrate the midguts of mosquitoes. This has been observed in other MF/mosquito species combinations. For example, Santos et al. (2006) showed that Litomosoides chagasfilhoi de Moraes Neto, Lanfredi and de Souza, a mite-borne filarioid of neotropical rodents, penetrated and disrupted the midgut of Cx. quinquefasciatus Say mosquitoes fed on microfilaremic rodents. Similarly, Hibler (1963) exposed two black-billed magpies (Pica pica L.), each with a quadruple infection of Splendidofilaria picacardina Hibler, S. caperata Hibler, Ch. striatospicula Hibler, and E. longicaudata Hibler MF, to a total of 2,000 co-indigenous Cx. tarsalis mosquitoes during peak MF periodicity (=midnight). He reported that; “no larval development occurred even though each mosquito obtained an average of 100 MF, many of which penetrated the midgut. Living MF were found in the abdomen and thorax up to 12 h after ingestion, after which all of the MF were dead.” These filarioid species only complete their development in Culicoides (Hibler 1963). Likewise, Vaughan et al. (2007) reported that Mansonella ozzardi (Manson), a Culiciodes-borne filarioid of humans, readily penetrated the midgut of a co-indigenous strain of Ae. aegypti (L.) mosquitoes fed directly on microfilaremic people. Thus, a mosquito species does not have to be a competent vector for the filarioid parasite to have a potential role in MF enhancement (Vaughan et al. 1999). The MF only have to penetrate the gut. Subsequent development is irrelevant.
Another requirement is that MF penetration of the midgut must introduce enough virus into the hemocoel to establish a disseminated infection. The appearance of dead and dying red Cx. pipiens mosquitoes produced by a high intensity of Ch. quiscali MF penetration (mean = 15 MF) clearly indicates that copious amounts of bloodmeal leakage can occur as the result of Ch. quiscali MF passage through the midgut. However, in these trials such a high intensity of MF penetration was rare and the more Ôtypical' intensity of MF penetration averaged only two MF per midgut (Table 7). Although this might seem low, it is comparable to penetration intensities reported in two previous trials (means ranged from 1 to 3 MF) wherein three Aedes spp. mosquitoes were fed on gerbils concurrently infected with Brugia spp. MF and different arboviruses (Vaughan and Turell 1996, Vaughan et al. 1999). In those trials, midgut penetration by even one or two Brugia MF was enough to produce significantly higher rates of disseminated infections in two of the three Aedes mosquitoes than when mosquitoes fed on singly infected gerbils with equivalent viremias but no microfilaremias. This suggests that if Cx. pipiens fed on a dually infected grackle (i.e., microfilaremic and viremic), one or two penetrating Ch. quiscali MF could potentially introduce enough co-ingested WNV into the hemocoel to result in disseminated infection and cause MF enhancement. Assuming that MF-induced introduction of virus into the hemocoel would reduce the EIP of WNV in Cx. pipiens by over half, as it does in the dengue virus/B. malayi MF/Ae. aegypti system (Turell et al. 1987, Vaughan et al. 2009), then the effect on viral amplification could be considerable. In our calculations, the theoretical vectorial capacity for WNV in Cx. pipiens feeding on grackle populations was over three times higher than would be expected if calculations were based on vector competence and EIP values derived from clean, nonmicrofilaremic grackles.
In this report, we identified a naturally occurring system where there appears to be sufficient interaction among the vertebrate (common grackle), parasite (Ch. quiscali MF), vector (Culex pipiens), and virus (WNV) to warrant further studies on MF enhancement. There may be many other naturally occurring systems in which MF enhancement could influence arboviral transmission. Indeed, the very first experimental model of MF enhancement (Mellor and Bore-ham 1980) alluded to a naturally occurring system that is both economically important and easily studied, that is, bluetongue virus in cattle. Over the past decade, Europe has experienced multiple invasions of different serotypes of bluetongue virus, all vectored by Culicoides midges. Throughout Europe, Culicoides (and notably, black flies) also transmit bovine onchocerciasis. The prevalence of Onchocerca spp. MF in the dermis of European cattle are substantial, that is, Austria (131/345; Safar-Hermann and Supperer 1983) Germany (175/438; Dohnal et al. 1990), Wales (130/463; Trees et al. 1987), and Finland (77/209; Solismaa et al. 2008). If bovine onchocerciasis were shown experimentally to accelerate the EIP of bluetongue virus in the midge vector, then antihelminthic treatment of cattle herds may prove to be an effective supplement to traditional bluetongue management strategies (i.e., vector control).
Studies of MF enhancement in natural systems may lead to the recognition of additional, important amplifying host species. For example, the American robin is considered a major amplifying host of WNV based on the disproportionate amount of Cx. pipiens feeding on this avian species (Kilpatrick et al. 2006, Hamer et al. 2009, Savage et al. 2007, Molaei et al. 2006). However, other bird species not as frequently fed upon but having high prevalence of MF infection (i.e., common grackle), may also be important contributors to viral amplification if vector competence and/or the EIP for WNV is enhanced in mosquitoes that feed on dually infected hosts. Likewise, MF enhancement may produce unanticipated vector species. For example, researchers in Louisiana amplified WNV RNA from three species of ornithophilic Culicoides midges at concentrations comparable to that found in Culex mosquito vectors (Sabio et al. 2006). The ability of Culicoides to transmit WNV remains unknown. However, even if it were found that Culicoides had impenetrable midgut barriers to WNV, it is very likely that their midguts would be penetrated by passerine MF because Culicoides are primary vectors for passerine MF (Anderson 2000). The first priority in defining the role of MF enhancement in this particular system would be to determine whether or not ornithophilic Culicoides possess salivary gland barriers to WNV, that is, conduct transmission studies with intrathoracically inoculated midges.
Without a fuller understanding of the potential role of MF enhancement in naturally occurring systems, cryptic, potentially important amplifying hosts, and vectors may be overlooked and our understanding of enzootic transmission of arboviruses may be incomplete.
Acknowledgments
We thank LeAnne Froese and Amber Basting for assistance in collecting birds and mosquitoes. We also thank Lisa Patrican for providing to us individual sets of lungs from 53 American crows taken in 2006 from upstate New York. This study was supported by grants from the National Institute of Allergy and Infectious Diseases (project numbers R01 AI049477, R03 AI092306 to J.A.V, and R01AI049724 to T.R.U.) and by grant from the University of North Dakota Research Seed Money Committee to J.A.V.
References Cited
- Anderson JF, Main AJ, Cheng G, Ferrandino FJ, Fikrig E. Horizontal and vertical transmission of West Nile virus genotype NY99 by Culex salinarius and genotypes NY99 and WN02 by Culex tarsalis. Am. J. Trop. Med. Hyg. 2012;86:134–139. doi: 10.4269/ajtmh.2012.11-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Brust RA. Field evidence for multiple host contacts during blood feeding by Culex tarsalis, Cx. restuans, and Cx. nigripalpus (Diptera: Culicidae) J. Med. Entomol. 1995;32:705–710. doi: 10.1093/jmedent/32.5.705. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Brust RA. Interrupted blood feeding by Culex (Diptera:Culicidae) in relation to individual host tolerance to mosquito attack. J. Med. Entomol. 1997;34:95–101. doi: 10.1093/jmedent/34.2.95. [DOI] [PubMed] [Google Scholar]
- Anderson RC. Their development and transmission. 2nd ed. CABI Publishing; New York, NY: 2000. Nematode parasites of vertebrates. [Google Scholar]
- Bartlett CM, Anderson RC. Filarioid nematodes (Filarioidea: Onchocercidae) of Corvus brachyrhynchos brachyrhynchos Brehm in southern Ontario, Canada and a consideration of the epizootiology of avian filariasis. Syst. Parasit. 1980;2:77–102. [Google Scholar]
- Bartlett CM. Filarioid nematodes. In: Atkinson CT, Thomas NJ, Hunter DB, editors. Parasitic Diseases of Wild Birds. Blackwell Publishing; Ames, IA: 2008. [Google Scholar]
- Beerntsen BT, C. A., Lowenberger, Klinkhammer JA, Christensen LA, Christensen BM. Influence of anesthetics on the peripheral blood microfilaremia of Brugia malayi in the Mongolian jird, Meriones unguiculatus. J. Parasitol. 1996;82:327–330. [PubMed] [Google Scholar]
- Blitvich BJ, Marlenee NL, Hall RA, Calisher CH, Bowen RA, Roehrig JT, Komar N, Langevin SA, Beaty BJ. Epitope-blocking enzyme-linked immunosorbent assays for the detection of serum antibodies to West Nile virus in multiple avian species. J. Clin. Microbiol. 2003;41:1041–1047. doi: 10.1128/JCM.41.3.1041-1047.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck OD, Cooper CL, Crites JL. Helminth parasites of the Common Grackle (Quiscalus quiscula versicolor) in central Ohio. J. Parasitol. 1975;61:380. [PubMed] [Google Scholar]
- Chamberlain RW, Sudia WD. Mechanism of transmission of viruses by mosquitoes. Annu. Rev. Entomol. 1961;6:371–390. doi: 10.1146/annurev.en.06.010161.002103. [DOI] [PubMed] [Google Scholar]
- Collins JD. The detection of microfilariae using the capillary hematocrit tube methods. Trop. Animal. Hlth. Prod. 1971;3:23–25. doi: 10.1007/BF02356680. [DOI] [PubMed] [Google Scholar]
- Darbro JM, Harrington LC. Bird-baited traps for surveillance of West Nile mosquito vectors: effect of bird species, trap height, and mosquito escape rates. J. Med. Entomol. 2006;43:83–92. doi: 10.1093/jmedent/43.1.83. [DOI] [PubMed] [Google Scholar]
- Dohm DJ, O'Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Dohnal J, Blinn J, Wahl G, Schulz-Key H. Distribution of microfilariae of Onchocerca lienalis and Onchocerca gutturosa in the skin of cattle in Germany and their development in Simulium ornatum and Culicoides nubeculosus following artificial infestation. Vet. Parasitol. 1990;36:325–332. doi: 10.1016/0304-4017(90)90044-c. [DOI] [PubMed] [Google Scholar]
- Granath WO. Fate of the wild avian filarioid nematode Chandlerella quiscali (Onchocercidae: Filarioidae) in the domestic chicken. Poultry Sci. 1980;59:996–1000. doi: 10.3382/ps.0590996. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am. J. Trop. Med. Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
- Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu. Rev. Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- Hassan HK, Cupp EW, Hill GE, Katholi CR, Klingler K, Unnasch TR. Avian host preference by vectors of Eastern Equine Encephalomyelitis virus. Am. J. Trop. Med. Hyg. 2003;69:641–647. [PubMed] [Google Scholar]
- Hibler CP. Onchocercidae (Nematoda: Filarioidea) of the American Magpie, Pica pica hudsonia (Sabine) in northern Colorado. Ph.D. dissertation. Colorado State University; Fort Collins, CO.: 1963. [Google Scholar]
- Highby PR. Mosquito vectors and larval development of Dipetalonema arbuta Highby (Nematoda) from the porcupine, Erethizon dorsatum. J. Parasitol. 1943;29:243–252. [Google Scholar]
- Homstad PR, Anwar A, Iezhova T, Skorping A. Standard sampling techniques underestimate prevalence of avian hematozoa in Willow Ptarmigan (Lagopus lagopus) J. Wildl. Dis. 2003;39:354–358. doi: 10.7589/0090-3558-39.2.354. [DOI] [PubMed] [Google Scholar]
- Johnson AA. Helminths of common grackles (Quiscalus quiscali-versicolor, Vieillot) in central Arkansas. Arkansas Acad. Sci. Proc. 1984;38:53–55. [Google Scholar]
- Jones CE, Lounibos LP, Marra PP, Kilpatrick AM. Rainfall influences survival of Culex pipiens (Diptera: Culicidae) in a residential neighborhood in the mid-Atlantic United States. J. Med. Entomol. 2012;49:467–473. doi: 10.1603/me11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proc. Biol. Sci. 2006;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LD, Hardy JL, Presser SB, Houk EJ. Dissemination barriers for western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low viral doses. Am. J. Trop. Med. Hyg. 1981;30:190–197. doi: 10.4269/ajtmh.1981.30.190. [DOI] [PubMed] [Google Scholar]
- Lura T, Cummings R, Velten R, De Collibus K, Morgan T, Nguyen K, Gerry A. Host (avian) biting preference of southern California Culex mosquitoes (Diptera: Culicidae) J. Med. Entomol. 2012;49:687–696. doi: 10.1603/me11177. [DOI] [PubMed] [Google Scholar]
- MacDonald G. The analysis of equilibrium in malaria. Trop. Dis. Bull. 1952;49:813–829. [PubMed] [Google Scholar]
- McHugh CP. Survivorship and gonotrophic cycle length of Culex tarsalis (Diptera: Culicidae) near Sheridan, Placer County, California. J. Med. Entomol. 1990;27:1027–1030. doi: 10.1093/jmedent/27.6.1027. [DOI] [PubMed] [Google Scholar]
- Mellor PS, Boorman J. Multiplication of blue-tongue virus in Culicoides nubeculosus (Meigen) simultaneously infected with the virus and the microfilariae of Onchocerca cervicalis (Railliet & Henry) Ann. Trop. Med. Parasitol. 1980;74:463–469. doi: 10.1080/00034983.1980.11687368. [DOI] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg. Inf. Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odetoyinbo JA. Biology of Splenididofilaria quiscali (von Linstow, 1904) n. comb. (Nematoda: Onchocercidae). Ph.D. dissertation. Iowa State University; Ames: 1960. [Google Scholar]
- Petney TN, Andrews RH. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int. J. Parasitol. 1998;28:377–393. doi: 10.1016/s0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- Pung OJ, Davis PH, Richardson DJ. Filariae of raccoons form southwest Georgia. J. Parasitol. 1966;82:849–851. [PubMed] [Google Scholar]
- Reisen WK, Milby MM, Meyer RP. Population dynamics of adult Culex mosquitoes (Diptera: Culicidae) along the Kern River, Kern County, California, in 1990. J. Med. Entomol. 1992;29:531–543. doi: 10.1093/jmedent/29.3.531. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Hardy JL. Bionomics of Culex tarsalis (Diptera: Culicidae) in relation to arbovirus transmission in southeastern California. J. Med. Entomol. 1995;32:316–327. doi: 10.1093/jmedent/32.3.316. [DOI] [PubMed] [Google Scholar]
- Robinson EJ. Observations on the epizootiology of filarioid infections in two species of the avian family Corvidae. J. Parasitol. 1955;41:209–214. [PubMed] [Google Scholar]
- Sabio IJ, Mackay AJ, Roy A, Foil LD. Detection of West Nile virus RNA in pools of three species of ceratopogonids (Diptera: Ceratopogonidae) collected in Louisiana. J. Med. Entomol. 2006;43:1020–1022. doi: 10.1603/0022-2585(2006)43[1020:downvr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Safar-Hermann N, Supperer R. Occurrence of Onchocerca lienalis (Stiles, 1892) in cattle in Austria. Tropenmed Parasitol. 1983;34:129–132. [PubMed] [Google Scholar]
- Santos JN, Lanfredi RM, Pimenta PFP. The invasion of the midgut of the mosquito Culex (Culex) quinquesfasciatus Say, 1823 by the helminth Litomosoides chagasfilhoi Moraes, Neto, Lanfredi and De Souza, 1997. J. Invertebr. Pathol. 2006;93:1–10. doi: 10.1016/j.jip.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, McMillen L, Unnasch EA, Unnasch TR. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector-Borne Zoon. Dis. 2007;7:365–386. doi: 10.1089/vbz.2006.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solismaa M, Laaksonen S, Nylund M, Pitkänen E, Airakorpi R, Oksanen A. Filarioid nematodes in cattle, sheep and horses in Finland. Acta Vet. Scand. 2008;16:50–53. doi: 10.1186/1751-0147-50-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa OE, Rossan RN, Baerg DC. The prevalence of trypanosomes and microfilariae in Panamanian monkeys. Am. J. Trop. Med. Hyg. 1974;23:862–868. doi: 10.4269/ajtmh.1974.23.862. [DOI] [PubMed] [Google Scholar]
- Thiemann TC, Wheeler SS, Barker CM, Reisen WK. Mosquito host selection varies seasonally with host availability and mosquito density. PLoS Negl. Trop. Dis. 2011;5:e1452. doi: 10.1371/journal.pntd.0001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach V, Pawlowski J. A new method of DNA extraction from the ethanol-fixed parasitic worms. Acta Parasitologica. 1999;44:147–148. [Google Scholar]
- Trees AJ, McCall PJ, Crozier SJ. Onchocerciasis in British cattle: a study of Onchocerca gutturosa and O. lienalis in North Wales. J. Helminthol. 1987;61:103–113. doi: 10.1017/s0022149x00009834. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Rossignol PA, Spielman A, Rossi CA, Bailey CL. Enhanced arboviral transmission by mosquitoes that concurrently ingested microfilariae. Science. 1984;225:1039–1041. doi: 10.1126/science.6474165. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Mather TN, Spielman A, Bailey CL. Increased dissemination of dengue 2 virus in Aedes aegypti associated with concurrent ingestion of microfi-lariae of Brugia malayi. Am. J. Trop. Med. Hyg. 1987;37:197–201. doi: 10.4269/ajtmh.1987.37.197. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O'Guinn M, Oliver J. Potential for New York mosquitoes to transmit West Nile virus. Am. J. Trop. Med. Hyg. 2000;62:413–414. doi: 10.4269/ajtmh.2000.62.413. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O'Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J. Med. Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O'Guinn ML, Dohm DJ, Webb JP, Jr., Sardelis MR. Vector competence of Culex tarsalis from Orange County, California, for West Nile virus. Vector-Borne Zoon. Dis. 2002;2:193–196. doi: 10.1089/15303660260613756. [DOI] [PubMed] [Google Scholar]
- Vaughan JA, Turell MJ. Dual host infections: enhanced infectivity of eastern equine encephalitis virus to Aedes mosquitoes mediated by Brugia microfilariae. Am. J. Trop. Med. Hyg. 1996;54:105–109. doi: 10.4269/ajtmh.1996.54.105. [DOI] [PubMed] [Google Scholar]
- Vaughan JA, Trpis M, Turell MJ. Brugia malayi microfilariae enhance the infectivity of Venezuelan equine encephalitis virus to Aedes mosquitoes. J. Med. Entomol. 1999;36:758–763. doi: 10.1093/jmedent/36.6.758. [DOI] [PubMed] [Google Scholar]
- Vaughan JA, Chadee DD, Turell MJ. Passage of ingested Mansonella ozzardi (Spirurida: Onchocercidae) microfilariae through the midgut of Aedes aegypti mosquitoes (Diptera: Culicidae) J. Med. Entomol. 2007;44:111–116. doi: 10.1603/0022-2585(2007)44[111:poimos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Vaughan JA, Focks DA, Turell MJ. Simulation models examining the effect of Brugian filariasis on dengue epidemics. Am. J. Trop. Med. Hyg. 2009;80:44–50. [PubMed] [Google Scholar]
- Weinmann CJ, Anderson JR, Longhurst WM, Connolly G. Filarioid worms of Columbian black-tailed deer in California. I. Observations in the vertebrate host. J. Wildl. Dis. 1973;9:213–220. doi: 10.7589/0090-3558-9.3.213. [DOI] [PubMed] [Google Scholar]
- Welker GW. Helminth parasites of the common grackle, Quiscalis quiscula versicolor Veillot in Indiana. Ph.D. dissertation. The Ohio State University; 1962. [as cited in: Robinson, E. J. 1971. Culicoides crepuscularis (Malloch) (Diptera:Ceratopogonidae) as a host for Chandlerella quiscali (Von Listow, 1904) comb. n. (Filarioidea: Onchocercidae) J. Parasitol. 57: 772-776.] [Google Scholar]
- Wirtz RA, Duncan JF, Njelesani EK, Schneider I, Brown AE, Oster CN, Were JB, Webster HK. ELISA method for detecting Plasmodium falciparum circumsporozoite antibody. Bull. W.H.O. 1989;67:535–542. [PMC free article] [PubMed] [Google Scholar]
- Zytoon EM, El–Belbasi HI, Matsumura T. Mechanism of increased dissemination of chikungunya virus in Aedes albopictus mosquitoes concurrently ingesting microfilariae of Dirofilaria immitis. Am. J. Trop. Med. Hyg. 1993;49:201–207. doi: 10.4269/ajtmh.1993.49.201. [DOI] [PubMed] [Google Scholar]