Summary
Mitochondrial biology is the sum of diverse phenomena from molecular profiles to physiological functions. A mechanistic understanding of mitochondria in disease development, and hence the future prospect of clinical translations, relies on a systems-level integration of expertise from multiple fields of investigation. Upon the successful completion of a recent National Institutes of Health, National Heart, Lung, and Blood Institute initiative on integrative mitochondrial biology in cardiovascular diseases, we reflect on the accomplishments made possible by this unique interdisciplinary collaboration effort and exciting new fronts on the study of these remarkable organelles.
Keywords: NHLBI, Mitochondria, Integrative Physiology
I. Introduction
Heart disease persists as a leading cause of mortality worldwide as the search for new diagnostic and therapeutic approaches continues. Recent research has converged on the importance of mitochondria in the life, death, and stress response of cardiac cells, spurring hope of new insights and therapies as we decipher the organizing principles of these organelles. Despite that the mitochondrial genome is but ~16,600-nucleotides long, and its proteome only ~1,500-protein large, mitochondria represent a complex physiological system – where genome, proteome, and metabolome interact to orchestrate key biological processes including energy transduction, Ca2+ flux, redox balance, and cell death. A comprehensive, mechanistic understanding of mitochondria in heart diseases will require systems-level approaches on how alterations of biomolecules (i.e., nucleotides, proteins, metabolites) trigger sophisticated interplay between bioenergetics, electrophysiology, Ca2+ homeostasis, and other subsystems.
Advances in systems biology over the past 15 years offer a tremendous opportunity to simultaneously monitor multiple biomolecules in disease. At the same time, it creates an enormous challenge to integrate and substantiate massive amounts of molecular data into a meaningful synthesis of mitochondrial pathophysiology. This challenge demands integrative thinking and collaborative relationships among researchers whose areas of expertise were traditionally under the purview of separate disciplines. In light of this challenge, a NHLBI Working Group on Modeling Mitochondrial Dysfunction in Cardiovascular Disease was convened in 2007 to outline the future goals of mitochondrial studies in cardiovascular diseases. The resulting Executive Summary identified three key areas of scientific opportunities: (i) advance understanding of mitochondrial functions in health and disease using integrated genetic, proteomic, and functional data, (ii) develop and manage new open-source computational models and experimental tools to enable cross-disciplinary investigations, and (iii) accelerate the translation of basic mitochondrial research to in vivo systems to identify novel therapeutic targets. Consequently, an NHLBI Request for Application entitled The Role of Cardiomyocyte Mitochondria in Heart Disease: An Integrated Approach (HL-10-002) was announced in 2008. The NHLBI initiative brought together 6 teams (led by centers at UCSD, Aurora Healthcare, Sanford-Burnham, Johns Hopkins, U. Washington, and UCLA), which were tasked to form interdisciplinary collaborations and employ innovative systems approaches to understand the role of cardiac mitochondria in health and disease.
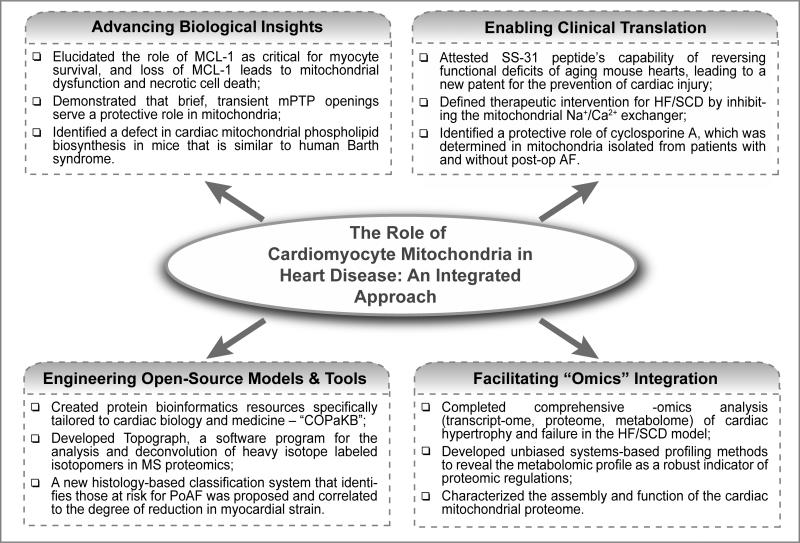
As the initiation concludes, we are pleased to report that the ensuing productivity has been impressive, resulting in 132 publications, one patent, and numerous conference presentations over the four-year award period. These works generated new projects that led to additional NIH-funded applications, the training of 35 trainees, and the inception of seven lasting collaborations. We highlight several key accomplishments and scientific innovations here (Fig.1).
Figure 1.
Schematic overview of accomplished milestones: characterizing the role of mitochondria in heart diseases using integrated approaches.
II. Advancing Cross-Disciplinary, Systems Biology Studies of Cardiac Mitochondria
A number of noteworthy discoveries were made through comprehensive omics analysis of various model systems, surveying the transcriptome, proteome, post-translational modifications (PTMs), and metabolome of mitochondria. Highlights include: (i) understanding cardiac energy production adaptations in response to physiological changes (e.g., development of heart failure; HF) by emphasizing the relationship between gene regulatory pathways, metabolite profiles, and energetics phenotypes1; (ii) integrating mass spectrometry and functional data to simultaneously measure metabolites, bioenergetics, and turnover to identify predictive/diagnostic metabolic disease states2; (iii) characterizing the mitochondrial proteome in normal vs. HF to reveal protective alterations under mitochondrial reactive oxygen species (mtROS) production; (iv) discovering new pathways that control cardiac mitochondrial phospholipid biosynthesis3; (v) interrogating the therapeutic potential of mitochondrial-targeted peptides to mitigate proteomic changes in transverse aortic constriction (TAC) models and enhance cardioprotection; and (vi) developing new methods to quantify mitochondrial phosphorylation signaling.
In parallel, integrated physiology combining molecular profiles and functional measurements have made great strides in revealing the regulatory principles of mitochondrial physiology, including: (i) the defensive role of transient mitochondrial permeability transition (MPT) in protective mitochondria against cardiac stress (e.g., elevated cytoplasmic Ca2+)4; (ii) the transcriptional regulatory circuitry controlling mitochondrial dynamics in cardiac development; (iii) the functional consequences of mtROS in mtDNA damage/decline on mitochondrial biogenesis signaling; (iv) the local dynamics of mitochondrial Ca2+ flux in disease5; (v) the pro-survival role of mitophagy following infarcts6; and (vi) the prevention of sudden cardiac death by modulating mitochondrial Na+/Ca2+ exchange7.
III. Building Novel Open-Source Tools, Models, and Platforms
A second scientific priority of the initiative was to promote the integration of computational and informatics methods into the central thought process and analysis pipelines of the investigations. This development has spurred the construction of several readily accessible open-source computational platforms and models for the scientific community to explore mitochondrial biology. Several mathematical and computational models emerged to synthesize molecular data into higher organizational structures of mitochondria, including: (i) computational models of mitochondrial pH regulation, electron transport, redox, Ca2+ flux, and Pi-dependent buffering; and (ii) models of mitochondria temporal events (e.g., Ca2+, MPT, mtROS) and spatial architecture of PKCε-Src (protein kinase C epsilon-Src kinase) signaling pathways in NO-mediated cardioprotection.
Moreover, two computational platforms materialized in response to the need for specific tools to facilitate integrative sciences: (i) Topograph – a freely available software program to improve mass spectrometry discovery of protein turnover rates8 (https://goo.gl/dysfqm); (ii) COPaKB – a cardiac protein knowledgebase that serves as a protein bioinformatics resource tailored to cardiac biology and medicine9 (http://heartproteome.org/copa). Looking ahead, we anticipate the continual development of bioinformatics resources to be instrumental for the study of cardiac mitochondria as integrated systems. A corollary of the -omics revolution is the massive amount of large-scale data that are being generated every day. The aggregation, re-analysis, and meta-analysis of these data will be an exciting new front for the integrated approach. It is foreseen that mechanisms to cross-reference existing datasets generated by these other NHLBI-funded projects will represent an upcoming area of intense research.
IV. Bridging the Divide between Basic Research and Clinical Intervention
In parallel, studies are making headway to bring itegrative mitochondrial biology to the clinic. Treatments are under trial or development that target redox, cell death, and mitophagy pathways, while our understanding of their mechanisms of action continue to refine. Highlights include:
-
(i)
Clinical studies of patient atrial samples following coronary bypass operations implicate mitochondrial alterations in the development of post-operative atrial fibrillation (AF). Atrial mitochondria are sensitized towards MPT in AF, suggesting new targets of intervention by cyclosporine A or metformin. Reduced respiratory complex I/II activities and mitochondrial metabolomic markers are being explored as features to complement blood biomarkers in predicting/stratifying patients at risk for post-operative paroxysmal/persistent AF. Newly defined roles of mitochondria in fibroblast proliferation/differentiation prompted further avenues of intervention, with the mitochondrial depolarizer 2,4-dinitrophenol shown to inhibit fibrotic growth in pre-clinical studies. These studies contributed to the ongoing development of a scoring system to monitor post-op AF.
-
(ii)
Bendavia/SS-31, a mitochondrial-protective tetrapeptide, is shown to protect cardiac function and reverse proteomic profiles in TAC and angiotensin (Ang) II mouse models of HF. Mechanistic studies are exploring its similarities and differences with the mitochondrial catalase model and reveal a role of the peptide in improving state III respiration and reversing age-induced respiratory dysfunctions. As a result of this work, a new patent has resulted for protection from HF (US Appl.-20110082084); the peptide is currently under Phase II trials for the prevention of injury following acute infarction, repair of renal artery stenosis, and improving skeletal muscle function in elderly subjects10 (NCT 01572909/01755858/02245620).
-
(iii)
A newly characterized BCL-2 family protein MCL-1 presents a promising diagnostic marker for cell death, mitochondrial dysfunction, and impaired autophagy11. Animal models and reagents are being generated to capitalize MCL-1 as a future therapeutic target to restore salutary mitophagy; whereas in non-cardiac systems, BCL-2 family inhibitors that specifically counter the anti-apoptotic properties of MCL-1 are under trials for cancer treatment.
-
(iv)
On the imaging front, novel-imaging modalities such as 3D speckle tracking echocardiography, were used to predict recurrence in AF patients12. A toolbox of fluorescent bioprobes was developed to track hexokinase isoforms and their translocation from mitochondria to cytoplasm in real time, which can be used for spatiotemporal dynamics studies in living cells.
V. Conclusion and Future Directions
The “mitochondria in heart diseases” initiative brought together 15 investigators whose expertise lies in diverse areas of redox biology, metabolism, transcriptional control, mass spectrometry, computational biology, clinical physiology, electrophysiology, and beyond. Many conceptual advances emerged from this work, which include the studies from multiple Centers have consistently shown that changes in myocardial fuel utilization per se are not sufficient to drive HF. This suggests that the adult mammalian heart is capable of remarkable fuel utilization plasticity in a manner reminiscent of adaptive metabolic shifts in cancer. The projects also established the paradigm that alterations in ion homeostasis in the cytoplasm and mitochondrial matrix are the mechanisms behind impaired mtROS handling in addition to impaired energy supply/demand matching in HF.
Most importantly, these investigations unequivocally confirmed cardiac mitochondria as a rich source of future therapeutic targets in diverse cardiac diseases. This theme is now receiving great attention by increasing numbers of investigators in the field in search for transformative diagnostic/therapeutic avenues. For instance, mechanistic knowledge on mitochondrial redox balance, Ca2+ flux and MPT permits new strategies to correct specific defects and arrest disease progression. Newly identified molecular targets mediating ion transport across mito-membranes are leading to genetically engineered mouse models from several labs, which enable the testing of new concepts and hypotheses. The acknowledgment that defects in fuel/energy metabolism are key to dysfunction and failure of multiple cardiac subsystems is particularly transformative and has contributed to major new emphases with relevance to the development of novel therapeutics aimed at early HF, diabetes, and I/R. Indeed, the once-esoteric field of bioenergetics has been transformed into a hot topic in cardiovascular research, drawing in diverse researchers interested in protein modification and turnover, organelle processing and autophagy, oxidative stress, necrotic and apoptotic cell death, energy balance, and ion homeostasis.
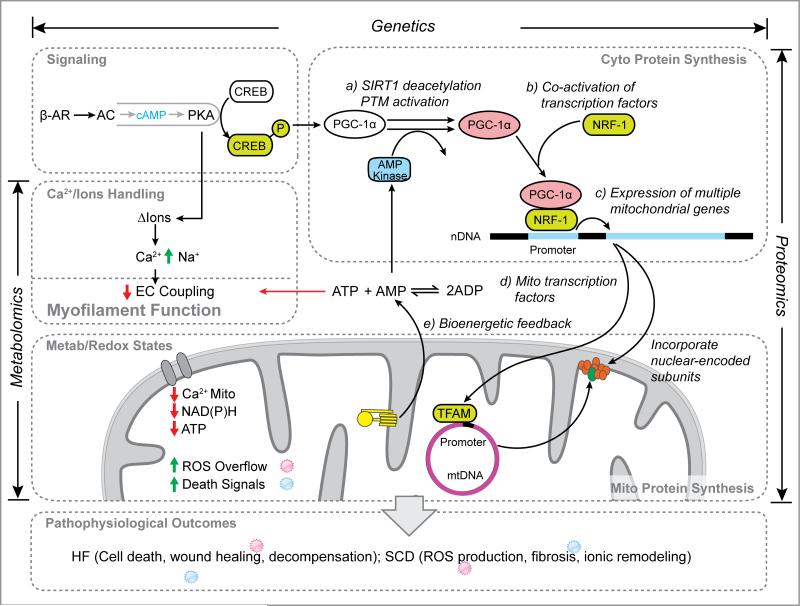
These works also reaffirm the power of systems-level investigations to identify unexpected hypotheses, pathogenic mechanisms, and therapeutic targets. Complex network relationships are emerging that encompass multiple layers of details and molecules in cardiac mitochondria (e.g., Fig.2). Comparative analyses of genomic, transcriptomic, proteomic, and metabolomic profiles consistently conclude that the mitochondrial derangements so frequently observed in disease models cannot be predicted from single pathways alone, and that orthogonal multi-omics data are necessary if a more complete picture of the post-transcriptional and post-translational regulations of heart disease are to be pursued. This realization now compels investigators to delve deeper into the plasticity and dynamics of mitochondrial biomolecules with respect to their modifications, interactions, and spatiotemporal distributions in search of causal disease drivers. As simultaneous characterizations of multiple molecular parameters become the norm, the integration of previously insulated disciplines will enable more sophisticated questions to be asked, e.g., whether global mitochondrial metabolite alterations could regulate post-translational modifications that contribute to vicious cycles of energy starvation,or whether a systems biology strategy can be devised to screen for induced pluripotent stem cell-derived cardiomyocyte lines with preserved mitochondrial functions and resistance to injury.
Figure 2.
Defining new network connections through multi-omics analysis, highlighting the role of mitochondria in heart failure.
The priority now is to work to translate the mechanistic insights obtained from the studies to new clinical interventions such as mitochondrial targeted therapeutics, including both antioxidants and agents that target cardiolipin to enhance stability and function of the electron transport system. This work will require continuous collaboration and support similar to those that materialized here. A consensus unanimously expressed by the investigators was that the organizational structure of the initiative has been especially propitious to foster cross-center/cross-disciplinary interactions. The frequent meetings and communications have stimulated conversations and organic exchanges of ideas that are driven by the common goal to understand a biological entity rather than the conventional boundaries of methodologies. By extension, these interactions created a fertile training environment, which conferred a collaborative mentality and scientific vision upon the trainees that might be sustained throughout their research career. Future endeavors will likely also benefit from formal coordination of computational experts (e.g., through specialized working groups) from the outset to promote the sharing of data science resources.
In summary, this initiative has resulted in opportunities extended to additional investigators of mitochondrial biology in both cardiovascular and non-cardiovascular fields. Many regulatory principles in mitochondrial biology are important not only to heart diseases, but also to cancer and neurodegenerative disorders, which often present common molecular targets for intervention. Prime examples include bidirectional modulations of BCL-2 proteins to confer tumor suppression (promoting apoptosis) or cardioprotection (inhibiting); inhibition of MPT to mitigate I/R and axonal degeneration; and the profound cardiac implications of mitophagy pathways originally expounded in familial Parkinsonism. Exciting opportunities exist that, through mutually beneficial exchange and collaborations with other mitochondrial experts, a new discipline of mitochondrial physiology can arise that has the same level of rigor and vigor as other current fields of biomedical sciences and that will make substantial contributions to the medicine of multiple common disorders.
Acknowledgments
The teams are grateful for helpful discussions and critiques from Dr. Edward Lau.
Sources of Funding: The investigators of cited works were supported by the NHLBI Mitochondria in Heart Diseases Initiative (RFA-HL-10-002); grant numbers R01-HL101217, R01-HL101240, R01-HL101189, R01-HL101235, R01-HL101228, R01-HL101186.
Nonstandard Abbreviations and Acronyms
- AF
Atrial fibrillation
- HF
Heart failure
- I/R
Ischemic/reperfusion (injury)
- MPT
Mitochondrial permeability transition
- mtROS
Mitochondrial reactive oxygen species
- PTM
Post-translational modification
- TAC
Transverse aortic constriction
- COPaKB
Cardiac Organellar Protein Knowledgebase
- SCD
sudden cardiac death
- MCL-1
myeloid cell leukemia 1
- TFAM
mitochondrial transcription factor A
- SIRT1
sirtuin-1
- EC
excitation-contraction
- NRF-1
nuclear respiratory factor 1
- PGC
peroxisome proliferator-activated receptor-gamma coactivator
- PKA
protein kinase A
Footnotes
A list of 132 publications originating from this initiative can be found at: http://goo.gl/oJZYrb
Disclosures: None.
Any views expressed are those of the authors and do not necessarily reflect those of the NIH. LS and RW are employees of NHLBI at NIH. Although their participation in the development of this manuscript was performed as an official duty activity, the views expressed do not necessarily represent those of the Institute.
References
- 1.Aubert G, Vega RB, Kelly DP. Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. Biochim Biophys Acta. 2012;1833:840–847. doi: 10.1016/j.bbamcr.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemutlu E, Zhang S, Gupta A, Juranic NO, Macura SI, Terzic A, Jahangir A, Dzeja P. Dynamic phosphometabolomic profiling of human tissues and transgenic models by 18O-assisted 31P NMR and mass spectrometry. Physiol Genomics. 2012;44:386–402. doi: 10.1152/physiolgenomics.00152.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, Attie AD, Muoio DM, Kelly DP. A role for peroxisome proliferator-activated receptor γ coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res. 2014;114:626–636. doi: 10.1161/CIRCRESAHA.114.302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korge P, Yang L, Yang JH, Wang Y, Qu Z, Weiss JN. Protective role of transient pore openings in calcium handling by cardiac mitochondria. J Biol Chem. 2011;286:34851–34857. doi: 10.1074/jbc.M111.239921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu X, Ginsburg KS, Kettlewell S, Bossuyt J, Smith GL, Bers DM. Measuring local gradients of intramitochondrial [Ca2+] in cardiac myocytes during sarcoplasmic reticulum Ca2+ release. Circ Res. 2013;112:424–431. doi: 10.1161/CIRCRESAHA.111.300501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, Jimenez R, Petrosyans S, Murphy AN, Gustafsson ÅB. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013;288:915–926. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Takimoto E, Dimaano VL, DeMazumder D, Kettlewell S, Smith G, Sidor A, Abraham TP, O'Rourke B. Inhibiting Mitochondrial Na+/Ca2+ Exchange Prevents Sudden Death in a Guinea Pig Model of Heart Failure. Circ Res. 2014;115:44–54. doi: 10.1161/CIRCRESAHA.115.303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh EJ, Shulman NJ, Dai DF, Vincow ES, Karunadharma PP, Pallanck L, Rabinovitch PS, MacCoss MJ. Topograph, a software platform for precursor enrichment corrected global protein turnover measurements. Mol Cell Proteomics. 2012;11:1468–1474. doi: 10.1074/mcp.O112.017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zong N, Li H, Lam MP, Jimenez RC, Kim CS, Deng N, Kim AK, Choi JH, Zelaya I, Liem DA, Meyer D, Odeberg J, Fang C, Lu HJ, Xu T, Weiss JN, Duan H, Uhlen M, Yates J, 3rd, Apweiler R, Ge J, Hermjakob H, Ping P. Integration of Cardiac Proteome Biology and Medicine by a Specialized Knowledgebase. Circ Res. 2013;113:1043–1053. doi: 10.1161/CIRCRESAHA.113.301151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai DF, Chen T, Szeto H, Nieves-Cintrón M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas RL, Roberts DJ, Kubli DA, Lee Y, Quinsay MN, Owens JB, Fischer KM, Sussman MA, Miyamoto S, Gustafsson ÅB. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes Dev. 2013;27:1365–1377. doi: 10.1101/gad.215871.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirza M, Caracciolo G, Khan U, Mori N, Saha SK, Srivathsan K, Altemose G, Scott L, Sengupta P, Jahangir A. Left atrial reservoir function predicts atrial fibrillation recurrence after catheter ablation: A two-dimensional speckle strain study. J Interv Card Electrophysiol. 2011;31:197–206. doi: 10.1007/s10840-011-9560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]