Abstract
p53 reactivation offers a broad-based strategy for cancer therapy. In this study we report the identification of prodigiosin that can reactivate p53 family-dependent transcriptional activity in p53 deficient human colon cancer cells. Prodigiosin and its structural analogue (compound R) induced the expression of p53 target genes accompanied by cell cycle arrest and apoptosis in p53 deficient cancer cells. Prodigiosin restored p53 signaling in cancer cells harboring hotspot p53 mutations, with little to no detectable cytotoxicity in normal human fibroblasts and with no genotoxicity. Prodigiosin induced the expression of p73 and disrupted its interaction with mutant p53, thereby rescuing p53 pathway deficiency and promoting anti-tumor effects. The disruption of mutant p53/p73 interaction was specific to prodigiosin and not related to mTOR inhibition. Our findings suggest that mutant p53 needs to be targeted in the context of p73 stimulation to allow efficient restoration of the p53 pathway. In exhibiting this capability, prodigiosin and its analogue provide lead compounds to rescue deficiencies in the p53 pathway in cancer cells by up-regulating p73 and targeting mutant p53/p73 interaction there.
Keywords: Colorectal cancer, p53 rescue, p73, Anti-tumor effect, Prodigiosin
Introduction
The p53 tumor suppressor is mutated in more than 50% of human tumors (1, 2). p53 activation can induce cell cycle arrest and apoptosis through its transcriptional regulation of specific target genes, such as p21, Gadd45, Bax, Noxa, DR5 and PUMA (3). Due to deficiencies in cell cycle control and apoptosis, functionally p53-deficient cells are susceptible to malignant transformation (4). It is well known that p53 knock-out mice develop spontaneous tumors (5). In clinical studies, p53 mutation is associated with resistance to radiotherapy and chemotherapy, poor patient survival and tumor progression (6, 7). Therefore, p53 pathway functional reactivation is an attractive strategy for cancer therapy development and may address an unmet need in the clinic for progressive therapy-refractory human tumors.
Although efforts have been devoted to searching for drugs with the ability to rescue a mutant p53 pathway, no p53-rescuing drugs have been approved for clinical use in the United States (8). p53, as a transcription factor, is not an easy drug target, since numerous mechanisms lead to p53 inactivation in human cancers, such as p53 mutations, p53 deletion and the disruption of p53 degradation (8). Thousands of p53 mutations have been detected in human cancers and so many different mutations could lead to different changes in p53 structure and function although there are hotspots within its DNA-binding domain (9). Therefore, p53 is not easily targeted directly and alternative p53-targeting approaches need to be developed.
p73, a p53 family protein, shares a high degree of sequence homology with p53 and regulates the expression of similar genes by binding to p53-responsive elements within gene promoters and performs similar functions to p53 (10, 11). However, unlike p53, p73 is not a target for mutational inactivation in human cancer (11). Recently, it has been found that some agents induce cell death via the up-regulation of p73, including NSC176327 (12), securinine (13) and RETRA (14). These studies indicate that these p73-inducing agents cause cell death in a p53-like manner by activating p53-responsive genes as well as inducing apoptosis. Furthermore, p73 function has been shown to be inhibited by the interaction with mutant p53 (15). The synthesized short peptides (Short Interfering Mutant p53 Peptides/SIMPs) (16) and a small molecule RETRA (14) disrupt the interaction of mutant p53 and p73, consequently leading to p73 release, p53 pathway activation and apoptosis induction. Therefore, p73 up-regulation and disruption of a mutant p53/p73 complex would be a promising strategy to activate the p53 pathway and induce cancer cell death.
Using a high throughput drug screen, we identified prodigiosin and a structural analogue (Compound R) with p53 rescue activity in p53-mutant and p53-null cancer cells. We found that prodigiosin and compound R inhibit tumor cell growth at very low concentration (nM range), and without cytotoxicity towards normal cells. Furthermore, we found that p73 up-regulation is involved in the mechanism of p53 pathway activation and apoptosis induction by prodigiosin and compound R. We also found that prodigiosin, but not the mTOR inhibitor rapamycin, is able to disrupt the interaction of mutant p53 and p73. Our study suggests that p73 could be a useful alternative target to reactivate the p53 pathway in p53 deficient cancer cells.
Materials and Methods
Cell-based p53 activity screening
Cell-based screening for p53-dependent transcriptional activity was performed by using noninvasive bioluminescence imaging. p53 mutant SW480 human colon cancer cells, stably expressing a p53-responsive luciferase reporter, were used for chemical library screening as previously described (17). Cells were treated with small molecule compounds from the NCI compound library (The NCI/DTP Open Chemical Repository). After the treatment, cells were imaged by using an IVIS imaging system (Xenogen, Alameda, CA) to detect luciferase activity. Positive hits with strong activity for luciferase induction were selected for the secondary screen. In the secondary screen, the compounds were tested at concentrations ranging from 0.01–10 μM for 2, 20 and 72 hr in p53 mutant human cancer cell lines SW480 (R273H and P309S) and DLD1 (S241F), as well as the p53 null HCT116 human cancer cell line.
Establishment of SW480 p53 reporter cell line with stable p73 knock-down (p73 KD)
The retroviral vector containing p73 siRNA (pSIREN-RetrolQ-p73 siRNA- blasticidin-retroviral vector) was described previously (17). Phoenix cells were transfected with the retroviral vector containing p73 siRNA. After 48 hr transfection, supernatant (packaged retrovirals) was collected, and then infected SW480 p53 reporter containing cells (17). Blasticidin at 5 μg/ml was added for selection. After two weeks of selection, blasticidin-resistant cells were collected for p73 knock-down validation.
Western blotting
After treatment, protein lysates were collected for Western blot. Twenty-five micrograms of protein was used for SDS-PAGE. After primary and secondary antibody incubations, the signal was detected by chemiluminescent detection kit, followed by autoradiography.
Immunoprecipitation of mutant p53 with p73
After treatment, cells were lysed with IP lysis buffer. 500 μg of whole-cell extracts were incubated with 1 μg of p53 antibody followed by rocking at 4°C for overnight. After incubation, 50 μl of protein A-Sepharose beads was added, and the samples were rocked at 4°C for 4 hr and then washed three times. Samples were resuspended in 20 μl of 2× sample buffer, boiled for 10 min and centrifuged for 1 min at 13,000 rpm followed by SDS-PAGE to detect p73 and p53.
Immunofluorescence
Cells were seeded in 4-chamber slides. After treatment, cells were fixed by Cytofix/Cytoperm (BD Biosciences) for 30 min. Fixed cells were blocked for 2 hr, followed by primary antibody incubation for 2 hr and secondary antibody incubation for 2 hr at room temperature. After washing, samples were stained with DAPI for 1 min, mounted and examined by fluorescence microscopy.
Flow cytometry assay
After treatment, cells were harvested, fixed by ethanol and stained by PI, then flow cytometry was performed as previously described (17).
Cell viability assay
Cancer cells were treated with different concentrations of prodigiosin or DMSO control for 72 hr. The cell viability was assessed by CellTiterGlo bioluminescent cell proliferation assay (Promega, Madison, WI). Percentage of cell viability (mean±SEM) at each dose was calculated against the respective DMSO control. The IC50 values were determined from the sigmoidal dose-response curves using PRISM4 software (GraphPad, La Jolla, CA).
In vivo assay
Animal experiments were carried out according to a protocol approved by Institutional Animal Care and Use Committee of Pennsylvania State University. Athymic nude mice were injected subcutaneously in the left and right dorsal flank, each with a 100 μL suspension of 1–4×106 cancer cells in an equal volume of Matrigel. When tumors grew to 4–5 mm in diameter, the mice were treated with the prodigiosin, compound R or DMSO control by i.p. injection. p53 transcriptional activity was detected using bioluminescence imaging at 5 minutes following i.p. injection of 60 μL D-luciferin (50 mg/ml). The induction of p53 responsive transcriptional activity was obtained by the comparison of the luciferase activity after 12 hr treatment with that before treatment. The tumor size was monitored by caliper measurements.
Xenograft tumor section analysis
Tumors were harvested from euthanized mice and fixed in 4% paraformaldehyde in PBS for 48 hours. Paraffin-embedding, sectioning and hematoxylin and eosin staining were performed by the Histology Core Facility at Penn State Hershey Medical Center. TUNEL staining was carried out according to the manufacturer’s protocol for ApopTag Peroxidase In Situ Apoptosis Detection Kit (Millipore). For immunohistochemistry analysis, slides were dewaxed in xylene, rehydrated in a decreasing gradient of ethanol and antigen retrieval was carried out by boiling in 10mM citric acid (pH 6.0) for 6 minutes. Samples were blocked with goat serum (Vector Laboratories). Primary antibody for Ki67 (Immunotech, 0505) was incubated overnight at 4°C in a humidity chamber. Incubation with biotinylated secondary antibody and DAB deposition was carried out according to the manufacturer’s protocol (Vector Laboratories DAB Substrate Kit for Peroxidase). Samples were counterstained with hematoxylin (DAKO) for 6 minutes, rinsed in dH2O for 5 minutes, rinsed with PBS, and dehydrated and sealed under cover slips. Images were recorded on an Axioskop microscope using QCapture software (QImaging).
Statistical analysis
All data were analyzed using PRISM4 Software (GraphPad Software, Inc., San Diego, CA, USA). Statistical analysis was performed using unpaired or paired t-test. Results were considered as statistically significant when p < 0.05.
Results
Prodigiosin and compound R can activate p53-like transcriptional activity in p53-mutant and p53-null human cancer cells
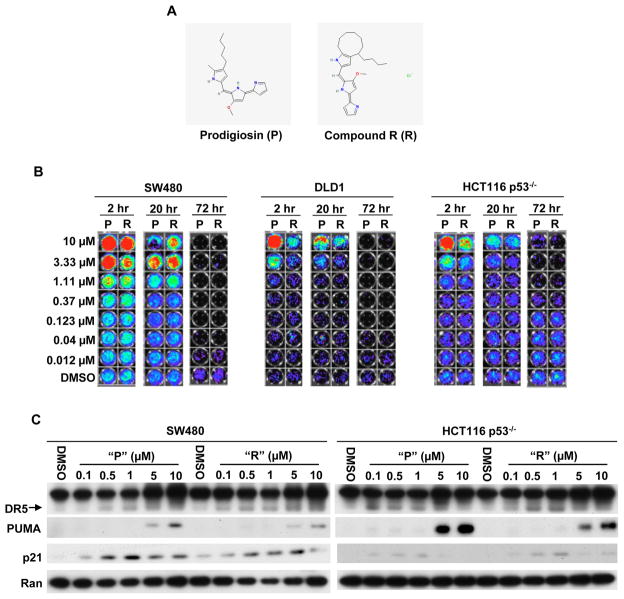
Using a mutant p53-expressing cancer cell line (SW480) with a stably integrated p53-responsive luciferase reporter, we sought to identify small molecules with p53 rescue activity by screening of the NCI compound library. Our screening system was previously established to identify small molecules that can activate p53 family-specific transcriptional activity coupled to loss of cell viability (17). In the screening, we identified prodigiosin, which can activate p53-like transcriptional activity in SW480 cells. We have also identified a structurally related analogue of prodigiosin (Compound R) in the NCI library. The structures of prodigiosin (P) and compound R (R) are shown in figure 1A.
Figure 1. Prodigiosin and compound R induce p53-like transcriptional activity in p53-mutant and p53-null human cancer cells.
(A), The structures of prodigiosin (P) and compound R (R). (B), SW480, DLD1 and HCT116 p53−/− cells with p53-responsive reporter were treated with different concentrations of “P”, “R” or DMSO control for 2, 20 and 72 hr. p53-responsive reporter activity was imaged by the IVIS imaging system after treatment. (red/green/blue color indicates bioluminescent signal from p53 responsive luciferase reporter, lack of signal (black) indicates absence of cells due to cell death). (C), Prodigiosin and compound R induce endogenous p53 target genes DR5, PUMA and p21. SW480 and HCT116 p53−/− cells were treated with the indicated concentrations of “P” or “R” for 12 hr. DR5, PUMA and p21 were detected by Western Blotting. Ran protein expression was used as the loading control.
We validated prodigiosin (P) and compound R (R) in a secondary screen. SW480, DLD1 and HCT116 p53−/− cells with p53 reporter were treated with different concentrations of “P”, “R” or DMSO control for 2, 20 and 72 hr. After treatment, p53-responsive luciferase reporter activity was imaged by an IVIS imaging system. As shown in figure 1B, “P” or “R” activated p53-responsive reporter activity at early time points (2 hr and 20 hr) in a dose dependent manner. Following at 72 hr, all cell lines exhibited the phenotypes of a dose-dependent cell death by the treatment of either “P” or “R”. These data indicate that prodigiosin and compound R can activate p53 responsive transcriptional activity in p53-mutant and p53-null cancer cells.
To further validate that prodigiosin (P) and compound R (R) can activate p53 signaling pathway in p53-mutant and p53-null cancer cells, we examined whether “P” or “R” can induce the expression of endogenous p53 target genes. Western blot indicated that “P” or “R” induced the expression of p53 target genes (p21, DR5 and PUMA) in a dose dependent manner in SW480 and HCT116 p53−/− cells (Figure 1C). The data demonstrate that p53 responsive signaling pathway is activated by prodigiosin and compound R in p53-mutant and p53-null cancer cells.
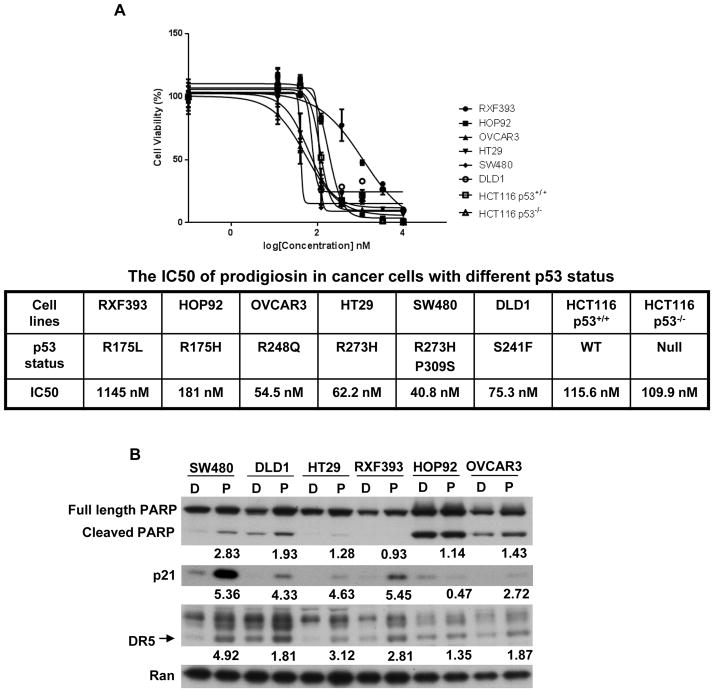
In a panel of cancer cell lines with p53 hotspot mutants, prodigiosin activates p53 pathway and induces cell death
Cancer cells harbor a variety of p53 mutations, and there are hotspot mutations occurring in DNA-binding domain. The diversity of mutations promoted us to investigate whether prodigiosin can active p53 pathway and induce cell death in cancer cells containing different p53 hotspot mutants. To test this, we selected a panel of cancer cell lines with p53 hotspot mutants (Figure 2), and treated these cells with prodigiosin. Cell viability assay indicates that prodigiosin induces cell death in all tested cancer cells with p53 hotspot mutants as well as cancer cells with wild type p53 and p53 null (Figure 2A). Among these p53 mutant cancer cells, SW480, DLD1, HT29 and OVCAR3 cells are all sensitive to prodigiosin with very low IC50 values, and PARP cleavage is also induced by prodigiosin in these cancer cells. However, HOP92 and RFX393 cells are less sensitive to prodigiosin with higher IC50 values, and PARP cleavage is not induced in these two cancer cell lines (Figure 2). Prodigiosin induces DR5 and p21 in the sensitive cancer cell lines (SW480, DLD1, HT29 and OVCAR3). In the less sensitive cancer cell lines (HOP92 and RFX393), HOP92 cells are five times more sensitive to prodigiosin than the RFX393 cells, but show no induction of p21 while the RFX393 cells do (Figure 2). HOP92 cells have a higher level of apoptotic rate, as indicated by the higher basal level of cleaved PARP (Figure 2B). Therefore, we consider that HOP92 cells could have undergone apoptosis at the 12 hr treatment of prodigiosin, so the expression of p21 (cell cycle arrest marker) is reduced. We suspected that p21 may be induced earlier than 12 hr in HOP92 cells and performed a time course experiment. The data show that p21 is induced by prodigiosin in HOP92 cell at the 4 hr time point. As the time extends, p21 expression is decreased gradually by the treatment of prodigiosin (Figure S1). Our study suggests that prodigiosin is effective to reactive the p53 pathway and to induce apoptosis in cancer cells with various p53 hotspot mutants.
Figure 2. In a panel of cancer cell lines with p53 hotspot mutants, prodigiosin activates p53 pathway and induces cell death.
(A), Dose-dependent growth inhibition by prodigiosin in a panel of cancer cell lines. Various cancer cells were treated with different concentrations (14 nM to 10 μM) of prodigiosin for 72 hr. The cell viability was assessed by CellTiterGlo assay. The IC50 values were determined from the sigmoidal dose-response curves using PRISM4 software. (B), The induction of p53 target proteins and PARP cleavage by prodigiosin. Various cancer cells were treated by 1 μM prodigiosin (P) or DMSO (D) for 12 hr. Full-length, cleaved PARP, DR5, p21 and loading control Ran were detected by Western Blotting. The density of the bands was quantified and normalized to loading control (Ran). Then, the fold induction of cleaved PARP, p21 and DR5 was calculated in each cell line by comparing normalized density in “P” to normalized density in “D”. The fold induction is shown under the band.
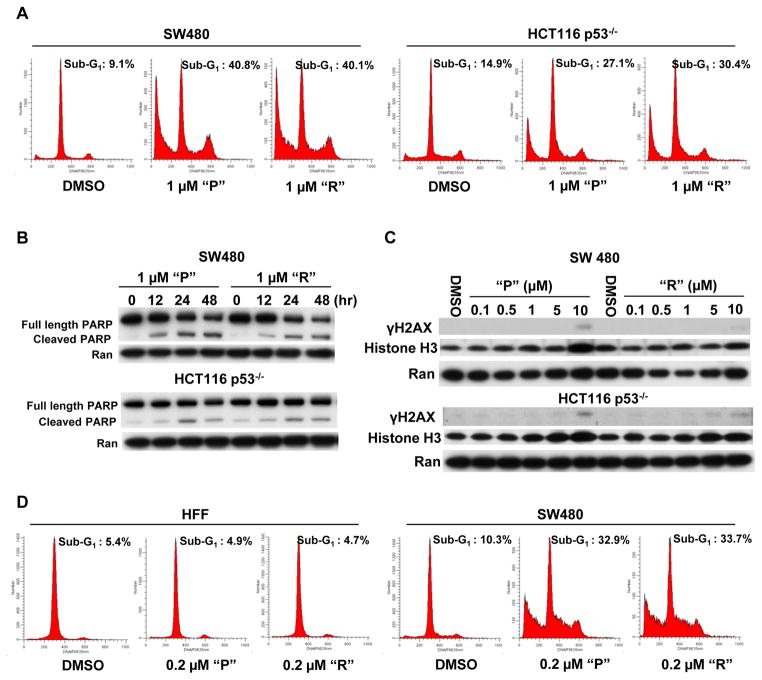
Prodigiosin and compound R induce cell cycle arrest and apoptosis in cancer cells, and exhibit less cytotoxicity towards normal cells at relatively low concentration
Once the p53 pathway is activated, mammalian cells respond by undergoing cell cycle arrest and/or apoptosis. In order to determine whether “P” or “R” induce cell cycle arrest and/or apoptosis following p53 pathway activation, we performed flow cytometric analysis and PARP cleavage analysis. The cell cycle analysis indicated that 1 μM of “P” or “R” induce G1 and/or G2 arrest at an early time point (12 hr) in SW480 and HCT116 p53−/− cells (Figure S2). When the cells were treated for a longer time (72 hr), 1 μM of “P” or “R” appeared to induce a remarkable sub-G1 peak (apoptotic peak) (Figure 3A). Western blot indicated that both “P” and “R” induce obvious PARP cleavage (a marker of apoptosis due to caspase activation) in SW480 and HCT116 p53−/− cells (Figure 3B). DNA damage can activate p53 pathway to induce cell cycle arrest and/or apoptosis. To indicate that the p53 pathway activation by prodigiosin and compound R is not due to DNA damage, we detected the γH2AX expression after the treatment. Western blot showed that “P” and “R” did not induce γH2AX in SW480 cells and HCT116 p53−/− cells at lower doses required for p53 pathway activation as well as cell cycle arrest and apoptosis induction (Figure 3C). By immunofluorescence staining, we detected γH2AX foci. The data showed that 1 μM prodigiosin does not induce γH2AX foci formation after the 12 hr treatment. The chemotherapeutic agent oxaliplatin (10 μg/ml) was used as the positive control, and dramatically induced γH2AX foci in SW480 and HCT116 p53−/− cells (Figure S3). These results indicate that the activation of the p53 pathway by prodigiosin or compound R is accompanied by the induction of cell cycle arrest and apoptosis without genotoxic effects in p53-mutant and p53-null cancer cells.
Figure 3. Prodigiosin and compound R induce apoptosis in human cancer cells, and exhibit less cytotoxicity towards normal cells.
(A), “P” and “R” induce apoptosis. SW480 and HCT116 p53−/− cells were treated with 1 μM “P”, “R” or DMSO control for 72 hr. After treatment, flow cytometry was performed. (B), “P” and “R” induce PARP cleavage. SW480 and HCT116 p53−/− cells were treated with 1 μM “P” or 1 μM “R” for 0, 12, 24 and 48 hr. Full-length, cleaved PARP and loading control Ran were detected by Western Blotting. (C), “P” and “R” do not induce γH2AX at the low concentrations. SW480 and HCT116 p53−/− cells were treated with the indicated concentrations of “P” or “R” for 12 hr. γH2AX, Histone H3 and loading control Ran were detected by Western Blotting. Histone H3 is used as a control of total Histone. (D), Prodigiosin and compound R exhibit less cytotoxicity towards normal cells. Tumor cells (SW480) and normal cells (HFF: Human Foreskin Fibroblasts) were treated with 0.2 μM “P”, “R” or DMSO control for 72 hr. After treatment, flow cytometry was performed.
Under physiological conditions, normal cells express very low levels of p53 in the absence of stressful stimulation. In order to investigate the impact of activation of the p53 pathway by prodigiosin (P) or compound R (R) on normal cells, we compared the effect of these two compounds on cellular apoptosis between cancer and normal cells. As shown in figure 3D, 0.2 μM of either “P” or “R” did not appear to induce a sub-G1 phase (apoptotic cells) following a 72 hr treatment in normal cells (HFF: Human Foreskin Fibroblasts), however the same concentration of “P” or “R” induced a significant increase in sub-G1 phase following a 72 hr treatment in cancer cells (SW480). But we also found that the higher concentration (1 μM) of “P” or “R” moderately induces cellular apoptosis at 72 hr treatment in HFF normal cells (Figure S4). We performed cell viability assays and obtained a dose-response curve of prodigiosin in cancer cells (SW480) and normal cells (HFF) (Figure S5). The dose-response curve demonstrates that 1.11 μM prodigiosin induces 83.90% growth inhibition in SW480 cells and 76.16% growth inhibition in HFF cells. However, at a lower dose (123 nM), prodigiosin does not induce growth inhibition in HFF normal cells, but dramatically induces growth inhibition in SW480 cancer cells. The IC50 of prodigiosin in HFF normal cells is 476 nM, which is 3–10 times higher than the IC50s of cancer cell lines, except for the RXF393 cancer cell line. In the curve, we observed that there is a large gap between HFF and SW480 at concentrations from 123 nM to 1.11 μM, which indicates that prodigiosin in the range of concentrations (100 nM – 1 μM) induces preferential killing of cancer cells as compared to normal cells (Figure S5). To indicate the effect of prodigiosin on the p53 pathway in normal cells, we treated normal cells HFF and MRC-5 (human lung fibroblasts) with 1 μM prodigiosin for 12 hr. Western blotting indicates that at 12 hr treatment, 1 μM prodigioin does not induce p21, and slightly induces DR5 expression in normal cells (HFF and MRC-5); But DR5 and p21 are dramatically induced in Fadu cancer cells, a p53 mutant head and neck cancer cell line (Figure S6). The data suggest that prodigiosin exhibits a preferential ability to induce the p53 signaling pathway in cancer cells compared to normal cells. These findings indicate that prodigiosin or compound R preferentially induce the death of cancer cells rather than normal cells. This is an interesting and important observation indicating there may be a therapeutic index for these small molecules screened to restore signaling in the p53 pathway within mutant p53-expressing cancer cells.
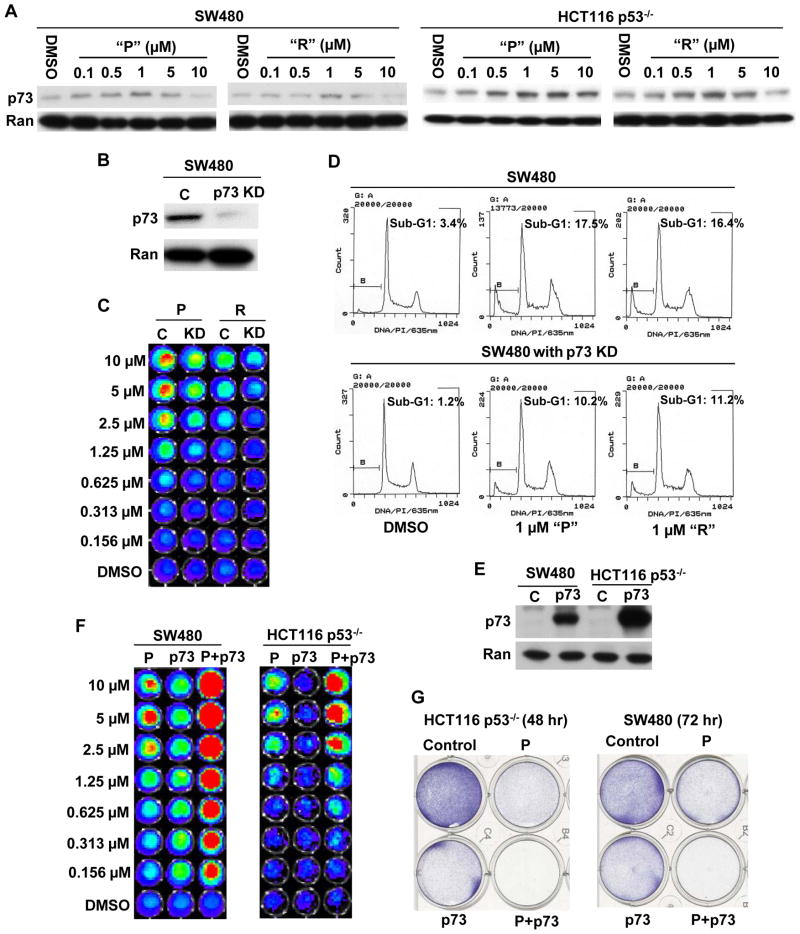
The p53 family member, p73 is involved in the mechanism of p53 pathway restoration by prodigiosin or compound R in p53-mutant and p53-null cancer cells
We observed that prodigiosin and compound R induce p53-like transcriptional activity and its downstream genes in p53-null cancer cells. p53 expression was not found to be induced by prodigiosin in SW480 (p53 mutant) and HCT116 (p53 wild type) cells, but p53 target proteins (DR5, p21 and PUMA) were induced by prodigiosin in SW480 and HCT116 cells (Figure S7). These results suggest that prodigiosin and compound R can restore p53 transcriptional responses in a p53-independent manner. p73, a p53 family protein, can induce cell cycle arrest and/or apoptosis in a p53-like manner and recognizes the same genomic DNA-binding responsive element as p53. Therefore, we hypothesized that prodigiosin and compound R induce p73 expression to activate p53 pathway signaling leading to cellular apoptosis in p53-deficient cancer cells. Indeed, as shown in figure 4A, “P” or “R” appear to induce p73 expression in mutant p53-expressing SW480 and p53−/− HCT116 cells. p73 expression is also induced in p53 wild type HCT116 cells (Figure S7). p63 is another p53 family protein, which can functionally replace p53. We examined the level of p63 when SW480 and HCT116 p53−/− cells were treated with prodigiosin. Western blotting shows that prodigiosin does not induce p63, and even decreases p63 level as concentrations increase in the SW480 and HCT116 p53−/− cells (Figure S8). These data suggest that among the p53 family members p53, p63 and p73, prodigiosin is specific to p73 in restoring the p53 signaling pathway.
Figure 4. p73 is involved in p53 pathway restoration and apoptosis induction by prodigiosin or compound R in p53-mutant and p53-null cancer cells.
(A), Prodigiosin and compound R stimulate expression of p73. SW480 and HCT116 p53−/− cells were treated with the indicated concentrations of “P” or “R” for 12 hr. p73 and loading control Ran were detected by Western Blotting. (B), p73 knock down was confirmed by Western blot. (C), The induction of p53-responsive transcriptional activity by either “P” or “R” was reduced in SW480 cells with stable p73KD. SW480 p53 reporter cells (C) and SW480 p53 reporter cells with stable p73KD (KD) were treated with different concentrations of “P”, “R” or DMSO control for 2 hr. p53-responsive reporter activity was imaged by the IVIS imaging system after treatment. (D), “P” or “R” induces less apoptosis in SW480 cells with stable p73KD as compared to parental SW480 cells. SW480 cells and SW480 cells with stable p73KD were treated with 1 μM “P”, “R” or DMSO control for 48 hr. After treatment, flow cytometry was performed. (E), p73 overexpression was confirmed by Western Blot. (F), p73 overexpression enhances prodigiosin-induced p53-dependent transcriptional activity. SW480 and HCT116 p53−/− cells with p53 reporter were treated with different concentrations of “P” or DMSO control with or without infection by a p73-expressing adenovirus. p53-responsive reporter activity was imaged by the IVIS imaging system after treatment. (G), p73 overexpression enhances prodigiosin-induced apoptosis. SW480 and HCT116 p53−/− cells were treated with DMSO, p73-expressing adenovirus, 0.1 μM “P” or p73-expressing adenovirus plus 0.1 μM “P” for 48 or 72 hr. After treatment, cells viability was examined by crystal violet staining.
To further confirm that p73 plays a role in p53 pathway activation and apoptosis induction by “P” or “R”, we knocked-down p73 by using p73 shRNA and overexpressed p73 by using p73-expressing adenovirus infection. When p73 is stably knocked-down in SW480 cells (Figure 4B), we found that the induction of p53-responsive transcriptional activity by “P” or “R” is reduced in p73-knockdown SW480 cells, as compared to parental SW480 cells (Figure 4C). The sub-G1 analysis demonstrates that “P” or “R” induce less apoptosis in SW480 cells with stable p73 knockdown than parental SW480 cells (Figure 4D). When p73 is over-expressed in either SW480 or HCT116 p53−/− cells (Figure 4E), bioluminescence analysis indicates that the induction of p53-responsive transcriptional activity by either “P” or “R” is enhanced (Figure 4F). Crystal violet staining demonstrates that p73 overexpression enhances prodigiosin-induced cell death (Figure 4G). Together, these findings indicate that p73 up-regulation is involved in the mechanism of p53 pathway activation and apoptosis induction by either prodigiosin or compound R.
Prodigiosin disrupts the physical interaction between mutant p53 and p73 independent of mTOR inhibition
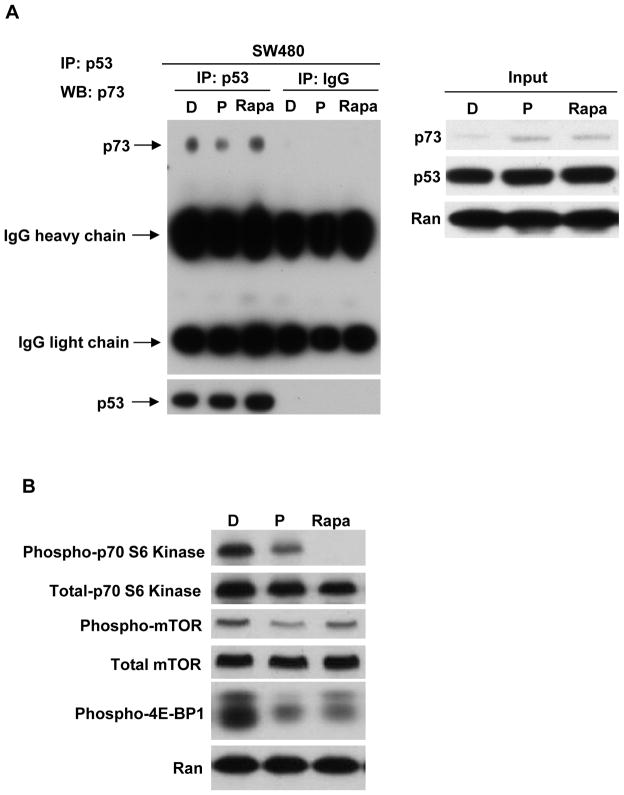
Previous studies have shown that mutant p53 can interact with p73 and abrogate p73 function (15). In order to indicate whether prodigiosin can disrupt the interaction of a mutant p53/p73 complex, we performed an immunoprecipitation assay. We observed that mutant p53 interacts with p73 and prodigiosin treatment disrupts the interaction in SW480 cells (Figure 5A).
Figure 5. Prodigiosin, but not a pure mTOR inhibitor rapamycin, disrupts the interaction of mutant p53 and p73.
(A), Immunoprecipitation of mutant p53 with p73. SW480 cells were treated with DMSO, 1 μM prodigiosin or 10 nM rapamycin for 12 hr. Cells were lysed in IP lysis buffer. Cellular extracts were immunoprecipitated with 1 μg anti-p53 antibody or normal IgG and blots were probed for p73 and p53 protein. The input was 5% of total protein used in immunoprecipitation. p73, p53 and Ran in 5% input were detected by western blot. (B), SW480 cells were treated with DMSO, 1 μM prodigiosin or 10 nM rapamycin for 12 hr. phospho-mTOR, total mTOR, phospho-p70 S6 Kinase, total p70 S6 Kinase and phospho-4E-BP1 and loading control Ran were detected by Western Blotting.
Prodigiosin has been reported to be able to bind to mTOR and inhibit mTOR activity (18). In order to determine whether prodigiosin disrupts the interaction of mutant p53/p73 complex through mTOR inhibition, we treated SW480 cells with the mTOR inhibitor rapamycin to assess whether mTOR inhibition can disrupt the interaction. As shown in figure 5A, prodigiosin disrupts the physical interaction of mutant p53 and p73, but rapamycin does not disrupt this interaction. Western blot confirmed that both prodigiosin and rapamycin inhibit the mTOR pathway as indicated by the decrease of phospho-mTOR, phospho-p70 S6 Kinase and phospho-4E-BP1 when SW480 cells are treated with either prodigiosin or rapamycin (Figure 5B). In order to inhibit the mTOR pathway sufficiently, we also treated SW480 cells with a higher concentration of rapamycin (100 nM). We compared the inhibition of the mTOR pathway between 1 μM prodigiosin and 100 nM rapamycin treatments. Western blotting shows that 100 nM rapamycin induces a greater inhibition of p-mTOR and p-p70 S6 kinase as compared to 1 μM prodigiosin. The inhibition of p-4E-BP1 is same between prodigiosin and rapamycin treatments (Figure S9A). Then we performed the immunoprecipitation assay. The result shows that 100 nM rapamycin still does not disrupt the interaction of mutant p53/p73, but prodigiosin does (Figure S9B). The result suggests that prodigiosin disrupts the interaction of mutant p53/p73 complex independent of mTOR inhibition.
Prodigiosin induces p53-dependent transcriptional activity and inhibits tumor growth in vivo in a p73-dependent manner
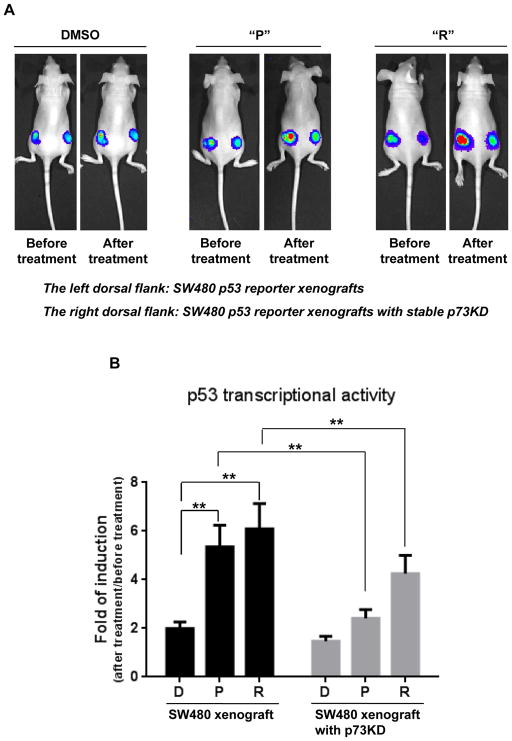
To evaluate the anti-tumor efficacy of prodigiosin (P) and compound R (R) in vivo, we established a human tumor xenograft model by subcutaneously injecting human colon cancer cells into nude mice. Tumor-bearing mice were treated with either “P” or “R” by intraperitoneal injection. We first investigated whether “P” or “R” could stimulate p53-responsive transcriptional activity in living tumor xenografts (SW480 xenografts with p53 reporter and SW480 p73 knockdown xenografts with p53 reporter). Before we performed the first drug treatment, we detected the baseline luciferase activity (p53 responsive transcriptional activity) by bioluminescence imaging. After 12 hr of DMSO, “P” or “R” treatment, the luciferase activity was detected again. As shown in figure 6, either “P” or “R” treatment significantly increases p53 responsive transcriptional activity as compared with DMSO treatment in SW480 xenografts, moreover the induction of p53 responsive transcriptional activity by either “P” or “R” treatment appears to be significantly reduced in SW480 xenografts with stable p73 knockdown. The in vivo results are consistent with our earlier in vitro results that show either prodigiosin or compound R induce less p53-responsive transcriptional activity in SW480 cell with stable p73 knockdown as compared to the parental SW480 cells.
Figure 6. Prodigiosin and compound R induce p53-dependent transcriptional activity in vivo in a mutant p53-expressing human colon tumor xenograft.
(A), Tumor cells (SW480 p53 reporter cells and SW480 p53 reporter cells with stable p73KD) were inoculated into nude mice. After 1 week, mice were treated with 5mg/kg of “P”, “R” or DMSO. Before treatment, bioluminescence imaging was performed using the IVIS imaging system. After 12 hr of drug or control treatment, imaging was performed again. (B), The bar graph indicates the quantitative data for luciferase signal from 6 mice in each group. The graph shows the fold of induction of p53 transcriptional activity (After treatment/before treatment) in the “P”, “R” or DMSO groups. The mean fold of induction ± SEM is shown. (** P < 0.01, “P” vs “D” or “R” vs “D” by unpaired t-test; ** P < 0.01, SW480 vs SW480 p73KD in “P” or “R” treatment by paired t-test).
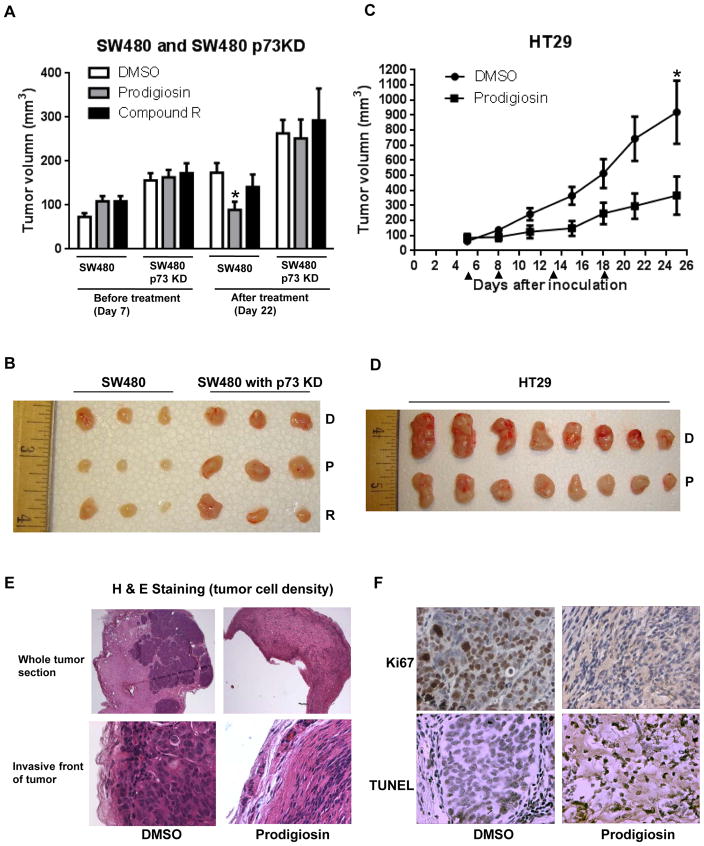
The anti-tumor efficacy of “P” and “R” was evaluated in vivo by measuring tumor volumes. In SW480 xenografts, tumor volume in prodigiosin-treated mice appeared to be significantly reduced as compared with the DMSO-treated mice (Figure 7A and 7B). However, compound R appears to be toxic due to unknown reasons. Three mice (from the total of eight mice in the “R” treated group) died after the second drug treatment. For compound R treatment, there was no significant anti-tumor growth effect observed at the end of the experiment (Figure 7A and 7B). Importantly, we found that tumor growth of SW480 p73 knockdown xenografts is enhanced as compared with SW480 xenografts (Figure 7A and 7B). Prodigiosin or compound R do not reduce tumor growth in SW480 p73 knockdown xenografts (Figure 7A and 7B). An additional human colon tumor xenograft, HT29 xenograft (p53 mutant R273H), was also used to test the antitumor efficacy of prodigiosin. As shown in figures 7C and 7D, prodigiosin significantly inhibits tumor growth of HT29 xenograft as compared with DMSO-treated control. H&E staining of tumor sections revealed that prodigiosin treatment decreases tumor cell density (Figure 7E). The tumor cell density is also reduced at the invasive front of the tumor upon prodigiosin treatment as compared to DMSO treatment (Figure 7E). Ki67 expression was found to be significantly decreased in prodigiosin-treated tumors as compared to DMSO-treated tumors (Figure 7F and Figure S10), while apoptosis (TUNEL staining) is significantly increased in the same prodigiosin-treated tumors as compared to the DMSO-treated tumors (Figure 7F and Figure S10). No significant difference in body weight was observed between DMSO control group and prodigiosin treatment group (Figure S11). The results indicate that prodigiosin inhibits tumor growth and that the anti-tumor effect of prodigiosin is associated with p73 expression and requires p73 in the mouse xenograft model.
Figure 7. Prodigiosin inhibits tumor growth in vivo.
(A), SW480 xenografts and SW480 p73KD xenografts were treated with 5mg/kg prodigiosin, compound R or DMSO every 4 days for a total of three treatments. The mean tumor volume ± SEM before treatment and after treatment is shown (* P < 0.05 by unpaired t-test). (B), Image of three representative tumors from each group (SW480 xenografts and SW480 p73KD xenografts). (C), HT29 xenografts were treated with 5mg/kg prodigiosin and DMSO control for four times. The arrows show the days of the treatment. The mean tumor volume ± SEM is shown (* P < 0.05 by unpaired t-test). (D), Imaging of tumors from each group (HT29 xenografts). The tumors were excised at the end of the experiment (day 25 after inoculation). (E), H & E staining shows that prodigiosin treatment decreases tumor cell density in the whole tumor section and the invasive front of tumor. (F), Ki67 and TUNEL staining.
Discussion
Rescue of deficient p53 function is an attractive strategy for cancer therapy. However, p53 is not an easy drug target due to many different mechanisms that result in its deficiency. An ideal compound for functional p53 rescue therapy is the one that can rescue the p53 pathway by overcoming different mechanisms of p53 loss of function in p53-deficient cancer cells. In our study, we discovered that either prodigiosin or compound R can rescue p53 signaling pathway and induce tumor cell death in mutant p53-expressing or p53-null cancer cells. Our data also show that prodigiosin possess the capability of p53 pathway activation as well as apoptosis induction in a panel of cancer cell lines with p53 hotspot mutations. Therefore, our study suggests prodigiosin or its structural analogues as candidates for p53 rescue-based drug development, regardless of p53 deficiency status. Since p53 has been a challenge to target directly by small molecules, several advantages make p73 an alternative target (8). First, p73 is expressed in tumor cells and is almost never mutated. Second, p73 induces cell cycle arrest and/or apoptosis in p53-like manner. Third, p73 can rescue the p53 pathway in various p53-deficient cancer cells. Our data indicate that both prodigiosin and compound R up-regulate p73 expression. p73 is required for the restoration of p53 signaling as well as observed in vitro and in vivo anti-tumor effects of either prodigiosin or compound R. Our study suggests that p73 induction could rescue p53 inactivation in almost all p53 deficient cancer cells, including p53-mutant and p53-deleted cancer cells.
p73 activity has been shown to be inhibited by mutant p53 in cancer cells (15). Thus, although p73 expression is up-regulated by prodigiosin, it is likely that the increased p73 is inhibited by interacting with the overexpressed mutant p53 in cancer cells. Our study indicates that prodigiosin not only up-regulates p73, but also prevents p73 from interacting with mutant p53. Therefore, the dual effects of prodigiosin make it a very promising candidate in anti-cancer drug development. Prodigiosin has been demonstrated to be able to inhibit mTOR activity (18) and a previous study has indicated that mTOR inhibition can up-regulate p73 (19). Therefore, it is possible that in our study prodigiosin may have up-regulated p73 through mTOR inhibition. However, it is interesting and uniquely important that mTOR inhibition by rapamycin does not disrupt the physical association between mutant p53 and p73, whereas prodigiosin efficiently disrupts this p53 pathway inhibitory interaction. Our study demonstrates that prodigiosin targets p73 by dual effects (up-regulation of p73 expression and disruption of mutant p53/p73 interaction), however a pure mTOR inhibitor rapamycin targets p73 only by up-regulation of its expression. Our study implies that p73 up-regulation by mTOR inhibition may not have a potent anti-tumor effect in p53 mutant cancers, as overexpressed mutant p53 would be expected to inhibit p73 function by protein-protein interaction.
Compared with other agents that disrupt the interaction of mutant p53 and p73, such as short interfering mutant p53 peptide/SIMPs (16) and RETRA (14), prodigiosin has an advantage because of its dual effects. SIMPs and RETRA target p73 only by disrupting its interaction with mutant p53 and therefore they are only effective in p53 mutant cancer cells; however, prodigiosin possesses the dual ability (up-regulating p73 expression and disrupting its interaction with mutant p53), and therefore prodigiosin is effective in p53 mutant and p53 null cancer cells. In other words, prodigiosin can restore the p53 pathway and induce anti-tumor effects in cancer cells, regardless of p53 status.
A major desirable characteristic of any potential anti-cancer drug is that it preferentially kills cancer cells leaving normal cells unaffected. Our study clearly shows that relatively low concentrations of prodigiosin or compound R do not induce cell death of normal cells in culture, while causing significant cell death of cancer cells. Our study is consistent with previous studies that reported a lack of toxicity of prodigiosin to normal cells. Montaner et al. reported that prodigiosin induces apoptosis in hematopoietic cancer cells with no marked toxicity in nonmalignant cells (20). Yamamoto et al. reported that a prodigiosin derivative, cycloprodigiosin hydrochloride inhibits the growth of liver cancer cells with very low IC50, while it does not inhibit the growth of isolated normal rat hepatocytes (21). Therefore, these published data together with our present results suggest that prodigiosin is a highly potent inhibitor of cancer cells with no marked toxicity toward normal cells at relatively low doses. It is important to note that no prior study has linked p53 or p73 to the mechanism of action of either prodigiosin or its related structures and no prior study has demonstrated the ability of these compounds to restore p53 pathway signaling in mutant p53 expressing or p53-null cancer cells.
We further investigated the effect of prodigiosin and compound R on tumor growth in vivo. We found that p53 responsive transcriptional activity is activated by prodigiosin and compound R in vivo. Prodigiosin treatment at very low dose (5 mg/kg) effectively suppresses tumor growth. Yamamoto et al. has reported that a prodigiosin derivative, cycloprodigiosin hydrochloride, significantly inhibits the growth of xenografted Huh-7 liver cancer cells at 1 or 10mg/kg concentrations (21). Our result shows that compound R is toxic in a mouse model, although it does not kill normal cell in vitro. It is possible that the in vivo toxicity of compound R may be due to a modification of the structure. Importantly, our in vivo experiments reveal that SW480 xenografts with stable p73 knockdown grow faster than SW480 xenografts. SW480 xenografts with stable p73 knockdown are also resistant to prodigiosin, suggesting that p73 down-regulation enhances tumorigenesis and confers resistance to prodigiosin. A synthetic prodigiosin derivative, GX15-070 (Obatoclax) has been in phase I and II clinical trials to treat various types of cancers through a mechanism unrelated to the p53 pathway and we have not evaluated Obatoclax in our experiments (22). Therefore, prodigiosin and its structural analogues have potential for further clinical development based on our reported findings.
We conclude that prodigiosin rescues a deficient p53 signaling pathway and induces anti-tumor effects via a dual mechanism involving p73 up-regulation and disruption of the mutant p53/p73 complex. Our study suggests that p73 is a viable and potent alternative target to reactivate the p53 pathway in p53-deficient cancers. We hypothesize that in mutant p53-expressing tumors it will be necessary to target mutant p53 as an important component of strategies aimed at stimulation of p73 to restore p53 pathway signaling and to achieve potent anti-tumor effects. Prodigiosin could serve as a promising lead compound for the development of anti-cancer drugs that rescue p53 activity through multiple effects on the complex p53 network, and offer a therapeutic index with demonstrated in vivo anti-tumor effects.
Supplementary Material
Acknowledgments
The authors thank Fang Chen for technical assistance with the early part of this work. The work was presented in part at the 15th international p53 workshop in Philadelphia (October, 2010) and the AACR 102nd and 103rd annual meetings in Orlando and Chicago (April, 2011 and April, 2012). The work was supported in part by NIH grants to W.S.E-D. W.S.E-D. is an American Cancer Society Research Professor.
Footnotes
Conflict of Interest Disclosure:
W.S.E-D. is a co-founder and share-holder of Oncoceutics, Inc., has disclosed his relationship with Oncoceutics including potential conflict of interest to his academic institution/employer and is fully compliant with institutional policy that is managing this potential conflict of interest.
References
- 1.Chen F, Wang W, El-Deiry WS. Current strategies to target p53 in cancer. Biochem Pharmacol. 2010;80(5):724–30. doi: 10.1016/j.bcp.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Prabhu VV, Allen JE, Hong B, Zhang S, Cheng H, El-Deiry WS. Therapeutic targeting of the p53 pathway in cancer stem cells. Expert Opin Ther Targets. 16(12):1161–74. doi: 10.1517/14728222.2012.726985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu C, El-Deiry WS. Targeting p53 for enhanced radio- and chemo-sensitivity. Apoptosis. 2009;14(4):597–606. doi: 10.1007/s10495-009-0330-1. [DOI] [PubMed] [Google Scholar]
- 4.Rivlin N, Brosh R, Oren M, Rotter V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer. 2011;2(4):466–74. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donehower LA, Harvey M, Slagle BL, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356(6366):215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 6.Kressner U, Inganas M, Byding S, et al. Prognostic value of p53 genetic changes in colorectal cancer. J Clin Oncol. 1999;17(2):593–9. doi: 10.1200/JCO.1999.17.2.593. [DOI] [PubMed] [Google Scholar]
- 7.Katkoori VR, Jia X, Shanmugam C, et al. Prognostic significance of p53 codon 72 polymorphism differs with race in colorectal adenocarcinoma. Clin Cancer Res. 2009;15(7):2406–16. doi: 10.1158/1078-0432.CCR-08-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leslie M. Brothers in arms against cancer. Science. 2011;331(6024):1551–2. doi: 10.1126/science.331.6024.1551. [DOI] [PubMed] [Google Scholar]
- 9.Hainaut P, Hernandez T, Robinson A, et al. IARC Database of p53 gene mutations in human tumors and cell lines: updated compilation, revised formats and new visualisation tools. Nucleic Acids Res. 1998;26(1):205–13. doi: 10.1093/nar/26.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jost CA, Marin MC, Kaelin WG., Jr p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 1997;389(6647):191–4. doi: 10.1038/38298. [DOI] [PubMed] [Google Scholar]
- 11.Bisso A, Collavin L, Del Sal G. p73 as a pharmaceutical target for cancer therapy. Curr Pharm Des. 2011;17(6):578–90. doi: 10.2174/138161211795222667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu C, Wang W, El-Deiry WS. Non-genotoxic anti-neoplastic effects of ellipticine derivative NSC176327 in p53-deficient human colon carcinoma cells involve stimulation of p73. Cancer Biol Ther. 2008;7(12):2039–46. doi: 10.4161/cbt.7.12.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rana S, Gupta K, Gomez J, et al. Securinine induces p73-dependent apoptosis preferentially in p53-deficient colon cancer cells. FASEB J. 2010;24(6):2126–34. doi: 10.1096/fj.09-148999. [DOI] [PubMed] [Google Scholar]
- 14.Kravchenko JE, Ilyinskaya GV, Komarov PG, et al. Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc Natl Acad Sci U S A. 2008;105(17):6302–7. doi: 10.1073/pnas.0802091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Como CJ, Gaiddon C, Prives C. p73 function is inhibited by tumor-derived p53 mutants in mammalian cells. Mol Cell Biol. 1999;19(2):1438–49. doi: 10.1128/mcb.19.2.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Agostino S, Cortese G, Monti O, et al. The disruption of the protein complex mutantp53/p73 increases selectively the response of tumor cells to anticancer drugs. Cell Cycle. 2008;7(21):3440–7. doi: 10.4161/cc.7.21.6995. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Kim SH, El-Deiry WS. Small-molecule modulators of p53 family signaling and antitumor effects in p53-deficient human colon tumor xenografts. Proc Natl Acad Sci U S A. 2006;103(29):11003–8. doi: 10.1073/pnas.0604507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espona-Fiedler M, Soto-Cerrato V, Hosseini A, et al. Identification of dual mTORC1 and mTORC2 inhibitors in melanoma cells: prodigiosin vs. obatoclax. Biochem Pharmacol. 83(4):489–96. doi: 10.1016/j.bcp.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbluth JM, Mays DJ, Pino MF, Tang LJ, Pietenpol JA. A gene signature-based approach identifies mTOR as a regulator of p73. Mol Cell Biol. 2008;28(19):5951–64. doi: 10.1128/MCB.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montaner B, Navarro S, Pique M, et al. Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br J Pharmacol. 2000;131(3):585–93. doi: 10.1038/sj.bjp.0703614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto C, Takemoto H, Kuno K, et al. Cycloprodigiosin hydrochloride, a new H(+)/Cl(−) symporter, induces apoptosis in human and rat hepatocellular cancer cell lines in vitro and inhibits the growth of hepatocellular carcinoma xenografts in nude mice. Hepatology. 1999;30(4):894–902. doi: 10.1002/hep.510300417. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104(49):19512–7. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.