Abstract
Peyronie’s Disease (PD) is a superficial fibrosing disorder of the penis resulting in plaque formation and penile deformity. Once considered rare, PD has more recently been found in up to 13% of men, and can negatively affect sexual and psychosocial function of both patients and their partners. While the etiology of PD is unclear, it is thought to result from an inciting traumatic event followed by aberrant fibrosis or dysregulated wound healing. The evaluation of men presenting with PD includes a detailed history and physical exam, focusing on the penis in both the flaccid and erect states. PD is often associated with erectile dysfunction (ED), as well as several other comorbidities. Laboratory testing is not needed to diagnose PD, although given the associations between PD and systemic diseases including hypogonadism, diabetes, and cardiovascular disease, screening and work-up for these conditions in men with PD may be warranted. Treatment modalities for PD are diverse and include oral, topical, intralesional, mechanical, and surgical therapies. Oral, topical, and mechanical therapies generally have little evidence supporting their efficacy. Several intralesional therapies, including interferon α2b and collagenase Clostridium hystiolyticum have demonstrated efficacy in the treatment of PD. Surgical treatment, indicated in men with significant, stable deformity, includes plication of the tunica albuginea, plaque incision/excision and grafting, and placement of inflatable penile prosthesis (IPP) with or without additional maneuvers to achieve desired results, and has high success rates.
Keywords: Peyronie’s disease, Duplex Doppler ultrasound, Tunical plication, Plaque incision and grafting, Intralesional therapy, Oral Peyronie’s disease therapy, Dupuytren’s disease, Ledderhose disease, Genetics
INTRODUCTION
Peyronie’s Disease (PD) is a superficial fibrosing condition of the penis characterized by the presence of fibrotic plaques often leading to penile deformity, with or without concomitant pain. Men with PD most commonly present in their sixth decade of life, with a mean age of 52-57 years old.1-4 PD impacts sexual function and is also associated with psychosocial distress in patients and their partners. Once thought to be rare, PD now has a reported prevalence of up to 13.1% in adult men.5
François Gigot de la Peyronie, the French physician and surgeon to King Louis XV, is credited with the initial description of the disease.6 However, several written reports precede la Peyronie’s seminal publication, dating back to Theoderic Borgogni of Bologne in the 13th century.7 Despite its long history, a complete understanding of the etiologies and natural history of PD remain elusive, and clear-cut patient treatment algorithms are lacking. La Peyronie treated his patients with topical mercury without success, then reported complete resolution in one patient after treatment in the holy water of a French thermal spa at Barèges.8 Since that time, a plethora of surgical and non-surgical treatments have been described, all with a scientific basis, although defining efficacy with most treatments has been challenging due to suboptimal or small studies.
Given the high prevalence of PD and its significant impact on affected men, a better understanding of PD is essential. The purpose of this review is to synthesize and summarize the current understanding of PD etiology, diagnosis, and management.
PREVALENCE AND SIGNIFICANCE
The prevalence of PD is variable between studies, ranging from 0.39% in a population of men from Rochester, Minnesota3 to 3.2% based on a survey of 4,432 subjects from the Cologne region of France.4 A large, web-based survey of 11,420 subjects estimated PD prevalence in the U.S. at 0.5-13.1%, with variability due to differences in PD diagnostic criteria.5 The prevalence of PD has also been studied in various sub-populations. In 2004, Mulhall et al. reported a PD prevalence of 8.9%, with PD diagnosed by the presence of a palpable plaque on physical exam, in a cohort of 534 men undergoing prostate cancer screening.9 Subsequently, Tal et al. reported PD in 15.9% of 1,011 men after radical prostatectomy.10 El-Sakka observed 7.9% of 1,440 men being evaluated for ED with concomitant PD,1 using strict PD diagnostic criteria including the presence of a palpable nodule or penile curvature observed after intracorporal injection of prostaglandin E1. Arafa et al. reported a high proportion (20.3%) of 206 diabetic men with ED as also having PD, identifying diabetes as a potential PD risk factor.11
In addition to physical symptoms, which can limit sexual function, PD can cause significant psychosocial distress for patients and their partners.12-14 Feelings of shame and embarrassment often accompany the physical symptoms of PD, with up to 81% of men reporting "emotional difficulties."13 These feelings may persist or worsen with progression of the disease; depression has been reported in up to 48% of men.12 The negative psychological impact of PD on patients and their partners is not always apparent to clinicians, and must be considered when approaching the patient with PD. Only recently has psychosocial function in men with PD been evaluated using validated methods. In 2008, Nelson et al. used two questionnaires to evaluate the psychosocial status of 92 men with PD: The Center for Epidemiological Studies Depression scale (CES-D) and the Mental Health subscale of the Short Form-36 (SF-36), which assesses quality of life.12 Remarkably, 48% of men in the cohort were depressed based on the CES-D, and the incidence of depression did not vary with length of time from PD diagnosis up to >18 months, suggesting a lack of adjustment to the PD diagnosis. The PD cohort also had a significantly lower average Mental Health subscale on the SF-36 compared to the general male population, indicating overall worse mental health in these men. The first PD-specific questionnaire, the Peyronie’s Disease Questionnaire (PDQ), was developed and validated by Hellstrom et al. in 2013.15 The PDQ assesses the severity of both physical and psychological symptoms of PD, as well as symptom bother. Such tools are invaluable in the comprehensive assessment of the patient with PD, fostering the multidisciplinary approach that is necessary to best treat the multifaceted nature of this disorder.
ETIOLOGY AND MODIFIABLE RISK FACTORS
The etiology of PD is multifactorial and incompletely understood. The most prevalent etiological theory implicates penile trauma, including both acute traumatic events as well as repetitive microtrauma such as that which occurs during intercourse, as the inciting event for PD.16 Multiple other risk factors can also predispose to development of PD (TABLE 1).
Table 1.
Modifiable Risk Factors for Peyronie’s Disease
| Acute penile trauma or microtrauma |
| Penile / genital trauma from urologic procedures (e.g. Foley catheterization, cystoscopy, transurethral resection of the prostate, radical prostatectomy) |
| History of nongonococcal urethritis |
| History of inflammatory diseases or fibromatous lesions of the genital tract in sexual partner(s) |
| Smoking |
| Hypogonadism |
| ?Diabetes |
| ?Hypertension and other cardiovascular disease risk factors |
| ?Alcohol use |
It is important to point out that data on modifiable PD risk factors come almost entirely from retrospective studies, limiting their generalizability. Modifiable risk factors for PD include a personal history of nongonococcal urethritis, genital and/or perineal trauma, and iatrogenic trauma such as urethral catheterization, cystoscopy, or transurethral resection of the prostate.16,17 Radical prostatectomy is also associated with increased risk of PD, with a 15.9% incidence of PD observed in men following radical prostatectomy.10 An increased risk of PD in men with sexual partners having a history of inflammatory diseases of the genital tract,16 fibromatous lesions of the genital tract,16 and surgery on the genital tract has also been reported.16,17 While a history of smoking has been implicated as a PD risk factor,16,18 it is unclear whether this is related to the amount of smoking. The impact of alcohol use in PD is unclear, with one study supporting an association,16 and another, larger study failing to show a relationship.18 Possible associations with diabetes, hypertension, and/or other cardiovascular conditions have been suggested,11,16 but other studies have failed to corroborate these findings.17,18
Hypogonadism is also a possible risk factor for PD.19 Recent work suggests a high prevalence of hypogonadism in men with PD, with one study observing hypogonadal testosterone (T) levels (<300 ng/dL) in 74.4% of 121 PD patients.19 In hypogonadal men with PD, penile curvature may be greater than in men with PD and normal T levels.19,20 While yet another study observed similarly high rates of low T levels in men with PD and ED, no correlation between low T and degree of penile curvature was observed.21 In contrast, yet another study failed to show a difference between T levels in men with PD compared to controls, although the study was small and likely underpowered.22 Interestingly, significantly lower adrenal androgen levels in the PD group compared to controls were observed, leading to a hypothesis that hypogonadism or androgen deficiency may play a role in PD pathogenesis because androgens modulate matrix metalloproteinases that are important in normal wound healing. If these androgens are deficient, the risk of PD increases.
Erectile dysfunction (ED) has been observed in up to 32% of men presenting with PD.23 However, it is unclear whether one condition precedes the other or if they occur synchronously. Of note, fibrosis of corporal cavernosal blood vessels has been implicated in both the development of vasculogenic ED as well as PD, providing a potentially unifying pathogenic mechanism.24-26 In men with both PD and ED, it is important to consider that both organic and psychogenic factors may contribute to both conditions, which can impact treatment approaches.27 In summary, while numerous risk factors have been implicated in the development of PD, additional work is required to strengthen these associations. Furthermore, a more complete understanding of the molecular pathways that may link these disease states is essential in establishing and expanding the growing network of interactions between PD and other conditions.
HISTOPATHOLOGY AND MOLECULAR BIOLOGY
The tunica albuginea, a fascial structure surrounding the penile corpora cavernosa, provides both the pliancy of the penis when flaccid as well as its rigidity when erect.28 Normal tunica albuginea consists of an organized lattice of elastin and collagen arranged around the penile corpora in inner circular and outer longitudinal layers, each consisting of multiple sublayers.28 PD is characterized by focal tunical fibrosis, resulting in plaque formation and penile curvature or deformity due to the decreased pliancy of the tunica albuginea involved with plaque, with or without penile pain.4 Ultrastructural and histologic changes in the tunica have been described in animal models of PD, as well as in biopsy and cadaveric specimens from patients with PD. These changes include collagen deposition in abnormally dense clumps and disordered, fragmented, sparse elastin fibers.28-30 The abnormal presence of fibrin28,29,31 or ossification in a linear configuration29 may also be seen. In one series, fibrin deposition was seen in 95% of PD plaque samples and in no controls.31 Increased peri-tunical cellularity has also been described in PD patients, which may manifest as a perivascular lymphocytic infiltrate surrounding the tunica or may involve the tunica itself.29 These histopathologic changes are thought to develop from abnormal inflammation and wound healing.4,32-36 Trauma to the microvasculature of the erect penis and subsequent fibrin extravasation may initiate wound healing, which in susceptible patients is thought to progress to fibrosis and plaque formation.31
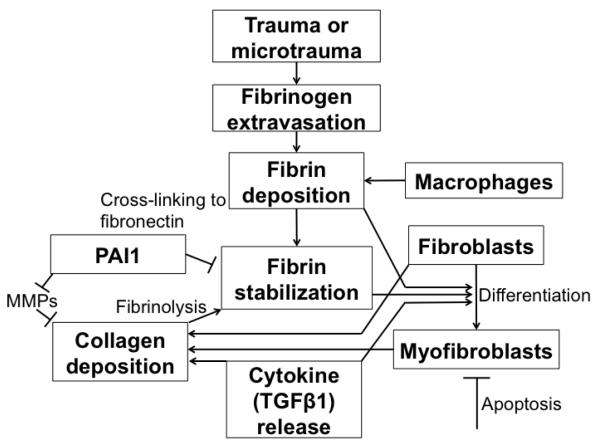
The cellular and biochemical changes that occur in normal wound healing and in PD have been studied in cell culture and in animal models. In the setting of trauma, extravasation of fibrin initiates the release of multiple cytokines, including transforming growth factor β (TGFβ) and plasminogen activator inhibitor 1 (PAI1), and an increase in reactive oxygen species (ROS), which stimulates differentiation of fibroblasts into myofibroblasts (FIGURE 1).37,38 TGFβ is a secreted protein that promotes tissue repair and inflammation, can induce monocyte infiltration and angiogenesis, and can modulate other inflammatory mediators.39 TGFβ also stimulates the synthesis of extracellular matrix components (ECM) including collagen, while concurrently inhibiting ECM degradation by proteases.39 Myofibroblasts synthesize collagen and are important in normal wound healing.37 During normal wound healing, myofibroblasts differentiate, help to repair the wound, and then undergo apoptosis. In abnormal wound healing, however, the persistence of myofibroblasts may cause continued accumulation of collagen, leading to fibrosis.37 While myofibroblasts are common in PD plaques from humans and animal models, they are rarely seen in normal human tunica albuginea.40 Nitric oxide (NO) is a potent antifibrotic mediator and can decrease collagen deposition and myofibroblast number in culture, possibly by inhibiting myofibroblast differentiation or by promoting apoptosis. 41
Figure 1.
Histopathology of Peyronie’s Plaque Formation (adapted from 37)
[Place text below underneath Figure 1]
MMPs – matrix metalloproteinases
PAI1 – plasminogen activator inhibitor 1
Rat PD models have been developed using injection of profibrotic agents, including cytomodulin (a synthetic heptapeptide with TGFβ-like activity)39 and fibrin42 into the tunica albuginea. A rat model developed by El-Sakka et al. compared incision of the tunica albuginea followed by suture repair to animals receiving a sham operation.43 Histopathological changes similar to those seen in the acute phase of PD were observed in rats undergoing tunical incision from 6 hours to 8 weeks after injury. Transient elevations in TGFβ1 were observed in some of the rats 6 hours to 3 days after injury, with resolution by 8 weeks after injury. These tunical changes in test rats were more consistent with normal wound healing than a Peyronie’s-like plaque formation, indicating that a single event in otherwise normal animals may not be sufficient to induce PD. Trauma alone may not be sufficient to induce plaque formation in an otherwise normal organism, and genetic factors have been linked to PD, suggesting that a predisposition to fibrosis may be necessary to cause disease.
GENETICS
While all sexually active men are exposed to some level of penile trauma as a result of sexual activity, few develop PD. Multiple small, retrospective studies showed an association between PD and various histocompatibility antigens (HLA-B7,44 HLA-A1, B8, Cw7, DR3, and DQw2,45 and HLA-DQ546), suggesting a genetic predisposition to PD. A variant of PD with autosomal dominant inheritance was described in 3 pedigrees by Nyberg et al., further supporting a genetic link.47 An association between the autosomal dominant form of PD and HLA-B7 was noted, with HLA-B7 present in 90% of men with PD in the study. However, other published reports, including a more recent prospective study of 154 consecutive PD patients,48 have failed to show an association between PD and HLA subtypes.49,50
PD has also been associated with Duputyren’s disease (DD), a superficial fibrotic diathesis that involves the palmar fascia, resulting in fibrotic nodules and hand contractures. The two conditions often occur coincidentally, with one study observing a 21% incidence of DD in a cohort of 134 PD patients, as compared with 0% in a control group of equivalent size.17 A strong association with DD was also noted in the autosomal dominant form of PD, in which 78% of PD-affected individuals concurrently suffered from DD.47 A more recent case-control study by Bjekic et al. revealed a 39% rate of DD in their PD population (n=82), compared to 1.2% in the 246 controls (p<0.01).16 Family history of DD was also a significant risk factor, present in 9.8% of cases compared with 0% of controls (p<0.001).
Multiple genes that promote fibrosis have increased expression in both PD and DD plaques when compared to normal control tissues using gene expression microarray analyses.51 These upregulated genes include matrix metalloproteinases (MMP2, MMP9), matrix metalloproteinase activators (thymosins TMβ10, TMβ4), osteoblast-specific factors (OSF-1), and genes involved in myofibroblast differentiation (RhoGDP dissociation inhibitor 1). These genes that are common to both conditions further suggest that both PD and DD may have a common etiology that is influenced by genetics.
MALIGNANT POTENTIAL
While no association between PD and malignancy in humans exists, a link between fibrotic conditions and malignancies has begun to emerge. Cell proliferation and chromosomal instability are well-known hallmarks of malignancy, playing an important role in every phase of cancer from malignant cell transformation and progression/invasion, to development of intra-tumor heterogeneity with clonal resistance to various cancer therapies. Fluorescence in situ hybridization (FISH) has demonstrated chromosomal instability in fibroblast cultures derived from DD lesions, with numerical (autosomal trisomies and monosomies, gain or loss of Y chromosome) and structural (insertion/deletion) chromosomal abnormalities described.52,53 FISH has also shown chromosomal instability in fibroblasts derived from human PD plaques.54 Perhaps most strikingly, fibroblasts derived from human PD lesions can result in tumors when injected into immunodeficient mice55. While not related directly to PD, a recent study observed an increased frequency of certain malignancies in DD families when compared with controls, but failed to establish any statistically significant differences in cancer rates between the groups.56 The same study also reported that of 20 polymorphisms in 12 cancer susceptibility genes studied, one (D312M variant of Xeroderma Pigmentosum complementation group D (XPD) gene, also known as Excision Repair Cross Complementing Rodent Repair Deficiency group 2 (ERCC2))57 was significantly associated with DD (OR 1.75, p=0.004).56 XPD/ERCC2 polymorphisms have also been linked to an increased risk of bladder cancer.58,59 Thus, dysregulation of cellular replication or senescence that predisposes to PD or DD plaque formation may result in malignancy, although further investigation is necessary to define this relationship in humans.
NATURAL HISTORY AND PRESENTATION
Although PD was initially described over 250 years ago,6 its natural history has only recently been studied in detail. Williams and Thomas were the first to report on the natural history of PD during the 1970s, observing spontaneous symptom resolution in 50% of the patients in a small cohort.60 This high PD resolution rate has not been confirmed in subsequent studies. Twenty years later, Gelbard et al. reported a spontaneous resolution rate of only 13%, with 47% of patients remaining stable and 40% worsening over time.61 Berookhim et al. reported a 12% improvement rate in untreated men with uniplanar PD, with time to presentation of ≤ 6 months and younger age as predictors of improvement.62 Perhaps more significantly, 67% of men in this cohort remained stable, and 21% worsened during the follow-up period of at least 12 months. Another study observed a 30% progression rate in 307 men with PD over 8 months, with resolution occurring in only 0.65% of cases.63
PD most commonly affects men in the sixth decade of life,23 with mean age at diagnosis ranging from 52-57 years.1-4 However, PD may present at any time in adulthood, and patients as young as 21 years old have been reported.2 The hallmark of PD is acquired penile deformity, which must be differentiated from congenital penile curvature and normal anatomic variants. PD-related deformity consists of curvature during erection, with associated findings including loss of flaccid stretched penile length, tunical indentations or hourglass deformity with erection, and buckling or penile instability on minimal axial loading despite maximal erection.23 Unlike congenital penile curvature, in which ventral curvature predominates, curvature in PD may be in any direction, and may be uni- or biplanar. However, PD-related penile curvature is most commonly dorsal, with one study reporting dorsal curvature in 72% of patients,23 and others citing lower dorsal curvature rates (30% dorsal + 12% dorsolateral).2 Patients with dorsal curvature tend to present with more severe curvature than other PD patients.2
The natural history of PD is often divided into acute and chronic phases. The acute phase is characterized by progression of penile deformity and may be associated with pain in the erect and/or flaccid states. The length of the acute phase varies from 6-18 months.23 In contrast, the chronic phase of PD is defined by stability of penile deformity for at least 3-6 months,64,65 with improvement in or resolution of pain.64 Mulhall et al. observed that pain improved in all patients and resolved in the majority within 12 months of PD presentation, with 89% of men being pain free at last follow-up with a mean follow-up of 18 months.23
Subjective loss of penile length is a common complaint, reported by 84% of patients undergoing expectant PD management. In one study, which followed 246 men with PD, mean stretched penile length decreased from 12.2 cm on initial assessment to 11.4 cm after a mean of 14.5 months follow-up (p=0.035).23
EVALUATION
Obtaining a detailed history and performing a thorough physical exam is the lynchpin of the PD diagnosis and lays the foundation for the treatment approach. The specific timing of symptom onset, presence of potential inciting incidents, including a history of penile trauma, progression or stability of the deformity, and interference with intercourse should be noted (TABLE 2). Since PD can negatively impact a man’s psychosocial status and relationships, obtaining information regarding mood and relationship status can prompt mutidisciplinary therapeutic approaches.
Table 2.
Essential Elements of the Peyronie’s Disease History and Physical Examination
| History | Flaccid Penile Exam | Erect Penile Exam (w/ Doppler US) |
|---|---|---|
| Onset and progression of symptoms: establish acute versus stable phase |
Palpable plaque(s)? Location, size. |
Palpable plaque(s)? Location, size. |
| Penile Deformity: Palpable plaque? Direction and degree of curvature. Impedes intercourse? Presence of hourglass deformity, hinge defect, other anomaly? |
Stretched penile length | Plaque(s) visible by US? Location, size, calcification. |
| Penile Pain? Location, timing (flaccid, erect and/or with penetration), severity/progression |
Tenderness to palpation? Location, severity. |
Penile Deformity: Degree and direction of curvature. Uniplanar or biplanar? Hourglass deformity? Hinge defect? Other anomaly? |
| Presence of ED? If yes, pre-existing or new with PD symptoms? Validated questionnaire: IIEF† |
Tenderness to palpation? Location, severity. |
|
| History of penile trauma? (urologic procedure/surgery, penile fracture, etc.) |
||
| History of sexually transmitted infections? |
||
| Other medical and surgical history. Diabetes, hypertension, cardiovascular disease. |
||
| Social history: Sexual history, smoking/tobacco history, other recreational drug-use |
||
| Family history of PD or DD? | ||
| Assess distress, overall mood Consider using PDQ |
IIEF: international Index of Erectile Function
Physical examination of the penis should be performed in both the flaccid and erect states to ensure that the physician understands the anatomic impact of the PD, and the patient corroborates what he has experienced outside of the clinical setting. Objective measurement of penile curvature is essential given that patient report of curvature is inaccurate.66 In the flaccid state, the clinician should determine stretched penile length and note palpable penile plaque location and size. Men should also be examined with the penis erect, which can be most effectively accomplished using intracavernosal injection of vasoactive substances67. Concurrent use of duplex Doppler penile ultrasound allows for objective evaluation of plaque size, location, and calcification, and aids in determination of ED etiology if ED is concurrently present. Ultrasonography after intracavernosal injection is the most accurate assessment tool to determine type and degree of PD deformity and is preferred over photographs or vacuum erectile device (VED)-assisted erection.23 Accurate assessment of penile deformity is invaluable in treatment planning and in evaluating treatment results. Furthermore, given the association of PD with DD, as well as Ledderhose disease, a fibrosing disorder of the plantar fascia, examination of the palms of the hands and soles of the feet, looking for nodules and/or contractures, can be helpful.
Laboratory testing is not necessary for PD diagnosis. However, given the possible association between PD, diabetes mellitus and cardiovascular disease,11,16 screening for these comorbidities should be considered in at-risk PD patients. In men with both PD and ED, laboratory evaluation for ED risk factors, as well as evaluation of serum hormones and the hypothalamic-pituitary-gonadal axis should be performed (reviewed in 68). Serum hormone evaluation in men presenting with PD and hypogonadal symptoms may also be useful.
TREATMENT
Treatment of PD utilizes both medical and surgical approaches, and includes a diverse group of systemic and locally administered drugs. Approaches to PD have included observation, small molecule and biologic drugs administered orally, topically, and intralesionally, mechanical therapies, and surgery. Counseling and observation alone may be appropriate for patients with minimal curvature that does not impede sexual intercourse and with no ED;69 other patients will elect to proceed with treatment. It is important to note that most treatment methods have little evidence in the literature supporting their efficacy. Clinicians should also consider the psychosocial impact of PD, and refer affected, as well as their partners if appropriate, to a mental health specialist and/or sex therapist.
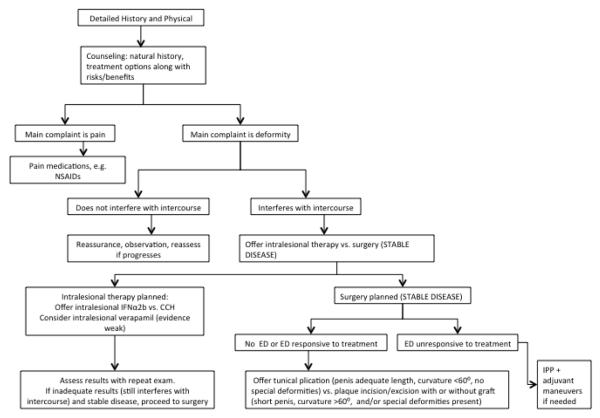
Surgical intervention should be avoided during the acute phase of PD, as the risk of progression or recurrence of curvature during this phase may interfere with optimal outcomes.65 Penile deformity and any associated ED should be stable for at least 3-6 months prior to surgical intervention to avoid progressive disease after surgical treatment.64 Resolution of penile pain should ideally be observed as well,65 as pain most frequently accompanies active disease and may indicate persistent inflammation. While medical or minimally invasive treatments have traditionally been viewed as most effective during the acute phase,69 these may be tried at any time in the disease course (FIGURE 2).
Figure 2.
Peyronie’s Disease Treatment Approach
Systemic Drugs
Numerous systemic drugs have been evaluated in the treatment of PD including procarbazine, vitamin E, propionyl-L-carnitine, acetyl-L-carnitine, tamoxifen, omega-3 fatty acids, interferon-α2a (IFNα2a), interferon-α2b (IFNα2b), pentoxifylline, L-arginine, sildenafil, colchicine, coenzyme Q10, and potassium paraaminobenzoate (POTABA). While most of the above have little proven efficacy , we highlight available evidence for each drug, with the intention of guiding clinicians towards the use of drugs that may have efficacy, and restrict our discussion to drugs that have been evaluated in humans (TABLE 3). It is important to note that many of these medications have been combined with others, and these combinations have not been thoroughly vetted or have also failed to show a beneficial effect.
Table 3.
Drugs and Biologic Agents Used in Peyronie’s Disease Treatment
| Treatment | Proposed Mechanism of Action in PD | Recommended | Recommended AGAINST? |
|---|---|---|---|
| Systemic (Oral) | |||
| Procarbazine | Cytotoxic alkylating agent (?anti-inflammatory) | No | Yes |
| Vitamin E | Anti-oxidant (↓ROS) | No | No |
| Propionyl-L-carnitine | ↓Ca2+ in endothelial cells | No | No |
| Acetyl-L-carnitine | ↓Ca2+ in endothelial cells | No | No |
| Tamoxifen | Modulation of TGF-β1 secretion by fibroblasts | No | Yes |
| Omega-3 fatty acids | Anti-inflammatory | No | No |
| Pentoxifylline | Non-specific PDE-inhibitor → ↑NO, ↓TGF-β1 expression | No | No |
| L-arginine | Precursor of NO | No | No |
| Sildenafil | PDE5-inhibitor; may ↓collagen and ↑apoptosis index within plaque |
No | No |
| Colchicine | Anti-microtubule (anti-inflammatory) | No | Yes |
| POTABA | ↑ tissue O2 uptake, ↑GAG† secretion, ↑MAO‡ activity | No | Yes |
| Coenzyme Q1083 | Anti-oxidant (↓ROS), ↓TGF-β1 expression | No | No |
| Transdermal | |||
| Verapamil (EMDA or topical gel) | Ca2+ channel antagonist; may ↓collagen synthesis and/or ↑collagenase activity |
No | No |
| Dexamethasone (EMDA w/ verapamil) | Steroid (anti-inflammatory) | No | Yes |
| Liposomal recombinant human SOD* (topical) |
Antioxidant (↓ROS) | No | No |
| Intralesional | |||
| IFNα2a | ↓fibroblast proliferation → ↓collagen synthesis | No | No |
| IFNα2b | ↓fibroblast proliferation → ↓collagen synthesis | Yes | NA |
| Verapamil | Ca2+ channel antagonist; may ↓collagen synthesis and/or ↑collagenase activity |
Yes | NA |
| Collagenase Clostridium histolyticum | Clostridial collagenase | Yes | NA |
| Liposomal recombinant human SOD | Antioxidant (↓ROS) | No | Yes |
| Non-pharmacologic, non-surgical | |||
| ESWT | Direct damage to plaque and/or ↑vascularity 2/2 heat → plaque lysis159 |
Yes | NA |
| Penile traction therapy | Mechanical straightening and/or lengthening | No | No |
| Vacuum erection device therapy | Mechanical straightening and/or lengthening | No | No |
| Radiotherapy (RT) | Unknown | No | Yes |
| Hyperthermia therapy | Unknown (possible modulation of heat-shock proteins,160 ?↑vascularity 2/2 heat159) |
No | No |
| Surgical | |||
| Tunical albugineal plication | Tunical shortening (opposite side of plaque) | Yes | NA |
| Plaque incision/excision and grafting | Tunical lengthening (side of plaque) | Yes | NA |
| IPP (with possible penile modeling, tunical plication, or plaque incision/excision and grafting) |
Mechanical straightening (w/ or w/o plaque manipulation, tunical shortening or lengthening procedures) |
Yes | NA |
GAG: glycosaminoglycans
MAO: monoamine oxidase
SOD: superoxide dismutase
Procarbazine was evaluated historically for PD treatment, but did not appear to have efficacy in improving curvature or reducing plaque.70-72 Adverse effects, including gastrointestinal symptoms, headache, and anxiety, are common.
Multiple studies have evaluated oral vitamin E in treatment of PD, alone or in combination with other treatments.71,73-76 The free-radical scavenging effect of vitamin E provides the theoretical basis for its use in PD77 although no strong evidence that it provides benefit exists. Hashimoto et al. saw no difference in pain relief, curvature improvement, plaque size reduction, or ED improvement between the vitamin E and no-medication groups in a retrospective review of 31 patients.74 A small, randomized prospective study by Inal et al. failed to show any difference between three treatment groups: vitamin E alone, intralesional IFNα2b alone, or combination therapy in 30 men.75 A larger double-blind, randomized, placebo-controlled study of 236 men by Safarinejad et al. revealed no significant improvements in pain, decreases in penile curvature or changes in plaque size between any of four treatment arms, including placebo, vitamin E alone, propionyl-L-carnitine alone, or vitamin E and propionyl-L-carnitine together.76 Only one study of combination therapy evaluating vitamin E and extracorporeal shock wave therapy (ESWT) in 35 patients observed a significant decrease in penile curvature compared to vitamin E alone.73 However, these results have not been reproduced. As such, vitamin E is not recommended treatment in the European Association of Urology (EAU) 2012 PD guideline.78
Tamoxifen may act through modulation of TGFβ1 secretion by fibroblasts.78-81 Its first application in PD was in 1992 in a small cohort of 35 men, with results demonstrating improvements in pain, penile deformity, and plaque size.80 However, this study was limited by the absence of a control group, and a subsequent randomized controlled trial (RCT) by Teloken et al. failed to show a difference in improvements in pain, curvature, or plaque size in the tamoxifen group relative to placebo.68 A randomized study comparing acetyl-L-carnitine to tamoxifen was published in 2001, suggesting that acetyl-L-carnitine was safer and more effective than tamoxifen, with a greater decrease in penile curvature in the acetyl-L-carnitine group (7.5 degrees vs. 0.5 degrees), and significantly more patients reporting improved pain (90% vs. 50%).79 However, only 48 men were included in the study, which lacked a placebo control group. The 2012 EAU guidelines do not recommend tamoxifen for PD treatment based on available data.78
Omega-3 fatty acids have been evaluated in the setting of PD in a recent large RCT.82 No differences in pain, penile curvature, or plaque size were observed between treatment and placebo groups.
Coenzyme Q10 has been evaluated in a single RCT with 93 patients in each arm (treatment vs placebo).83 Plaque size and penile curvature both decreased in the treatment group and slightly increased in the placebo group (both p=0.001). Additional studies are needed to confirm efficacy.
Pentoxifylline is a non-specific cyclic adenosine monophosphate (cAMP) phosphodiesterase inhibitor78,84 that attenuates TGFβ1-stimulated collagen deposition in cultured cells derived from human PD plaques.85 L-Arginine, a precursor of NO synthesis, pentoxyfylline, and sildenafil all decrease collagen I expression in fibroblast cultures derived from human PD plaques as well as in rats.86 Pentoxifylline use for PD treatment in humans has only been described in a single case report, which suggested that the patient’s penile curvature improved as a result of pentoxyfylline use rather than the sildenafil he was concomitantly taking.87 Despite promising results in cell culture and animal models, L-Arginine and sildenafil have not yet been evaluated for efficacy in PD patients.
Colchicine is an antimicrotubule agent88 that decreases collagen deposition and elastic fiber fragmentation in a rat model of PD.89 A small pilot study in 24 PD patients treated with colchicine appeared promising,90 as did a subsequent study in 60 patients.91 However, neither of these studies included comparison or control groups. A more recent RCT conducted in 84 patients failed to show any significant differences between colchicine and placebo in improvement in pain, penile curvature, or plaque size.92
Potassium Paraaminobenzoate (POTABA) may have an anti-inflammatory effect by enhancing oxygen uptake, fibroblast glycosaminoglycan secretion, and monoamine oxidase activity.78,84 It was first introduced as a possible PD treatment in 1959, based on in vitro studies showing decreased collagen expression in treated fibroblasts.93 The effects of POTABA were more recently studied in a RCT of 103 patients, which reported a response rate of 74.3% in the POTABA group and 50% in the placebo group (p=0.016), with significantly larger decrease in plaque size observed in the treatment group during the 12 month study period.94 Pre-existing penile curvature did not improve with POTABA, but the authors concluded that the drug appeared to stabilize the disease, as curvature did not worsen in treated patients, whereas it did in placebo patients. There were no differences in penile pain between the two groups. This study is difficult to interpret, however, given the small sample size in each arm, the variability of PD symptom progression in the absence of treatment in men with PD more generally, and the significant improvements observed in placebo groups in other studies. As a result, confirmatory studies supporting POTABA are needed.
Transdermal And Intralesional Therapy
Verapamil has been studied in men with PD using electromotive drug administration (EMDA; either alone or in combination with dexamethasone),95-98 via injection into PD plaque (with or without concurrent oral therapies),98-108 via EMDA and injection together,109,110 and as a topical gel.111 The results of these studies are mixed and difficult to interpret, and in spite of the numerous studies investigating verapamil in PD, evidence of its efficacy remains weak. Multiple observational studies support efficacy of intralesional verapamil in stabilizing or reducing curvature, although all of these studies lacked control groups.99,102-105 However, two RCTs both failed to demonstrate a significant difference in outcomes between intralesional verapamil and placebo control.107,108 The EAU guidelines state that intralesional, EMDA, and topical verapamil therapies may be efficacious in treatment of PD.78
Intralesional112-114 liposomal human recombinant superoxide dismutase (lhrSOD, a.k.a. orgotein) use in PD has only been decribed in observational studies.112-114 Topical lhrSOD has been described in one observational study115 and one crossover RCT,116 with promising results. In the test phase, pain improved significantly in the treatment group compared to placebo. Curvature and plaque size both improved; however, these outcomes were not evaluated until after crossover. Therefore, no statement can be made comparing lrhSOD to placebo for these outcomes. Only 59 patients received the treatment between both studies. Thus further work is needed to confirm efficacy.
Multiple observational studies of intralesional IFNα2b and IFNα2a have supported a possible benefit in patients with stable PD with noncalcified plaques.117-124 Stronger evidence for the use of IFNα2b comes from a RCT, which demonstrated significant improvement in penile curvature, plaque size, penile pain, and penile hemodynamics in the treatment arm compared to placebo control.125,126 A second RCT compared results of patients treated with IFNα2b alone, vitamin E alone and combination therapy.75 No significant differences were seen between any of these groups, although the study may have not been adequately powered, with only 10 patients per arm. Intralesional IFNα2b is considered potentially effective in PD treatment by the EAU guidelines.78
Intralesional collagenase Clostridium histolyticum (CCH) is a biologic agent consisting of 2 syngergistic microbial collagenases, and was recently approved by the U.S. Food and Drug Administration for the treatment of PD in men with palpable plaque and stable penile curvature of >30 degrees.127 Evidence for the efficacy and safety of this agent is strong, with multiple large RCTs supporting the clinical benefit of CCH in PD,127-131 including the Investigation for Maximal Peyronie’s Reduction Efficacy and Safety Studies (IMPRESS) Trial128 and a subsequent subgroup analysis.127 These studies demonstrate significantly greater improvement in penile curvature and PD symptom bother score in the treatment arm compared to placebo, with effects on pain and erectile function similar to placebo. An average improvement of 34% in penile curvature, equivalent to −17.0±14.8 degrees of curvature, was observed in the treatment arm, in comparison with 18% (−9.3±13.6 degrees) in the placebo arm (p <0.0001). Inclusion criteria for the IMPRESS trial included stable disease, dorsal curvature between 30 and 90 degrees, and intact erectile function with or without use of phosphodiesterase 5 inhibitors. Patients with isolated hourglass deformity, calcified plaque, plaque proximal to penile base, or ventral penile curvature were excluded. Adverse effects were relatively uncommon, and few serious adverse events including penile hematoma and penile rupture were reported, supporting the overall safety of CCH in the treatment of PD. To maintain optimal outcomes and safety, CCH can only be administered by physicians who receive specialized training.
Mechanical and Other Non-Surgical Therapies
A diverse group of non-surgical therapies have been studied in the setting of PD, including ESWT, penile traction therapy, vacuum erectile device (VED) therapy, radiation therapy, and hyperthermia therapy.
ESWT has been evaluated in numerous observational studies and several randomized trials, both placebo controlled132-134 and uncontrolled.135 No randomized studies support a benefit of ESWT in improving penile curvature or plaque size. Two RCTs of ESWT reported decreased pain in the treatment group compared to placebo/sham.133,134 A randomized, uncontrolled trial showed similar large improvements in pain using a visual analog score (baseline score of 5 decreasing to 0.4 after treatment) in both the ESWT and ESWT + tadalafil treatment groups.135 Another RCT showed similar improvements in pain in the treatment and placebo groups. However, both groups had a low baseline pain level, which limited the ability to distinguish differences after treatment.132 These studies suggest that while ESWT may not effectively treat penile deformity in PD, it may expedite the resolution of PD-related pain. However, it is unclear whether the trauma associated with extracorporeal shock waves may resulting in lasting damage or progressive penile deformity over time.
Potential benefits of penile traction therapy regimens of 2-8 hours per day have been investigated in observational studies, with decreases in penile curvature in treatment groups over time.136,137 However, these studies were small (10 and 15 patients, respectively) and lacked control groups. In addition, penile traction therapy may be uncomfortable and inconvenient, given the overall long duration of daily treatment needed.
In 2010, Raheem et al. reported a decrease in penile curvature, improvement in penile length, and decrease in pain associated with a 12 week course of VED therapy in 31 PD patients.138 The authors concluded that VED use may stabilize or improve penile curvature and decrease the need for surgical treatment. An earlier study evaluated use of the VED for penile lengthening after cirumferential tunical incision and venous grafting.139 This study included only 4 patients who used a VED daily for 6 months starting one month after surgery, with increases in penile length in all four patients. One patient was noncompliant with the VED, but nonetheless gained 1 inch in penile length 6 months after surgery. The remaining 3 patients gained 2 inches each at 18-month follow-up. Interestingly, de novo PD following VED use has been described in two case reports, and is thought to be secondary to trauma associated with VED use.140,141 While relatively few studies evaluating the use of VED in men with PD are available, the risks associated with VED use are few. Thus, a trial of VED therapy in men with PD, with or without other treatments, is reasonable.
Local hyperthermia therapy may be effective in men with PD, though the mechanism of action is unknown. Perugia et al. evaluated local hyperthermia therapy compared with intralesional verapamil in a randomized uncontrolled trial with 30 patients in each of two arms, and no control group.106 Hyperthermia therapy consisted of 30 minutes of local treatment biweekly for 5 weeks, with significantly reduced plaque size and penile curvature observed when compared with intralesional verapamil. While these preliminary data are promising, additional studies are needed to confirm a benefit of hyperthermia therapy in men with PD.
Radiation therapy in treating PD has been evaluated only with observational studies, with only one including a control group, and with varying dosing regimens between studies.142-144 These studies observed response rates of 29-47% of patients with improved curvature and 50%-69% with pain improvement.143,144 However, these results are of limited value in the absence of comparison groups. The only study with a comparison group was performed by Furlow et al. in 1975 and observed more symptomatic improvement in the radiotherapy group, with no difference in overall outcomes between radiotherapy and nontreatment groups.142 Given the known risks of radiation exposure and the lack of compelling evidence for benefit, radiotherapy is not recommended in the treatment of PD.
Surgical Treatment
Surgical intervention is indicated in men with bothersome penile deformity that limits sexual intercourse and has been stable for at least 3-6 months, with options including tunical plication, plaque incision or excision with grafting, and insertion of inflatable penile prosthesis (IPP) with or without concurrent modeling, plication, or plaque incision/grafting.
Tunical plication is considered first-line surgical treatment to correct curvature in the stable PD patient with good erectile function with or without the use of pharmacologic or vacuum aids, adequate penile length, curvature <60 degrees, and absence of hourglass, hinge, or other atypical deformities.78 Use of plication in patients with shaft narrowing or hinge defects, as well as curvatures ≥60 degrees, has nevertheless been reported.66 Success rates of tunical plication, as defined by improvement in or resolution of penile curvature, range from 92%-99%, and serious adverse events are rare.66,145,146 Patients must be appropriately counseled to expect penile shortening after plication.66,146,147 In a series of 68 PD and 34 chordee patients, Greenfield et al. reported an average loss of 0.36 cm in penile length (2.4% of preoperative length), with a range of 0 to 2.5 cm.66 Mean loss of penile length was directly related to degree of curvature, with greater loss of length in men with more significant curvature (4% in men with ≥60 degree curvature, 2% in men with curvature 45-59 degrees, and 1% in men with <45 degree curvature). The potential for altered penile sensation postoperatively should also be discussed, and has been reported in up to 8.8% of men.66,148 While often transient, altered sensation may persist in the minority of men.148
Plaque incision or excision with grafting is another option in patients with good erectile function, and may be performed concurrently with tunical plication. Grafting has been reported with several materials, including cadaveric pericardium,146,148-150 autologous temporalis fascia,151 autologous buccal mucosa,152 autologous venous patch,147,153 autologous dermis,148 autologous rectus fascia,147 and porcine small intestinal submucosa.148 No evidence is available to support efficacy of one graft material over another. Reported success rates, as defined by improvement in or resolution of penile curvature, range from 60-100%, and serious adverse events are rare.146-148,152,153 Penile shortening was reported in 22.4% of patients in one series153 and transient glans anesthesia in approximately 20% of patients.148 Erectile function may be compromised postoperatively in a relatively small proportion of patients, with one study observing an 8.6% rate of de novo ED postoperatively.147
While men with good erectile function benefit from direct address of the PD plaque, in men with PD and concurrent ED unresponsive to medical therapy, placement of an IPP with penile modeling or plaque incision/excision and grafting is the preferred approach. Penile modeling is performed after placement of the IPP by grasping the penis and bending it in the direction opposite the curvature for 90 seconds while the IPP is inflated.154 Significant improvements in curvature can be achieved after 1-3 repetitions of this sequence. Reported success rates are 88-100%.147,149,150 Penile plication may also be performed concurrently if needed to obtain desired results.147,155
CONCLUSIONS AND FUTURE DIRECTIONS
Peyronie’s disease was once considered rare, but is now known to be more common than previously appreciated. PD is associated with significant psychosocial distress in patients and their partners, and consideration to both the physical and psychological ramifications of PD should be given during evaluation and treatment. Diagnosis of PD is based on detailed history and physical exam, and workup includes penile assessment in the flaccid and erect states to accurately assess plaques and curvature. The etiology of PD is not fully understood, but is likely related to trauma followed by abnormal wound healing. Interestingly, the predisposition to fibrosis and abnormal wound healing has a genetic basis in some affected men. Myriad nonsurgical modalities have been evaluated for PD treatment including pharmacologic, biologic, and mechanical therapies, the majority with unclear efficacy. Of the medications currently in use, CCH is the only FDA-approved drug for treatment of PD, and improves penile curvature and symptom bother. Surgical repair is indicated in men with significant, stable penile curvature, generally after failure of nonsurgical treatment approaches, and includes penile plication and plaque incision/excision and grafting with or without the placement of an IPP. Alternative nonsurgical treatments are continuously being investigated. Recently, intralesional decorin (proteoglycan)156 and human adipose-derived stem-cell therapy157,158 have shown promising results in animal models. Further research is needed to determine whether these agents will prove to be safe and effective in humans. An improved understanding of the factors predisposing to PD, including genetic factors, and the links between ED and other comorbid conditions in humans, including malignancy, is necessary to better inform patient risk stratification, as well as long- and short-term approaches to health maintenance.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Aylin N. Bilgutay declares no conflict of interest.
Alexander W. Pastuszak declares that he is a NIH Men’s Reproductive Health Research (MRHR) K12 scholar (HD073917), and a Urology Care Foundation Russell Scott, Jr., MD, Resident Research Award recipient.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.El-Sakka AI. Prevalence of Peyronie’s disease among patients with erectile dysfunction. European urology. 2006;49(3):564–569. doi: 10.1016/j.eururo.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Kadioglu A, Sanli O, Akman T, et al. Factors affecting the degree of penile deformity in Peyronie disease: an analysis of 1001 patients. Journal of andrology. 2011;32(5):502–508. doi: 10.2164/jandrol.110.011031. [DOI] [PubMed] [Google Scholar]
- 3.Lindsay MB, Schain DM, Grambsch P, Benson RC, Beard CM, Kurland LT. The incidence of Peyronie’s disease in Rochester, Minnesota, 1950 through 1984. The Journal of urology. 1991;146(4):1007–1009. doi: 10.1016/s0022-5347(17)37988-0. [DOI] [PubMed] [Google Scholar]
- 4.Schwarzer U, Sommer F, Klotz T, Braun M, Reifenrath B, Engelmann U. The prevalence of Peyronie’s disease: results of a large survey. BJU international. 2001;88(7):727–730. doi: 10.1046/j.1464-4096.2001.02436.x. [DOI] [PubMed] [Google Scholar]
- 5.Dibenedetti DB, Nguyen D, Zografos L, Ziemiecki R, Zhou X. A Population-Based Study of Peyronie’s Disease: Prevalence and Treatment Patterns in the United States. Advances in urology. 2011;2011:282503. doi: 10.1155/2011/282503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Peyronie F. Sur Quelques Obstacles Qui S’opposent a L’ejaculation Naturelle de la Semence. Mem Acad Chir. 1743:1. [Google Scholar]
- 7.Borgogni T. Cyrugia edita et compilata. Venice1498 (written 1265-1275)
- 8.Akkus E. Historical review of Peyronie’s disease: de la Peyronie to Devine. In: Levine LA, editor. Peyronie’s Disease: A Guide to Clinical Management. Humana Press; Totowa, NJ: 2007. pp. 1–8. [Google Scholar]
- 9.Mulhall JP, Creech SD, Boorjian SA, et al. Subjective and objective analysis of the prevalence of Peyronie’s disease in a population of men presenting for prostate cancer screening. The Journal of urology. 2004;171(6 Pt 1):2350–2353. doi: 10.1097/01.ju.0000127744.18878.f1. [DOI] [PubMed] [Google Scholar]
- 10.Tal R, Heck M, Teloken P, Siegrist T, Nelson CJ, Mulhall JP. Peyronie’s disease following radical prostatectomy: incidence and predictors. The journal of sexual medicine. 2010;7(3):1254–1261. doi: 10.1111/j.1743-6109.2009.01655.x. [DOI] [PubMed] [Google Scholar]
- 11.Arafa M, Eid H, El-Badry A, Ezz-Eldine K, Shamloul R. The prevalence of Peyronie’s disease in diabetic patients with erectile dysfunction. International journal of impotence research. 2007;19(2):213–217. doi: 10.1038/sj.ijir.3901518. [DOI] [PubMed] [Google Scholar]
- 12 *.Nelson CJ, Diblasio C, Kendirci M, Hellstrom W, Guhring P, Mulhall JP. The chronology of depression and distress in men with Peyronie’s disease. The journal of sexual medicine. 2008;5(8):1985–1990. doi: 10.1111/j.1743-6109.2008.00895.x. [DOI] [PubMed] [Google Scholar]; Important work that emphasizes the significant impact that PD has on a man’s mental and psychosocial well-being and the need for a multidisciplinary approach to treatment.
- 13.Nelson CJ, Mulhall JP. Psychological impact of Peyronie’s disease: a review. The journal of sexual medicine. 2013;10(3):653–660. doi: 10.1111/j.1743-6109.2012.02999.x. [DOI] [PubMed] [Google Scholar]
- 14.Hartzell R. Psychosexual Symptoms and Treatment of Peyronie’s Disease Within a Collaborative Care Model. Sexual medicine. 2014;2(4):168–177. doi: 10.1002/sm2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15 *.Hellstrom WJ, Feldman R, Rosen RC, Smith T, Kaufman G, Tursi J. Bother and distress associated with Peyronie’s disease: validation of the Peyronie’s disease questionnaire. The Journal of urology. 2013;190(2):627–634. doi: 10.1016/j.juro.2013.01.090. [DOI] [PubMed] [Google Scholar]; Describes the first quality of life questionnaire validated for use specifically in PD.
- 16.Bjekic MD, Vlajinac HD, Sipetic SB, Marinkovic JM. Risk factors for Peyronie’s disease: a case-control study. BJU international. 2006;97(3):570–574. doi: 10.1111/j.1464-410X.2006.05969.x. [DOI] [PubMed] [Google Scholar]
- 17.Carrieri MP, Serraino D, Palmiotto F, Nucci G, Sasso F. A case-control study on risk factors for Peyronie’s disease. Journal of clinical epidemiology. 1998;51(6):511–515. doi: 10.1016/s0895-4356(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 18.La Pera G, Pescatori ES, Calabrese M, et al. Peyronie’s disease: prevalence and association with cigarette smoking. A multicenter population-based study in men aged 50-69 years. European urology. 2001;40(5):525–530. doi: 10.1159/000049830. [DOI] [PubMed] [Google Scholar]
- 19.Moreno SA, Morgentaler A. Testosterone deficiency and Peyronie’s disease: pilot data suggesting a significant relationship. The journal of sexual medicine. 2009;6(6):1729–1735. doi: 10.1111/j.1743-6109.2009.01250.x. [DOI] [PubMed] [Google Scholar]
- 20.Nam HJ, Park HJ, Park NC. Does testosterone deficiency exaggerate the clinical symptoms of Peyronie’s disease? International journal of urology : official journal of the Japanese Urological Association. 2011;18(11):796–800. doi: 10.1111/j.1442-2042.2011.02842.x. [DOI] [PubMed] [Google Scholar]
- 21.Kirby EW, Verges D, Matthews J, Carson CC, Coward RM. Low Testosterone Has a Similar Prevalence among Men with Sexual Dysfunction Due to Either Peyronie’s Disease or Erectile Dysfunction and Does Not Correlate with Peyronie’s Disease Severity. The journal of sexual medicine. 2015;12(3):690–696. doi: 10.1111/jsm.12805. [DOI] [PubMed] [Google Scholar]
- 22.Karavitakis M, Komninos C, Simaioforidis V, et al. The relationship between androgens, regulators of collagen metabolism, and Peyronie’s disease: a case control study. The journal of sexual medicine. 2010;7(12):4011–4017. doi: 10.1111/j.1743-6109.2010.01915.x. [DOI] [PubMed] [Google Scholar]
- 23 *.Mulhall JP, Schiff J, Guhring P. An analysis of the natural history of Peyronie’s disease. The Journal of urology. 2006;175(6):2115–2118. doi: 10.1016/S0022-5347(06)00270-9. discussion 2118. [DOI] [PubMed] [Google Scholar]; Provides an excellent account of the natural history of PD, debunking the misconception of spontaneous resolution being common.
- 24.Azadzoi KM, Schulman RN, Aviram M, Siroky MB. Oxidative stress in arteriogenic erectile dysfunction: prophylactic role of antioxidants. The Journal of urology. 2005;174(1):386–393. doi: 10.1097/01.ju.0000161209.39959.67. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Cadavid NF, Rajfer J. Therapy of erectile dysfunction: potential future treatments. Endocrine. 2004;23(2-3):167–176. doi: 10.1385/ENDO:23:2-3:167. [DOI] [PubMed] [Google Scholar]
- 26.Ferrini MG, Kovanecz I, Sanchez S, et al. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biology of reproduction. 2007;76(5):915–923. doi: 10.1095/biolreprod.106.059642. [DOI] [PubMed] [Google Scholar]
- 27.Levine LA. Preface. In: Levine LA, editor. Peyronie’s Disease: A Guide to Clinical Management. Humana Press; Totowa, NJ: 2007. pp. vii–ix. [Google Scholar]
- 28.Brock G, Hsu GL, Nunes L, von Heyden B, Lue TF. The anatomy of the tunica albuginea in the normal penis and Peyronie’s disease. The Journal of urology. 1997;157(1):276–281. [PubMed] [Google Scholar]
- 29.Davis CJ., Jr The microscopic pathology of Peyronie’s disease. The Journal of urology. 1997;157(1):282–284. [PubMed] [Google Scholar]
- 30.El-Sakka AI, Hassan MU, Nunes L, Bhatnagar RS, Yen TS, Lue TF. Histological and ultrastructural alterations in an animal model of Peyronie’s disease. British journal of urology. 1998;81(3):445–452. doi: 10.1046/j.1464-410x.1998.00529.x. [DOI] [PubMed] [Google Scholar]
- 31.Somers KD, Dawson DM. Fibrin deposition in Peyronie’s disease plaque. The Journal of urology. 1997;157(1):311–315. [PubMed] [Google Scholar]
- 32.Devine CJ, Jr., Somers KD, Jordan SG, Schlossberg SM. Proposal: trauma as the cause of the Peyronie’s lesion. The Journal of urology. 1997;157(1):285–290. doi: 10.1016/s0022-5347(01)65361-8. [DOI] [PubMed] [Google Scholar]
- 33.Diegelmann RF. Cellular and biochemical aspects of normal and abnormal wound healing: an overview. The Journal of urology. 1997;157(1):298–302. [PubMed] [Google Scholar]
- 34.Gholami SS, Gonzalez-Cadavid NF, Lin CS, Rajfer J, Lue TF. Peyronie’s disease: a review. The Journal of urology. 2003;169(4):1234–1241. doi: 10.1097/01.ju.0000053800.62741.fe. [DOI] [PubMed] [Google Scholar]
- 35.Jalkut M, Gonzalez-Cadavid N, Rajfer J. New discoveries in the basic science understanding of Peyronie’s disease. Current urology reports. 2004;5(6):478–484. doi: 10.1007/s11934-004-0074-y. [DOI] [PubMed] [Google Scholar]
- 36.Mulhall JP, Thom J, Lubrano T, Shankey TV. Basic fibroblast growth factor expression in Peyronie’s disease. The Journal of urology. 2001;165(2):419–423. doi: 10.1097/00005392-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Cadavid NF, Rajfer J. Mechanisms of Disease: new insights into the cellular and molecular pathology of Peyronie’s disease. Nature clinical practice. Urology. 2005;2(6):291–297. doi: 10.1038/ncpuro0201. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Cadavid NF, Rajfer J. Experimental models of Peyronie’s disease. Implications for new therapies. The journal of sexual medicine. 2009;6(2):303–313. doi: 10.1111/j.1743-6109.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- 39.El-Sakka AI, Hassoba HM, Chui RM, Bhatnagar RS, Dahiya R, Lue TF. An animal model of Peyronie’s-like condition associated with an increase of transforming growth factor beta mRNA and protein expression. The Journal of urology. 1997;158(6):2284–2290. doi: 10.1016/s0022-5347(01)68236-3. [DOI] [PubMed] [Google Scholar]
- 40.Vernet D, Nolazco G, Cantini L, et al. Evidence that osteogenic progenitor cells in the human tunica albuginea may originate from stem cells: implications for peyronie disease. Biology of reproduction. 2005;73(6):1199–1210. doi: 10.1095/biolreprod.105.041038. [DOI] [PubMed] [Google Scholar]
- 41.Vernet D, Ferrini MG, Valente EG, et al. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie’s fibrotic plaque and in its rat model. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2002;7(4):262–276. doi: 10.1016/s1089-8603(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 42.Davila HH, Ferrini MG, Rajfer J, Gonzalez-Cadavid NF. Fibrin as an inducer of fibrosis in the tunica albuginea of the rat: a new animal model of Peyronie’s disease. BJU international. 2003;91(9):830–838. doi: 10.1046/j.1464-410x.2003.04224.x. [DOI] [PubMed] [Google Scholar]
- 43.El-Sakka AI, Selph CA, Yen TS, Dahiya R, Lue TF. The effect of surgical trauma on rat tunica albuginea. The Journal of urology. 1998;159(5):1700–1707. doi: 10.1097/00005392-199805000-00097. [DOI] [PubMed] [Google Scholar]
- 44.Willscher MK, Cwazka WF, Novicki DE. The association of histocompatibility antigens of the B7 cross-reacting group with Peyronie’s disease. The Journal of urology. 1979;122(1):34–35. doi: 10.1016/s0022-5347(17)56238-2. [DOI] [PubMed] [Google Scholar]
- 45.Rompel R, Weidner W, Mueller-Eckhardt G. HLA association of idiopathic Peyronie’s disease: an indication of autoimmune phenomena in etiopathogenesis? Tissue antigens. 1991;38(3):104–106. doi: 10.1111/j.1399-0039.1991.tb02021.x. [DOI] [PubMed] [Google Scholar]
- 46.Nachtsheim DA, Rearden A. Peyronie’s disease is associated with an HLA class II antigen, HLA-DQ5, implying an autoimmune etiology. The Journal of urology. 1996;156(4):1330–1334. [PubMed] [Google Scholar]
- 47.Nyberg LM, Jr., Bias WB, Hochberg MC, Walsh PC. Identification of an inherited form of Peyronie’s disease with autosomal dominant inheritance and association with Dupuytren’s contracture and histocompatibility B7 cross-reacting antigens. The Journal of urology. 1982;128(1):48–51. doi: 10.1016/s0022-5347(17)52751-2. [DOI] [PubMed] [Google Scholar]
- 48.Hauck EW, Hauptmann A, Weidner W, Bein G, Hackstein H. Prospective analysis of HLA classes I and II antigen frequency in patients with Peyronie’s disease. The Journal of urology. 2003;170(4 Pt 1):1443–1446. doi: 10.1097/01.ju.0000076488.89748.e1. [DOI] [PubMed] [Google Scholar]
- 49.Leffell MS, Devine CJ, Jr., Horton CE, et al. Non-association of Peyronie’s disease with HLA B7 cross-reactive antigens. The Journal of urology. 1982;127(6):1223–1224. doi: 10.1016/s0022-5347(17)54302-5. [DOI] [PubMed] [Google Scholar]
- 50.Noss MB, Day NS, Christ GJ, Melman A. The genetics and immunology of Peyronie’s disease. International journal of impotence research. 2000;12(Suppl 4):S127–132. doi: 10.1038/sj.ijir.3900591. [DOI] [PubMed] [Google Scholar]
- 51 *.Qian A, Meals RA, Rajfer J, Gonzalez-Cadavid NF. Comparison of gene expression profiles between Peyronie’s disease and Dupuytren’s contracture. Urology. 2004;64(2):399–404. doi: 10.1016/j.urology.2004.04.006. [DOI] [PubMed] [Google Scholar]; Provides evidence that PD and DD may share a common pathophysiology.
- 52.Casalone R, Mazzola D, Meroni E, et al. Cytogenetic and interphase cytogenetic analyses reveal chromosome instability but no clonal trisomy 8 in Dupuytren contracture. Cancer genetics and cytogenetics. 1997;99(1):73–76. doi: 10.1016/s0165-4608(96)00430-x. [DOI] [PubMed] [Google Scholar]
- 53.Dal Cin P, De Smet L, Sciot R, Van Damme B, Van den Berghe H. Trisomy 7 and trisomy 8 in dividing and non-dividing tumor cells in Dupuytren’s disease. Cancer genetics and cytogenetics. 1999;108(2):137–140. doi: 10.1016/s0165-4608(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 54.Mulhall JP, Nicholson B, Pierpaoli S, Lubrano T, Shankey TV. Chromosomal instability is demonstrated by fibroblasts derived from the tunica of men with Peyronie’s disease. International journal of impotence research. 2004;16(3):288–293. doi: 10.1038/sj.ijir.3901170. [DOI] [PubMed] [Google Scholar]
- 55 *.Mulhall JP, Martin DJ, Lubrano T, et al. Peyronie’s disease fibroblasts demonstrate tumorigenicity in the severe combined immunodeficient (SCID) mouse model. International journal of impotence research. 2004;16(2):99–104. doi: 10.1038/sj.ijir.3901183. [DOI] [PubMed] [Google Scholar]; Reveals a link between PD and malignancy, which warrants further investigation.
- 56.Zyluk A, Paszkowska-Szczur K, Gupta S, Scott RJ, Lubinski J, Debniak T. Dupuytren’s disease and the risk of malignant neoplasms. Hereditary cancer in clinical practice. 2014;12(1):6. doi: 10.1186/1897-4287-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiong T, Yang J, Wang H, et al. The association between the Lys751Gln polymorphism in the XPD gene and the risk of bladder cancer. Molecular biology reports. 2014;41(4):2629–2634. doi: 10.1007/s11033-014-3121-x. [DOI] [PubMed] [Google Scholar]
- 58.Gao W, Romkes M, Zhong S, et al. Genetic polymorphisms in the DNA repair genes XPD and XRCC1, p53 gene mutations and bladder cancer risk. Oncology reports. 2010;24(1):257–262. doi: 10.3892/or_00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sobti RC, Kaur S, Sharma VL, Singh SK, Hosseini SA, Kler R. Susceptibility of XPD and RAD51 genetic variants to carcinoma of urinary bladder in North Indian population. DNA and cell biology. 2012;31(2):199–210. doi: 10.1089/dna.2011.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams JL, Thomas GG. The natural history of Peyronie’s disease. The Journal of urology. 1970;103(1):75–76. doi: 10.1016/s0022-5347(17)61894-9. [DOI] [PubMed] [Google Scholar]
- 61.Gelbard MK, Dorey F, James K. The natural history of Peyronie’s disease. The Journal of urology. 1990;144(6):1376–1379. doi: 10.1016/s0022-5347(17)39746-x. [DOI] [PubMed] [Google Scholar]
- 62.Berookhim BM, Choi J, Alex B, Mulhall JP. Deformity stabilization and improvement in men with untreated Peyronie’s disease. BJU international. 2014;113(1):133–136. doi: 10.1111/bju.12346. [DOI] [PubMed] [Google Scholar]
- 63.Kadioglu A, Tefekli A, Erol B, Oktar T, Tunc M, Tellaloglu S. A retrospective review of 307 men with Peyronie’s disease. The Journal of urology. 2002;168(3):1075–1079. doi: 10.1016/S0022-5347(05)64578-8. [DOI] [PubMed] [Google Scholar]
- 64.Jordan GHM KA. Peyronie’s Disease. In: Kavoussi LRNACPCAWAJ, editor. Campbell-Walsh Urology. 10 ed Vol 1. Elsevier Saunders; Philadelphia: 2012. pp. 792–809. [Google Scholar]
- 65.Wilson SKaC CC. Surgical Straightening with Penile Prosthesis. In: Levine LA, editor. Peyronie’s Disease: A Guide to Clinical Management. Humana Press; Totowa, NJ: 2007. pp. 249–258. [Google Scholar]
- 66 **.Greenfield JM, Lucas S, Levine LA. Factors affecting the loss of length associated with tunica albuginea plication for correction of penile curvature. The Journal of urology. 2006;175(1):238–241. doi: 10.1016/S0022-5347(05)00063-7. [DOI] [PubMed] [Google Scholar]; Provides important outcomes data for tunical plication and also provides evidence that patients’ description of their own deformity is inaccurate, supporting objective measurement of deformity on erect penile exam.
- 67.Ohebshalom M, Mulhall J, Guhring P, Parker M. Measurement of penile curvature in Peyronie’s disease patients: comparison of three methods. The journal of sexual medicine. 2007;4(1):199–203. doi: 10.1111/j.1743-6109.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 68.Pastuszak AJ. Current Diagnosis and Management of Erectile Dysfunction. Curr Sex Health Rep. 2014;6(3):164–176. doi: 10.1007/s11930-014-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mirone V. Combination Nonsurgical Therapy. In: Levine LA, editor. Peyronie’s Disease: A Guide to Clinical Management. Humana Press; Totowa, NJ: 2007. pp. 111–119. [Google Scholar]
- 70.Bystrom J. Induration penis plastica. Experience of treatment with procarbazine Natulan. Scandinavian journal of urology and nephrology. 1976;10(1):21–25. doi: 10.3109/00365597609179649. [DOI] [PubMed] [Google Scholar]
- 71.Morgan RJ, Pryor JP. Procarbazine (Natulan) in the treatment of Peyronie’s disease. British journal of urology. 1978;50(2):111–113. doi: 10.1111/j.1464-410x.1978.tb03038.x. [DOI] [PubMed] [Google Scholar]
- 72.Oosterlinck W, Renders G. Treatment of Peyronie’s disease with procarbazine. British journal of urology. 1975;47(2):219–220. doi: 10.1111/j.1464-410x.1975.tb03951.x. [DOI] [PubMed] [Google Scholar]
- 73.Claro JA, Passerotti CC, Figueiredo Neto AC, Nardozza A, Jr., Ortiz V, Srougi M. An alternative non-invasive treatment for Peyronie’s disease. International braz j urol: official journal of the Brazilian Society of Urology. 2004;30(3):199–204. doi: 10.1590/s1677-55382004000300004. discussion 204. [DOI] [PubMed] [Google Scholar]
- 74.Hashimoto K, Hisasue S, Kato R, Kobayashi K, Shimizu T, Tsukamoto T. Outcome analysis for conservative management of Peyronie’s disease. International journal of urology : official journal of the Japanese Urological Association. 2006;13(3):244–247. doi: 10.1111/j.1442-2042.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 75.Inal T, Tokatli Z, Akand M, Ozdiler E, Yaman O. Effect of intralesional interferon-alpha 2b combined with oral vitamin E for treatment of early stage Peyronie’s disease: a randomized and prospective study. Urology. 2006;67(5):1038–1042. doi: 10.1016/j.urology.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 76.Safarinejad MR, Hosseini SY, Kolahi AA. Comparison of vitamin E and propionyl-L-carnitine, separately or in combination, in patients with early chronic Peyronie’s disease: a double-blind, placebo controlled, randomized study. The Journal of urology. 2007;178(4 Pt 1):1398–1403. doi: 10.1016/j.juro.2007.05.162. discussion 1403. [DOI] [PubMed] [Google Scholar]
- 77.Ahuja SK, Sikka SC, Hellstrom WJ. Stimulation of collagen production in an in vitro model for Peyronie’s disease. International journal of impotence research. 1999;11(4):207–212. doi: 10.1038/sj.ijir.3900414. [DOI] [PubMed] [Google Scholar]
- 78 **.Hatzimouratidis K, Eardley I, Giuliano F, et al. EAU guidelines on penile curvature. European urology. 2012;62(3):543–552. doi: 10.1016/j.eururo.2012.05.040. [DOI] [PubMed] [Google Scholar]; Summarizes the EAU guidelines for management of PD, which provided much-needed guidance that was previously lacking.
- 79.Biagiotti G, Cavallini G. Acetyl-L-carnitine vs tamoxifen in the oral therapy of Peyronie’s disease: a preliminary report. BJU international. 2001;88(1):63–67. doi: 10.1046/j.1464-410x.2001.02241.x. [DOI] [PubMed] [Google Scholar]
- 80.Ralph DJ, Brooks MD, Bottazzo GF, Pryor JP. The treatment of Peyronie’s disease with tamoxifen. British journal of urology. 1992;70(6):648–651. doi: 10.1111/j.1464-410x.1992.tb15836.x. [DOI] [PubMed] [Google Scholar]
- 81.Teloken C, Rhoden EL, Grazziotin TM, Ros CT, Sogari PR, Souto CA. Tamoxifen versus placebo in the treatment of Peyronie’s disease. The Journal of urology. 1999;162(6):2003–2005. doi: 10.1016/S0022-5347(05)68087-1. [DOI] [PubMed] [Google Scholar]
- 82.Safarinejad MR. Efficacy and safety of omega-3 for treatment of early-stage Peyronie’s disease: A prospective, randomized, double-blind placebo-controlled study. The journal of sexual medicine. 2009;6(6):1743–1754. doi: 10.1111/j.1743-6109.2009.01235.x. [DOI] [PubMed] [Google Scholar]
- 83.Safarinejad MR. Safety and efficacy of coenzyme Q10 supplementation in early chronic Peyronie’s disease: a double-blind, placebo-controlled randomized study. International journal of impotence research. 2010;22(5):298–309. doi: 10.1038/ijir.2010.20. [DOI] [PubMed] [Google Scholar]
- 84.Trost LW, Gur S, Hellstrom WJ. Pharmacological Management of Peyronie’s Disease. Drugs. 2007;67(4):527–545. doi: 10.2165/00003495-200767040-00004. [DOI] [PubMed] [Google Scholar]
- 85.Shindel AW, Lin G, Ning H, et al. Pentoxifylline attenuates transforming growth factor-beta1-stimulated collagen deposition and elastogenesis in human tunica albuginea-derived fibroblasts part 1: impact on extracellular matrix. The journal of sexual medicine. 2010;7(6):2077–2085. doi: 10.1111/j.1743-6109.2010.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valente EG, Vernet D, Ferrini MG, Qian A, Rajfer J, Gonzalez-Cadavid NF. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie’s fibrotic plaque and related fibroblast cultures. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2003;9(4):229–244. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Brant WO, Dean RC, Lue TF. Treatment of Peyronie’s disease with oral pentoxifylline. Nature clinical practice. Urology. 2006;3(2):111–115. doi: 10.1038/ncpuro0409. quiz 116. [DOI] [PubMed] [Google Scholar]
- 88.Jordan GH, Carson CC, Lipshultz LI. Minimally invasive treatment of Peyronie’s disease: evidence-based progress. BJU international. 2014;114(1):16–24. doi: 10.1111/bju.12634. [DOI] [PubMed] [Google Scholar]
- 89.El-Sakka AI, Bakircioglu ME, Bhatnagar RS, Yen TS, Dahiya R, Lue TF. The effects of colchicine on a Peyronie’s-like condition in an animal model. The Journal of urology. 1999;161(6):1980–1983. [PubMed] [Google Scholar]
- 90.Akkus E, Carrier S, Rehman J, Breza J, Kadioglu A, Lue TF. Is colchicine effective in Peyronie’s disease? A pilot study. Urology. 1994;44(2):291–295. doi: 10.1016/s0090-4295(94)80155-x. [DOI] [PubMed] [Google Scholar]
- 91.Kadioglu A, Tefekli A, Koksal T, Usta M, Erol H. Treatment of Peyronie’s disease with oral colchicine: long-term results and predictive parameters of successful outcome. International journal of impotence research. 2000;12(3):169–175. doi: 10.1038/sj.ijir.3900519. [DOI] [PubMed] [Google Scholar]
- 92.Safarinejad MR. Therapeutic effects of colchicine in the management of Peyronie’s disease: a randomized double-blind, placebo-controlled study. International journal of impotence research. 2004;16(3):238–243. doi: 10.1038/sj.ijir.3901185. [DOI] [PubMed] [Google Scholar]
- 93.Zarafonetis CJ, Horrax TM. Treatment of Peyronie’s disease with potassium para aminobenzoate (potaba) The Journal of urology. 1959;81(6):770–772. doi: 10.1016/S0022-5347(17)66108-1. [DOI] [PubMed] [Google Scholar]
- 94.Weidner W, Hauck EW, Schnitker J, Urologists PsDSGoAGoG Potassium paraaminobenzoate (POTABA) in the treatment of Peyronie’s disease: a prospective, placebo-controlled, randomized study. European urology. 2005;47(4):530–535. doi: 10.1016/j.eururo.2004.12.022. discussion 535-536. [DOI] [PubMed] [Google Scholar]
- 95.Di Stasi SM, Giannantoni A, Capelli G, et al. Transdermal electromotive administration of verapamil and dexamethasone for Peyronie’s disease. BJU international. 2003;91(9):825–829. doi: 10.1046/j.1464-410x.2003.04242.x. [DOI] [PubMed] [Google Scholar]
- 96.Di Stasi SM, Giannantoni A, Stephen RL, et al. A prospective, randomized study using transdermal electromotive administration of verapamil and dexamethasone for Peyronie’s disease. The Journal of urology. 2004;171(4):1605–1608. doi: 10.1097/01.ju.0000116450.82816.2c. [DOI] [PubMed] [Google Scholar]
- 97.Greenfield JM, Shah SJ, Levine LA. Verapamil versus saline in electromotive drug administration for Peyronie’s disease: a double-blind, placebo controlled trial. The Journal of urology. 2007;177(3):972–975. doi: 10.1016/j.juro.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 98.Mehrsai AR, Namdari F, Salavati A, Dehghani S, Allameh F, Pourmand G. Comparison of transdermal electromotive administration of verapamil and dexamethasone versus intra-lesional injection for Peyronie’s disease. Andrology. 2013;1(1):129–132. doi: 10.1111/j.2047-2927.2012.00018.x. [DOI] [PubMed] [Google Scholar]
- 99.Bennett NE, Guhring P, Mulhall JP. Intralesional verapamil prevents the progression of Peyronie’s disease. Urology. 2007;69(6):1181–1184. doi: 10.1016/j.urology.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 100.Cavallini G, Modenini F, Vitali G. Open preliminary randomized prospective clinical trial of efficacy and safety of three different verapamil dilutions for intraplaque therapy of Peyronie’s disease. Urology. 2007;69(5):950–954. doi: 10.1016/j.urology.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 101.Chung E, Garcia F, Young LD, Solomon M, Brock GB. A comparative study of the efficacy of intralesional verapamil versus normal saline injection in a novel Peyronie disease animal model: assessment of immunohistopathological changes and erectile function outcome. The Journal of urology. 2013;189(1):380–384. doi: 10.1016/j.juro.2012.08.191. [DOI] [PubMed] [Google Scholar]
- 102.Heidari M, Nejadi JR, Ghate A, Delfan B, Iran-Pour E. Evaluation of intralesional injection of verapamil in treatment of Peyronie’s disease. JPMA. The Journal of the Pakistan Medical Association. 2010;60(4):291–293. [PubMed] [Google Scholar]
- 103.Levine LA, Goldman KE, Greenfield JM. Experience with intraplaque injection of verapamil for Peyronie’s disease. The Journal of urology. 2002;168(2):621–625. doi: 10.1016/s0022-5347(05)64691-5. discussion 625-626. [DOI] [PubMed] [Google Scholar]
- 104.Levine LA, Merrick PF, Lee RC. Intralesional verapamil injection for the treatment of Peyronie’s disease. The Journal of urology. 1994;151(6):1522–1524. doi: 10.1016/s0022-5347(17)35291-6. [DOI] [PubMed] [Google Scholar]
- 105.Moskovic DJ, Alex B, Choi JM, Nelson CJ, Mulhall JP. Defining predictors of response to intralesional verapamil injection therapy for Peyronie’s disease. BJU international. 2011;108(9):1485–1489. doi: 10.1111/j.1464-410X.2010.10029.x. [DOI] [PubMed] [Google Scholar]
- 106.Perugia G, Liberti M, Vicini P, Colistro F, Gentile V. Role of hyperthermia in the treatment of Peyronie’s disease: a preliminary study. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2005;21(4):367–374. doi: 10.1080/02656730500133892. [DOI] [PubMed] [Google Scholar]
- 107.Rehman J, Benet A, Melman A. Use of intralesional verapamil to dissolve Peyronie’s disease plaque: a long-term single-blind study. Urology. 1998;51(4):620–626. doi: 10.1016/s0090-4295(97)00700-0. [DOI] [PubMed] [Google Scholar]
- 108.Shirazi M, Haghpanah AR, Badiee M, Afrasiabi MA, Haghpanah S. Effect of intralesional verapamil for treatment of Peyronie’s disease: a randomized single-blind, placebo-controlled study. International urology and nephrology. 2009;41(3):467–471. doi: 10.1007/s11255-009-9522-4. [DOI] [PubMed] [Google Scholar]
- 109.Paulis G, Brancato T, D’Ascenzo R, et al. Efficacy of vitamin E in the conservative treatment of Peyronie’s disease: legend or reality? A controlled study of 70 cases. Andrology. 2013;1(1):120–128. doi: 10.1111/j.2047-2927.2012.00007.x. [DOI] [PubMed] [Google Scholar]
- 110.Paulis G, Cavallini G, Brancato T, Alvaro R. Peironimev-Plus(R) in the treatment of chronic inflammation of tunica albuginea (Peyronie’s disease). results of a controlled study. Inflammation & allergy drug targets. 2013;12(1):61–67. doi: 10.2174/1871528111312010009. [DOI] [PubMed] [Google Scholar]
- 111.Fitch WP, 3rd, Easterling WJ, Talbert RL, Bordovsky MJ, Mosier M. Topical verapamil HCl, topical trifluoperazine, and topical magnesium sulfate for the treatment of Peyronie’s disease--a placebo-controlled pilot study. The journal of sexual medicine. 2007;4(2):477–484. doi: 10.1111/j.1743-6109.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 112.Lenk S, Schonberger B. Penile deviation--our therapeutic concept. Acta chirurgica Hungarica. 1994;34(1-2):189–194. [PubMed] [Google Scholar]
- 113.Bartsch G, Menander-Huber KB, Huber W, Marberger H. Orgotein, a new drug for the treatment of Peyronie’s disease. European journal of rheumatology and inflammation. 1981;4(2):250–259. [PubMed] [Google Scholar]
- 114.Gustafson H, Johansson B, Edsmyr F. Peyronie’s disease: experience of local treatment with Orgotein. European urology. 1981;7(6):346–348. doi: 10.1159/000473262. [DOI] [PubMed] [Google Scholar]
- 115.Riedl CR, Plas E, Vorauer K, Vcelar B, Wagner A, Pfluger H. Pilot study on liposomal recombinant human superoxide dismutase for the treatment of Peyronie’s disease. European urology. 2001;40(3):343–348. doi: 10.1159/000049797. discussion 348-349. [DOI] [PubMed] [Google Scholar]
- 116.Riedl CR, Sternig P, Galle G, et al. Liposomal recombinant human superoxide dismutase for the treatment of Peyronie’s disease: a randomized placebo-controlled double-blind prospective clinical study. European urology. 2005;48(4):656–661. doi: 10.1016/j.eururo.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 117.Ahuja S, Bivalacqua TJ, Case J, Vincent M, Sikka SC, Hellstrom WJ. A pilot study demonstrating clinical benefit from intralesional interferon alpha 2B in the treatment of Peyronie’s disease. Journal of andrology. 1999;20(4):444–448. [PubMed] [Google Scholar]
- 118.Astorga R, Cantero O, Contreras D, et al. Intralesional recombinant interferon alpha-2b in Peyronie’s disease. Archivos espanoles de urologia. 2000;53(7):665–671. [PubMed] [Google Scholar]
- 119.Brake M, Loertzer H, Horsch R, Keller H. Treatment of Peyronie’s disease with local interferon-alpha 2b. BJU international. 2001;87(7):654–657. doi: 10.1046/j.1464-410x.2001.02139.x. [DOI] [PubMed] [Google Scholar]
- 120.Dang G, Matern R, Bivalacqua TJ, Sikka S, Hellstrom WJ. Intralesional interferon-alpha-2B injections for the treatment of Peyronie’s disease. Southern medical journal. 2004;97(1):42–46. doi: 10.1097/01.smj.0000056658.60032.d3. [DOI] [PubMed] [Google Scholar]
- 121.Judge IS, Wisniewski ZS. Intralesional interferon in the treatment of Peyronie’s disease: a pilot study. British journal of urology. 1997;79(1):40–42. doi: 10.1046/j.1464-410x.1997.02849.x. [DOI] [PubMed] [Google Scholar]
- 122.Polat O, Gul O, Ozbey I, Ozdikici M, Bayraktar Y. Peyronie’s disease: intralesional treatment with interferon alpha-2A and evaluation of the results by magnetic resonance imaging. International urology and nephrology. 1997;29(4):465–471. doi: 10.1007/BF02551115. [DOI] [PubMed] [Google Scholar]
- 123.Trost LW, Ates E, Powers M, Sikka S, Hellstrom WJ. Outcomes of intralesional interferon-alpha2B for the treatment of Peyronie disease. The Journal of urology. 2013;190(6):2194–2199. doi: 10.1016/j.juro.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 124.Wegner HE, Andresen R, Knipsel HH, Miller K. Treatment of Peyronie’s disease with local interferon-alpha 2b. European urology. 1995;28(3):236–240. doi: 10.1159/000475057. [DOI] [PubMed] [Google Scholar]
- 125.Hellstrom WJ, Kendirci M, Matern R, et al. Single-blind, multicenter, placebo controlled, parallel study to assess the safety and efficacy of intralesional interferon alpha-2B for minimally invasive treatment for Peyronie’s disease. The Journal of urology. 2006;176(1):394–398. doi: 10.1016/S0022-5347(06)00517-9. [DOI] [PubMed] [Google Scholar]
- 126.Kendirci M, Usta MF, Matern RV, Nowfar S, Sikka SC, Hellstrom WJ. The impact of intralesional interferon alpha-2b injection therapy on penile hemodynamics in men with Peyronie’s disease. The journal of sexual medicine. 2005;2(5):709–715. doi: 10.1111/j.1743-6109.2005.00110.x. [DOI] [PubMed] [Google Scholar]
- 127.Lipshultz LI, Goldstein I, Seftel AD, et al. Clinical Efficacy of Collagenase Clostridium Histolyticum in the Treatment of Peyronie’s Disease by Subgroups: Results From Two Large, Double-Blind, Randomized, Placebo-Controlled, Phase 3 Studies. BJU international. 2015. [DOI] [PubMed]
- 128 **.Gelbard M, Goldstein I, Hellstrom WJ, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. The Journal of urology. 2013;190(1):199–207. doi: 10.1016/j.juro.2013.01.087. [DOI] [PubMed] [Google Scholar]; Findings of the IMPRESS trial, demonstrating safety and efficacy of clostridial collagenase in PD. This biologic agent is the only FDA-approved medical treatment for PD.
- 129.Gelbard M, Lipshultz LI, Tursi J, Smith T, Kaufman G, Levine LA. Phase 2b study of the clinical efficacy and safety of collagenase Clostridium histolyticum in patients with Peyronie disease. The Journal of urology. 2012;187(6):2268–2274. doi: 10.1016/j.juro.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 130.Gelbard MK, James K, Riach P, Dorey F. Collagenase versus placebo in the treatment of Peyronie’s disease: a double-blind study. The Journal of urology. 1993;149(1):56–58. doi: 10.1016/s0022-5347(17)35998-0. [DOI] [PubMed] [Google Scholar]
- 131.Levine LA, Cuzin B, Mark S, et al. Clinical safety and effectiveness of collagenase clostridium histolyticum injection in patients with Peyronie’s disease: a phase 3 open-label study. The journal of sexual medicine. 2015;12(1):248–258. doi: 10.1111/jsm.12731. [DOI] [PubMed] [Google Scholar]
- 132.Chitale S, Morsey M, Swift L, Sethia K. Limited shock wave therapy vs sham treatment in men with Peyronie’s disease: results of a prospective randomized controlled double-blind trial. BJU international. 2010;106(9):1352–1356. doi: 10.1111/j.1464-410X.2010.09331.x. [DOI] [PubMed] [Google Scholar]
- 133.Hatzichristodoulou G, Meisner C, Gschwend JE, Stenzl A, Lahme S. Extracorporeal shock wave therapy in Peyronie’s disease: results of a placebo-controlled, prospective, randomized, single-blind study. The journal of sexual medicine. 2013;10(11):2815–2821. doi: 10.1111/jsm.12275. [DOI] [PubMed] [Google Scholar]
- 134.Palmieri A, Imbimbo C, Longo N, et al. A first prospective, randomized, double-blind, placebo-controlled clinical trial evaluating extracorporeal shock wave therapy for the treatment of Peyronie’s disease. European urology. 2009;56(2):363–369. doi: 10.1016/j.eururo.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 135.Palmieri A, Imbimbo C, Creta M, Verze P, Fusco F, Mirone V. Tadalafil once daily and extracorporeal shock wave therapy in the management of patients with Peyronie’s disease and erectile dysfunction: results from a prospective randomized trial. International journal of andrology. 2012;35(2):190–195. doi: 10.1111/j.1365-2605.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- 136.Gontero P, Di Marco M, Giubilei G, et al. Use of penile extender device in the treatment of penile curvature as a result of Peyronie’s disease. Results of a phase II prospective study. The journal of sexual medicine. 2009;6(2):558–566. doi: 10.1111/j.1743-6109.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 137.Levine LA, Newell M, Taylor FL. Penile traction therapy for treatment of Peyronie’s disease: a single-center pilot study. The journal of sexual medicine. 2008;5(6):1468–1473. doi: 10.1111/j.1743-6109.2008.00814.x. [DOI] [PubMed] [Google Scholar]
- 138.Raheem AA, Garaffa G, Raheem TA, et al. The role of vacuum pump therapy to mechanically straighten the penis in Peyronie’s disease. BJU international. 2010;106(8):1178–1180. doi: 10.1111/j.1464-410X.2010.09365.x. [DOI] [PubMed] [Google Scholar]
- 139.Lue TF, El-Sakka AI. Lengthening shortened penis caused by Peyronie’s disease using circular venous grafting and daily stretching with a vacuum erection device. The Journal of urology. 1999;161(4):1141–1144. [PubMed] [Google Scholar]
- 140.Kim JH, Carson CC., 3rd Development of Peyronie’s disease with the use of a vacuum constriction device. The Journal of urology. 1993;149(5 Pt 2):1314–1315. doi: 10.1016/s0022-5347(17)36378-4. [DOI] [PubMed] [Google Scholar]
- 141.Hakim LS, Munarriz RM, Kulaksizoglu H, Nehra A, Udelson D, Goldstein I. Vacuum erection associated impotence and Peyronie’s disease. The Journal of urology. 1996;155(2):534–535. [PubMed] [Google Scholar]
- 142.Furlow WL, Swenson HE, Jr., Lee RE. Peyronie’s disease: a study of its natural history and treatment with orthovoltage radiotherapy. The Journal of urology. 1975;114(1):69–71. doi: 10.1016/s0022-5347(17)66945-3. [DOI] [PubMed] [Google Scholar]
- 143.Incrocci L, Hop WC, Slob AK. Current sexual functioning in 106 patients with Peyronie’s disease treated with radiotherapy 9 years earlier. Urology. 2000;56(6):1030–1034. doi: 10.1016/s0090-4295(00)00805-0. [DOI] [PubMed] [Google Scholar]
- 144.Niewald M, Wenzlawowicz KV, Fleckenstein J, Wisser L, Derouet H, Rube C. Results of radiotherapy for Peyronie’s disease. International journal of radiation oncology, biology, physics. 2006;64(1):258–262. doi: 10.1016/j.ijrobp.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 145.Adibi M, Hudak SJ, Morey AF. Penile plication without degloving enables effective correction of complex Peyronie’s deformities. Urology. 2012;79(4):831–835. doi: 10.1016/j.urology.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 146.Taylor FL, Levine LA. Surgical correction of Peyronie’s disease via tunica albuginea plication or partial plaque excision with pericardial graft: long-term follow up. The journal of sexual medicine. 2008;5(9):2221–2228. doi: 10.1111/j.1743-6109.2008.00941.x. discussion 2229-2230. [DOI] [PubMed] [Google Scholar]
- 147.Kadioglu A, Sanli O, Akman T, Cakan M, Erol B, Mamadov F. Surgical treatment of Peyronie’s disease: a single center experience with 145 patients. European urology. 2008;53(2):432–439. doi: 10.1016/j.eururo.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 148 *.Kovac JR, Brock GB. Surgical outcomes and patient satisfaction after dermal, pericardial, and small intestinal submucosal grafting for Peyronie’s disease. The journal of sexual medicine. 2007;4(5):1500–1508. doi: 10.1111/j.1743-6109.2007.00453.x. [DOI] [PubMed] [Google Scholar]; Describes use of various grafts in plaque incision/excision for PD and reports outcomes.
- 149 **.Mulhall J, Ahmed A, Anderson M. Penile prosthetic surgery for Peyronie’s disease: defining the need for intraoperative adjuvant maneuvers. The journal of sexual medicine. 2004;1(3):318–321. doi: 10.1111/j.1743-6109.04046.x. [DOI] [PubMed] [Google Scholar]; Describes IPP placement for the surgical management in the PD patient with ED unresponsive to medical therapy and describes how to decide whether adjuvant maneuvers are necessary, as well as how to perform them.
- 150.Usta MF, Bivalacqua TJ, Sanabria J, Koksal IT, Moparty K, Hellstrom WJ. Patient and partner satisfaction and long-term results after surgical treatment for Peyronie’s disease. Urology. 2003;62(1):105–109. doi: 10.1016/s0090-4295(03)00244-9. [DOI] [PubMed] [Google Scholar]
- 151.Gelbard MK, Hayden B. Expanding contractures of the tunica albuginea due to Peyronie’s disease with temporalis fascia free grafts. The Journal of urology. 1991;145(4):772–776. doi: 10.1016/s0022-5347(17)38447-1. [DOI] [PubMed] [Google Scholar]
- 152.Cormio L, Zucchi A, Lorusso F, et al. Surgical treatment of Peyronie’s disease by plaque incision and grafting with buccal mucosa. European urology. 2009;55(6):1469–1475. doi: 10.1016/j.eururo.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 153.Akkus E, Ozkara H, Alici B, et al. Incision and venous patch graft in the surgical treatment of penile curvature in Peyronie’s disease. European urology. 2001;40(5):531–536. doi: 10.1159/000049831. discussion 537. [DOI] [PubMed] [Google Scholar]
- 154.Yafi FA, Sangkum P, McCaslin IR, Hellstrom WJ. Strategies for Penile Prosthesis Placement in Peyronie’s Disease and Corporal Fibrosis. Current urology reports. 2015;16(4):491. doi: 10.1007/s11934-015-0491-0. [DOI] [PubMed] [Google Scholar]
- 155.Chung PH, Scott JF, Morey AF. High patient satisfaction of inflatable penile prosthesis insertion with synchronous penile plication for erectile dysfunction and Peyronie’s disease. The journal of sexual medicine. 2014;11(6):1593–1598. doi: 10.1111/jsm.12530. [DOI] [PubMed] [Google Scholar]
- 156.Akman T, Tefekli A, Armagan A, et al. Decorin as a new treatment alternative in Peyronie’s disease: preliminary results in the rat model. Andrologia. 2013;45(2):101–106. doi: 10.1111/j.1439-0272.2012.01318.x. [DOI] [PubMed] [Google Scholar]
- 157.Castiglione F, Hedlund P, Van der Aa F, et al. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie’s disease. European urology. 2013;63(3):551–560. doi: 10.1016/j.eururo.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gokce A, Abd Elmageed ZY, Lasker GF, et al. Adipose tissue-derived stem cell therapy for prevention and treatment of erectile dysfunction in a rat model of Peyronie’s disease. Andrology. 2014;2(2):244–251. doi: 10.1111/j.2047-2927.2013.00181.x. [DOI] [PubMed] [Google Scholar]
- 159.Husain J, Lynn NN, Jones DK, Collins GN, O’Reilly PH. Extracorporeal shock wave therapy in the management of Peyronie’s disease: initial experience. BJU international. 2000;86(4):466–468. doi: 10.1046/j.1464-410x.2000.00827.x. [DOI] [PubMed] [Google Scholar]
- 160.Ballerini M, Baronzio GF, Capit, et al. Androtherm Application for the Peyronie’s Disease. Conference Papers in Medicine.2013. p. 6. [Google Scholar]