SUMMARY
Major advances in pathology, molecular biology, patient diagnosis and care, as well as the advent of personalized therapy, have resulted in a greatly increased role for the pathologist, who has emerged as a key member of the lung cancer management team. A new multidisciplinary, clinically relevant classification of pulmonary adenocarcinoma has resulted in a paradigm shift in how we view and practice lung cancer pathology. In the future, the role of the pathologist will continue to grow and become fully integrated with clinical care.
Lung cancer remains the major cause of cancer deaths in the USA and the world [1–4], and despite major advances in early diagnosis, smoking cessation and treatment, its prognosis remains dismal and the worldwide incidence is increasing rapidly. Despite these gloomy statistics, there are glimmers of hope based on the tremendous strides in understanding the genome-wide molecular and biological changes present in lung cancer, and the evolution and application of individualized therapy based on rational identification of targets has generated considerable interest and promise [5,6]. As our concepts of therapy have evolved, so has the role of the pathologist, who has emerged as a key player in decision-making, clinical management and therapy selection. A brief timeline of lung cancer pathology as it relates to clinical practice is presented in Table 1.
Table 1.
A brief timeline of lung cancer pathology as it relates to clinical practice.
| Time period | Observation/discovery/application | Ref. |
|---|---|---|
| Late 1800s | NSCLC types recognized after the establishment of Virchow’s cell theory | |
| Mid 1920s | SCLC distinguished from ‘mediastinal sarcoma’ | [45] |
| 1960s | The concept of noninvasive ADC and the term BAC introduced by Averill Liebow | [27] |
| 1970s | Therapeutic benefit of distinguishing SCLC from NSCLC discovered | [45] |
| Mid 1990s | Importance of noninvasive ADC recognized | [29] |
| Mid 1990s–present | Importance of identifying SCC and ADC types of NSCLC Application of immunostains for NSCLC typing (see Table 2) General recognition of large-cell neuroendocrine carcinoma as an entity |
[13,15,16] [46] |
| Mid 2000s–present | Widespread usage of molecular testing for the identification of targets for individualized therapy | [47–49]† |
| 2010 | Revised classification of ADC having multidisciplinary input and clinical relevance (see text) accompanied by the timely death of the term BAC | [8] |
| Predictions for the near future | Revised classification of SCC as having clinical relevance Importance of ADC subtyping recognized and its application to clinical practice Improved immunostain panel for NSCLC typing The term NSCLC-NOS, now on the endangered species list, will become extinct |
These references contain further details on the indications and methodologies for molecular testing for clinically actionable targets.
ADC: Adenocarcinoma; BAC: Bronchioloalveolar carcinoma; NSCLC: Non-small-cell lung cancer; NSCLC-NOS: Non-small-cell lung cancer – not otherwise specified; SCC: Squamous cell carcinoma; SCLC: Small-cell lung cancer.
With the development of modern combination chemotherapy regimens, initial interest focused on small-cell lung cancer (SCLC). SCLC was usually metastatic at diagnosis, and unlikely to be cured by conventional therapies (surgical resection or localized radiotherapy). However, it was found to be more responsive to combination chemotherapy (at least initially) than non-small-cell lung cancer (NSCLC). Thus, curative-intent surgical resection was often limited to NSCLC, and chemotherapy became the front-line therapy for SCLC. Until approximately 10 years ago, the role of the pathologist was largely limited to determining whether the lung cancer was SCLC or NSCLC. There was little or no prognostic or therapeutic decision-making based on NSCLC typing. That situation has completely changed for a number of reasons. These reasons include the development of drugs that target specific NSCLC type(s) and the discovery of drugs that are contraindicated in specific NSLCLC type(s) and mutation-based targets that are usually or always associated with specific NSCLC types or subtypes. The pathologist’s armamentarium has been greatly enhanced by the identification and validation of relatively simple immunostaining methodologies and algorithms that aid histologic typing, as well as the development of more clinically relevant pathologic classifications (Table 2). As discussed in detail below, these subjects form the basis of this management perspective.
Table 2.
Select list of commonly used immunostains for the identification of the major non-small-cell lung cancer types.
| NSCLC type | Gene | Function | Comment |
|---|---|---|---|
| Squamous cell carcinoma | TP63 | Member of TP53 family of transcriptional factors | Expressed in bronchial basal cells and metaplastic squamous cells |
| P40 (δNp63) | One of the two major isoforms of TP63 | More specific than TP63 | |
| Sox2† | Transcription factor for stem cell renewal | Frequently amplified and over expressed in squamous cell carcinoma | |
| High-molecular-weight keratins, CK5/6 | Lineage-specific intermediate filaments | Characteristic of squamous cells | |
| Adenocarcinoma | TITF1 (also known as TTF1 or NKX2-1) | Master transcription factor for the peripheral airways | Marker for peripheral adenocarcinomas |
| Napsin A | Aspartic proteinase involved in the maturation of surfactant proteins | May be more sensitive than TITF1 | |
The importance of NSCLC typing
NSCLC consists of two major forms of differentiated cancers (adenocarcinoma [ADC] and squamous cell carcinoma [SCC]), one undifferentiated form (large-cell carcinoma) and several minor or rare types. Large-cell carcinoma may consist of poorly differentiated forms of the other types or represent truly undifferentiated NSCLC [7,8]. Thus, for practical purposes, the differentiation of the two major forms of NSCLC – ADC and SCC – is of paramount interest. Several recent observations and developments underscore the importance of this distinction, with more to surely follow.
The recent introduction in the USA and some other countries of pemetrexed in the first-line and maintenance treatment of advanced nonsquamous NSCLC represents an important step forward in the treatment of NSCLC [9–11]. Pemetrexed is an inhibitor of the folate-dependent enzyme thymidylate synthase. Thymidylate synthase is differentially expressed among the histological subtypes of lung cancer, being lower in ADC and higher in SCC and SCLC. While some studies have not confirmed these findings [12], maintenance therapy with pemetrexed is well tolerated and offers improved progression-free and overall survival compared with placebo in patients with advanced NSCLC [13].
While platinum-based doublet therapy is the ‘gold standard’ as the first-line therapy for NSCLC, the addition of bevacizumab may increase the efficacy of doublet therapy [11,14]. Bevacizumab, an anti-VEGF monoclonal antibody, is the only US FDA-approved antiangiogenic agent for advanced NSCLC [15]. However, squamous histology is a contraindication for bevacizumab therapy based on reports of severe hemorrhage following therapy, possibly related to the bulky size of centrally located SCCs. Thus, drug administration is usually limited to NSCLC tumors that have nonsquamous histologies. Small-molecule kinase inhibitors against angiogenesis targets are also associated with hemorrhage in NSCLC tumors having squamous histologies [16].
Thus, histology may be useful for both selection as well as exclusion of therapy. In addition, as described below, histology may also play a crucial role in testing for molecular targets and for the selection of individualized therapies based on such findings.
Identification of NSCLC types
The importance of accurate identification of NSCLC types has been discussed. This process has been greatly aided by the development and application of reliable immunostains for this purpose, and algorithms for their interpretation [17–20]. However, although the stains and the algorithms are reliable, they are not totally sensitive or specific, hence the continuing search for the ‘perfect’ immunostain. Recently, my colleagues and I have developed a microarray expression signature that distinguishes the major NSCLC types with great precision [Gazdar AF, Unpublished Data]. The signature has identified many previously unknown markers for SCC and ADC. Hopefully, some of these will lead to the introduction and validation of improved immunostains and algorithms. A selected group of commonly used and reliable immunostains is listed in Table 2.
Molecular targets for NSCLC
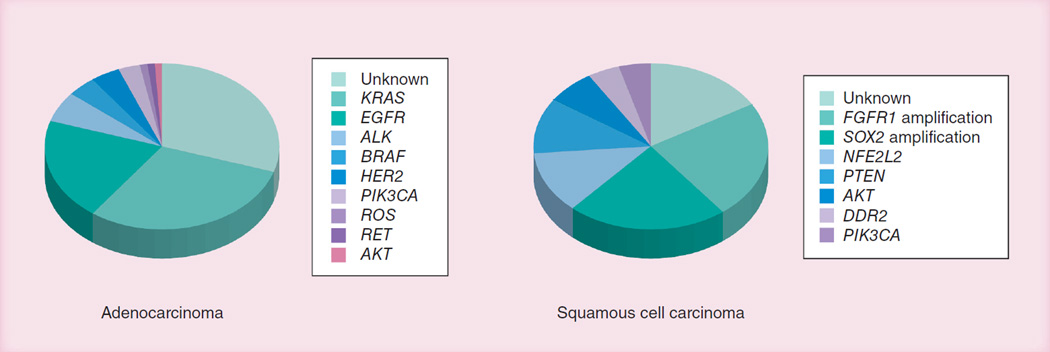
Lung cancers demonstrate remarkable intertumor heterogeneity and, to varying extents, intratumoral heterogeneity. ADC and, to a lesser extent, SCC tumors can be subdivided into multiple pathological and molecular subtypes [21,22]. Current cancer therapy is based on the premise that patients with similar types and stages of cancer should be treated using standardized protocols. However, recent advances in pharmacology, genomics and the molecular biology of tumors have permitted the development of individualized selection of treatment, as determined by the individualized features of the patient and the tumor [5,6,23]. Classic examples of such successful applications include imatinib for ABL-activated chronic myelogenous leukemia [24] and tyrosine kinase inhibitors for EGFR-mutant NSCLC [25]. There are three requirements for the successful application of personalized therapy: identification of a suitable target, usually molecular; identification of a suitable drug that inhibits or kills target-containing tumor cells; and identification of a subset of the tumor type that carries the target. There has been an enormous effort for the identification of driver mutations in lung cancers for the successful application of targeted therapies. Until recently, much of the work has centered on lung ADCs. Recently, much new knowledge regarding SCCs has been discovered, although its characterization remains a work in progress. Little new knowledge has emerged regarding SCLC, in part, because of the difficulty in obtaining tumor tissue for molecular analyses. A summary about driver mutations in NSCLC is presented in Figure 1. The almost completely different mutation patterns for ADC and SCC reflect the different origins and pathogenesis of these major NSCLC types.
Figure 1. Dominant driver mutation patterns for the major forms of non-small-cell lung cancer.
The pie chart values represent, in many cases, the median values of the ranges reported. ‘Unknown’ frequencies assume that all mutations are mutually exclusive and the true figure may be higher.
Data are from multiple sources in the literature, but in particular from [58,59].
Currently acknowledged effective therapies are available for certain ADC markers, including EGFR and ALK translocations, but not for many other theoretically promising markers, including KRAS mutations for ADC and all of the SCC markers. However, with approximately 800 targeted therapies in development or trial, many other effective therapies for ADC and SCC will almost certainly develop in the near future.
The ability to identify molecular targets is essential for the application of effective therapies. Some academic institutions test for multiple mutations, even though effective therapies are not currently available for all of them. At some institutions, the diagnosis of lung ADC triggers ‘reflex testing’ for mutations (i.e., testing dictated by histological diagnosis or clinical setting rather than by physician). Testing is often performed in commercial or reference laboratories; however, large centers with heavy lung cancer-patient loads may perform in-house testing. Such testing often falls to the pathology laboratory for a number of reasons: the pathologist ‘controls’ the tissue and can make judgments regarding selection of tumor-rich viable samples for both diagnosis and testing; and the pathology laboratory often has other established laboratories for molecular testing that are Clinical Laboratory Improvement Amendments certified and thus suitable for clinical testing [101].
A clinically relevant pathological classification of ADC
The WHO sponsored a series of classifications of cancers including lung cancer, the latest of which was published in 2004 [26]. These classification series were determined by lung cancer pathologists, with limited input from clinicians and molecular biologists. Thus, they could be regarded as ‘pathology classifications by pathologists for pathologists’. While some aspects of the classification were, and remain, clinically relevant, others, especially the subtyping of ADCs, were either ignored or misapplied or had no apparent clinical relevance. It became apparent that a more clinically relevant classification was required, with the greatest need existing for ADC. A series of conferences were held in New York City under the auspices of the International Association for the Study of Lung Cancer, the American Thoracic Society and the European Respiratory Society. The international group consisted of approximately 50 experts representing the fields of pathology, thoracic surgery, radiology, molecular biology, pulmonology and medical oncology. Although histology remains as the basis of the new classification, the major input resulting from a mutltidisciplinary approach has resulted in a much improved, evidence-based classification with clinical relevance [8]. Another important aspect of the new classification is that it addressed the problem of small specimens, while previous classifications only addressed resected tumors. The classification and the panel’s recommendations go well beyond pathologic typing and encompass a number of important points regarding the crucial clinical role of the pathologist in the management of lung cancer. The major new points raised by the new classification and their impact are discussed below.
▪ Preinvasive lesions
Over the past several years, it has been recognized that, in addition to ADC in situ (AIS), peripheral lesions known as atypical adenomatous hyperplasias (AAHs) are potential precursors of invasive ADC. AAH lesions are the equivalent of squamous dysplastic lesions in the larger airways for SCC and may progress to AIS (see below) and, eventually, to invasive ADC. Most AAH lesions are small (<1 cm). Both AIS and AAH lesions may appear as ground glass opacities without solid components on computed tomography examination. Minimally invasive and other invasive cancers may have extensive ground glass components, but also have solid foci.
▪ Noninvasive & minimally invasive ADC
It was recommended that the term AIS replace bronchioloalveolar carcinoma (BAC). This is perhaps the most controversial of the recommended changes as it proposes replacing one of the most widely used (and misused) terms in pulmonary pathology. The term was coined by the famous pulmonary pathologist Averill Liebow of Yale University in 1960 for a noninvasive form of peripheral ADC with prominent lepidic growth along alveoli and bronchioles [27]. He postulated that BAC, if extensive, would almost certainly have focal areas of invasion. As a pathological term, it initially referred to a noninvasive form, but was widely misquoted, misspelled and misused. Another problem was that ‘BAC-like’ tumors were often regarded as a distinct form of NSCLC, rather than a noninvasive form of ADC. The ADC panel reluctantly came to the conclusion that the only way to salvage its meaning as a strictly noninvasive form of ADC was to discard the term BAC and to replace it with AIS. The clinical importance of the strictly noninvasive form of ADC was highlighted in a series of papers from a Japanese team of pathologists who demonstrated that small (<3 cm) noninvasive ADCs, if completely resected, had an excellent chance of being cured [28,29], while tumors with minimal regions of invasion also enjoyed a prognosis that was nearly as good. A new category of ‘minimally invasive ADC’ was established for small tumors having a prominent noninvasive growth. Nearly all of these patients survive if their tumors are completely resected.
▪ Invasive ADC & its subtypes
An invasive component is present in up to 90% of resected ADC. While the WHO 1999/2004 classifications had recognized four subtypes of ADC, almost all tumors were of the mixed subtype, containing components of two or more of the individual subtypes. This led to a disillusionment with subtyping, which was rarely used except for ‘BAC-like’ tumors. In addition, the panel recommended that for invasive ADCs, the predominant pattern should be reported, as well as the percentages of any subtypes also present. A micropapillary subtype associated with poor prognosis was added to the original subtypes (BAC pattern or noninvasive, acinar, papillary and solid with mucin). It was also recognized that certain genetic changes may correspond to specific subtypes or histological variants. For instance, EGFR mutation-containing tumors often have AIS or papillary components, mucinous cancers frequently contain KRAS mutations (and almost never have EGFR mutations), and ALK translocations often have a mucinous or signet ring appearance. Signet ring cancers were not recognized as a distinct subtype because such cells may be seen in more than one subtype.
▪ The virtual disappearance of ‘NSCLC – not otherwise specified’ cancers
The term NSCLC – not otherwise specified (NSCLC-NOS) was coined to describe small biopsies or cytology specimens of lung cancers that were clearly not SCLC, but could not be classified into SCC or ADC types [30]. Resected specimens of NSCLC that cannot be classified are termed ‘large-cell carcinomas’. However, the term could not be applied to small specimens because the availability of more material may have led to type identification. Until recently, it was a moot point, as NSCLC typing did not have much effect on lung cancer management. However, as previously discussed, this is no longer the situation, and NSCLC typing is becoming of increasing importance. Fortunately, the application of the simple immunostaining panel as previously described (Table 2) has led to the vast majority of tumors with scant material previously classified as NSCLC-NOS to be accurately typed as SCC or ADC. Thus, the term NSCLC-NOS is on the endangered species list and, hopefully, will become extinct in the not-too-distant future. The pathology panel recognized the importance of accurate NSCLC typing whenever possible.
▪ The problem of multifocal lung cancers
It has been recognized for some years that many lung cancers present as multiple primaries, as was noted by Slaughter et al. in his original description of the field cancerization process for head and neck cancers [31]. In addition to field effects, multifocal cancers may represent metastases from a single primary cancer [32]. The widespread introduction of computed tomography screening methods for the early diagnosis of lung cancer has highlighted this problem and demonstrated its high prevalence [33]. Both genomic and histological methods may be used to distinguish between these two possibilities [32,34]. However, the prognosis of lesions having these very different origins and spread is similar, provided they are treated aggressively [32]. These facts have resulted in a downstaging of multifocal cancers without distant metastases [35].
▪ Validation
A recent international multi-institutional study demonstrated that, for the majority of ADC cases, “there is good reproducibility in identifying a predominant pattern and fair reproducibility distinguishing invasive from in situ (wholly lepidic) patterns” [36]. However, the report indicated that more precise definitions and better education regarding the interpretation of existing terminology were of importance.
▪Management of tissue for molecular studies & protocol requirements is critical
With the successful application of individualized therapy for tumors with specific mutations, and with many lung cancer patients entering clinical trials at academic centers, the pathologist must resolve the dilemma of satisfying diagnostic needs with those for molecular testing and other protocol requirements. In addition, the majority (~70%) of diagnostic specimens are small biopsies or cytology specimens. In other words, ‘more is expected of less’. Thus, the satisfaction of these competing requirements may present a dilemma for the pathologist. In general, decisions on optimal usage of scant specimens require multidisciplinary input for specific protocols or other individual situations. Good general practice would indicate preservation of snap-frozen tumor and adjacent non-malignant lung whenever possible, and preparation of a cell pellet from cytological specimens. Identification of suitable blocks for molecular studies would be of great utility. Such blocks should contain a relatively high proportion of viable tumor cells.
▪ Prognostic & predictive markers
A large body of literature has addressed the issue of the identification of prognostic and predictive markers. Staging has been demonstrated to be of prognostic importance. However, in general, predictive markers have been of greater utility than prognostic markers. The use of histology and molecular testing as a predictive marker has been described. In addition, a large number of individual genes [37], as well as genome-wide molecular signatures, have been proposed as having predictive or prognostic value. Most of these have not been validated in large multicenter clinical trials and have fallen by the wayside. Optimizing treatment according to tumor status for DNA-repair biomarkers, such as ERCC1, BRCA1 or RRM1, could predict response to platinum-, taxane- and gemcitabine-based therapies, respectively, and might substantially improve the response of individual patients’ tumors [38]. Perhaps the best-studied DNA marker is ERCC1, but a recent meta-analysis indicated the need for further study [39]. Most gene signatures for lung cancer described in the literature are flawed by intrinsic design or data analysis [40,41] and fail to meet the minimal requirements for such studies [42]. However, some studies appear to have avoided these pitfalls [21,43,44], but still require validation. The role of the pathologist in this arena is crucial for biomarker discovery, validation and application.
Conclusion & future perspective
The major changes in pathologic practice, coupled with the vastly different landscape of clinical diagnosis, care and the advent of individualized therapy, have brought about a paradigm shift in the role of the pathologist in lung cancer management. This paradigm shift has been aided by the emergence of a new, multidisciplinary, clinically relevant classification system for pulmonary ADC. No longer is the pathologist only responsible for making the broadest of pathological diagnoses, now being an intrinsic part of the lung cancer management team, playing a key role in many aspects, including diagnosis, tumor typing and subtyping, molecular testing, analyses for prediction and prognosis and for the fulfillment of protocol requirements. The pathologist must closely interact with the other members of the clinical team in order to fulfill these functions.
The successful emergence of the new, paradigm-changing multidisciplinary classification of pulmonary ADC will spur the development of similar classifications for all of the major lung cancer types. Advances in molecular biology will bring further changes, with molecular testing replacing some of the common and routine procedures, such as conventional histological diagnosis, for some special cases or applications. Our concept of the evolving role of the pathologist in clinical decision-making regarding the management of lung cancer is presented in Box 1. However, pathological examination will remain the cornerstone of cancer diagnosis, and the role of the pathologist will continue to evolve into an integral, essential component of the lung cancer clinical management team.
Box 1. The evolving role of the pathologist in clinical decision-making regarding the management of lung cancer.
Then
-
▪
‘Is the cancer SCLC or NSCLC?’
Now
-
▪
‘What type of NSCLC is the cancer?’
‘What is the subtype?’
‘Is there invasion present?’
‘Is the lesion unifocal or multifocal?’
‘Have you identified any predictive or prognostic markers?’
‘Are there preneoplastic lesions present?’
‘Is there sufficient material for diagnosis and for molecular testing?’
‘Is there sufficient material for satisfying protocol study requirements?’
This box is presents as a series of frequently asked questions of the pathologist at two time points. ‘Then’ refers to the period prior to the year 2000 and ‘Now’ refers to the post-2000 period.
NSCLC: Non-small-cell lung cancer; SCLC: Small-cell lung cancer.
Practice Points.
-
▪
Major developments in conventional (cytotoxic) and individualized (targeted) therapy have made accurate typing of non-small-cell lung cancer (NSCLC) essential.
-
▪
The application of a relatively simple set of immunostains has resulted in the accurate typing of most NSCLC specimens.
-
▪
Using these immunostains, even most small biopsies and cytological specimens of lung cancer can be typed, greatly reducing the need for the term ‘NSCLC – not otherwise specified’.
-
▪
Previous pathological classifications were devised ‘by pathologists for pathologists’ and had only modest clinical relevance.
-
▪
A new multidisciplinary classification of lung adenocarcinoma is of clinical and radiological relevance, and some of the subtypes are correlated with specific mutational patterns.
-
▪
Because of widespread misuse and misinterpretation of the term ‘bronchioloalveolar carcinoma’, the Adenocarcinoma Panel recommended that it be replaced by the term ‘adenocarcinoma in situ’ and its use be strictly limited to noninvasive carcinomas.
-
▪
The pathologist has developed into a key player in the lung cancer management team, having a crucial role in diagnosis, therapy selection, clinical management, biomarker testing and satisfaction of protocol requirements.
Acknowledgement
The author is supported by grants from the Canary Foundation and the National Cancer Institute, Bethesda, MD, USA (The University of Texas SPORE in Lung Cancer P50CA70907 and the Early Detection Research Network U0CA084986). The author is also a consultant/lecturer for AstraZeneca.
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur. J. Cancer. 2010;46(4):765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM. 2. Tobacco-attributable cancer burden in the UK in 2010. Br. J. Cancer. 2011;105(Suppl. 2):S6–S13. doi: 10.1038/bjc.2011.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gazdar AF. Personalized medicine and inhibition of EGFR signaling in lung cancer. N. Engl. J. Med. 2009;361(10):1018–1020. doi: 10.1056/NEJMe0905763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok TS. Personalized medicine in lung cancer. What we need to know. Nat. Rev Clin. Oncol. 2011;8(11):661–668. doi: 10.1038/nrclinonc.2011.126. ▪ Good practical review of personalized medicine.
- 7.Pardo J, Martinez-Penuela AM, Sola JJ, et al. Large cell carcinoma of the lung: an endangered species? Appl. Immunohistochem. Mol. Morphol. 2009;17(5):383–392. doi: 10.1097/PAI.0b013e31819bfd59. [DOI] [PubMed] [Google Scholar]
- 8. Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/ European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. ▪▪ Revised classification of lung adenocarcinoma that is a ‘must read’ for all clinicians involved in the diagnosis or management of lung cancer.
- 9.Gridelli C, Maione P, Rossi A, et al. Pemetrexed in advanced non-small cell lung cancer. Expert Opin. Drug Saf. 2011;10(2):311–317. doi: 10.1517/14740338.2011.553281. [DOI] [PubMed] [Google Scholar]
- 10.Galvani E, Peters GJ, Giovannetti E. Thymidylate synthase inhibitors for non-small cell lung cancer. Expert Opin. Investig. Drugs. 2011;20(10):1343–1356. doi: 10.1517/13543784.2011.617742. [DOI] [PubMed] [Google Scholar]
- 11.Selvaggi G, Scagliotti GV. Histologic subtype in NSCLC. Does it matter? Oncology. 2009;23(13):1133–1140. [PubMed] [Google Scholar]
- 12.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist. 2009;14(3):253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 13.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, Phase 3 study. Lancet. 2009;374(9699):1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 14.Rossi G, Pelosi G, Graziano P, Barbareschi M, Papotti M. A reevaluation of the clinical significance of histological subtyping of non-small-cell lung carcinoma. diagnostic algorithms in the era of personalized treatments. Int. J. Surg. Pathol. 2009;17(3):206–218. doi: 10.1177/1066896909336178. [DOI] [PubMed] [Google Scholar]
- 15.Planchard D. Bevacizumab in non-small-cell lung cancer: a review. Expert Rev. Anticancer Ther. 2011;11(8):1163–1179. doi: 10.1586/era.11.80. [DOI] [PubMed] [Google Scholar]
- 16.Langer CJ, Besse B, Gualberto A, Brambilla E, Soria JC. The evolving role of histology in the management of advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28(36):5311–5320. doi: 10.1200/JCO.2010.28.8126. [DOI] [PubMed] [Google Scholar]
- 17.Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod. Pathol. 2011;24(10):1348–1359. doi: 10.1038/modpathol.2011.92. [DOI] [PubMed] [Google Scholar]
- 18.Cagle PT, Allen TC, Dacic S, et al. Revolution in lung cancer: new challenges for the surgical pathologist. Arch. Pathol. Lab. Med. 2011;135(1):110–116. doi: 10.5858/2010-0567-RA.1. [DOI] [PubMed] [Google Scholar]
- 19.Mukhopadhyay S, Katzenstein AL. Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. Am. J. Surg. Pathog. 2011;35(1):15–25. doi: 10.1097/PAS.0b013e3182036d05. [DOI] [PubMed] [Google Scholar]
- 20.Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod. Pathol. 2012;25(3):405–415. doi: 10.1038/modpathol.2011.173. [DOI] [PubMed] [Google Scholar]
- 21.Bryant CM, Albertus DL, Kim S, et al. Clinically relevant characterization of lung adenocarcinoma subtypes based on cellular pathways: an international validation study. PLoS One. 2010;5(7):e11712. doi: 10.1371/journal.pone.0011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkerson MD, Yin X, Hoadley KA, et al. Lung squamous cell carcinoma mRNA expression subtypes are reproducible, clinically important, and correspond to normal cell types. Clin. Cancer Res. 2010;16(19):4864–4875. doi: 10.1158/1078-0432.CCR-10-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nat. Med. 2012;18(3):349–351. doi: 10.1038/nm.2697. [DOI] [PubMed] [Google Scholar]
- 24.Druker BJ. Perspectives on the development of imatinib and the future of cancer research. Nat. Med. 2009;15(10):1149–1152. doi: 10.1038/nm1009-1149. [DOI] [PubMed] [Google Scholar]
- 25.Gazdar AF. Epidermal growth factor receptor inhibition in lung cancer: the evolving role of individualized therapy. Cancer Metastasis Rev. 2010;29(1):37–48. doi: 10.1007/s10555-010-9201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travis WD, Brambilla E, Muller-Herelink HK, Harris CC. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. France: IARC Press; 2004. [Google Scholar]
- 27.Liebow AA. Bronchiolo-alveolar carcinoma. Adv. Intern. Med. 1960;10:329–358. [PubMed] [Google Scholar]
- 28. Noguchi M. Stepwise progression of pulmonary adenocarcinoma – clinical and molecular implications. Cancer Metastasis Rev. 2010;29(1):15–21. doi: 10.1007/s10555-010-9210-y. ▪ Update and summary of the concept (originally described in [29]) that small noninvasive adenocarcinomas have an excellent prognosis if completely resected.
- 29.Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844–2852. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Gazdar AF. Should we continue to use the term non-small-cell lung cancer? Ann. Oncol. 2010;21(Suppl. 7):vii225–vii229. doi: 10.1093/annonc/mdq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slaughter DP, Southwick HW, Smejkal W. ‘Field cancerization’ in oral stratified squamous epithelium. Clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Gazdar AF, Minna JD. Multifocal lung cancers – clonality vs field cancerization and does it matter? J. Natl Cancer Inst. 2009;101(8):541–543. doi: 10.1093/jnci/djp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flieder DB, Vazquez M, Carter D, et al. Pathologic findings of lung tumors diagnosed on baseline CT screening. Am. J. Surg. Pathol. 2006;30(5):606–613. doi: 10.1097/01.pas.0000202040.51967.d0. [DOI] [PubMed] [Google Scholar]
- 34.Girard N, Deshpande C, Lau C, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am. J. Surg. Pathol. 2009;33(12):1752–1764. doi: 10.1097/PAS.0b013e3181b8cf03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J. Thorac. Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. ▪ Important update of lung cancer staging.
- 36.Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod. Pathol. 2012 doi: 10.1038/modpathol.2012.106. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coate LE, John T, Tsao MS, Shepherd FA. Molecular predictive and prognostic markers in non-small-cell lung cancer. Lancet Oncol. 2009;10(10):1001–1010. doi: 10.1016/S1470-2045(09)70155-X. [DOI] [PubMed] [Google Scholar]
- 38. Postel-Vinay S, Vanhecke E, Olaussen KA, Lord CJ, Ashworth A, Soria JC. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat. Rev. Clin. Oncol. 2012;9(3):144–155. doi: 10.1038/nrclinonc.2012.3. ▪ Comprehensive review of DNA-repair proteins as predictive and prognostic markers.
- 39.Hubner RA, Riley RD, Billingham LJ, Popat S. Excision repair cross-complementation group 1 (ERCC1) status and lung cancer outcomes: a meta-analysis of published studies and recommendations. PLoS One. 2011;6(10):e25164. doi: 10.1371/journal.pone.0025164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Subramanian J, Simon R. Gene expression-based prognostic signatures in lung cancer. ready for clinical use? J. Natl Cancer Inst. 2010;102(7):464–474. doi: 10.1093/jnci/djq025. ▪ Critical analysis of gene expression prognostic signatures.
- 41.Gazdar AF, Schiller JH. Predictive and prognostic factors for non-small cell lung cancer – potholes in the road to the promised land. J. Natl Cancer Inst. 2011;103(24):1810–1811. doi: 10.1093/jnci/djr497. [DOI] [PubMed] [Google Scholar]
- 42. Baggerly KA, Coombes KR. What information should be required to support clinical ‘omics’ publications? Clin. Chem. 2011;57:688–690. doi: 10.1373/clinchem.2010.158618. ▪ Clearly states the essential components required for disclosure when gene prognostic signatures are published.
- 43.Chen DT, Hsu YL, Fulp WJ, et al. Prognostic and predictive value of a malignancy-risk gene signature in early-stage non-small cell lung cancer. J. Natl Cancer Inst. 2011;103(24):1859–1870. doi: 10.1093/jnci/djr420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kratz JR, He J, Van Den Eeden SK, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet. 2012;379(9818):823–832. doi: 10.1016/S0140-6736(11)61941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haddadin S, Perry MC. History of small-cell lung cancer. Clin. Lung Cancer. 2011;12(2):87–93. doi: 10.1016/j.cllc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Travis WD. Advances in neuroendocrine lung tumors. Ann. Oncol. 2010;21(Suppl. 7):vii65–vii71. doi: 10.1093/annonc/mdq380. [DOI] [PubMed] [Google Scholar]
- 47.D’Angelo SP, Park B, Azzoli CG, et al. Reflex testing of resected stage I through III lung adenocarcinomas for EGFR and KRAS mutation: report on initial experience and clinical utility at a single center. J. Thorac. Cardiovasc. Surg. 2010;141(2):476–480. doi: 10.1016/j.jtcvs.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taylor BS, Ladanyi M. Clinical cancer genomics: how soon is now? J. Pathol. 2011;223(2):319–327. doi: 10.1002/path.2794. ▪ Useful summary of the clinical applications of cancer genomics.
- 49.Heist RS, Engelman JA. SnapShot. Non small cell lung cancer. Cancer Cell. 2012;21(3):448.e2. doi: 10.1016/j.ccr.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Hussenet T, Dali S, Exinger J, et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One. 2010;5(1):e8960. doi: 10.1371/journal.pone.0008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mccaughan F, Pole JC, Bankier AT, et al. Progressive 3q amplification consistently targets SOX2 in preinvasive squamous lung cancer. Am. J. Resp. Crit. Care Med. 2010;182(1):83–91. doi: 10.1164/rccm.201001-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sholl LM, Long KB, Hornick JL. SOX2 expression in pulmonary non-small cell and neuroendocrine carcinomas. Appl. Immunohistochem. Mol. Morphol. 2010;18(1):55–61. doi: 10.1097/PAI.0b013e3181b16b88. [DOI] [PubMed] [Google Scholar]
- 53.Maier S, Wilbertz T, Braun M, et al. SOX2 amplification is a common event in squamous cell carcinomas of different organ sites. Hum. Pathol. 2011;42(8):1078–1088. doi: 10.1016/j.humpath.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology. Strategic management of tissue for molecular testing. Semin. Respir. Crit. Care Med. 2011;32(1):22–31. doi: 10.1055/s-0031-1272866. [DOI] [PubMed] [Google Scholar]
- 55. Sigel CS, Moreira AL, Travis WD, et al. Subtyping of non-small cell lung carcinoma. A comparison of small biopsy and cytology specimens. J. Thorac. Oncol. 2011;6(11):1849–1856. doi: 10.1097/JTO.0b013e318227142d. ▪ Demonstrates the practicality of using small biopsies and cytology specimens for non small-cell lung cancer typing.
- 56.Rekhtman N, Brandt SM, Sigel CS, et al. Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing. J. Thorac. Oncol. 2011;6(3):451–458. doi: 10.1097/JTO.0b013e31820517a3. [DOI] [PubMed] [Google Scholar]
- 57.Loo PS, Thomas SC, Nicolson MC, Fyfe MN, Kerr KM. Subtyping of undifferentiated non-small cell carcinomas in bronchial biopsy specimens. J. Thorac. Oncol. 2010;5(4):442–447. doi: 10.1097/JTO.0b013e3181d40fac. [DOI] [PubMed] [Google Scholar]
- 58.Drilon A, Rekhtman N, Ladanyi M, Paik P. Squamous cell carcinomas of the lung. Emerging biology, controversies, and the promise of targeted therapy. Lancet Oncol. 2012;13(10):e418–e426. doi: 10.1016/S1470-2045(12)70291-7. [DOI] [PubMed] [Google Scholar]
- 59. Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12(2):175–180. doi: 10.1016/S1470-2045(10)70087-5. ▪ Summarizes the driver mutations (and thus potential targets for therapy) in non-small-cell lung cancer. However, recent major sequencing studies have added many new drivers.
- 101.Global Resource for Advancing Cancer Education. ‘Reflex testing’ in lung cancer: who to test and when? http://cancergrace.org/lung/2011/06/25/reflex-testing-in-lung-cancer-who-to-test-and-when.