Abstract
Across taxa, males employ a variety of mating strategies, including sexual coercion and the provision, or trading, of resources. Biological market theory (BMT) predicts that trading of commodities for mating opportunities should exist only when males cannot monopolize access to females and/or obtain mating by force, in situations where power differentials between males are low; both coercion and trading have been reported for chimpanzees (Pan troglodytes). Here, we investigate whether the choice of strategy depends on the variation in male power differentials, using data from two wild communities of East African chimpanzees (Pan troglodytes schweinfurthii): the structurally despotic Sonso community (Budongo, Uganda) and the structurally egalitarian M-group (Mahale, Tanzania). We found evidence of sexual coercion by male Sonso chimpanzees, and of trading—of grooming for mating—by M-group males; females traded sex for neither meat nor protection from male aggression. Our results suggest that the despotism–egalitarian axis influences strategy choice: male chimpanzees appear to pursue sexual coercion when power differentials are large and trading when power differentials are small and coercion consequently ineffective. Our findings demonstrate that trading and coercive strategies are not restricted to particular chimpanzee subspecies; instead, their occurrence is consistent with BMT predictions. Our study raises interesting, and as yet unanswered, questions regarding female chimpanzees’ willingness to trade sex for grooming, if doing so represents a compromise to their fundamentally promiscuous mating strategy. It highlights the importance of within-species cross-group comparisons and the need for further study of the relationship between mating strategy and dominance steepness.
Keywords: Pan troglodytes, Mating strategy, Social grooming, Aggression, Dominance rank, Biological market theory
Introduction
Mating strategies can be defined as those behaviours that males and females use to maximize their reproductive success (Newton-Fisher 2014). Since males commonly invest less in the production and care of offspring than do females (Trivers 1972), they have higher rates of reproduction (Clutton-Brock and Parker 1992) and consequent intra-sexual competition over access to mates. Across taxa, adult males show a variety of mating strategies, including displays to elicit female mate choice (e.g. male coloration in sticklebacks, Gasterosteus aculeatus: Milinski and Bakker 1990), aggressive herding and other forms of coercion (e.g. dolphins, Tursiops sp.: Connor et al. 1992), as well as controlling resources of value to females, either via territory (e.g. pied flycatcher, Ficedula hypoleuca: Alatalo et al. 1986), or directly: in a broad range of arthropods (e.g. nursery-web spider, Pisaura mirabilis: Bilde et al. 2007), for instance, males offer material donations (or nuptial gifts) to the females during mating (Gwynne 2008), providing females with an important food source (Voigt et al. 2005).
In a number of primate species, there is evidence that males similarly trade commodities such as food, or a service such as grooming, with females for mating access (chimpanzees, Pan troglodytes: Hemelrijk et al. 1992; Gomes and Boesch 2009, 2011; long-tailed macaque, Macaca fascicularis: Gumert 2007; sifaka, Propithecus verreauxi: Norscia et al. 2009; whitehanded gibbon, Hylobates lar: Barelli et al. 2011; snub-nosed monkey, Rhinopithecus roxellana: Yu et al. 2013). Such trading can be understood using biological market theory (BMT), a model of natural selection under which behaviours are shaped by market forces (Noë and Hammerstein 1994, 1995; Noë 2001; Barrett and Henzi 2001; Kaburu and Newton-Fisher 2015). BMT predicts that trading patterns should break down when commodities can be forcibly appropriated, and so trading systems in the form of mating markets (exchanging commodities or services for mating access: Gumert 2007; Clarke et al 2010) are expected when males cannot monopolize access to sexually receptive females and/or cannot obtain mating by force (Noë and Hammerstein 1995).
By contrast, where males have substantially greater resource holding potential (RHP: Parker 1974) than females, and where RHP differs drastically amongst males, socially dominant males do not need to ‘buy’ a female’s acquiescence by offering a commodity in return: they can obtain mating by force (e.g. orangutan, Pongo pygmaeus, Mitani 1985; chacma baboon, Papio ursinus: Clarke et al. 2010; hamadryas baboon, Papio hamadryas: Colmenares et al. 2002) and monopolize sexually receptive females (as long as synchronicity in female fertility is limited). In this situation, the assumption is that the coercive strategy has a higher benefit to cost ratio for males than does the trading strategy; otherwise, we would expect males to trade for mating opportunities rather than taking them by force (i.e. using sexual coercion: Smuts and Smuts 1993). Where males are socially dominant to females but power differentials between males are small, intra-male competition over females is expected to be intense (Clarke et al. 2010), with males able to challenge one another more effectively. If this makes it harder for them to coerce and/or gain exclusive control over sexually receptive females, the costs of a coercive strategy may increase relative to trading (so decreasing net benefits) making the latter more viable as an alternative, and this may be exacerbated by any female resistance to male mating efforts (Cox and Le Boeuf 1977; Oda and Masataka 1995; Wong and Candolin 2005).
Therefore, the use of either a coercive or trading strategy should be contingent upon male power differentials, such that trading is absent where these are strong, and present where they are weak. Such power differentials may vary as a result of social and demographic processes (Cowlishaw and Dunbar 1991, 1992; Pawłowski et al. 1998; Mitani et al. 2002; Kutsukake and Nunn 2006), raising the possibility that both strategies may be present within the same species, or even the same social group under different conditions (e.g. Gross 1996; Setchell 2008; Neff and Svensson 2013).
In chimpanzees (P. troglodytes), there is evidence for both coercion and trading as male mating strategies, although controversy exists over interpretations of the latter (cf. Gilby et al. 2010). In East African chimpanzees (Pan troglodytes schweinfurthii), there is good evidence that males pursue a sexually coercive mating strategy, showing both direct (where males target aggression at females) and indirect (where they use aggression to stop other males mating with those females, e.g. mate guarding) forms of coercion (Goodall 1965; Tutin 1979; Muller et al. 2007, 2009; Muller and Wrangham 2009; Feldblum et al. 2014) with resulting fitness benefits (Feldblum et al. 2014). Coercive aggression is costly for females (Muller et al. 2007), and there is little evidence that proximate female mate choice (through both resistance and reluctance) has functional consequences (Klinkova et al. 2005; Muller et al. 2011). Female chimpanzees pursue a promiscuous mating strategy aimed at maximizing offspring survival by confusing paternity and reducing infanticide risk (Wrangham 1993, 2002; van Schaik 2000; Muller et al. 2007; Watts 2007): they show extended receptive periods with pronounced perianal swellings and long (3–5 days) periovulatory periods (Emery Thompson 2005; Stumpf and Boesch 2005). Female promiscuity also limits the usefulness of intra-male competition for high social rank as a male mating strategy: in the absence of coercion, high rank alone is unlikely to be enough to stop females from mating with rivals.
With regard to trading, whilst Tutin (1979) found evidence that males were more likely to initiate a consortship with females to whom they had given more grooming and with whom they shared meat more often, specific investigation has failed to find evidence that East African male chimpanzees trade meat for mating opportunities with cycling females (Mitani and Watts 2001; Gilby et al. 2010; cf. Stanford 1998). By contrast, in the West African subspecies (Pan trog lodytes verus), there is evidence that male chimpanzees trade commodities with females in return for mating. In the Taï Forest (Côte D’Ivoire), female chimpanzees mated more with those males who shared meat with them than with those who did not (Gomes and Boesch 2009, 2011); in Guinea, male chimpanzees were more likely to engage successfully in consortship with cycling females with whom they shared fruits more frequently (Hockings et al. 2007). Similarly in captive chimpanzees, often fully or partially of West African descent (Ely et al. 2005; Hvilsom et al. 2013), female chimpanzees mated more frequently with males that groomed them more (Hemelrijk et al. 1992), whilst the market forces of supply and demand influenced the amount of grooming that males directed to cycling females: males groomed females showing sexual swellings more when the availability of such females decreased (Koyama et al. 2012).
Although this variation in mating strategy is sometimes seen as a fundamental difference between subspecies (coercion amongst East African chimpanzees; trading amongst West African chimpanzees), this is a difficult hypothesis to test. Fortunately, the framework provided by BMT allows for an alternative: that the choice of strategy is a consequence of male power differentials, with trading—a mating market— appearing where these are small. Power differentials between males are captured by the steepness of their dominance hierarchy: when males have greater power differentials, hierarchies are steeper, whilst hierarchies are shallow when males differ little in their relative power (van Schaik 1989; Henzi and Barrett 1999). Hierarchy steepness measures the outcome of intra-male competition and so reflects the interplay of multiple possible causative ecological, demographic and social variables that drive the power differentials amongst males; whilst it is typical to regard hierarchy steepness as a species-level trait, we have shown previously (Kaburu and Newton-Fisher 2015) that, at least for chimpanzees, steepness varies over time within as well as between social groups (‘communities’: Goodall 1973).
Here, we test predictions derived from each of these two male mating strategies using data from two communities of wild East African chimpanzees: the Sonso community of the Budongo Forest Reserve, Uganda, in 2003/2004 and M-group from the Mahale Mountains National Park, Tanzania, in 2011. Specifically, we predict that if male chimpanzees use sexual coercion: (1) male aggression against females will be a significant predictor of male mating success; (2) males will direct more aggression to cycling than non-cycling females (cf. Feldblum 2014); (3) more aggressive males will gain more, and a greater proportion of, copulations; and (4) male rank will be associated with mating frequency, as high ranking males should be able to exploit their greater RHP to direct aggression against females without risking aggression from other males or female retaliation (cf. Newton-Fisher 2006), thereby achieving greater mating success than lower ranking males. Whilst such a rank–mating association might also be seen if females preferentially mated with high-ranking males without any influence of male aggression, female mate choice is thought to have little impact in East African chimpanzees (Muller et al. 2009, 2011)
Conversely, if males trade services or commodities in return for mating opportunities, cycling females will mate more with males who provide them with more meat and/or grooming, and so we predict that (5) grooming and/or meat transfer will be a significant predictor of male mating success; (6) males will groom cycling females more frequently when they are maximally swollen; and (7) cycling females will receive more grooming from males than they give. We include meat as a commodity as exchanges of meat for mating have been reported previously for chimpanzees (see above), but our focus here is on grooming as (a) it is a ubiquitous behaviour that any individual can perform (and thus a service that can be offered), and (b) male chimpanzees of both communities trade grooming amongst themselves (Kaburu and Newton-Fisher 2015); we take as a working assumption that grooming has inherent value, through parasite removal (Saunders and Hausfater 1988; Tanaka and Takefushi 1993; Zamma 2002) and/or stress reduction (Keverne et al. 1989; Feh and Demazieres 1993; Aureli et al. 1999).
We also investigate whether any exchange of grooming for sex is restricted to the immediate mating context (‘short-term’ trading). Hemelrijk et al. (1992) proposed that male chimpanzees groom females in order to suppress their tendency to flee, thereby allowing the male to mate. Whilst they contrasted this with a trading system, the two are not necessarily contradictory: their hypothesis refers to a proximate mechanism linking grooming with mating, whereas BMT is concerned with ultimate processes. That said, Hemelrijk et al.’s (1992) hypothesis specifically excludes long-term investment by males to lower females’ wariness. Instead, it predicts that (8) males direct grooming to cycling females primarily in a mating context, and that (9) grooming by males precedes, rather than follows, mating, in order to ensure that females do not run away before copulation occurs. Whilst these predictions alone are not sufficient to demonstrate the mechanism, both must hold if the Hemelrijk et al.’s (1992) hypothesis is valid.
Finally, we test whether male power differentials affect mating strategies. From our discussion of BMT, we predict (10) that males of the structurally despotic Sonso community (steep hierarchy, large male power differentials) will show evidence of a sexually coercive mating strategy, whilst those of the more structurally egalitarian M-group (shallow hierarchy, limited male power differentials) will show evidence of trading (a ‘mating market’). This relationship between variation in hierarchy steepness and male mating strategies has not been explored previously, and our approach allows us to avoid possible confounds due to subspecies differences, as well applying a common set of definitions and methodologies to the collection and analysis of data from both communities.
Material and methods
Study subjects and field sites
We collected behavioural data from two chimpanzee communities: the Sonso community of the Budongo Forest Reserve, a semi-deciduous tropical forest in western Uganda (Newton-Fisher 1997; Reynolds 2005), and M-group from the semi-evergreen Kasoje forest of the Mahale Mountains National Park in western Tanzania (Nishida 1990, 2012; Nakamura and Nishida 2012). The Budongo forest is situated between latitudes 1° 35′ and 1° 55′ North, and longitudes 31° 18′ and 31° 42′ East, with an average altitude of 1100 m (Newton-Fisher 1997; Reynolds 2005), whilst the Mahale Mountains are located at latitude 6° 15′ South and longitude 29° 55′ East with the highest peak exceeding 2500 m (Nakamura and Nishida 2012). Sonso chimpanzees were habituated by late 1994 (Newton-Fisher 1997) and have been continuously studied ever since (Reynolds 2005), whilst M-group chimpanzees have been studied for over 30 years, since the late 1970s (Nishida 1990, 2012; Nakamura and Nishida 2012).
Data collection
NEN-F collected behavioural data on Sonso chimpanzees between December 2003 and August 2004, during which time the community contained 63 individuals in total, including eight adult males (≥16 years old) and 21 adult females (≥14 years old). This community contained a total of 13 cycling females: 11 adults and 2 adolescents. SSKK collected data on M-group chimpanzees between February and November 2011. At the beginning of these observations, M-group contained 60 individuals in total, including 10 adult males and 23 adult females, with 11 cycling females in total: 6 adults and 5 adolescents (Kaburu and Newton-Fisher 2013). Specifically, we collected data on grooming interactions, aggression, meat transfers and copulations between males and females, defined as
(a) Grooming: visual examination, search and manipulation of the skin and hair with one or both hands. A grooming bout was considered ended when both individuals engaged in other activities, including simply resting, for more than 30 s. We consider a bout as an interaction, rather than the behaviour of a single individual (Barrett et al. 1999; Newton-Fisher and Lee 2011; Kaburu and Newton-Fisher 2013).
(b) Aggression: instances in which an individual attacked a community member either through physical contact (e.g. push, bite, slap) or by chase or charging display (Kaburu and Newton-Fisher 2013).
(c) Meat transfer: when one or more individuals were allowed to take the meat under the control of the owner, defined as the individual who had the carcass in the mouth, or in the hand or in close proximity (Nishida et al. 1999).
(d) Copulations: heterosexual interaction that included at least one intromission (Tutin 1979).
We regarded females as cycling if they showed a regular sexual swelling and elicited sexual interest amongst the adult males. In addition, all but two females designated as cycling had no infants under the age of 5 years (in the Sonso community, two such females had infants aged 4 years). Reproductive status was noted each day (if encountered) based on visual inspection of the sexual swelling, using a 3-point scale: I = no swelling; II = medium size; III = maximum swelling (Hasegawa and Hiraiwa-Hasegawa 1983). Male chimpanzees tend to mate with females preferentially around the period of maximum tumescence (Hasegawa and Hiraiwa-Hasegawa 1983), and we use the term ‘fully swollen’ to refer to cycling females at stage III of their swelling. All references to males or females refer to adult animals.
We used all-occurrence sampling within focal parties (i.e. all occurrences of these interactions that occurred in a party that contained a nominal focal animal, where party is defined as a sub-group produced by the fluid fission–fusion social system). We followed parties from first encounter until the focal individual built a night nest (sleeping platform); focal animals were identified to allow unbiased decisions on which animals to observe when parties fissioned. In the Sonso community, we identified, as focal animals, six adult males and six adult females; in M-group, eight adult males and seven adult females. If contact with chimpanzees was lost due to terrain and/or chimpanzee movement patterns, we searched for and observed the next party encountered that contained one of the predetermined focal animals.
Behavioural observations were recorded through audio narration, by pen and paper, or on videotape. We recorded a total of 1109 h and 30 min of observation of the Sonso community over 159 days/follows (median observation per day= 7 h; in 84 of these observation days, there were fully swollen females in the community); we conducted 141 focal follows of the M-group chimpanzees (in 109 of these days, the community contained fully swollen females) for a total of 800 h and 53 min of observation (median observation per day=6 h 20 min). Total hours of observation of the Sonso and M-group conducted in days when there were fully swollen females in the community were 606 h (median observation per day=7 h 22 min) and 640 h and 04 min (median observation per day= 6 h 40 min), respectively. Individual focal animals were under observation for a median duration of 80 h and 15 min (Sonso community), or 48 h and 43 min (M-group).
Data analysis
We tallied the number of days on which at least one female was fully swollen, and of these, the number of days in which more than one female was fully swollen. We compared availability of fully swollen (i.e. potentially fertilizable) females between communities using a Mann–Whitney test. To clarify the way in which copulations were distributed amongst males, we determined both the number of mating partners and the standardized Shannon–Weiner diversity index (H′: Krebs 1999; Newton-Fisher and Lee 2011) for mating effort, for each female. We calculated rates of interaction for mating, aggression and grooming as dyadic rates (Muller et al. 2007; Feldblum et al. 2014). For mating rates, we used the number of copulations achieved by males when the female partner in the dyad was fully swollen. We calculated grooming and aggression rates separately for females when fully swollen and when not fully swollen but still cycling.
We tested predictions 1 and 5 using generalized linear mixed model analysis (GLMM) with Poisson distributions and log link functions. We used the glmmADMB package (Bolker et al. 2012) as this handles zero-inflated data, and we had some male–female dyads that were not recorded copulating (therefore creating zeros in the dependent variable). In each model (model 1 for Sonso; model 2 for M-group), the number of copulations, entered as count data, was set as the dependent variable, whilst continuous data on both grooming effort (duration) and aggression received (number of interactions) from males, both corrected for dyadic observation time, were fixed factors, with the identities of males and females included as random factors with crossed structure. Including individual identity allowed us to control for differential individual-level effects such as a particularly aggressive male or attractive female. We also included meat received as fixed factor (binary variable) in model 2 (M-group) but not model 1 (Sonso): hunting of vertebrate prey is historically rare at Sonso (Newton-Fisher et al. 2002; Newton-Fisher 2015), and observations of meat transfer during this study were too few for formal analysis (n=4 sessions: 3 × Cephalophus monticola, 1 × Colobus guereza), although sharing between males and from males to both fully swollen and females at other reproductive stages was seen (NEN-F, unpublished data).
Simple regressions of both rates of aggression and grooming by males towards females against number of copulations (results not presented) indicated that the slopes of these relationships differed between males. We therefore included by-male random slopes for these variables. Our GLMMs were thus random intercept, random slope models. To control for a possible influence on male behaviour, we included grooming of males by females as a fixed factor. We also included the mean number of fully swollen females as a fixed factor, calculated separately for each male–female dyad across days for which they were observed mating, as males’ ability to monopolize mating access may be affected by the number of females who are fully swollen at the same time. We analysed our models twice, first using interactions between males and cycling females, and second, restricting analysis to the subset of interactions involving only fully swollen females. All the models met the assumptions of lack of overdispersion and collinearity (Zuur et al. 2013).
In addition to running full models (i.e. models containing all the variables of interest), we conducted a model selection procedure to identify those models that included only the variables that best predicted the number of copulations. We ranked models with differing combinations of predictors on the basis of AICc (the AIC value for small samples) and Δ, using the MuMIn package (Barton 2014). Models that fit the data well have low Δ and AICc values (Burnham et al. 2011). We selected and present the best model for each community, as well as other models with Δ<2 as this criterion distinguishes those with strong empirical support (Burham and Anderson 2002).
We examined whether males directed more aggression to cycling than to non-cycling females (prediction 2), using within-male paired t tests. We used by-female daily interaction rates to test whether females received more aggression when cycling (vs. non-cycling) using Mann–Whitney tests for a between-female comparison, and when fully swollen (vs. otherwise cycling) using Wilcoxon signed-ranks tests for a within-female comparison. To determine whether males who were more aggressive gained more, and a greater share of, copulations (prediction 3), we calculated the correlation between individual males’ aggression and mating rates. We also classified the males based on whether they gained high or low proportions of copulations (i.e. above and below the median for each community) and used t tests to compare aggression rates between those with proportionately high and proportionately low mating success. We conducted a similar analysis for grooming rates in both communities.
To test whether there was an association between mating rates and male dominance rank (prediction 4), we determined rank following our previous approach (Kaburu and Newton-Fisher 2015) using decided, directed aggressive interactions to derive Elo ratings (Albers and de Vries 2001) using the R function elo.sequence (Neumann et al. 2011). We preferred the Elo rating to other methods of assessing dominance rank such as I&SI (de Vries 1998) or David’s score (David 1988), since it more accurately detects rank changes, is not influenced by variation in group size, and is more reliable when the proportion of unknown relationships is high (Neumann et al. 2011). We confirmed that the assigned ordinal ranks were consistent with the direction of pant–grunt vocalizations (performed by subordinates towards dominants: Bygott 1979; Goodall 1986). Following convention, we assigned a value of 1 to the highest ranked individual (the alpha male), with numerically larger values indicating lower ranked individuals. We used Spearman rank correlations to test the association with mating rates for both communities.
In order to test whether males directed grooming to cycling females more when these females were fully swollen than when they were not, in each of the two communities (prediction 6), we used Wilcoxon matched-pairs signed-rank tests to compare rates of grooming when the female was and was not fully swollen for each male-cycling female dyad that was seen to groom; we excluded grooming between known relatives (M-group: N=2). We used the same test to assess whether the number of male-to-female grooming bouts was greater than the number of female-to-male grooming bouts (prediction 7), again, in each of the two communities.
We used binomial tests to test the predictions of Hemelrijk et al.’s (1992) hypothesis (predictions 8 and 9) comparing (a) the number of male–female grooming bouts in a mating context (i.e. within 30 s either before or after a copulation), with those the occurred at other times and (b) the number of these grooming bouts observed within 30 s before copulation and the number of bouts occurring within 30 s after copulation.
All analyses were conducted in R 3.0.3 (R Development Core Team 2012).
Results
We recorded 177 copulations and 364 aggressive interactions directed from males to cycling females in the Sonso community; equivalent figures for M-group were 105 copulations and 68 aggressive interactions. Females in both communities distributed mating across multiple partners (medians: H′Sonso = 0.88, H′M-group =0.71; Mann–Whitney U =27.5; p=0.267), with a median number of partners of 6 (of 8 possible mates) for Sonso, and 5 (of 10) for M-group (Fisher’s exact test, p= 0.367). Including additional ad lib observations brought the indices closer to 1 (medians: H′Sonso =0.96; H′M-group =0.83) and increased the median number of partners to 7 (of 8) for Sonso and 7 (of 10) for M-group.
The number of females who were fully swollen on any particular day varied between the two communities. In Sonso, we recorded 65 days when only a single female was fully swollen, and a further 19 days when more than one female was fully swollen (median number of females across days on which at least one female was fully swollen = 1; range = 1–3); in M-group, a single female was fully swollen on 32 days, whereas on 77 days more than one female was fully swollen (median number of females across days = 2; range = 1–8). On average, more females were fully swollen at the same time in M-group than in Sonso (U = 7090.5, p < 0.0001), but Sonso chimpanzees displayed higher copulation rates (0.14/h) than did those of M-group (0.07/h; U =53, p = 0.028).
The Sonso community also had higher rates of male–female aggression (median 0.20/h) than did M-group (median 0.07/h; Mann–Whitney U=72; p=0.005), and mating success of Sonso males was predicted by the frequency of aggression towards cycling females (GLMM 1: β±SE=2.329±0.662, z= 3.52, p<0.001, Table 1) together with the number of simultaneously fully swollen females (β±SE=0.345±0.15, z=2.30, p=0.021, Table 1, Fig. 1). The best model (i.e. with the lowest AICc value) retained only these two predictors (Table 2). Restricting the analysis to fully swollen females produced essentially the same result (model 1: male aggression z = 3.65, p<0.001; number of simultaneously fully swollen females z=2.16, p=0.031; Tables 1 and 2).
Table 1.
Variables in and results of model 1, a random slope, random intercept Poisson generalized linear mixed model (GLMM) explaining male mating success (number of copulations) amongst Sonso chimpanzees
| β | SE | z | p | |
|---|---|---|---|---|
| Dataset: cycling females | ||||
| Intercept | 0.059 | 0.333 | 0.18 | 0.860 |
| Aggression received by female | 2.329 | 0.662 | 3.52 | <0.001 |
| Grooming received by female | 0.015 | 0.016 | 0.95 | 0.341 |
| Grooming given by female | −0.010 | 0.020 | −0.51 | 0.611 |
| No. of fully swollen females | 0.345 | 0.150 | 2.30 | 0.021 |
| Dataset: fully swollen females only | ||||
| Intercept | 0.040 | 0.354 | 0.11 | 0.910 |
| Aggression received by female | 1.348 | 0.369 | 3.65 | <0.001 |
| Grooming received by female | 0.012 | 0.009 | 1.28 | 0.199 |
| Grooming given by female | −0.006 | 0.010 | −0.65 | 0.514 |
| No. of fully swollen females | 0.328 | 0.152 | 2.16 | 0.031 |
GLMM analysis conducted using glmmADMB (Bolker et al. 2012) in R 3.03, and analysis was conducted using two datasets: one including all cycling females, the other restricted to the subset of those females showing full anogenital swelling. Significant (p<0.05) predictors of mating are shown in italics
Fig. 1.
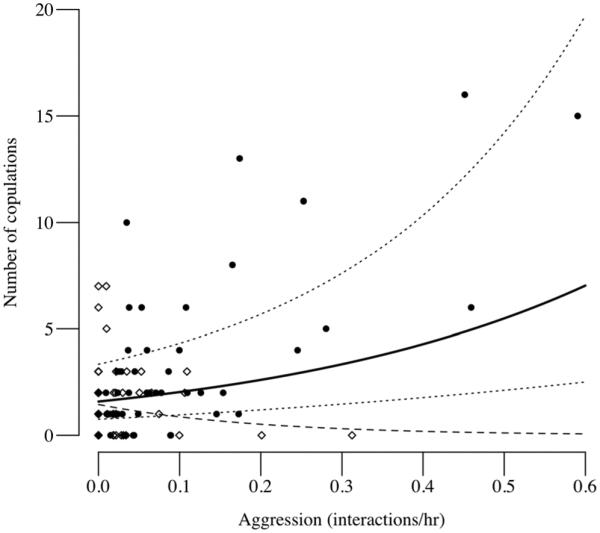
Relationship between dyadic rates of male aggression (number of interactions/h) towards cycling females and male mating success (number of copulations) amongst two communities of East African chimpanzees. Data points (black circle indicates Sonso; white diamond indicates M-group) represent unique male–female dyads. The solid line shows the relationship for Sonso community males predicted by generalized linear mixed modelling (Table 1) using the model with the lowest Δ value (Table 2) and so controlling for mate availability (number of fully swollen females). Dotted lines show the 95 % CI. The dashed line shows the (non-significant) relationship between aggression and mating for the M-group males
Table 2.
Generalized linear mixed models with AICc Δ<2 that best explain the number of copulations amongst Sonso chimpanzees
| Variables in the model | z | AICc | Δ | Weight | |
|---|---|---|---|---|---|
| Dataset: cycling females | |||||
| i. | Aggression received by female No. of fully swollen females |
4.04 2.61 |
233.77 | 0.00 | 0.49 |
| Dataset: fully swollen females only | |||||
| i. | Aggression received by female No. of fully swollen females |
3.78 2.48 |
236.25 | 0.00 | 0.55 |
See Table 1 for the full model and the “Material and methods” for details of the analysis
Males of both communities directed more aggression to cycling than to non-cycling females (paired t test: Sonso 364 vs. 83 interactions, t = 5.70, p < 0.001; M-group 68 vs. 38 interactions, t = 2.54, p = 0.032) and cycling females received higher rates of aggression than non-cycling females (Sonso, median 1.74 vs. 1.22 interactions/female/day: Mann–Whitney U =9, p = 0.004; M-group, median 1.267 vs. 1.00 interactions/female/day: U = 34.5, p = 0.008). However, only Sonso males directed more aggression to fully swollen than to other cycling females (2.44 vs. 1.09 interactions/female/day: Wilcoxon signed-rank test V =63; df =7; p = 0.001; M-group: 1.125 vs 1.00 interactions/female/day; V = 70.5, df =9, p = 0.115), and males who were more aggressive had higher mating rates in Sonso (r =0.814, p = 0.014) but not in M-group (r = −0.258, p = 0.472). Similarly, Sonso males who achieved a higher proportion of copulations showed significantly higher rates of aggression towards fully swollen females (mean rates: 0.19 interactions/h) than did males who achieved a low proportion of copulations (mean rates: 0.06 interactions/h; t =2.945; p = 0.005), but there was no difference amongst males of M-group (t =0.680; p = 0.50). We found an effect of male dominance rank on mating frequency in the Sonso community (rs = −0.738, N =8, p =0.046, Fig. 2), but not in M-group (rs =0.381, N =8, p = 0.360, Fig. 2). Since male chimpanzees show strongest competition over parous females (Muller et al. 2006), we re-ran these analyses excluding nulliparous females and found the same pattern with a more marked difference between the communities (Sonso: rs =−0.762, N=8, p=0.036; M-group: rs =0.119, N=8, p=0.793).
Fig. 2.
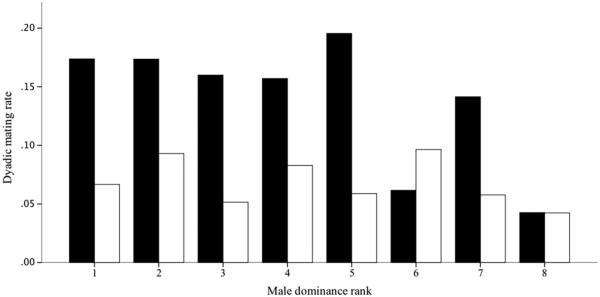
Relationship between mating rates (number of copulations/h) and dominance rank amongst the male chimpanzees from two communities: Sonso, in the Budongo Forest Reserve, Uganda (2003–2004) and M-group, in the Mahale Mountains National Park, Tanzania (2011). Male ranks were derived from agonistic interactions using Elo ratings (Albers and de Vries 2001) calculated with the R function elo.sequence (Neumann et al. 2011)
By contrast, aggression was not a significant predictor of mating success for M-group males; instead, mating success was predicted by the rate of grooming given by males to cycling females (GLMM 2: β±SE =0.979 ± 0.462, z=2.12, p=0.034, Table 3, Fig. 3), as well as the number of simultaneously fully swollen females (β±SE=0.224±0.060, z=3.72, p<0.001). Restricting the analysis to fully swollen females produced essentially the same results (model 2: male grooming of fully swollen females z=2.63, p=0.008; number of simultaneously fully swollen females z=2.57, p=0.010; Table 3). The best model for cycling females included both these predictors, as well as a negative effect of male aggression; the best model for fully swollen females retained only grooming received and the mean number of fully swollen cycling females (Table 4).
Table 3.
Variables in and results of model 2, a random slope, random intercept Poisson generalized linear mixed model (GLMM) explaining male mating success (number of copulations) amongst M-group chimpanzees
| β | SE | z | p | |
|---|---|---|---|---|
| Dataset: cycling females | ||||
| Intercept | −0.220 | 0.243 | −0.91 | 0.365 |
| Aggression received by female | −4.871 | 3.536 | −1.38 | 0.168 |
| Grooming received by female | 0.979 | 0.462 | 2.12 | 0.034 |
| Grooming given by female | 1.248 | 1.092 | 1.14 | 0.253 |
| Meat received by female | −0.154 | 0.344 | −0.45 | 0.655 |
| No. of fully swollen females | 0.224 | 0.060 | 3.72 | <0.001 |
| Dataset: fully swollen females only | ||||
| Intercept | 0.025 | 0.256 | 0.10 | 0.922 |
| Aggression received by female | −3.692 | 2.194 | −1.68 | 0.092 |
| Grooming received by female | 0.440 | 0.167 | 2.63 | 0.008 |
| Grooming given by female | 0.314 | 0.482 | 0.65 | 0.515 |
| Meat received by female | −0.106 | 0.353 | −0.30 | 0.765 |
| No. of fully swollen females | 0.670 | 0.065 | 2.57 | 0.010 |
GLMM analysis conducted using glmmADMB (Bolker et al. 2012) in R 3.03, and analysis was conducted using two datasets: one including all cycling females, the other restricted to the subset of those females showing full anogenital swelling. Significant (p<0.05) predictors of mating are shown in italics
Fig. 3.
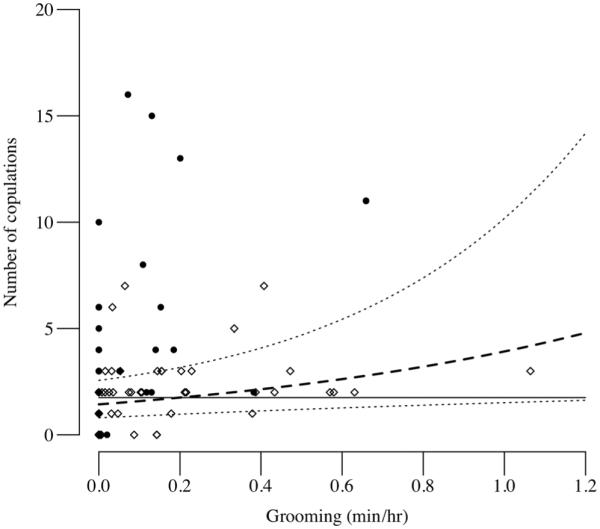
Relationship between dyadic rates of male grooming (min/h) of cycling females and male mating success (number of copulations) amongst two communities of East African chimpanzees. Data points (black circle indicates Sonso; white diamond indicates M-group) represent unique male–female dyads. The dashed line shows the relationship for M-group community males predicted by generalized linear mixed modelling (Table 3), using the model with the lowest Δ value (Table 4) and so controlling for mate availability (number of fully swollen females) and male aggression. Dotted lines show the 95 % CI. The solid line shows the (non-significant) relationship between grooming and mating for the Sonso males
Table 4.
Generalized linear mixed models with AICc Δ <2 that best explain the number of copulations amongst M-group chimpanzees
| Variables in the model | z | AICc | A | Weight | |
|---|---|---|---|---|---|
| Dataset: cycling females | |||||
| i. | Aggression received by female Grooming received by female |
−1.62 2.49 |
195.51 | 0.00 | 0.31 |
| No. of fully swollen females | 4.08 | ||||
| ii. | Aggression received by female Grooming received by female |
−1.38 2.13 |
197.02 | 1.51 | 0.15 |
| Grooming given by female No. of fully swollen females |
1.22 3.80 |
||||
| iii. | Grooming received by female No. of fully swollen females |
2.28 4.28 |
197.27 | 1.77 | 0.13 |
| Dataset: fully swollen females only | |||||
| i. | Grooming received by female No. of fully swollen females |
2.37 2.84 |
187.92 | 0.00 | 0.30 |
| ii. | Aggression received by female Grooming received by female |
−1.71 2.75 |
188.51 | 0.59 | 0.22 |
| Grooming given by female No. of fully swollen females |
0.64 2.59 |
||||
| iii. | Aggression received by female Grooming received by female |
−1.87 2.91 |
188.64 | 0.72 | 0.21 |
See Table 3 for the full model and the “Material and methods” for details of the analysis
From the M-group chimpanzees, we recorded 340 bouts (1273 min of grooming) between males and cycling females, whilst for Sonso, we recorded 86 bouts (305 min of grooming). These bouts were largely unidirectional—i.e. only one individual groomed within a bout—and of similar duration for both communities (M-group, 280 of 340 (82 %); mean duration±SD=225±332 s; median=107 s; Sonso, 61 of 86 bouts (71 %); mean duration ± SD = 213 ± 256 s; median = 115 s). In M-group, these bouts consisted largely of males grooming females (median number of male-to-female bouts=2.5 bouts; median number of female-to-male bouts= 1 bout; V=1408, p<0.001), whereas in the Sonso community the number of male-to-female grooming bouts did not significantly differ from the number of female-to-male bouts (median number of male-to-female bouts = 1.5 bouts; median number of female-to-male bouts = 1 bout; V =208.5, p = 0.393). Males of M-group directed more grooming bouts to fully swollen females (median rates=0.06 bouts/h) than to other cycling females (median rates=0.02 bouts/h; Wilcoxon signed ranks V=740, p=0.011); this was not the case for the Sonso community where there was no difference (median rates: grooming fully swollen females = 0.041 bouts/h; grooming other cycling=0.042 bouts/h; V=78, p=0.632). In both communities, grooming effort between males and females was unbalanced, with males grooming females for longer than females groomed males, but the skew was substantially greater in M-group (median duration: M-group, 7.63 vs. 0.56 min, V=266.5, p<0.001; Sonso, 6.33 vs. 1.56 min; V= 117, p=0.02). Males of M-group who achieved a higher proportion of copulations directed significantly higher rates of grooming to fully swollen females (mean rates: 0.407 min/h) than did males with low proportion of copulations (mean rates: 0.001 min/h; t=4.593; p<0.001), whereas there was no difference in grooming rates amongst the Sonso males (t=1.888; p=0.065).
We found no support for Hemelrijk et al.’s (1992) hypothesis, which we tested only with M-group as grooming of females did not predict mating success for the Sonso males. Most grooming of cycling females by M-group males (254/290 bouts, 88 %) occurred outside mating context (binomial test, p<0.001), and of the 36 bouts within a mating context, 32 occurred after mating and only four before (p<0.001). Thus, the grooming that M-group males gave to females was not a short-term exchange for mating access and did not appear to be the result of a male strategy to prevent females from fleeing.
Overall, these results indicate that Sonso males gained mating success through the use of aggression, whereas for M-group males mating success was associated with the provision of grooming, but not meat: these males hunted successfully on 30 occasions (0.054/h) and shared meat with females 54 times, of which 11 (20 %) involved cycling (fully swollen) females, but meat transfer was not a significant predictor of the number of copulations obtained and meat was not traded for sex in this community (Table 3).
This evidence for a mating market in M-group chimpanzees is a novel finding for East African chimpanzees but, as we discuss below, it is puzzling given the potential differences in value between mating and grooming. We therefore decided to test an additional hypothesis for the M-group chimpanzees: that the real commodity offered by males was protection (Wrangham 1979; van Schaik and Dunbar 1990), with the exchange mediated by grooming. Overt protection of females against male aggression was rare (NEN-F and SSKK, personal observations) so, allowing for this to be more subtle, we tested two predictions from this hypothesis: females should receive less aggression from males from whom they receive more grooming, and females who receive more grooming overall should also receive less aggression from males. We found no support for the first prediction (LMM analysis: β ±SE= −0.071 ± 0.122; t = −0.585; p = 0.548): females did not receive less aggression from the males who groomed them more. This LMM used dyadic data, with aggression received by females as a continuous dependent variable and grooming received as continuous fixed factor, together with individual identities as random factors and by-female random slopes for grooming received. To test the second prediction, we examined the total amount of both grooming and aggression each female received when fully swollen, and similarly found no significant relationship (rs = 0.475; N =9; p = 0.197): females who received more grooming did not receive less aggression.
Discussion
Previous studies of chimpanzee mating strategies have suggested that males make use of at least two different strategies to increase their mating success: wild male East African chimpanzees pursue sexual coercion (Muller et al. 2007, 2009; Muller and Wrangham 2009; Feldblum et al 2014), whereas West African (P. t. verus) and captive chimpanzees (often largely drawn from West African chimpanzees, or hybrids with this subspecies) trade commodities such as meat (Gomes and Boesch 2009, 2011) or grooming (Hemelrijk et al. 1992). Our results (Table 5), from two communities of the same subspecies, provide the first evidence that East African chimpanzees can resort to a trading strategy. Consistent with findings from other East African chimpanzee communities (Mitani and Watts 2001; Gilby et al. 2010), and contrary to the suggestion made for Taï’s South-group of West African chimpanzees (Gomes and Boesch 2009, 2011), M-group males did not trade meat for sex. Instead, they appeared to trade grooming: (a) provision of grooming significantly predicted male mating success; (b) males groomed cycling females more when they were fully swollen; (c) cycling females received more grooming from males than they gave; (d) grooming directed from males to cycling females was not restricted to mating contexts. We tested an alternative possibility that the commodity really offered by males was protection from aggression. Whilst intuitively more valuable to females, we found no support for protection as a traded commodity.
Table 5.
Summary of predictions tested for each of two communities of East African chimpanzees, the structurally despotic Sonso community and the more structurally egalitarian M-group
| Prediction | Sonso | M-group | Prediction 10 |
|---|---|---|---|
| 1. Male aggression is a significant predictor of mating success | Yes | No | Yes |
| 2. Males direct more aggression to cycling females | Yes | Yes | No |
| 3. More aggressive males gain more matings | Yes | No | Yes |
| 4. Male rank is associated with mating frequency | Yes | No | Yes |
| 5a. Provision of meat significantly predicts male mating success | – | No | No |
| 5b. Provision of grooming significantly predicts male mating success | No | Yes | Yes |
| 6. Males groom cycling females more when they are fully swollen | No | Yes | Yes |
| 7. Cycling females receive more grooming from males than they give | Yes | Yes | Partiala |
| Tests of Hemelrijk et al.’s (1990) hypothesis (M-group only) | |||
| 8. Males groom females primarily in a mating context | – | No | – |
| 9. Male grooming of females precedes mating | – | No | – |
| Tests of the male protector hypothesis (M-group only) | |||
| a. Females receive less aggression from males from whom they receive more grooming |
– | No | – |
| b. Females who receive more grooming overall receive less aggression from males |
– | No | – |
We predicted that males of M-group should trade grooming and/or meat for mating access, whilst this trade should be absent amongst the Sonso males who should use sexual coercion instead. This was our prediction 10 and follows from biological market theory
Although cycling females received from males more grooming than they gave in both communities this was more pronounced in M-group, supporting prediction 10
Conversely, Sonso chimpanzee males appeared to use sexual coercion to increase mating success: (a) male aggression significantly predicted mating success; (b) males directed more aggression to cycling than non-cycling females, and more to those who were fully swollen; (c) males who were more aggressive gained more mating; and (d) male rank was associated with mating success. It is important to note that the priority-of-access model (PoA: Altmann 1962; Suarez and Ackermann 1971) does not provide an alternative explanation for these findings. PoA models the relationship between male dominance rank and mating success, allowing for variation in the number of available mates; in itself, it is not a model of mating strategies. At most, it could be taken to imply that competition amongst males for social rank secures access to mates (with rank-competition then being the mating strategy) but in species such as chimpanzees where females are promiscuous, such a strategy alone would do nothing to counter female efforts to mate with other males, which is precisely the goal of a coercive strategy. Whilst our results for Sonso are therefore consistent with priority-of-access, the model does not account for the pattern of aggression against females and has been shown to have limited value as an explanation for the distribution of paternity in this community (Newton-Fisher et al. 2010).
Our results highlight the variability in behaviour between chimpanzee communities and refute the idea that the differential use of trading and coercion as mating strategies by chimpanzees is a sub-specific difference between West and East Africa, respectively. We suggest instead that sexual coercion and trading represent two alternative strategies exhibited under specific sociodemographic conditions. We have shown previously that, during our periods of data collection, Sonso males displayed a despotic social organization with a steep dominance hierarchy whilst M-group males showed an egalitarian dominance structure with flatter rank relationships (Kaburu and Newton-Fisher 2015) and our finding of a mating mark et in M-group, and its absence in Sonso, therefore matches the predictions of BMT. Ours is the first demonstration that strategy choice may be related to differences in male dominance steepness between communities. We note, however, that aggression appeared to have a larger effect on mating success for Sonso males than grooming did for M-group males. Whilst these effect sizes are not strictly comparable, this difference suggests that a grooming–mating exchange might represent a fall-back or ‘best-of-a-bad-job’ (Dawkins 1980; Dunbar 1982) strategy for male chimpanzees. If true, this could explain why evidence for a mating market in chimpanzees has not be forthcoming from communities in which sexual coercion appears to be a successful strategy.
Our results raise two particularly interesting questions. First, why were the male chimpanzees of the structurally egalitarian M-group not pursuing sexual coercion, given that this appears to be the more effective strategy, and that used by male chimpanzees in other communities (Kanyawara, Kibale Forest, Uganda: Muller et al. 2007; Kasekela, Gombe National Park, Tanzania: Feldblum et al. 2014; Sonso, Budongo Forest, Uganda: this study), and second, why should females allow their promiscuous mating strategy to be compromised in exchange for nothing more than grooming?
In answer to the first, we show that M-group males in fact directed significantly more aggression towards cycling than non-cycling females, behaviour indicative of sexual coercion and linked to paternity success amongst chimpanzees elsewhere (Feldblum et al. 2014), but that such aggression did not lead to increased mating success. The implication is that M-group males were attempting to use sexually coercive aggression but were unable to generate any variance in the impact of this aggression on female behaviour, and so were unsuccessful: by definition, sexual coercion (Smuts and Smuts 1993) requires that aggression leads to an increase in the likelihood that a female will mate with the aggressive male rather than another. The dominance hierarchy amongst these males was very shallow, probably because a large proportion of the adult males had similar competitive abilities (Kaburu and Newton-Fisher 2015), which suggests that these males posed similar levels of coercive threat to females and perhaps that they were able to thwart one another’s efforts to pursue a strategy of sexual coercion (clearly beneficial if this prevented rivals gaining a mating-share bias). If M-group males were equally successful in using aggression to influence female mating decisions, then the likelihood that a female mated with any particular male, relative to another, is the same as it would have been in the absence of aggression. The lack of a net mating bias (males who were more aggressive did not have higher mating success than those who were less aggressive) meant they failed to coerce females in a functional sense.
We also found that the number of simultaneously fully swollen females was significantly greater in M-group than in Sonso, and that whilst the number of simultaneously fully swollen females was a significant predictor of male mating success for both communities, the effect was stronger for M-group. Increased numbers of simultaneously fully swollen females should reduce male mating competition, and M-group males were less aggressive towards females than were Sonso males. However, with 10 adult males in M-group, the median of two fully swollen females should still allow for significant competition between males (Cowlishaw and Dunbar 1992; Pawłowski et al. 1998), particularly as female chimpanzees increase their gregariousness and association with males when fully swollen (Goodall 1986; Matsumoto-Oda 1999a).
We suggest that these two factors—greater availability of mates, together with more evenly matched males—are responsible for the lower effort level and lack of success for a sexual coercion strategy amongst M-group males. Whilst we cannot exclude the possibility that females in M-group were able to mount substantial and effective resistance to male aggression around mating (we have no systematic data on female mating resistance for M-group), this seems implausible.
Across communities, female chimpanzees typically show varying levels of resistance to male mating attempts, but not enough to overcome male coercive aggression (Muller et al. 2011), and despite M-group being one of the best studied communities of chimpanzees, including investigations of male choice by females (Matsumoto-Oda 1999b), strong and effective female resistance has not been reported. Similarly, M-group females are not hyper-dispersed relative to Sonso, and therefore no easier for males to monopolize: foraging party sizes are markedly similar across chimpanzee communities, despite other social and ecological variation (Itoh and Nishida 2007).
The second question—why females should compromise their mating strategy for grooming—is perhaps more intriguing. Female promiscuity is thought to function as a counter-strategy to infanticidal behaviour by males, by providing all potential fathers with a nonzero probability of paternity and so creating ‘paternity confusion’ (Wrangham 1993, 2002; van Schaik 2000; Muller et al. 2007; Watts 2007); allowing males to establish mating biases decreases this confusion and so, at least in principle, increases infanticide risk. Whilst a coercive male mating strategy imposes costs on females to force such a compromise, a sex-for-grooming trade suggests an exchange of benefits. Females may have been allowing males to gain additional shares of copulations at times when fertilization was unlikely and so at minimal cost to the females (selling sex on the cheap), but this seems doubtful: most grooming of females by males, and most mating, occurred when females were maximally swollen (although few of the male–female grooming bouts occurred in a strict mating context so we can also exclude temporally proximate—short-term— trading).
It seems more plausible, therefore, that M-group males who gained additional mating success through providing grooming may have benefited from an increased likelihood of achieving paternity. How much of a cost to the female this would represent is unclear; we simply do not know enough about the inter-male variation in mating success that females can accept without it compromising their promiscuous strategy (i.e. the degree of tolerance in the strategy) or how either the number or proportion of copulations (or more precisely, the probability of achieving paternity) obtained by males correlates with the likelihood of committing infanticide. It is unlikely that all adult males would ever achieve equal mating success with any particular female, or that individual males would know how their share of copulations compared with that of rivals, so it would seem reasonable to assume at least some leeway that females could exploit. It is also likely that males benefit from some degree of paternity confusion, as this provides protection from infanticide for their own offspring (Boyko and Marshall 2009), which should offset some of the costs of inequitable paternity opportunities. Females may, therefore, be able to garner additional direct benefits from grooming that they would not otherwise receive, at—if the strategy of ‘promiscuity for paternity confusion’ has a reasonable tolerance to inequitable mating—relatively little cost.
Another way of interpreting these results is in terms of indirect benefits. We cannot exclude the possibility that the apparent exchange of mating for grooming was the product of long-term relationships, or ‘friendships’, between particular male–female dyads, but this is unlikely. Whilst there may be an advantage for males in establishing relationships that provide preferential mating access, the benefit to females from such relationships is far from clear: we have no evidence that these grooming–mating–exchange dyads result in enhanced protection for females against male aggression, for instance, and females did not receive less aggression from the males from whom they received grooming, contrary to what might be expected if these dyads were ‘friends’. Furthermore, dominance steepness changes over time: M-group has been more despotic in the past (Kaburu and Newton-Fisher 2015), which raises the possibility that the lack of successful coercion in M-group was only transitory, in turn questioning why males would allocate time to establishing long-term relationships with females.
A final possibility is that females in M-group were attempting to exert mate choice by biasing their mating effort towards favoured males. Our results show that even though females mated with multiple males, on average and for both communities, they did not mate with all available mates and mating was not distributed completely evenly across these partners. With a relatively flat male hierarchy, social rank may be a poor indicator of male quality and females might instead consider grooming, perhaps as a marker of social competence given its importance in the interactions between males (Wrangham 1986; Nishida and Hiraiwa-Hasegawa 1987; Watts 2000; Newton-Fisher 2002; Mitani 2009; Newton-Fisher and Lee 2011; Kaburu and Newton-Fisher 2015). The relevance of female mate choice in chimpanzees is currently debated, however (Matsumoto-Oda 1999b; Stumpf and Boesch 2005, 2006; Pieta 2008; Muller et al. 2009, 2011). If such choice is only possible (due to the ineffectiveness of male coercion) or useful when male hierarchies are flat, any effort to resolve this debate may benefit from an explicit consideration of hierarchy steepness and variation in the degree of structural despotism across study communities. Although Clarke et al. (2010) cited chimpanzees as an example species in which intersexual cooperation (i.e. ‘trading’) persisted in the face of coercion contrary to the predictions of BMT, our study shows that once within-species variation in dominance steepness (and so power differentials) is recognized, chimpanzee behaviour conforms to the predictions of BMT: trading, here in the form of mating markets, does not persist when individuals have the ability to forcibly obtain commodities. Rather than assuming, as Clarke et al. (2010) propose, that indirect female mate choice is responsible for increasing male competition and decreasing power differentials, we suggest instead that males’ ability to establish large power differentials is regulated by demography (Cowlishaw and Dunbar 1991; Mitani et al. 2002; Kutsukake and Nunn 2006), this having a limiting effect when males find themselves against a number of similarly matched competitors (as appears to be the case for M-group: Kaburu and Newton-Fisher 2015). Thus, indirect female mate choice becomes potentially more effective as a consequence of small male power differentials, rather than being responsible for generating such differentials.
The difference between our two study communities in the steepness of the male dominance hierarchy suggests that the despotism–egalitarian axis influences the mating strategies adopted by male chimpanzees. The sexual coercion strategy appears ineffective when the male hierarchy is very flat (egalitarian), and males instead appear to attempt to bias female mating behaviour by offering grooming services. Why females should be swayed by this is unclear, and remains a topic for future work. Our study highlights the importance of within-species, cross-group comparisons in order to gain a full understanding of mating strategies, and indicates that studies of other chimpanzee communities are needed to explore further the relationship between mating strategy and dominance steepness.
Acknowledgments
This work was funded by the Wenner-Gren foundation (grant no. 8216), the Leverhulme Trust (grant no. F/00236/Z) and the H.F. Guggenheim Foundation. We thank the Uganda National Council for Science and Technology, the President’s Office, the Forest Department, and Vernon Reynolds for granting permission to work in the Budongo forest and the Tanzania Commission for Science and Technology, the Tanzania Wildlife Research Institute and the Mahale Mountains Wildlife Research Centre for allowing research in the Mahale Mountains National Park. We are also very grateful to Geresomu Muhumuza and the other Ugandan and Tanzanian field assistants for their fundamental help during data collection both in Budongo and Mahale. Finally, we would like to thank Robin Dunbar and two anonymous reviewers for insightful comments on a previous draft of the article.
Footnotes
Ethical standards This research complied with the regulations set by the Ethics Committee of University of Kent, the protocols of both the Budongo Forest Project (now BCFS) and the Mahale Mountains Wildlife Research Center, and the legal requirements of both Uganda and Tanzania.
References
- Alatalo RV, Lundberg A, Glynn C. Female pied flycatchers choose territory quality and not male characteristics. Nature. 1986;323:152–153. [Google Scholar]
- Albers PCH, de Vries H. Elo-rating as a tool in the sequential estimation of dominance strengths. Anim Behav. 2001;61:489–495. [Google Scholar]
- Altmann SA. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann N Y Acad Sci. 1962;102:338–435. doi: 10.1111/j.1749-6632.1962.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Aureli F, Preston SD, de Waal FBM. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J Comp Psychol. 1999;113:59–65. doi: 10.1037/0735-7036.113.1.59. [DOI] [PubMed] [Google Scholar]
- Barelli C, Reichard UH, Mundry R. Is grooming used as a commodity in wild white-handed gibbons, Hylobates lar? Anim Behav. 2011;82:801–809. [Google Scholar]
- Barrett L, Henzi SP. The utility of grooming in baboon troops. In: Noë R, van Hoof JARAM, Hammerstein P, editors. Economics in nature. Cambridge University Press; Cambridge: 2001. pp. 119–145. [Google Scholar]
- Barrett L, Henzi S, Weingrill T, Lycett J, Hill R. Market forces predict grooming reciprocity in female baboons. Proc R Soc Lond B. 1999;266:665–670. [Google Scholar]
- Barton K. MuMIn: multi-model inference. 2014 R package version 1.12.1, http://CRAN.R project.org/package=MuMIn.
- Bilde T, Tuni C, Elsayed R, Pekar S, Toft S. Nuptial gifts of male spiders: sensory exploitation of the female’s maternal care instinct or foraging motivation? Anim Behav. 2007;73:267–273. [Google Scholar]
- Bolker B, Skaug H, Magnusson A, Nielson A. Getting started with the glmmADMB package. 2012 http://glmmadmb.r forge.r project.org/glmmADMB.html.
- Boyko RH, Marshall AJ. The willing cuckold: optimal paternity allocation, infanticide and male reproductive strategies in mammals. Anim Behav. 2009;77:1397–1407. [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference. Springer; New York: 2002. [Google Scholar]
- Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol. 2011;65:23–35. [Google Scholar]
- Bygott JD. Agonistic behaviour, dominance, and social structure in wild chimpanzees of the Gombe National Park. In: Hamburg DA, McCown ER, editors. The great apes. Benjamin/Cummings Publishing Co; Menlo Park: 1979. pp. 405–427. [Google Scholar]
- Clarke PMR, Halliday JEB, Barrett L, Henzi SP. Chacma baboon mating markets: competitor suppression mediates the potential for intersexual exchange. Behav Ecol. 2010;21:1211–1220. [Google Scholar]
- Clutton Brock TH, Parker GA. Potential reproductive rates and the operation of sexual selection. Q Rev Biol. 1992;67:437–456. [Google Scholar]
- Colmenares F, Zaragoza F, Hernandez-Lloreda MV. Grooming and coercion in one-male units of hamadryas baboons: market forces or relationship constraints? Behaviour. 2002;139:1525–1553. [Google Scholar]
- Connor RC, Smolker RA, Richards AF. Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.) Proc Natl Acad Sci U S A. 1992;89:987–90. doi: 10.1073/pnas.89.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowlishaw G, Dunbar RIM. Dominance rank and mating success in male primates. Anim Behav. 1991;41:1045–1056. [Google Scholar]
- Cowlishaw G, Dunbar RIM. Dominance and mating success: a reply to Barton & Simpson. Anim Behav. 1992;44:1162–1163. [Google Scholar]
- Cox CR, le Boeuf BJ. Female incitation of male competition: a mechanism in sexual selection. Am Nat. 1977;111:317–335. [Google Scholar]
- David HA. The method of paired comparisons. Hafner; New York: 1988. [Google Scholar]
- Dawkins R. Good strategy or evolutionarily stable strategy? In: Silverberg J, editor. Sociobiology: beyond nature/nurture? Westview Press; Boulder: 1980. pp. 331–367. [Google Scholar]
- de Vries H. Finding a dominance order most consistent with a linear hierarchy: a new procedure and review. Anim Behav. 1998;55:827–843. doi: 10.1006/anbe.1997.0708. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria; 2012. www.Rproject.org. [Google Scholar]
- Dunbar RIM. Intraspecific variations in mating strategy. In: Bateson PPG, Klopfer PH, editors. Perspectives in ethology. Plenum Press; New York: 1982. pp. 385–431. [Google Scholar]
- Ely JJ, Dye B, Frels WI, Fritz J, Gagneux P, Khun HH, Switzer WM, Lee DR. Subspecies composition and founder contribution of the captive US chimpanzee (Pan troglodytes) population. Am J Primatol. 2005;67:223–241. doi: 10.1002/ajp.20179. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M. Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): methodological considerations and the role of hormones in sex and conception. Am J Primatol. 2005;67:137–158. doi: 10.1002/ajp.20174. [DOI] [PubMed] [Google Scholar]
- Feh C, Demazieres J. Grooming at a preferred site reduces heart-rate in horses. Anim Behav. 1993;46:1191–1194. [Google Scholar]
- Feldblum JT, Wroblewski EE, Rudicell RS, Hahn BH, Paiva T, Cetinkaya-Rundel M, Pusey AE, Gilby IC. Sexually coercive male chimpanzees sire more offspring. Curr Biol. 2014;24:2855–2860. doi: 10.1016/j.cub.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC, Emery Thompson M, Ruane JD, Wrangham RW. No evidence of short-term exchange of meat for sex amongst chimpanzees. J Hum Evol. 2010;59:44–53. doi: 10.1016/j.jhevol.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Gomes CM, Boesch C. Wild chimpanzees exchange meat for sex on a long-term basis. PLoS ONE. 2009;4:e5116. doi: 10.1371/journal.pone.0005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes CM, Boesch C. Reciprocity and trades in wild West African chimpanzees. Behav Ecol Sociobiol. 2011;65:2183–2196. [Google Scholar]
- Goodall J. Chimpanzees of the Gombe Stream Reserve. In: de Vore I, editor. Primate behavior: field studies of monkeys and apes. Holt, Rinehart and Winston; New York: 1965. pp. 425–473. [Google Scholar]
- Goodall J. Cultural elements in a chimpanzee community. In: Menzel E, editor. Precultural primate behaviour. Karger; Basel: 1973. pp. 144–184. [Google Scholar]
- Goodall J. The chimpanzees of Gombe: patterns of behaviour. Belknap; Cambridge: 1986. [Google Scholar]
- Gross MR. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. [DOI] [PubMed] [Google Scholar]
- Gumert MD. Payment for sex in a macaque mating market. Anim Behav. 2007;74:1655–1667. [Google Scholar]
- Gwynne DT. Sexual conflict over nuptial gifts in insects. Annu Rev Entomol. 2008;53:83–101. doi: 10.1146/annurev.ento.53.103106.093423. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Hiraiwa-Hasegawa M. Opportunistic and restrictive matings among wild chimpanzees in the Mahale Mountains, Tanzania. J Ethol. 1983;1:75–85. [Google Scholar]
- Hemelrijk CK, van Laere GJ, van Hooff JARAM. Sexual exchange relationships in captive chimpanzees? Behav Ecol Sociobiol. 1992;30:269–275. [Google Scholar]
- Henzi SP, Barrett L. The value of grooming to female primates. Primates. 1999;40:47–59. doi: 10.1007/BF02557701. [DOI] [PubMed] [Google Scholar]
- Hockings KJ, Humle T, Anderson JR, Biro D, Sousa C, Ohashi G, Matsuzawa T. Chimpanzees share forbidden fruit. PLoS ONE. 2007;2:e886. doi: 10.1371/journal.pone.0000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvilsom C, Frandsen P, Børsting C, Carlsen F, Sallè B, Simonsen BT, Siegismund R. Understanding geographic origins and history of admixture among chimpanzees in European zoos, with implications for future breeding programmes. Heredity. 2013;110:586–593. doi: 10.1038/hdy.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Nishida T. Chimpanzee grouping patterns and food availability in Mahale Mountains National Park, Tanzania. Primates. 2007;48:87–96. doi: 10.1007/s10329-006-0031-0. [DOI] [PubMed] [Google Scholar]
- Kaburu SSK, Newton-Fisher NE. Social instability raises the stakes during social grooming among wild male chimpanzees. Anim Behav. 2013;86:519–527. [Google Scholar]
- Kaburu SSK, Newton-Fisher NE. Egalitarian despots: hierarchy steepness, reciprocity and the grooming-trade model in wild chimpanzees, Pan troglodytes. Anim Behav. 2015;99:61–71. doi: 10.1016/j.anbehav.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinol. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Klinkova E, Hodges JK, Fuhrmann K, de Jong T, Heistermann M. Male dominance rank, female mate choice and male mating and reproductive success in captive chimpanzees. Int J Primatol. 2005;26:357–484. [Google Scholar]
- Koyama NF, Caws C, Aureli F. Supply and demand predict male grooming of swollen females in captive chimpanzees, Pan troglodytes. Anim Behav. 2012;84:1419–1425. [Google Scholar]
- Krebs CJ. Ecological methodology. 2nd Harper Collins; New York: 1999. [Google Scholar]
- Kutsukake N, Nunn CL. Comparative tests of reproductive skew in male primates: the roles of demographic factors and incomplete control. Behav Ecol Sociobiol. 2006;60:695–706. [Google Scholar]
- Matsumoto-Oda A. Mahale chimpanzees: grouping patterns and cycling females. Am J Primatol. 1999a;47:197–207. doi: 10.1002/(SICI)1098-2345(1999)47:3<197::AID-AJP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Oda A. Female choice in the opportunistic mating of wild chimpanzees (Pan troglodytes schweinfurthii) at Mahale. Behav Ecol Sociobiol. 1999b;46:258–266. [Google Scholar]
- Milinski M, Bakker TCM. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature. 1990;344:330–333. [Google Scholar]
- Mitani JC. Mating behaviour of male orangutans in the Kutai Game Reserve, Indonesia. Anim Behav. 1985;33:392–402. [Google Scholar]
- Mitani JC. Male chimpanzees form enduring and equitable social bonds. Anim Behav. 2009;77:633–640. [Google Scholar]
- Mitani JC, Watts DP. Why do chimpanzees hunt and share meat? Anim Behav. 2001;61:915–924. [Google Scholar]
- Mitani JC, Watts DP, Pepper JW, Merriwether DA. Demographic and social constraints on male chimpanzee behaviour. Anim Behav. 2002;64:727–737. [Google Scholar]
- Muller MN, Wrangham RW. Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Harvard University Press; Cambridge: 2009. [Google Scholar]
- Muller MN, Thompson ME, Wrangham RW. Male chimpanzees prefer mating with old females. Curr Biol. 2006;16:2234–8. doi: 10.1016/j.cub.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW. Male coercion and the costs of promiscuous mating for female chimpanzees. Proc R Soc Lond B. 2007;274:1009–1014. doi: 10.1098/rspb.2006.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Wrangham RW. Male aggression and sexual coercion in chimpanzees. In: Muller MN, Wrangham RW, editors. Sexual coercion in primates and humans: an evolutionary perspective on male aggression against females. Harvard University Press; Cambridge: 2009. pp. 3–22. [Google Scholar]
- Muller MN, Emery Thompson M, Kahlenberg SM, Wrangham RW. Sexual coercion by male chimpanzees shows that female choice may be more apparent than real. Behav Ecol Sociobiol. 2011;65:921–933. [Google Scholar]
- Nakamura M, Nishida T. Long-term field studies of chimpanzees at Mahale Mountains National Park, Tanzania. In: Kappeler PM, Watts DP, editors. Long-term field studies of primates. Springer; Berlin: 2012. pp. 339–356. [Google Scholar]
- Neff BD, Svensson EI. Polyandry and alternative mating tactics. Philos T Roy Soc B. 2013;368:20120045. doi: 10.1098/rstb.2012.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C, Duboscq J, Dubuc C, Ginting A, Irwa AM, Agil M, Widdig A, Engelhardt A. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim Behav. 2011;82:911–921. [Google Scholar]
- Newton-Fisher NE. Tactical behaviour and decision making in wild chimpanzees. University of Cambridge; Cambridge: 1997. [Google Scholar]
- Newton-Fisher NE. Male chimpanzee relationships in the Budongo Forest, Uganda. In: Boesch C, Hohmann G, Marchant L, editors. Behavioural diversity in chimpanzees and bonobos. Cambridge University Press; Cambridge: 2002. pp. 125–137. [Google Scholar]
- Newton-Fisher NE. Female coalitions against male aggression in wild chimpanzees of the Budongo Forest. Int J Primatol. 2006;27:1589–1599. [Google Scholar]
- Newton-Fisher NE. Roving females and patient males: a new perspective on the mating strategies of chimpanzees. Biol Rev. 2014;89:356–374. doi: 10.1111/brv.12058. [DOI] [PubMed] [Google Scholar]
- Newton-Fisher NE. The hunting behavior and carnivory of wild chimpanzees. In: Henke W, Tattersall I, editors. Handbook of paleoanthropology. 2nd Springer; Berlin: 2015. pp. 1661–1691. [Google Scholar]
- Newton-Fisher NE, Lee PC. Grooming reciprocity in wild male chimpanzess. Anim Behav. 2011;81:439–446. [Google Scholar]
- Newton-Fisher NE, Notman H, Reynolds V. Hunting of mammalian prey by Budongo Forest chimpanzees. Folia Primatol. 2002;73:281–283. doi: 10.1159/000067454. [DOI] [PubMed] [Google Scholar]
- Newton-Fisher NE, Emery Thompson M, Reynolds V, Boesch C, Vigilant L. Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. Am J Phys Anthropol. 2010;142:417–428. doi: 10.1002/ajpa.21241. [DOI] [PubMed] [Google Scholar]
- Nishida T. The chimpanzees of the Mahale Mountains: sexual and life history strategies. Tokyo University Press; Tokyo: 1990. [Google Scholar]
- Nishida T. Chimpanzees of the lakeshore. Cambridge University Press; Cambridge: 2012. [Google Scholar]
- Nishida T, Hiraiwa-Hasegawa M. Chimpanzees and bonobos: cooperative relationships among males. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker T, editors. Primate societies. University of Chicago Press; Chicago: 1987. pp. 165–177. [Google Scholar]
- Nishida T, Kano T, Goodall J, et al. Ethogram and ethnography of Mahale chimpanzees. Anthropol Sci. 1999;107:141–188. [Google Scholar]
- Noë R, Noë R, van Hooff JARAM, Hammerstein P. Economics in nature. Cambridge University Press; Cambridge: 2001. Biological market: partner choice as the driving force behind the evolution of mutualisms; pp. 93–118. [Google Scholar]
- Noë R, Hammerstein P. Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav Ecol Sociobiol. 1994;35:1–12. [Google Scholar]
- Noë R, Hammerstein P. Biological markets. Trends Ecol Evol. 1995;10:336–340. doi: 10.1016/s0169-5347(00)89123-5. [DOI] [PubMed] [Google Scholar]
- Norscia I, Antonacci D, Palagi E. Mating first, mating more: biological market fluctuation in a wild prosimian. PLoS ONE. 2009;4:e4679. doi: 10.1371/journal.pone.0004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda R, Masataka N. Function of copulatory vocalizations in mate choice by females of Japanese Macaques (Macaca fuscata) Folia Primatol. 1995;64:132–139. doi: 10.1159/000156843. [DOI] [PubMed] [Google Scholar]
- Parker G. Assessment strategy and the evolution of fighting behavior. J Theor Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. [DOI] [PubMed] [Google Scholar]
- Pawłowski B, Lowen CB, Dunbar RIM. Neocortex size, social skills and mating success in primates. Behaviour. 1998;135:357–368. [Google Scholar]
- Pieta K. Female mate preferences among Pan troglodytes schweinfurthii of Kanyawara, Kibale National Park, Uganda. Int J Primatol. 2008;29:845–864. [Google Scholar]
- Reynolds V. The chimpanzees of the Budongo forest: ecology, behaviour, and conservation. Oxford University Press; Oxford: 2005. [Google Scholar]
- Saunders CD, Hausfater G. The functional significance of baboon grooming behavior. Ann N Y Acad Sci. 1988;525:430–432. [Google Scholar]
- Setchell JM. Alternative reproductive tactics in primates. In: Oliveira RF, Taborsky M, Brockmann HJ, editors. Alternative reproductive tactics: an integrative approach. Cambridge University Press; Cambridge: 2008. pp. 373–398. [Google Scholar]
- Smuts BB, Smuts RW. Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv Study Behav. 1993;22:1–63. [Google Scholar]
- Stanford CB. Chimpanzee and red colobus. Harvard University Press; Cambridge: 1998. [Google Scholar]
- Stumpf RM, Boesch C. Does promiscuousmating preclude female choice? Female sexual strategies in chimpanzees (Pan troglodytes verus) of the Taï National Park, Côte d’Ivoire. Behav Ecol Sociobiol. 2005;57:511–524. [Google Scholar]
- Stumpf RM, Boesch C. The efficacy of female choice in chimpanzees of the Taï Forest, Côte d’Ivoire. Behav Ecol Sociobiol. 2006;60:749–765. [Google Scholar]
- Suarez B, Ackermann DR. Social dominance and reproductive behaviour in male rhesus monkeys. Am J Phys Anthropol. 1971;35:219–222. doi: 10.1002/ajpa.1330350209. [DOI] [PubMed] [Google Scholar]
- Tanaka I, Takefushi H. Elimination of external parasites (lice) is the primary function of grooming in free-ranging Japanese macaques. Anthropol Sci. 1993;101:187–193. [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aldine; Chicago: 1972. pp. 136–179. [Google Scholar]
- Tutin CEG. Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii) Behav Ecol Sociobiol. 1979;6:29–38. [Google Scholar]
- van Schaik CP. The ecology of social relationships amongst female primates. In: Standen V, Foley RA, editors. Comparative socioecology: the behavioural ecology of humans and other mammals. Blackwell Scientific Publications; Oxford: 1989. pp. 195–218. [Google Scholar]
- van Schaik CP. Social counterstrategies against infanticide by males in primates and other mammals. In: Kappeler PM, editor. Primate males: causes and consequences of variation in group composition. Cambridge University Press; Cambridge: 2000. pp. 34–52. [Google Scholar]
- van Schaik CP, Dunbar RIM. The evolution of monogamy in large primates: a new hypothesis and some crucial tests. Behaviour. 1990;115:30–62. [Google Scholar]
- Voigt CC, Michener R, Kunz TH. The energetics of trading nuptial gifts for copulations in katydids. Physiol Biochem Zool. 2005;78:417–423. doi: 10.1086/430224. [DOI] [PubMed] [Google Scholar]
- Watts DP. Grooming between male chimpanzees at Ngogo, Kibale National Park. II. Influence of male rank and possible competition for partners. Int J Primatol. 2000;21:211–238. [Google Scholar]
- Watts DP. Effects of male group size, parity, and cycle stage on female chimpanzee copulation rates at Ngogo, Kibale National Park, Uganda. Primates. 2007;48:222–31. doi: 10.1007/s10329-007-0037-2. [DOI] [PubMed] [Google Scholar]
- Wong BBM, Candolin U. How is female mate choice affected by male competition? Biol Rev. 2005;80:559–571. doi: 10.1017/S1464793105006809. [DOI] [PubMed] [Google Scholar]
- Wrangham RW. On the evolution of ape social systems. Soc Sc Inform. 1979;18:335–368. [Google Scholar]
- Wrangham RW. Ecology and social evolution in two species of chimpanzees. In: Rubenstein DI, Wrangham RW, editors. Ecology and social evolution: birds and mammals. Princeton University Press; Princeton: 1986. pp. 352–378. [Google Scholar]
- Wrangham RW. The evolution of sexuality in chimpanzees and bonobos. Hum Nat. 1993;4:47–79. doi: 10.1007/BF02734089. [DOI] [PubMed] [Google Scholar]
- Wrangham RW. The cost of sexual attraction: is there a trade-off in female Pan between sex appeal and received coercion? In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural diversity in chimpanzees and bonobos. Cambridge University Press; Cambridge: 2002. pp. 204–215. [Google Scholar]
- Yu Y, Xiang Z-F, Yao H, Grueter CC, Li M. Female snub-nosed monkeys exchange grooming for sex and infant handling. PLoS ONE. 2013;8:e74822. doi: 10.1371/journal.pone.0074822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamma K. Grooming site preferences determined by lice infection among Japanese macaques in Arashiyama. Primates. 2002;43:41–49. doi: 10.1007/BF02629575. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Hilbe JM, Ieno EN. A beginner’s guide to GLM and GLMM with R. Highland Statistics Ltd.; Newburgh: 2013. [Google Scholar]


