Abstract
Drug resistance is a major factor that limits the efficacy of targeted cancer therapies. In this review, we discuss the main known mechanisms of resistance to receptor tyrosine kinase inhibitors, which are the most prevalent class of targeted therapeutic agent in current clinical use. Here we focus on bypass track resistance, which involves the activation of alternate signaling molecules by tumor cells to bypass inhibition and maintain signaling output, and consider the problems of signaling pathway redundancy and how the activation of different receptor tyrosine kinases translates into intracellular signal transduction in different cancer types. This information is presented in the context of research strategies for the discovery of new targets for pharmacological intervention, with the goal of overcoming resistance in order to improve patient outcomes.
Keywords: targeted therapy, drug resistance, receptor tyrosine kinases, cancer
Introduction
Targeted therapy is defined as the treatment of cancer using a pharmacological agent designed to inhibit a specific molecule, resulting in reduced tumor progression. The rationale for this approach is based on the concept of oncogene addiction, put forward by Bernard Weinstein, which posits that cancer cells become dependent on a small number of key proteins whose activity is required to maintain cancer cell growth and proliferation [1]. Oncogenic proteins for which targeted therapies have been developed and entered widespread clinical use include the estrogen receptor in breast cancer, BRAF in melanoma, as well as a number of tyrosine kinases active across multiple cancers including ABL, ALK, EGFR, HER2, and VEGFR [2]. Notably, a large proportion of current drug targets belong to the receptor tyrosine kinase (RTK) family of cell surface receptors, which contains 58 members with different subsets being expressed in different human cell types. Binding of secreted growth factors to the extracellular domain of a RTK triggers a conformational change that promotes receptor homo- or hetero-dimerization, tyrosine phosphorylation, and activation of numerous intracellular signaling cascades [3]. In cancer, RTKs can be selectively targeted using either small molecule ATP analogs (e.g., erlotinib for EGFR-positive non-small cell lung cancer (NSCLC)) or inhibitory monoclonal antibodies (e.g., trastuzumab for HER2-positive breast cancer).
Different targeted therapies have achieved various degrees of success in cancer treatment, as measured by increased progression-free survival and patient response rate [4]. However, in many cases patient response is limited by either primary or secondary drug resistance. Primary (intrinsic) resistance refers to a tumor that fails to respond to targeted therapy despite harboring the molecular alteration that would be predicted to render the tumor susceptible to treatment. Alternatively, secondary (acquired) resistance occurs when a patient that initially responds to therapy relapses after a period of time, resulting in cancer progression via the resumption of primary tumor growth or metastasis. Primary resistance mechanisms are largely unknown, but could result from a small subset of cells harboring primary drug target mutations, amplifications, or bypass mechanisms similar to those known to occur during the development of secondary resistance (discussed below).
Several comprehensive reviews on the topic of targeted cancer therapy have recently appeared elsewhere [4–8]. Here, we survey the major known mechanisms underlying resistance to RTK inhibition while focusing on a few specific unresolved issues in the field, including the identification of RTKs mediating drug resistance in a given cancer type, how RTK activity translates into downstream signaling output, and therapeutic strategies designed to circumvent or overcome the problem of drug resistance.
Resistance mediated by the primary drug target
Probably the best understood mechanism of resistance to targeted therapy involves genetic mutation of the primary drug target such that the mutant RTK is impervious to inhibition. This likely occurs via a process of positive selection for tumor cells expressing the mutated variant. Such so-called “gatekeeper” mutations have been found to occur in imatinib-resistant chronic myeloid leukemia (CML, ABL T315I), crizotinib-resistant lung cancer (ALK L1196M and others), as well as lung cancers resistant to EGFR inhibition (most prominently EGFR T790M, which occurs in approximately half of these tumors). Current therapies are largely ineffective at inhibiting RTKs containing these mutations. In addition, gene amplification of both EGFR and ALK has been identified in patients with drug resistant NSCLC, and amplification is known to occur concurrently with gatekeeper point mutations [9–12]. Biochemical studies suggest that the EGFR T790M mutation confers resistance by increasing the kinase’s affinity for ATP [13]. Improved knowledge of the structural basis underlying the insensitivity of mutant RTKs to clinically available inhibitors could potentially aid in the development of new drugs that preferentially target mutant RTKs over the wild-type proteins.
Bypass via activation of other RTKs
Bypass track (compensatory) resistance refers to the ability of tumor cells to activate parallel pathways in order to maintain signaling output in the presence RTK inhibition [14]. As is true for resistance involving gatekeeper mutations, most of the research on bypass mechanisms has been carried out in EGFR and ALK positive lung cancers, while the molecular details in other resistant cancer types are generally less understood.
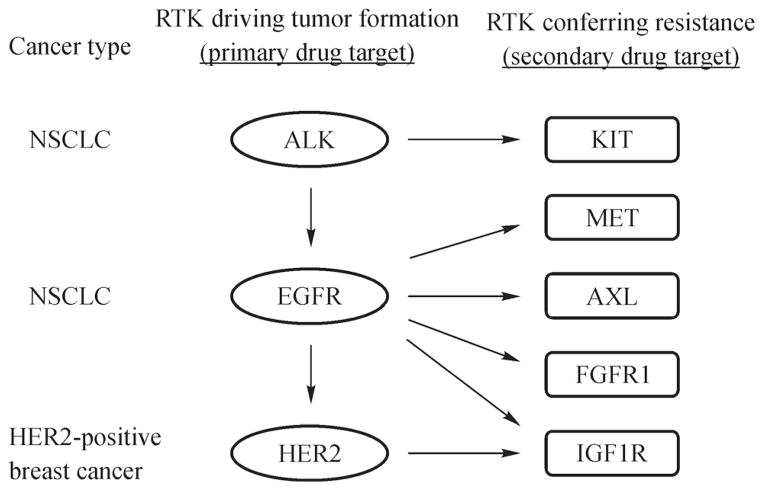
Most commonly, bypass mechanisms of resistance involve amplification of an alternative RTK that is not inhibited by the primary drug. For example, MET amplification occurs in approximately 5%–22% of lung cancers resistant to EGFR inhibition [15,16]. However, activation of other RTKs including HER2, IGF1R, AXL, and FGFR1 can also mediate this type of resistance [14]. In contrast, crizotinib-resistant lung cancers harboring ALK translocations instead upregulate EGFR or KIT [14], whereas IGF1R plays a role in some cases of trastuzumab-resistant breast cancer [17]. Extracellular RTK ligands produced by either tumor or stromal cells also have the potential to promote resistance to anticancer therapy. For example, a recent study demonstrated that elevated levels of the MET ligand HGF can rescue lung cancer cells from sensitivity to EGFR inhibition [18]. Clearly, a variety of RTKs have the ability to compensate for loss of signaling resulting from targeted therapy, and the specific RTK utilized for this purpose seems to depend to some extent on tumor type and drug treatment (Fig. 1).
Fig. 1.
Summary of RTKs known to mediate bypass resistance to RTK targeted cancer therapy. Ovals indicate oncogenes responsible for initial tumor formation and rectangles indicate RTKs involved in drug resistance. Arrows denote known bypass resistance mechanisms. Note that EGFR and HER2 activation can induce initial tumor formation and also mediate resistance.
A major unanswered question in the field of targeted therapy is why a given tumor upregulates a specific RTK to achieve resistance rather than one of the dozens of other family members that could theoretically also bring about compensatory signaling. Although the overall intracellular signaling network is thought to be shared among the RTK family, some RTKs might preferentially signal through a certain pathway over others. For example, some evidence indicates that in lung cancer cells EGFR and MET might activate both the MAPK and PI3K/AKT pathways, whereas IGF1R appears to preferentially favor PI3K/AKT [19]. In agreement with this, MacBeath et al. have used network analysis to identify distinct classes of RTKs, with IGF1R belonging to a different class than EGFR, FGFR1, and MET [20]. However, this explanation seems inconsistent with the observations that IGF1R can compensate for loss of EGFR signaling in lung cancer [4]. Alternatively, the relative expression levels of various downstream signaling proteins or transcription factors might underlie the bias resistant tumors show for one RTK over another. As a possible mechanism for this, Settleman et al. have shown that drug-tolerant lung cancer cells contain altered chromatin modifications compared to sensitive cell lines, and these epigenetic marks are important for maintaining the resistant state [21]. Clearly, more research is needed to better understand the factors responsible for determining the specific bypass mechanism favored by a given tumor.
To further our knowledge of resistance mechanisms involving RTK bypass, we believe that two considerations are important for future studies. First, when possible, identification of RTKs mediating resistance should be performed in an unbiased manner. In a large scale RNA interference study undertaken to identify kinases critical for cell proliferation and survival across multiple cell types, Harlow et al. found that relatively unknown and poorly studied kinases were as likely to play important roles in these process as well studied kinases with a large record of publications [22]. This “bias of familiarity” appears relevant to drug resistance because many current studies seem to focus on only a few well-studied kinases at the exclusion of others [4]. We believe that unbiased profiling of RTK expression in resistant cells and tumors as well as drug screening methodology will reveal under-appreciated roles in drug resistance for less studied members of the RTK family. In addition, for the majority of resistance mechanisms identified we still do not know the prevalence of occurrence in cancer patients. Therefore, quantitative assessment is needed to determine the frequency of RTK activation in different tumor types. If it is determined that a high percentage of tumors achieve resistance by activating a specific RTK, then it might be possible to design combination therapies that can be used prior to the development of resistance.
Downstream signaling pathways
RTK activity is known to stimulate signal transduction through a number of major intracellular cascades. Notably, RTK activation results in increased flux through both the RAS/RAF/MAPK and PI3K/AKT pathways, which are important for the proliferation and/or survival of many mammalian cell types, including cancer cells. Indeed, RAS, RAF, and PI3K activating mutations are known to occur in certain human cancers, and numerous pharmacological agents targeting these pathways are currently in clinical use or being developed.
The widespread belief that these two signaling axes account for the majority of the oncogenicity attributed to RTKs represents a conundrum for the field. This is because, if this is indeed the case, then one would predict that effectively blocking both of these pathways would be efficacious for the treatment of all tumors dependent on RTK signaling, regardless of the specific identity of the RTK(s) mediating tumor progression. In support of this, Engelman et al. have demonstrated that combined PI3K/MEK inhibition leads to apoptosis in gefitinib-resistant NSCLC cells [23]. Unfortunately, other cancers appear to have ways to circumvent such approaches. For example, one mechanism by which colon cancers expressing oncogenic BRAF resist BRAF inhibition is through feedback activation of EGFR [24]. Therefore, inhibition of intracellular pathways might not always be preferable to direct targeting of the RTK. In addition to these types of feedback mechanisms, a further consideration to take into account when targeting intracellular pathways is that, because of the redundancy and crosstalk built into these signaling networks, it is conceivable that similar resistance mechanisms could develop for intracellular kinases as those known to occur during the development of resistance to RTK inhibitors. It is also possible that increased toxicity could result from targeting downstream signaling due to pathway inhibition in normal (non-cancerous) cells.
Since all RTKs are thought to activate similar downstream signaling pathways, why is the activity of some RTKs required for the growth of a given tumor whereas others are relatively less important in determining disease progression? We propose that those receptors important for mediating tumor development might produce a similar signaling output, whereas the activity of RTKs that are less important for the growth of a given tumor, even if expressed at high levels, might have a lesser impact on downstream signaling. In support of this, unpublished data from our laboratory indicates that in breast cancer cells HER2 inhibition combined with inhibition of either IGF1R or MST1R/RON, which have been implicated in trastuzumab or lapatinib resistance [17,25], results in strongly reduced signaling through both the MAPK and AKT pathways. In contrast, inhibition of RTKs not predicted to be involved in resistance had no measurable effect on either of these pathways. Moreover, this decreased signaling output resulted in a reduced proliferative capacity of trastuzumab-resistant breast cancer cells treated with either IGF1R or MST1R inhibitors combined with trastuzumab. Thus, in trastuzumab-resistant breast cancer, those RTKs important for driving tumor cell growth appear to be major determinants of intracellular signal transduction. If this phenomenon holds true for other cancer types, then it might be possible to utilize downstream signaling flux as a readout in order to predict cancer cell vulnerability to specific RTK inhibitors.
Future prospects for treatment
Several strategies are currently being pursued to overcome the problem of targeted therapy resistance. To combat resistance caused by mutation or amplification of the primary target, next-generation RTK inhibitors targeting BCR-ABL, ALK, and EGFR are being developed and tested in clinical trials [4–6]. In contrast to first-generation (reversible) inhibitors, these drugs irreversibly block kinase activity by forming covalent bonds at or near the kinase active site. Significantly, some next-generation compounds have shown efficacy in inhibiting RTK isoforms associated with resistance, including EGFR T790M [26]. However, since EGFR activity is important for normal tissue homeostasis [27], the use of many next-generation RTK inhibitors appears to be limited by toxicity stemming from the irreversible inhibition of wild-type EGFR in normal cells. Because of this, efforts are currently underway to develop inhibitors preferentially targeting T790M over wild-type EGFR [28].
For bypass resistance, the most common strategy is to develop combination therapies that inhibit one or more RTKs or downstream signaling proteins in addition to the primary target [29]. The success of combination therapy first requires the correct identification of signaling molecules responsible for mediating resistance in an individual tumor. One approach for this is to sequence DNA from resistant biopsies or circulating tumor cells, with the aim of identifying mutated genes that could then be targeted to overcome resistance. While genetic analysis can detect some types of resistance such as those associated with gatekeeper mutations, sequencing can miss important drivers of resistance that are highly active in tumor cells but not mutated, and can also misidentify mutations that are not actually contributing to resistance. As an alternative to genetic approaches, lung cancer cell lines from patient biopsies have recently been established and screened against a panel of drugs to identify combinations effective against resistance [30]. This strategy uncovered combinations, such as combined ALK and SRC inhibition, that could not have been predicted from genetic analysis. Due to our limited knowledge of the molecular mechanisms underlying resistance, unbiased approaches like this might be most effective in discovering new resistance mechanisms and treatment options. As basic knowledge increases, it might eventually be possible to assess a more limited number of biomarkers in order to rapidly choose those combination therapies with the highest likelihood of success in treating a resistant tumor.
Acknowledgments
This study was supported by National Institutes of Health Grants CA186800 (X.F.W.) and CA059365 (P.B.A.).
Footnotes
Compliance with ethics guidelines
Peter B. Alexander and Xiao-Fan Wang declare that they have no conflict of interest. This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
References
- 1.Weinstein IB, Joe A, Felsher D. Oncogene addiction. Cancer Res. 2008;68(9):3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. discussion 3080. [DOI] [PubMed] [Google Scholar]
- 2.Sawyers C. Targeted cancer therapy. Nature. 2004;432(7015):294–297. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- 3.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19(11):1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13(10):714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 6.Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol. 2013;31(31):3987–3996. doi: 10.1200/JCO.2012.45.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groenendijk FH, Bernards R. Drug resistance to targeted therapies: déjà vu all over again. Mol Oncol. 2014;8(6):1067–1083. doi: 10.1016/j.molonc.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun C, Bernards R. Feedback and redundancy in receptor tyrosine kinase signaling: relevance to cancer therapies. Trends Biochem Sci. 2014;39(10):465–474. doi: 10.1016/j.tibs.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Ercan D, Zejnullahu K, Yonesaka K, Xiao Y, Capelletti M, Rogers A, Lifshits E, Brown A, Lee C, Christensen JG, Kwiatkowski DJ, Engelman JA, Jänne PA. Amplification of EGFR T790M causes resistance to an irreversible EGFR inhibitor. Oncogene. 2010;29 (16):2346–2356. doi: 10.1038/onc.2009.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, Engelman JA. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, Jessop NA, Wain JC, Yeo AT, Benes C, Drew L, Saeh JC, Crosby K, Sequist LV, Iafrate AJ, Engelman JA. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, Kondo KL, Linderman DJ, Heasley LE, Franklin WA, Varella-Garcia M, Camidge DR. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, Meyerson M, Eck MJ. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA. 2008;105(6):2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niederst MJ, Engelman JA. Bypass mechanisms of resistance to receptor tyrosine kinase inhibition in lung cancer. Sci Signal. 2013;6(294):re6. doi: 10.1126/scisignal.2004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Jänne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 16.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, Balak M, Chang WC, Yu CJ, Gazdar A, Pass H, Rusch V, Gerald W, Huang SF, Yang PC, Miller V, Ladanyi M, Yang CH, Pao W. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104(52):20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93(24):1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 18.Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487(7408):505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, Toschi L, Rogers A, Mok T, Sequist L, Lindeman NI, Murphy C, Akhavanfard S, Yeap BY, Xiao Y, Capelletti M, Iafrate AJ, Lee C, Christensen JG, Engelman JA, Jänne PA. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17(1):77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner JP, Wolf-Yadlin A, Sevecka M, Grenier JK, Root DE, Lauffenburger DA, MacBeath G. Receptor tyrosine kinases fall into distinct classes based on their inferred signaling networks. Sci Signal. 2013;6(284):ra58. doi: 10.1126/scisignal.2003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong KK, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grueneberg DA, Degot S, Pearlberg J, Li W, Davies JE, Baldwin A, Endege W, Doench J, Sawyer J, Hu Y, Boyce F, Xian J, Munger K, Harlow E. Kinase requirements in human cells: I. Comparing kinase requirements across various cell types. Proc Natl Acad Sci USA. 2008;105(43):16472–16477. doi: 10.1073/pnas.0808019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, Lifshits E, Chen Z, Maira SM, García-Echeverría C, Wong KK, Engelman JA. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci USA. 2009;106(46):19503–19508. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A, Bernards R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Quan H, Zhao J, Xie C, Wang L, Lou L. RON confers lapatinib resistance in HER2-positive breast cancer cells. Cancer Lett. 2013;340(1):43–50. doi: 10.1016/j.canlet.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov GN, Bradner JE, Althaus IW, Gandhi L, Shapiro GI, Nelson JM, Heymach JV, Meyerson M, Wong KK, Jänne PA. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67(24):11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 27.Alexander PB, Yuan L, Yang P, Sun T, Chen R, Xiang H, Chen J, Wu H, Radiloff DR, Wang XF. EGF promotes mammalian cell growth by suppressing cellular senescence. Cell Res. 2015;25(1):135–138. doi: 10.1038/cr.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HJ, Schaefer G, Heffron TP, Shao L, Ye X, Sideris S, Malek S, Chan E, Merchant M, La H, Ubhayakar S, Yauch RL, Pirazzoli V, Politi K, Settleman J. Noncovalent wild-type-sparing inhibitors of EGFR T790M. Cancer Discov. 2013;3(2):168–181. doi: 10.1158/2159-8290.CD-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kummar S, Chen HX, Wright J, Holbeck S, Millin MD, Tomaszewski J, Zweibel J, Collins J, Doroshow JH. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements. Nat Rev Drug Discov. 2010;9(11):843–856. doi: 10.1038/nrd3216. [DOI] [PubMed] [Google Scholar]
- 30.Crystal AS, Shaw AT, Sequist LV, Friboulet L, Niederst MJ, Lockerman EL, Frias RL, Gainor JF, Amzallag A, Greninger P, Lee D, Kalsy A, Gomez-Caraballo M, Elamine L, Howe E, Hur W, Lifshits E, Robinson HE, Katayama R, Faber AC, Awad MM, Ramaswamy S, Mino-Kenudson M, Iafrate AJ, Benes CH, Engelman JA. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346(6216):1480–1486. doi: 10.1126/science.1254721. [DOI] [PMC free article] [PubMed] [Google Scholar]