Abstract
Objective
To examine the experience, comprehension and perceptions of learning of a parent’s BRCA mutation during adolescence and early adulthood, and explore the impact on offspring’s physical and psychosocial well-being.
Methods
Semi-structured interviews were completed with 22 adult offspring who learned of their parent’s BRCA mutation prior to age 25 years. Data were summarized using qualitative methods and response proportions.
Results
Offspring reports of the content shared varied; discussion of cancer risks and offspring genetic testing were described more frequently than risk modification strategies. The majority of offspring reported a good understanding of the information shared and no negative aspects for learning this information. Some offspring reported changing their health behaviors after learning of the familial mutation; many tobacco users stopped smoking. Offspring interest in genetic counseling surrounding parent disclosure and genetic testing during adulthood were high.
Conclusions
Some offspring understand and respond adaptively to early communication of a genetic risk for cancer, and disclosure may foster improved health behaviors during adolescence and young adulthood. Further research is necessary to evaluate how offspring conceptualize and utilize genetic risk and to identify the biopsychosocial factors predictive of adaptive/maladaptive responses to early disclosure of hereditary risk for adult cancer.
Keywords: cancer, oncology, BRCA, genetic testing, psychosocial, communication
Introduction
With the discovery of two hereditary breast–ovarian cancer genes (BRCA1 and BRCA2) predictive genetic testing has become an increasingly utilized clinical service. Female carriers of BRCA mutations have significantly increased lifetime risks of breast (up to 85%) and ovarian cancer (up to 40%) [1–3] and are encouraged to consider a broad spectrum of risk reductions strategies, ranging from early breast cancer screening to chemoprevention, and prophylactic surgeries [4,5]. Studies evaluating the psychosocial impact of undergoing BRCA testing and learning of a personal risk for cancer among adult women have been favorable to date; indications of negative psychosocial sequelae among women have been quite limited [6–8]. The psychosocial impact of BRCA testing on young adult women, men and offspring in BRCA families has not been well described.
As BRCA-related cancers are rare before 25 years of age and there are potential negative aspects to the early application of risk reduction interventions, genetic testing for BRCA1/2 mutations is not routinely offered to children [9–11] and remains controversial between the ages of 18 and 25 [12]. However, several studies have suggested that many BRCA carriers inform their children of the familial mutation [13,14], and that the majority of adolescent and adult offspring learn of their parent’s BRCA mutation [15,16].
What children, adolescents and young adults understand of the concepts of cancer as a genetic disease and predictive cancer genetic testing in general, what they perceive the relevance of this information to be for themselves, and how they will choose to utilize this information remains un-known. What effect this information may have on subsequent psychological well-being, social and familial relationships and the performance of health behaviors is also unknown. A better understanding of these effects is crucial for both healthcare providers in their risk management of families with an identified hereditary risk for cancer and for policymakers in the ongoing debate over broadening genetic testing services and the risks and benefits of offering BRCA testing to minors.
To better understand the content and method of parental disclosure of hereditary risk to offspring, and the experiences and impact of parent to offspring communication of genetic risk, we are developing a cohort of BRCA mutation carriers with offspring under 25 years old at parent genetic testing. We interviewed adult offspring in this developing cohort who learned of their parent’s BRCA mutation to better understand the content and method of disclosure, their understanding and perceptions of hereditary risk and the psychosocial and health-related impact of this communication. As these constructs have not been previously described, the primary intent of this work was exploratory and is expected to inform future research regarding the optimal communication of a genetic risk for cancer.
Methods
Sample
Offspring were recruited through parent participants in a study examining how and when BRCA mutation carriers disclose their genetic test results to offspring. This ongoing study involves interviewing BRCA mutation carriers recruited from the University of Chicago Cancer Risk Clinic. Parents complete a semi-structured interview exploring their opinions regarding communication of test results to offspring and experiences with disclosure.
After obtaining Institutional Review Board approval, parents who reported communicating their test results to their offspring were re-contacted. A research assistant described the offspring study and invited parents to share information regarding this study with their eligible adult offspring. Parents subsequently provided verbal consent and contact information for the research assistant to contact the offspring. Offspring were contacted directly and the research assistant obtained oral informed consent from all offspring participants.
Among 52 parent participants, there were 44 eligible adult offspring. Six parents refused to introduce the study to their offspring and four could not be reached despite repeated attempts, excluding 14 offspring. Of the remaining 30 adult offspring, three refused to participate after their parent introduced the study. Five offspring agreed to contact, but did not complete the survey. The remaining 22 offspring who completed the survey represent 50% of eligible offspring in the cohort with a participation rate of 73% among offspring who learned of the study from a parent.
Procedure
A semi-structured interview was developed to elicit personal experiences, while ensuring coverage of all salient domains. Pre-selected domains were developed from parent interviews, reports of the disclosure process and their perceptions of offspring experiences [16] and were guided by a theoretical model grounded in the self-regulation theory of health behavior [17]. The initial semi-structured interview was pilot tested with five participants and modified accordingly. There was no attempt to educate participants during interviews and open-ended questions allowed for prompts and exploration of offspring responses. Interviews were conducted by a research assistant (K. P.) over a 6-month time period (September 2006–March 2007) and lasted 15–40 min. Participant responses were transcribed and entered into a database for coding and analysis.
A thematic analysis of the transcripts and responses was completed using the method of constant comparison [18]. Investigators (K. P., A. B., L. P. M.) intensively reviewed descriptive responses to open-ended questions and developed coding schemas through open coding. Two investigators (A. B., L. P. M.) independently assigned categorical codes to all descriptive responses. The mean inter-coder reliability was 89% (range 81–100%) for all coding schema (open-ended questions). Agreement was then established for all responses. Response proportions to structured questions and to the categorically coded open-ended questions are utilized to summarize results. Representative individual responses have been selected to characterize the data.
Offspring age at parental genetic testing was calculated using the parent’s genetic test result disclosure date and the offspring birth date. Estimated offspring age at disclosure was derived from the calculated offspring age at parental genetic testing and the parent’s report of how long after genetic testing they disclosed their genetic test results to their offspring.
Results
Sample characteristics
The 22 offspring included in this report are from 13 unrelated parents. The characteristics of all participants are described in Table 1. Parents were reporting on 1–3 offspring; the two fathers reporting on male offspring.
Table 1.
Participant characteristics
| Offspring (n=22) No. (%) |
Parents (n=13) No. (%) |
|
|---|---|---|
| Age at interview (year), median (range) | 26 (18–33) | 48 (43–66) |
| Daughters | 24.5 (18–33) | – |
| Sons | 27 (20–33) | – |
| Gender | ||
| Women | 12 (55) | 11 (85) |
| Men | 10 (45) | 2 (15) |
| Race | ||
| White | 20 (91) | 11 (85) |
| Black | 1 (5) | 1 (8) |
| Hispanic | 1 (5) | 1 (8) |
| Personal history of cancer | 0 (0) | 10 (77) |
| Marital status | ||
| Never married | 13 (59) | 0 (0) |
| Married | 9 (41) | 10 (77) |
| Divorced | 0 (0) | 2 (15) |
| Widowed | 0 (0) | 1 (8) |
| Education | ||
| High school only | 2 (10) | 4 (31) |
| Some college or in college | 8 (36) | 2 (15) |
| Completed college | 7 (32) | 4 (31) |
| Graduate education | 5 (23) | 3 (27) |
| Have children | 7 (32) | 13 (100) |
| Among women | ||
| Had prophylactic mastectomy | 0 (0) | 8 (73) |
| Had prophylactic oophorectomy | 0 (0) | 11(100) |
| Result of BRCA1/2 testing | ||
| Has mutation | 3 (14) | 13 (100) |
| No mutation | 4 (18) | 0 (0) |
| Not tested | 15 (68) | – |
Parent reports of offspring age at disclosure were generally consistent with the ‘estimated’ offspring age at disclosure; those who were not consistent were within 1–2 years of the estimated offspring age at disclosure (Table 2). Offspring reports of their age at disclosure were less frequently consistent with parent reports and ‘estimated’ offspring ages at disclosure. Those who were not consistent more frequently reported ages younger than the estimated disclosure age (within 1–4 years). Some offspring reported ages older than the estimated disclosure age (within 1–3 years).
Table 2.
Ages and gender of offspring participants
| Offspring (O) # | Gender | Age at parent GT | Estimated age at disclosurea | Parent reported disclosure age | Offspring reported disclosure age | Interview age |
|---|---|---|---|---|---|---|
| 1 | Son | 24 | 24 | 24 | 24 | 28 |
| 2 | Daughter | 11 | 19 | 18 or 19 | 19 | 21 |
| 3 | Daughter | 21 | 21 | 21 | 19 | 24 |
| 4 | Daughter | 22 | 22 | 22 | 22 or 23 | 33 |
| 5 | Daughter | 17 | 17 | 17 | 15 or 16 | 28 |
| 6 | Son | 14 | 14 | 14 | 12 or 13 | 20 |
| 7 | Daughter | 11 | 11 | 11 | 11 or 12 | 18 |
| 8 | Daughter | 16 | 16 | 16 | 17 or 18 | 20 |
| 9 | Son | 23 | 23 | 23 | 23 | 33 |
| 10 | Son | 23 | 23 | 23 | 23 or 24 | 30 |
| 11 | Daughter | 19 | 19 | 19 | 19 | 26 |
| 12 | Son | 17 | 17 | 17 | 16 | 24 |
| 13 | Son | 21 | 21 | 21 | 20 | 28 |
| 14 | Daughter | 17 | 17 | 17 | 16 or 17 | 25 |
| 15 | Daughter | 24 | 24 | 24 | 23 or 24 | 27 |
| 16 | Daughter | 24 | 24 | 24 | 24 or 25 | 29 |
| 17 | Son | 20 | 23 | 20 | 23 | 26 |
| 18 | Son | 19 | 19 | 19 | 16 | 28 |
| 19 | Son | 17 | 17 | 17 | 20 | 26 |
| 20 | Son | 14 | 14 | 14 | 15 | 21 |
| 21 | Daughter | 11 | 18–19 | 18 | 16 | 18 |
| 22 | Daughter | 9 | 17–18 | 17 | 13 | 19 |
Estimated age at disclosure was derived from the calculated age at parent testing (using parent test date and offspring birthdate) and the parent’s report of how long after genetic testing they disclosed their genetic test results to their offspring.
Content and method of parental disclosure
According to offspring reports, the majority of parents informed their offspring of their BRCA mutation. In three cases the genetic mutation was not initially described, although the parents (all mothers) did discuss the hereditary risk of cancer in the family. Based on parent reports, the majority of parents disclosed to their offspring immediately after receiving their genetic test results. All sons in the cohort were disclosed to immediately, the youngest at 14 years. Three mothers reported delaying communication with their four daughters. One waited until her 17-year-old daughter returned home from college so she could share the information in person. The other mothers delayed disclosure to their 9–11-year-old daughters until they were 17–19 years old because they felt they were too young to understand the information or that it would not be meaningful to them until an older age.
All offspring reported that the parent with the mutation shared the result; the majority alone and in eight cases with their spouses. Daughters were more likely to learn of the familial mutation from a mother alone (7/12) than sons (4/10). In some cases, offspring’s siblings or other family members were present during disclosure. Two offspring reported learning the information by telephone. In some cases (6/22), parents provided written materials. All offspring who received written materials indicated that they were helpful. Among offspring who did not receive written materials, the majority felt that written materials could have been helpful, either for themselves or for others.
Offspring descriptions of the content shared varied; some mentioned multiple content areas, while others described more limited communication. The topics offspring most frequently reported included who had genetic testing, the implications of the test result for the risk of developing cancer (for the parent, other family members or for the offspring) and the ability for the offspring to have genetic testing (Figure 1).
She [mother] explained that she had had breast cancer and we would have to be more diligent in checking for breast cancer, do monthly breast exams, etc. She discussed the [genetic test] results, what it meant for us, that we are at increased risk if we have gene. She also told us we could get tested and the possible effects on our insurance if the company found out (daughter, O4).
He [dad] said that testing had been done on his side of the family and a gene was found that makes women prone to breast cancer. He had testing and then brought it up over dinner one evening. He basically said that he is a carrier and at increased risk for cancer in family. Also, that we could be carriers (son, O19).
Figure 1.
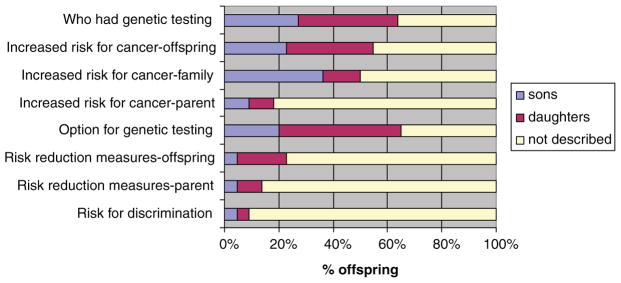
Offspring reports of the content disclosed
In addition, nine offspring reported a specific numerical risk of inheriting the genetic mutation.
There is a ‘cancer gene’ in family and I could be at increased risk for cancer. She told me she has it, so there’s a 50% chance for me having it. She said I could get tested for the gene and if you have the gene you could get certain cancers more easily. She also told me I should get testing [screening] for prevention of certain cancers (son, O12).
She tested positive for the BRCA gene, so I have a 50% chance of having the gene. After grandma tested positive, she met with a geneticist and tested positive. She said one day I can get tested, but not until I have kids (daughter, O14).
Risk reduction strategies (for the parent and/or offspring) were less frequently described. These included the potential for earlier cancer screening for the offspring (five references), prophylactic surgeries completed by the parent (three references) and general risk reduction measures such as avoiding tobacco (three references).
My mom explained that she wasn’t sick, but she was getting preventative surgeries so she doesn’t get breast or ovarian cancer in the future. She was doing it for us because she wants to be around, unlike her mother who died of ovarian cancer (daughter, O7).
Offspring understanding of, and response to, parental disclosure
Most offspring (17/22) reported that they felt they had a good understanding of the information shared, although many stated that they had developed a better understanding over time. Of the five who reported that they felt they had a poor understanding initially, four stated that they developed a better understanding over time.
Now I know the name of the genetic mutation and how it may affect my [future] family. I have done a little research on the gene on the Internet (son, O18).
Several offspring sought additional information after disclosure (42% of daughters, 20% of sons). The Internet was the most frequently utilized source; others included health-care providers, family, school and books.
When asked to describe their initial reaction when their parent shared this information, few offspring reported being surprised.
I felt ok with it. I suspected that my mother had it due to the family history of cancer. It was not a shock, more like common knowledge (daughter, O11).
Some offspring reported a concern about the increased risk of receiving a cancer diagnosis, either for themselves or among their parents and other family members. Nevertheless, the majority of offspring reported an adaptive response to the information.
It was surprising, but helped explain all the breast cancer in the family. I wasn’t worried about myself, because I am male, but thought it was useful if I have daughters (son, O19).
I knew mom had cancer and [I] just had to deal with the information. It wasn’t a big deal because I had already dealt with her cancer (daughter, O15).
Some offspring reported feeling frightened or disturbed (offspring 2, 8, 9, 12, 14, 16).
I was shocked, scared. I wondered if I was going to get the gene and realized I could pass it to my [future] kids. I would feel like it was my fault if they got cancer (daughter, O8).
Other descriptions representing this theme included ‘feeling doomed’ or ‘denying’ the presence of the mutation in hopes that it would ‘go away’. Offspring and parent characteristics (i.e. age, gender, parent history of cancer) predictive of distress at the time of parental disclosure were not clearly identifiable.
Many offspring reported developing a more mature response, e.g. acceptance of the familial risk or a deeper understanding of the implications over time.
Knowing what’s going on medically with my body is better than not. I am more mature now and have come to respect certain things. I want to learn more (son, O12).
I accept it as a fact of my life and my attitude has improved now about it. I spoke with a genetic counselor and have more information about the gene and preventative information (daughter, O16. She reported ‘shock and ‘denial’ at disclosure).
In many cases (42%) offspring reports of their initial reaction at disclosure were not consistent with parent reports of offspring initial reactions. Some parents reported that their offspring appeared to understand and accept the information without any detectable distress, yet their offspring reported shock, fear or distress on initially learning their parent’s results. The converse was also reported; a parent reporting distress in their offspring while the offspring reported acceptance or that they ‘were not surprised’ in learning their parent’s results. This differed markedly by offspring gender; 80% of son’s reports versus 33% of daughter’s reports were consistent with parent reports.
When asked what they currently understood to be the impact of their parent’s BRCA mutation, offspring most frequently cited the increased risk for the development of cancer for themselves and family members. Six sons specifically cited the possible increased risk for cancer in their offspring. Fewer offspring described the potential to engage in risk reduction interventions.
Offspring perceptions of the genetic risk for cancer
As many offspring reported concerns about developing cancer, offspring were asked which cancers, if any, were they concerned about developing. All female offspring reported concerns about developing cancer, most frequently breast cancer, and less frequently ovarian cancer. Three male offspring reported being unconcerned about developing cancer; the remainder reported concerns for prostate, testicular, breast and mouth cancer. When asked why they were concerned about these particular cancers, the majority identified the family history as the explanation for their concerns.
[I am most worried about] breast and ovarian cancer because they are prevalent on my mom’s side of the family (daughter, O7).
Others described the risks associated with the known genetic mutation and exposures such as tobacco.
[I am] most concerned about testicular, prostate and colon cancer, but also very concerned about lung, mouth, and brain cancer because I am male, cancer runs in my family and I smoked for 10 years (son, O12).
Offspring were asked what they think they could do now to avoid cancer. The most frequent responses were to exercise regularly, eat a healthy diet and avoid tobacco. Seeing a doctor regularly, avoiding alcohol and getting adequate sleep were reported less frequently. Some offspring felt that they could avoid cancer by engaging in these health behaviors. More frequently offspring shared some uncertainty, reporting that engaging in these health behaviors might help them avoid cancer. Some described these behaviors as helpful in improving general health, but not in reducing their risk of developing cancer.
Yes, I hope this will help, but we don’t know why cancer occurs right now. I want to believe that if you try to stay healthy, you can avoid cancer, but I don’t know for sure (son, O20).
It won’t completely stop cancer, but will keep risk low though, and it can be caught early (daughter, O21).
[If I do these things] I will stay healthy, but not necessarily avoid cancer, but it will increase my chances (son, O10).
Impact of disclosure
The majority (77%) of offspring reported that they felt that the disclosure had had no significant impact on their emotional health. Four daughters (offspring 4, 11, 15 and 16) reported a negative impact on their emotional health (cancer worry, stress or anger). Offspring uniformly reported no negative impact on relationships within or outside their family. The majority of offspring reported no impact on their childbearing plans; however, two sons reported that it might change their plans to have children.
Many offspring reported that the information their parent shared influenced their personal health behaviors (seven daughters, two sons). The most frequently cited behavior changes were smoking cessation and generally ‘staying healthy’. Of seven offspring with a tobacco history, five reported quitting smoking after their parent shared the familial risk for cancer. Two of the current smokers who had not quit reported that they did not think that changing health behaviors could impact their risk of developing cancer.
No [it won’t help me to stay healthy or avoid cancer]. If God wants you to have it you will get it regardless (daughter, O3).
Interest in genetic services
As all offspring were 18 or older at the time of their interview, all were eligible for genetic testing, although none were directly invited in for services. Genetic counselors routinely discuss the implications of parent genetic test results for family members, including the option for genetic testing for adult offspring and the recommendation for female mutation carriers to begin clinical breast exam and screening at the age of 25 [4]. Many offspring indicated that someone had suggested that they undergo genetic testing (70% of daughters versus 30% of sons). All but one recommendation came from parents; the exception was a health-care provider. Seven offspring (five daughters, two sons) had undergone genetic testing at the time of the interview (all>25 years at genetic testing). Those who had completed genetic testing were more likely to be female, older and to have children than those that had not had genetic testing. Among the 15 offspring who had not received genetic counseling and testing, the majority (87%) reported plans to undergo genetic testing in the future. Female offspring generally reported plans to undergo genetic testing between 18 and 25 years. The majority of male offspring indicated plans to undergo genetic testing prior to or surrounding childbearing.
Prior to having kids, to maybe see if we even want children (son, O1).
I would get it [genetic testing] if and when I have children, so that I can be aware of the potential effects for them [his children] (son, O20)
All offspring thought it would be helpful to meet with a genetic counselor or other expert in general, and the majority indicated that they thought it would have been helpful when they learned of their parent’s BRCA mutation. They indicated an interest in learning more about genetics and hereditary disease in general and the opportunity to ask specific questions.
Reported positive and negative aspects of parental disclosure
Offspring were asked to describe the potential benefits and negative aspects of someone, in general, learning of a parental mutation prior to age 25 years old, as well as the positive and negative aspects for themselves in particular (Table 3). Offspring frequently identified the potential to engage in preventive health behaviors, as a general benefit to learning of a familial risk at an early age.
Knowing that the gene exists and that you have the ability to get tested. Individuals can stay on top of things. Also, they will know that they can’t assume that they won’t get cancer at a young age. They will also be able to inform their physicians, so their physicians can be aware of this and more cautious (son, O10).
It could prepare them emotionally. It depends on the person, but it gives them the option to possibly lead their life differently in terms of childbearing, education, etc. (daughter, O11).
Table 3.
Reports of the positive and negative aspects of learning of the familial BRCA mutation prior to age 25 years old
| In general | For self | |
|---|---|---|
| Benefits of learning prior to 25 year old | ||
| To change health behaviors | 11 | 7 |
| To consider changing life plans | 5 | 2 |
| To be aware of the option for genetic testing | 4 | 7 |
| To explain the cancer in the family | 1 | 3 |
| For general knowledge or awareness of personal and family health | 4 | 10 |
| Emotional benefits | 2 | 3 |
| None | 1 | 2 |
| Negative aspects of learning prior to 25 years old | ||
| Could cause/has caused fear or worry of cancer | 11 | 6 |
| Could cause/has caused fatalistic attitude toward developing cancer | 4 | 2 |
| Could be/has been harmful if not mature enough at disclosure | 5 | 1 |
| Could impact/has impacted life plans or relationships | 3 | 0 |
| No potential negative aspects | 8 | 15 |
When considering the personal benefits, they most frequently reported benefits related to an increased awareness of their family and personal risk.
Knowing options, being able to catch something early, being more aware (daughter, O16).
Although many offspring reported potential negative aspects to learning of a familial mutation in general, the majority (15/22) reported no personal negative aspects for themselves.
[An individual] could have a fatalistic approach to things. It may make you change your life plans. Also, it could spook young kids out (son, O1).
[An individual] may not be mentally mature, it could affect the direction of [their] life in terms of childbearing, etc. (daughter, O4)
[It could cause] confusion, concern. Someone may be too young to understand the information. Also, individuals may feel this is a death warrant (son, O19).
Discussion
This qualitative study provides an initial understanding of how young adults and adolescents in BRCA families learn of the familial mutation and conceptualize their genetic risk. It also begins to examine the potential risks and benefits of learning this information during adolescence or early adulthood. Our results suggest that while the content and extent of the parent communication are variable, many offspring understand the complexities and implications of the familial risk. In addition, despite concerns for adverse psychological responses [19,20], some adolescent and young adult offspring appear to adapt well to this information. Equally important, these results suggest that some offspring may effectively change their general health behaviors in response to learning their familial risk.
While prior studies have demonstrated that parental disclosure to offspring is common [13–16], to our knowledge, none have reported what specifically parents share with their offspring or what offspring perceive they were told. A study evaluating proband communication to sisters in BRCA families reported that content varied among proband-sister pairs [21]. We also found considerable variability in the reported content communicated between parents and their offspring, suggesting that parents may disclose the hereditary risk for cancer and the option of genetic testing more frequently than risk reduction options. These findings suggest a need for further evaluation of what parents choose to discuss with offspring, the factors that influence those choices and the impact of those choices on the physical health and psychological well-being of their offspring. The reported content variability observed may reflect age appropriate, or offspring appropriate communication by parents to foster adaptive psychosocial and behavioral responses. Continued study of these issues could lead to the development of evidence-based guidelines and communication tools to facilitate health-care providers and parents in deciding when, how and what familial risk information can be best shared with young individuals in order to maximize adaptive responses.
Although preliminary, these results suggest that some offspring may adjust well to this information. In fact, many offspring in this study reported no negative aspects to learning this information. Few offspring reported adverse effects on family and social relationships, life plans or emotional health. Additionally, our results suggest potential health benefits related to early communication of hereditary risk. Many offspring reported that learning of their hereditary risk prompted them to engage in healthier behaviors, including tobacco cessation. It has been argued that because cancer surveillance measures are not recommended until 25 years old, there is no medical indication to sharing this information at a young age [12]. Our data suggest that there could be health benefits for both men and women related to the early communication of hereditary risk. Thus, further investigation of age-appropriate communication of risk may be indicated. Many offspring in our study reported written materials had been, or would have been useful, and that they sought additional information after parental disclosure, most frequently from the Internet. Many indicated an interest in and saw potential value of meeting with a genetic counselor surrounding parental disclosure. Although many offspring reported feeling they had a good understanding of the information shared, objective measures of their knowledge would be useful. Additionally, further research regarding which features of disclosure timing, method and content are associated with adaptive responses could be informative for parents considering disclosure. Such research could also assist health-care professionals to develop novel educational resources to supplement parent–offspring communication and tailoring clinical services, such as age appropriate genetic counseling, which may not include the opportunity to undergo genetic testing at that time.
Although the majority of offspring reported that learning of the hereditary risk had not changed their childbearing plans, the majority of sons expressed interest in undergoing genetic testing at or around the time of childbearing. Lower fertility intentions among BRCA mutation carriers after receipt of their test results has been previously described [22], and our findings suggest that this is an area that requires further study. Although several sons mentioned concerns regarding the risk for their offspring, they may have learned very little during parental disclosure about the risk reduction measures available. When parents undergoing genetic testing express concern about the implications for the next generation, health-care professionals often reassure them that the field is rapidly evolving and there are likely to be additional risk reduction options available in the future. The sons in this study may not have received this reassurance. Nonetheless, their concerns for the next generation and their reported interest in genetic services surrounding childbearing suggest there may be interest for reproductive advances such as pre-implantation genetic diagnosis (PGD). This controversial issue has been increasingly recognized in the field of cancer genetics [23,24]. PGD was not uniformly discussed with parents, but our findings suggest that further research is needed to evaluate the interest in and risks and benefits of incorporating such technology in the setting of hereditary cancer syndromes.
Several limitations of this study that are inherent in the methodology must be acknowledged. First, the study cohort represents a select group who may not be the representative of all BRCA families as they may be higher functioning than average families. As the offspring were ascertained through their parents, an already select group participating in a larger research study, it is possible that they are more likely to have had positive experiences and more adaptive responses. Additionally, some offspring (seven) had already had genetic testing and thus may have differed in their knowledge and perception based on the genetic counseling and testing experience. It is important to note that the offspring were reporting retrospectively and the qualitative method of data collection may not have been precise enough to accurately capture the content shared between parent and offspring. Although offspring reported feeling they had a good understanding of the information shared and descriptive responses of the content shared reflected accurate communication, objective detailed knowledge of numerical risks of inheritance, developing cancer and efficacy of risk reduction were not measured. Ultimately, it is offspring perceptions that influence their thoughts, feelings and behaviors and, therefore, their adaptation to the disclosed information. Thus, perhaps arguably, it is the long-term offspring perceptions that are the most relevant endpoints. Additionally, having a parent with cancer during adolescence may impact psychological responses to learning of a familial risk of cancer [25]. The majority of parents in this study did have a personal history of cancer and further enrollment of offspring without a parent with a history of cancer could be informative.
Conclusions
These findings suggest that adolescent and young adult offspring may understand and respond adaptively to the early communication of a hereditary risk for adult-onset cancer. In addition, parental disclosure of genetic risk may foster improved general health behaviors during adolescence and young adulthood. Further research is necessary to evaluate relationships among content and methods of disclosure, adaptive psychological responses and engagement in risk-reducing health behaviors among both high functioning and more distressed families. Such research can provide guidance to health-care professionals counseling hereditary cancer families and to parents considering how and when to share information with their offspring. In addition, there may be an increasing interest in and role for genetic counseling and emerging reproductive options, for both men and women in early adulthood. Further research can better define how best to incorporate these services into the care of hereditary cancer families.
Acknowledgments
This study was supported by American Cancer Society, grant MRSG-07-014-01-CPPB.
References
- 1.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 2.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94:1365–1372. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 4.Daly MB, Axilbund JE, Bryant E, et al. The National Comprehensive Cancer Network Clinical Practive Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast and Ovarian. (2007) 2007;1 [Google Scholar]
- 5.Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. J Am Med Assoc. 1997;277:997–1003. [PubMed] [Google Scholar]
- 6.Butow PN, Lobb EA, Meiser B, Barratt A, Tucker KM. Psychological outcomes and risk perception after genetic testing and counselling in breast cancer: a systematic review. Med J Aust. 2003;178:77–81. doi: 10.5694/j.1326-5377.2003.tb05069.x. [DOI] [PubMed] [Google Scholar]
- 7.Broadstock M, Michie S, Marteau T. Psychological consequences of predictive genetic testing: a systematic review. Eur J Hum Genet. 2000;8:731–738. doi: 10.1038/sj.ejhg.5200532. [DOI] [PubMed] [Google Scholar]
- 8.Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2004;96:122–133. doi: 10.1093/jnci/djh017. [DOI] [PubMed] [Google Scholar]
- 9.Clarke A. The genetic testing of children. Working party of the clinical genetics society (UK) J Med Genet. 1994;31:785–797. doi: 10.1136/jmg.31.10.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. American Society of Human Genetics Board of Directors, American College of Medical Genetics Board of Directors. Am J Hum Genet. 1995;57:1233–1241. [PMC free article] [PubMed] [Google Scholar]
- 11.Collins F. Commentary on the ASCO statement on genetic testing for cancer susceptibility. J Clin Oncol. 1996;14:1738–1740. [Google Scholar]
- 12.Kodish ED. Testing children for cancer genes: the rule of earliest onset. J Pediatr. 1999;135:390–395. doi: 10.1016/s0022-3476(99)70142-3. [DOI] [PubMed] [Google Scholar]
- 13.Hughes C, Lynch H, Durham C, et al. Communication of BRCA1/2 test results in hereditary breast cancer families. Cancer Res Ther Control. 1999;8:51–59. [Google Scholar]
- 14.Tercyak KP, Hughes C, Main D, et al. Parental communication of BRCA1/2 genetic test results to children. Patient Educ Couns. 2001;42:213–224. doi: 10.1016/s0738-3991(00)00122-1. [DOI] [PubMed] [Google Scholar]
- 15.Patenaude AF, Dorval M, DiGianni LS, Schneider KA, Chittenden A, Garber JE. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. J Clin Oncol. 2006;24:700–706. doi: 10.1200/JCO.2005.01.7541. [DOI] [PubMed] [Google Scholar]
- 16.Bradbury AR, Dignam JJ, Ibe CN, et al. How often do mutation carriers tell their young children of the family’s risk for cancer? A study of parental disclosure of BRCA mutations to minors and young adults. J Clin Oncol. 2007;25(24):3705–3711. doi: 10.1200/JCO.2006.09.1900. [DOI] [PubMed] [Google Scholar]
- 17.Leventhal H, Benyamini Y, Brownlee S, et al. Perceptions of Health and Illness: Current Research and Applications. Harwood; Amsterdam: 1997. Illness representations: theoretical foundations; pp. 19–46. [Google Scholar]
- 18.Strauss A, Corbin J. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Sage Publications; Newbury Park, London, UK: 1990. [Google Scholar]
- 19.Grosfeld FJ, Lips CJ, Beemer FA, van Spijker HG, Brouwers-Smalbraak GJ, ten Kroode HF. Psychological risks of genetically testing children for a hereditary cancer syndrome. Patient Educ Couns. 1997;32:63–67. doi: 10.1016/s0738-3991(97)00063-3. [DOI] [PubMed] [Google Scholar]
- 20.Fanos JH. Developmental tasks of childhood and adolescence: implications for genetic testing. Am J Med Genet. 1997;71:22–28. doi: 10.1002/(sici)1096-8628(19970711)71:1<22::aid-ajmg4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Hughes C, Lerman C, Schwartz M, et al. All in the family: evaluation of the process and content of sisters’ communication about BRCA1 and BRCA2 genetic test results. Am J Med Genet. 2002;107:143–150. doi: 10.1002/ajmg.10110. [DOI] [PubMed] [Google Scholar]
- 22.Smith KR, Ellington L, Chan AY, Croyle RT, Botkin JR. Fertility intentions following testing for a BRCA1 gene mutation. Cancer Epidemiol Biomarkers Prev. 2004;13:733–740. [PubMed] [Google Scholar]
- 23.Offit K, Kohut K, Clagett B, et al. Cancer genetic testing and assisted reproduction. J Clin Oncol. 2006;24:4775–4782. doi: 10.1200/JCO.2006.06.6100. [DOI] [PubMed] [Google Scholar]
- 24.Menon U, Harper J, Sharma A, et al. Views of BRCA gene mutation carriers on preimplantation genetic diagnosis as a reproductive option for hereditary breast and ovarian cancer. Hum Reprod. 2007 doi: 10.1093/humrep/dem055. [DOI] [PubMed] [Google Scholar]
- 25.van Oostrom I, Meijers-Heijboer H, Duivenvoorden HJ, et al. Experience of parental cancer in childhood is a risk factor for psychological distress during genetic cancer susceptibility testing. Ann Oncol. 2006;17:1090–1095. doi: 10.1093/annonc/mdl069. [DOI] [PubMed] [Google Scholar]


