Abstract
Introduction
Children with myelomeningocele (MMC) are usually subjected to multiple surgeries. However, the number and type of surgeries are not the same in every patient with MMC over time. This report summarizes the surgical interventions in a cohort of several ages.
Materials and methods
Data on all of the patients with MMC, aged from 1 year and 10 months to 21 years and 11 months, were retrospectively reviewed at the Dona Estefânia Hospital in Lisbon, Portugal. Data were collected by chart review and individual interviews. The factors analyzed were demographics, ambulatory status, neurological level of involvement, shunt status, Arnold–Chiari malformation type II, surgical history, and occurrence of fracture. The surgical interventions were categorized as neurosurgical, orthopedic, urinary, ulcer repair and others.
Results
A total of 84 alive were eligible and enrolled. The average age was 14 years and six months. A total of 59 patients received shunts (all but one ventriculoperitoneal). In the study group, the 84 patients required 663 surgeries. Neurosurgical interventions were the most frequent surgical procedure and predominated during the first 2 years of life. Surgical interventions related to shunts were the most common neurosurgical interventions. Orthopedic surgeries were more frequent in the age group 6–12 years. Urological surgeries were done mainly after 6 years of age. Surgical repair of pressure ulcers was more common after 12 years of age.
Conclusions
Our study brings to light the complexity of this condition, with multiple surgeries among patients with MMC.
Keywords: Myelomeningocele, Surgery, Quality of life
Introduction
When a newborn child presents with myelomeningocele (MMC), one of the main questions from parents to physicians is about the child's future. The survival, quality, and functional abilities of patients with MMC have improved due to advances in the neurosurgical, urological, and orthopedic areas.1 Longer survival has meant the need for multiple surgeries.1 Surgical treatment of MMC has been mired in some controversy, with several topics of debate, such as the timing of MMC closure, shunt placement, and clinical indications for orthopedic surgery. There has not yet appeared a definitive guideline with universal orientations in all fields of surgeries with regard to MMC. The lack of outcomes studies on this subject is related to the complexity of many patients with MMC and the difficulty in designing clinical trials because of the overall decrease in the prevalence of MMC in most industrialized countries.2,3 The rarity of MMC could cause physicians to encounter it less often, consequently promoting less research and a lack of interest in this disease. Moreover, funds for research are likely more available for more prevalent diseases and many hurdles are inherent in multi-centered, randomized clinical trials for pediatric diseases.4
This study was designed to define the number and type of surgeries performed in patients with MMC. Considering that the functional motor level of a patient with spina bifida (SB) is the primary determinant of neurological alterations, major orthopedic deformities, ambulatory status, treatment, outcome, and ultimate prognosis,5 we intended to define a pattern of surgeries according to the neurological level of involvement. We also undertook a review to find indications for surgery for MMC, attempting to select areas in which additional knowledge has been lacking, in order to instigate worldwide discussion. To our knowledge, this is the only study worldwide that presents a complete picture of the natural history of surgical interventions in all organic systems in a cohort of patients with MMC, as well the association of MMC surgeries with motor level across several ages.
Materials and methods
Study location
Dona Estefânia Hospital (Lisbon, Portugal) is a pediatric tertiary care hospital, with a center specializing in following patients with SB. This hospital is a national referral center for patients with SB from central and south Portugal and the Portuguese islands. It also receives patients from Africa, mainly Cape Verde, Guinea-Bissau, and Sao Tome and Principe, which are former Portuguese colonies.
Study design
A retrospective clinical study of all patients with MMC followed from January 22, 1990, to December 31, 2011, at Dona Estefânia Hospital was performed. After obtaining the Ethics Committee approval from our hospital, a written informed consent was obtained from all the patients, with parents accepting participation on behalf of their children.
Data collection
We consulted the clinical records in paper and electronic formats, the latter of which has been available since 1998. At the time of visiting our center for routine observations, all the parents or guardians of the patients and the patients 16 years old and older underwent face-to-face, structured interviews about all surgical interventions, in an attempt to detect surgical interventions lacking in our database because some interventions might have been performed in other institutions. Call-back examinations were requested for the patients followed in our center when the medical records were insufficient. For patients who changed their center of follow-up, we contacted by phone all the parents or guardians of each patient.
Eligibility criteria
The eligibility requirements for enrollment in this study included children alive of both sexes, a diagnosis of MMC, age 0–21 years, medical records available, and followed up for 12 months or longer in our center, with at least one consultation between 2008 and 2011 at our center. For patients who did not meet these criteria, we included them if we were able to schedule a consultation during 2012 to obtain missing information. We excluded patients with diagnoses of closed neural defect, meningocele, sacral agenesis, and encephalocele, and patients with unreliable or unavailable medical records, patients who changed centers for whom it was not possible to contact their parents to obtain missing information or who declined to participate or to provide missing medical record information. The cohort was stratified into four subgroups according to age: 0–2 years, 2–6 years, 6–12 years, and >12 years. We selected the following variables, obtained using a standardized protocol for analysis: data on demographics, vital status, and clinical aspects (including ambulatory status, level of motor involvement, and occurrence of fractures) as well as all surgical procedures. We evaluated available X-rays and magnetic resonance imaging from the brain to sacrum for each patient, seeking the presence of hydrocephalus, Arnold–Chiari malformation type II (ACMII) and hydrosyringomyelic cavitations. All of this information was recorded in a computerized database. For this study, the motor level, obtained after analytic manual motor testing, was graded from 0 to 5 (Medical Research Council Scale)6 by consultants from the departments of Pediatric Neurology and Physical Medicine and Rehabilitation at our institution. We referred to the motor level as the muscle corresponding to the lowest level at which the patient had strength of not less than grade 3 within the available range of joint motion.7–9 The patients were grouped according to the neurological motor level into four categories: (1) thoracic level, (2) upper lumbar level (L1–L2), (3) lower lumbar level (L3–L5), and (4) sacral level (S1–S3). For patients with different neuromotor levels on the left and right, the higher (worse) level was assigned.9 A comprehensive fracture history was obtained, including dates and sites of fractures and the fracture mechanism involved. Fracture mechanisms were categorized according to a previously published study10: spontaneous fracture (without known trauma), minor trauma (trivial injury), and major stress. Spontaneous fractures and those due to minor trauma were classified as pathological.10 Ambulatory status was assessed, according to a modified version of the assessment described by Hoffer et al.11 and already used by Swank and Dias,12 as community ambulators, household ambulators (partial ambulators), and non-ambulators (wheelchair bound).
Surgical interventions were divided into neurosurgical interventions, orthopedic interventions, urological interventions, intestinal interventions, pressure sore interventions, and others. For each procedure, we evaluated the number, type, location, and data of the interventions that were made.
Neurosurgical interventions
In the neurosurgical group, we categorized the procedures into MMC closure, shunt placement, shunt revision, posterior fossa decompression, spinal cord untethering, and others. For shunts, we analyzed the type of shunt, the patient age at initial shunt insertion, the number and date of shunt revisions, and the duration of each shunt. Revision surgery was performed for infection of the shunt, obstruction of the system, disconnection of a derivative system, malpositioning or overdrainage, and elective lengthening of the shunt.
Orthopedic surgeries
In the orthopedic group, we categorized interventions on the spine, hip, knee and ankle/foot, fractures, and others. Surgeries on fractures were considered an individualized subgroup of orthopedic interventions. Thus, surgeries on fractures were not integrated into the other orthopedic subgroup according to the anatomic site. The count of removals of osteosynthetic material following surgical interventions was undertaken at the corresponding anatomic site, with the exception of removals of osteosynthetic material following surgical interventions on fractures, which were integrated into the subgroup of fractures. Rotational deformity surgeries, such as tibial derotational osteotomy, were included in the group of interventions on the ankle/foot.
Surgeries on pressure sores
For surgeries on pressure sores, we evaluated the distribution and overall patient recurrence. We divided the ulcer sites into four groups: spinal, pelvic (buttock, sacrum, ischial tuberosities, and the greater trochanter), leg, and ankle/foot. Patients with surgeries for skin breakdown secondary to delayed operative wound healing, improper cast techniques, burns, or fracture malalignments were excluded so that isolated surgeries on pressure sores could be evaluated.13
Dehiscence of sutures after surgery followed by another surgery was counted in the major group for the corresponding topographic region. Other types of surgical interventions not categorized into any group of surgeries previously introduced were also obtained and integrated into the other major items.
During the same surgical period, if two anatomic regions were simultaneously operated on, we counted both surgeries. If more than one procedure was performed in an anatomic region during the same operative period, we considered only one surgery, although in the tables we summarize the different procedures, indicating that the number was higher than the total number of surgeries for each subgroup of surgeries. In our count of surgeries, we excluded interventions on teeth, post-traumatic wound cutaneous sutures, and interventions on nails.
As primary study endpoints, we considered the evolution pattern of each group of surgeries across the age groups. As secondary endpoints, we considered the occurrence of surgical interventions relative to the neurological level of involvement, the occurrence of fractures with regard to surgical interventions and evaluation of changes in demographics over time.
Statistical analysis
Analysis of contingency tables, proportions testing, the two-sample t-test, and the two-sample Kolmogorov–Smirnov (KS) test were all performed using the Statistical toolbox functions in the MATLAB software, version R2010b (Mathworks, Inc., Natick, MA, USA). The Fisher–Freeman–Halton test was performed with the StatXact software (Cytel, Inc., Cambridge, MA, USA). P values <0.05 were considered statistically significant.
Results
Total study sample characteristics
In this retrospective study, a total of 158 patients were identified with MMC, 84 living (53.60%) patients identified over a period of 21 years and 11 months who fulfilled the inclusion criteria and were selected for the study.
Study sample characteristics of patients
Within this study group, 32 patients were male and 52 were female. Our study group was born between January 22, 1990 and February 6, 2010. The age at inclusion was within the range of 1 year and 10 months old to 21 years and 11 months old, with a median of 15 years and 10 months and a mean of 14 years and 6 months. The cohort studied was distributed as follows: 1 patient (1.19%) was younger than 2 years of age; 9 (10.71%) were between 2 and 6 years old; 18 (21.43%) were between 6 and 12 years old, and 56 (66.67%) were older than 12 years. Seventy patients were natives of Portugal, 2 were from Brazil, and 12 were from PALOP (African Countries of Portuguese Official Language). Thoracic, upper lumbar, lower lumbar, and sacral levels of lesions were present in 10, 16, 42, and 16 children, and adolescents with MMC, respectively. ACM II was present in 73 patients, and in 9, 16, 37 and 11 cases at the thoracic, upper lumbar, lower lumbar and sacral levels, respectively. Forty-three patients had hydrosyringomyelic cavitations. In our study group, 37 were community ambulators, 13 were partial ambulators, and 34 were non-ambulators.
Surgical interventions
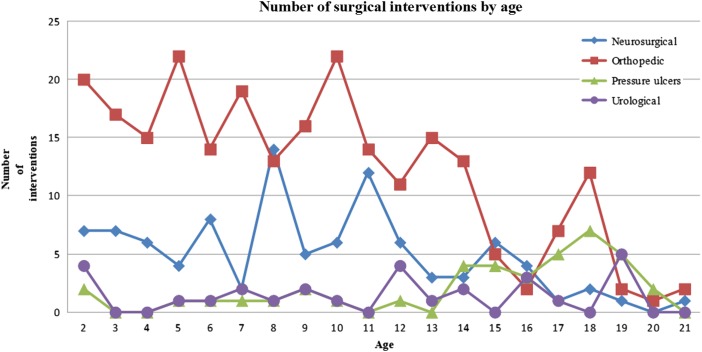
All were submitted to at least one neurosurgical procedure, followed by procedures for orthopedic problems (58/84; 69.05%), ulcers (20/84;23.81%) and finally, urological issues (16/84;19.05%). In the study group, the 84 patients required 663 surgeries. The average number of surgeries per patient was 7.89, and the median was 7 (range: 1–28). In the subgroup 0–2 years old, neurosurgical interventions (n = 209) were the most common, followed by orthopedic (n = 26), urological procedures (n = 6), and pressure ulcers (n = 2). In the subgroup 2–6 years old, 25 surgeries were neurosurgical, 68 were orthopedic, 2 were pressure ulcers, and 2 were urological. In the subgroup 6–12 years old, there were 45 neurosurgical, 95 orthopedic, 11 urological, and 6 pressure ulcer procedures. In the group >12 years old, we found 21 neurosurgical, 60 orthopedic, 30 pressure ulcer, and 12 urological procedures (Fig. 1).
Figure 1 .
Graph showing the distribution of the number and type of surgical interventions according to the age group.
The number of surgeries for each level of neurological involvement was 102 at the thoracic level, 181 at the upper lumbar level, 297 at the lower lumbar level, and 83 at the sacral level.
Neurosurgical interventions
According to each category, the total number of neurosurgical interventions was 300, with an average number of 3.57 neurosurgical interventions for each patient (range 1–14).
Closure of MMC defects
All the patients were submitted to closure of MMC postnatally. Closure of MMC defects was performed within 24 hours in 48 newborns (57.14%); in the 24–48 hour subgroup, there were 12 patients (14.28%); in the 48–72 hour subgroup, there were 5 patients; in the 72 hour to 1 week group, there were 3 patients (1 Portuguese, 1 Brazilian, and 1 PALOP); in the 1 week to 1 month group, there were 5 patients, all of whom were Portuguese; in the 1–6 month group, there were 8 patients (7 from PALOP countries and 1 from Portugal); in the 6 months to 1 year group, there were 2 patients (1 from a PALOP country and 1 from Portugal); and in the >1 year group, there was 1 patient (from a PALOP country).
Shunt placement
Fifty-nine of 84 (70.24%) MMC patients had shunted hydrocephalus. Most of the patients had a ventriculoperitoneal shunt (58/59; 98.31%), while only one had a ventriculoatrial shunt.
The placement of the first shunt occurred mostly during the first year of life, in 91.53% of cases (54/59).
The first shunt was inserted on the first day of life in 4 cases; at 1–7 days, there were 2 cases; at 7–30 days, there were 28 cases; and after 30 days, there were 25 cases. Nine patients received shunt insertion and closure of an MMC defect on the same day.
Forty patients (40/59; 67.79%) underwent at least 1 revision of the shunt, for a total of 93 revisions (mean: 1.11; range: 0–9). The median time to first shunt revision was 561 days. Twenty-two patients (37.29%) required at least one revision during the first year of life. Forty-three cases (51.19%) of shunt revision occurred during the first 6 months of life, 10 revisions (11.90%) occurred between 6 months and 1 year, and 40 revisions (47.62%) occurred after the first year (Figs. 2 and 3). Twenty-one patients suffered a second shunt failure, six patients had a third failure, four patients had four failures, and three patients had five failures. The time between the first shunt and second shunt was less than 1 week in 2 cases, between 1 week and 1 month in 8 cases, between 1 month and 6 months in 10 cases, and after 6 months in 20 cases. With a 5% level of confidence, we rejected the hypothesis that the occurrence of shunt insertion occurred in equal proportion at all levels of neurological involvement (P < 0.0045) (Table 1).
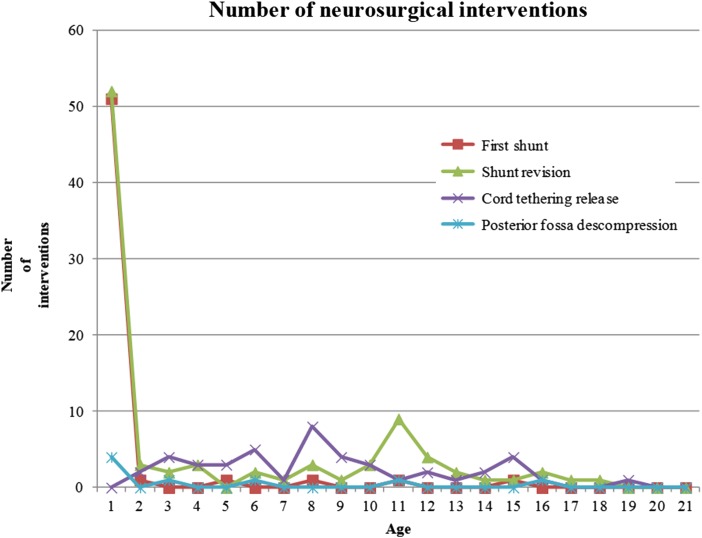
Figure 2 .
Distributions of neurosurgical interventions according to age.
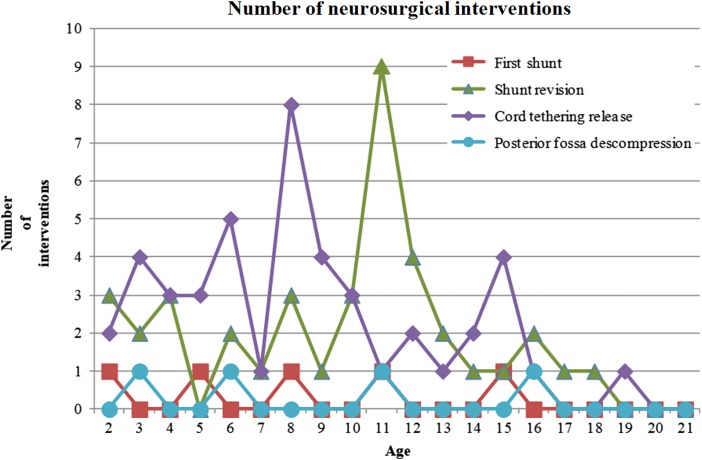
Figure 3 .
Distribution of neurosurgical interventions in patients older than 1 year of age.
Table 1 .
Number of patients undergoing surgery according to type of surgery and level of neurological involvement
| Neurosurgical patients (0.0069) |
Orthopedic patients (<0.0001) |
Pressure ulcer interventions | Urinary interventions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First shunt | Shunt revision | Cord tethering release | Posterior fossa decompression | Fracture interventions | Hip interventions | Knee interventions | Operative procedures on spine | Foot interventions | |||
| Sacral | 9 | 2 | 11 | 0 | 0 | 0 | 1 | 0 | 7 | 2 | 3 |
| Lower lumbar | 26 | 12 | 23 | 4 | 2 | 11 | 2 | 2 | 21 | 10 | 5 |
| Upper lumbar | 15 | 6 | 8 | 1 | 1 | 12 | 2 | 3 | 9 | 5 | 5 |
| Thoracic | 9 | 1 | 3 | 2 | 1 | 1 | 1 | 7 | 4 | 3 | 3 |
| Total | 60 | 21 | 45 | 7 | 4 | 24 | 6 | 12 | 41 | 20 | 16 |
| Proportion test P-value | 0.0045 | 0.0026 | 0.0002 | 0.1718 | 0.5724 | 0.0001 | 0.8810 | 0.0341 | 0.0010 | 0.0550 | 0.8013 |
| Global table χ2 P-value | <0.0001 | ||||||||||
ACMII decompression
In our study group, a total of 8 (8/84; 9.52%) patients presented with signs and symptoms of brainstem dysfunction requiring ACMII decompression. All the patients submitted to ACMII decompression presented with a shunt for hydrocephalus. One patient underwent a second hindbrain decompression. There were no patients with sacral-level involvement submitted to this procedure. There was no statistical evidence to reject the hypothesis that decompression of the brain stem occurred differently according to the lesion level (P = 0.1718) (Table 1).
Spinal cord untethering
In this series, 38 patients (38/84; 45.24%) developed a symptomatic tethering of the cord and underwent 45 tethered cord releases. Five patients were submitted to two surgical procedures, and one patient underwent a third intervention. The mean age to tethered cord release was 7 years and 11 months old, ranging from 1 year and 4 months to 18 years and 5 months with a median of 7 years and 9 months. Two patients were 0–2 years old, 15 patients (37.78%) were 2–6 years old, 19 patients (42.00%) were 6–12 years old, and 9 patients (20.00%) were older than 12 years. There was a peak between the ages of 6 and 10 years of age (Figs. 2 and 3). A total of 44.44% surgeries were performed on patients between 6 and 13 years old. Tethered cord release occurred in all levels of lesions. We rejected the hypothesis that occurrence of a tethered cord release was the same at all lesion levels (P < 0.0003) (Table 1).
Orthopedic interventions
Regarding orthopedic interventions, the total number of surgeries was 249, with an average of 2.96 per patient and a range of 0–20. A total of 89.16% of the orthopedic interventions were performed on patients between 0 and 15-year old, with a decrease after 15 years of age (Fig. 1). The numbers of orthopedic interventions were 47, 79, 106, and 17 for the thoracic, upper lumbar, lower lumbar, and sacral levels, respectively. Two contingency tables were created to cross two periods of a patient's life: before and after 9 years of age. The row and column variables coded the neurological level of the lesion and the location of the orthopedic surgery. We applied the Fisher–Freeman–Halton test to investigate the independence between the locations of the orthopedic surgeries before and after 9 years of age and the neurological level of the lesion. The table shows statistical dependence between the hip and spine surgeries and the neurological level of the lesion before and after the age of 9 years. This dependence was not found for the remaining surgery locations (Table 2).
Table 2 .
Number of orthopedic surgeries before and after 9 years of age
| Younger than 9 years old |
Older than 9 years old |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ankle/foot | Knee | Hip | Spine | Fractures | Ankle/foot | Knee | Hip | Spine | Fractures | |
| Sacral | 6 | 0 | 0 | 0 | 0 | 10 | 1 | 0 | 0 | 0 |
| Lower lumbar | 35 | 0 | 18 | 1 | 1 | 31 | 3 | 8 | 5 | 3 |
| Upper lumbar | 23 | 2 | 31 | 0 | 0 | 9 | 0 | 5 | 7 | 2 |
| Thoracic | 6 | 1 | 0 | 17 | 0 | 3 | 0 | 2 | 15 | 2 |
| P-value (Fisher– Freeman–Halton test) before and after 9 years old | 0.1059 | 0.0857 | 0.0121 | 0.0089 | 0.4859 | 0.1059 | 0.0857 | 0.0121 | 0.0089 | 0.4859 |
Subtype of orthopedic interventions over time
Interventions on the spine
Forty-five surgical interventions on the spine were performed in 12 patients, of whom 7 were female and 5 were male. Fourteen procedures were fusions, two procedures were to implant a vertical expandable prosthetic titanium rib device (VEPTR) and six were extensions of VEPTR. In our cohort, 10 patients (10/12; 83.33%) submitted to spine surgeries suffered post-surgical complications. Four procedures were revisions, with or without extension of fusion, one was a revision of VEPTR, six were removals of implants due to hardware complications secondary to mechanical complications or infection, and nine were procedures due to wound-healing problems and infections (7 due to debridement/fistulectomy, 1 myocutaneous flap, and 1 plastia) (Table 3). Only one case of pseudoarthrosis was documented. The average age at spinal surgery was 9 years and 11 months (range: 3 years and 8 month to 16 years and 11 months) (Fig. 4). The proportion of patients submitted to surgical intervention on the spine for each level decreased progressively at lower levels of neurological involvement: 7/10 (70.00%) thoracic (total surgeries: 32); 3/16 (18.75%) on upper lumbar (7 surgeries); and 2/42 (4.76%) on lower lumbar (6 surgeries). A total of 86.67% (39/45) of surgeries occurred in patients with thoracic and upper lumbar lesions. None of the patients with sacral-level involvement were submitted to surgical interventions on the spine (Table 1). The data implied a significant association between the number of patients with operative procedures on the spine and the neurological level of the lesion (P = 0.0341) (Table 1).
Table 3 .
Number of orthopedic procedures per anatomic site
| Site | Type of procedures | Total |
|---|---|---|
| Hip | Proximal femoral osteotomy | 23 |
| Extraction of material osteosynthesis | 14 | |
| Iliopsoas transfer | 11 | |
| Pelvic osteotomy | 11 | |
| Unspecified | 9 | |
| Tenotomy and lengthening (adductor tenotomy; rectus femoris tenotmy; tensor fascia lata tenotomy; iliopsoas tenotomy; sartorius lengthening) | 7 | |
| Hip osteotomy (unspecified) | 4 | |
| Knee | Tenotomy/lengthening and hamstring lengthening | 3 |
| Unspecified | 2 | |
| Extraction of material osteosynthesis | 1 | |
| Supracondylar extension osteotomy | 1 | |
| Foot and ankle | Tenotomy/lengthening | 44 |
| Unspecified | 28 | |
| Osteotomy | 26 | |
| Arthrodesis | 14 | |
| Extraction of material osteosynthesis | 14 | |
| Tendon transfer/transposition | 13 | |
| Steindler operation | 8 | |
| External fixator (Ilizarov; multiplanar; Taylor spatial frame) | 4 | |
| Tarsectomy | 3 | |
| Epiphysiodesis | 2 | |
| Reduction of astragalus dislocation | 2 | |
| Foot amputation/surgery for polydactyly | 2 | |
| Bone graft | 1 | |
| Cuboid resection | 1 | |
| Spine | Wound healing problems and infection (debridement/fistulectomy/myocutaneous flap/plastia) | 9 |
| Spinal fusion surgery | 7 | |
| Revision and/or extension of vertical expandable prosthetic titanium rib device | 7 | |
| Hardware complications with implant removal (mechanical complications/instrumentation failure; chronic infection; skin/ wound issues) | 6 | |
| Posterior fusion with instrumentation | 5 | |
| Unspecified | 5 | |
| Instrumentation revision/extension of fusion | 4 | |
| Anterior fusion with instrumentation | 2 | |
| Vertical expandable prosthetic titanium rib device | 2 | |
| Thoracoplasty | 1 |
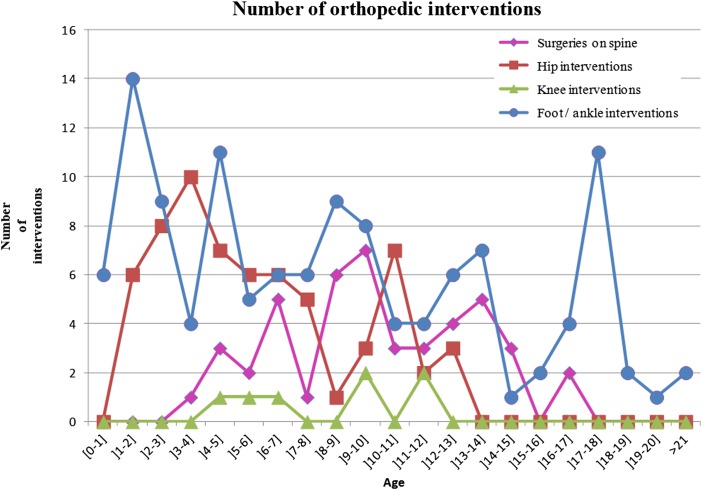
Figure 4 .
Distribution of orthopedic intervention according to age.
Interventions on the hip
We detected a total of 84 surgical interventions on the hip among 24 patients. The hip interventions predominated among patients aged 2–8 years, with 75.00% of surgeries (48 surgeries) (Fig. 4). Regarding the procedures, we identified 23 proximal femoral osteotomies, 11 pelvic osteotomies, 11 iliopsoas transfers and 7 tenotomies (Table 3). There was a biphasic peak in hip surgeries at 4 and 11 years of age (Fig. 4). Surgical interventions on the hip were more frequent among patients with lower lumbar and upper lumbar lesions, 26.19% (11/42) and 75.00% (12/16), respectively. None of the patients with sacral lesions were submitted to a surgical procedure on the hip, and only one patient with a thoracic level of involvement (1/10; 10.00%) underwent hip surgery (Table 1). Most of the surgical interventions at the upper lumbar level were performed before 9 years of age (Table 2). The frequency of children receiving treatment of the hip, i.e., surgery, was significantly associated with the level of lesion (P < 0.0002) (Table 1).
Interventions on the knee
Only six patients required interventions on the knee, with a total of seven surgeries. We did not detect an association between the number of patients receiving knee surgery and the level of lesion (P = 0.8810) (Table 1).
Interventions on the ankle/foot
A total of 123 surgical interventions on the ankle/foot were detected in 41 patients with MMC, ranging from 0 to 10 interventions for each patient in the data set, with an average of 1.46 (Table 1). The ankle/foot interventions were more frequent in patients between 0 and 12-year old, at 69.92% (86 surgeries) (Fig. 4). Regarding procedures, we detected 44 tenotomy/lengthening procedures, 26 osteotomies, and 14 arthrodeses (Table 3). The surgical correction of foot deformities in absolute numbers was more frequent at the lower lumbar level (n = 66 surgeries), followed by the upper lumbar level (32 surgeries), sacral level (16 surgeries), and thoracic level (9 surgeries). The proportions of number of operated individuals for each level/total number of patients in the data set for each level of lesion were 43.75% (7/16) for the sacral level, 50.00% (21/42) for the lower lumbar level, 56.25% (9/16) for the upper lumbar level, and 40.00% (4/10) for the thoracic level. After 9 years of age, there was a decrease in surgical interventions on the feet at higher levels of neurological involvement, and there was a persistence in surgical interventions at lower levels (Table 2). There was a relationship between surgical interventions on the foot and the level of lesion (P < 0.0010) (Table 1).
Interventions on fractures
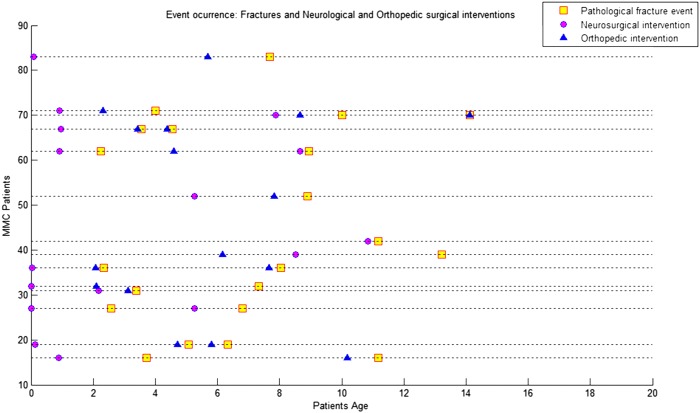
We detected 46 fractures in 24 patients. Four patients were submitted to eight surgical interventions following fractures. There was no statistical evidence for an association between the number of patients submitted to surgeries following fractures and the level of lesion (P = 0.5724) (Table 1). The data suggested that there was a shorter time interval between a pathological fracture succeeded by an orthopedic intervention compared to a pathological fracture succeeded by a neurosurgical procedure. The average time interval between a pathological fracture succeeded by an orthopedic procedure was 1 year and 7 months, while the difference between a pathological fracture succeeded by a neurosurgical intervention was 3 years. To investigate this claim, we created two vectors: one vector contained the difference, in days, between the pathological fracture occurrence date and the immediately previous neurosurgical intervention; the other vector contained the same difference regarding orthopedic procedures. Applying the KS test to both sample vectors, we rejected the hypothesis that these differences came from an identical continuous distribution (P = 0.0231 with two-sample t-test) (Fig. 5), indicating that these differences in the time intervals were distinct.
Figure 5 .
Distribution of pathological fractures and neurosurgical and orthopedic interventions over time.
Urinary interventions
There were 16 patients submitted to 31 urological interventions, for an average of 0.39 per patient over all the data (range 0–5). In this cohort, we identified eight bladder augmentations in six patients between 1 and 19 years old; two patients needed a second bladder augmentation. There were three radical nephrectomies/ureterectomies, three Monti ileal conduit surgeries, and two Mitrofanoff procedures. Only one renal transplant was identified (Table 4). Urinary interventions occurred at all lesion levels and were more frequent after 6 years of age (75.00% of urological surgeries) (Table 1 and Fig. 1). There was no evidence of an association between the number of individuals submitted to urinary surgery and the level of lesion (P = 0.80) (Table 1).
Table 4 .
Number of urinary procedures
| Type of procedures | Total |
|---|---|
| Bladder augmentation and re-implantation of the ureter | 8 |
| Re-implantation of the ureter | 4 |
| Radical nephrectomy/ureterectomy | 3 |
| Monti ileal conduit surgery | 3 |
| Ureteroneocystostomy/cysto-urethroplasty/reconstruction (plastic surgery) of the bladder neck | 2 |
| Mitrofanoff surgery | 2 |
| Partial nephrectomy | 1 |
| Kidney transplant | 1 |
| Intradetrusor botulinum toxin injection | 1 |
| Introduction of ureteral stent | 1 |
| Urethral stricture dilatation | 1 |
| Vesical lithotomy | 1 |
| Laparoscopic pyelolithotomy and ureteral stent repositioning | 1 |
| Post-operative complication of Monti surgery with conduit necrosis and relaparotomy with remotion of Monti | 1 |
| Operative procedure for post-operative complication of bladder augmentation with urinary ascites secondary to vesicoperitoneal fistula | 1 |
| Failed attempt to restore continence of Mitrofanoff | 1 |
| Ureteral stent removal | 1 |
Intestinal intervention
We had one patient submitted to a Malone antegrade continence enema (ACE) procedure.
Pressure ulcer interventions
The total number of surgeries was 40 in 20 individuals, of whom 8 were male and 12 were female, for an average for each patient of 0.48 surgeries (range 0–8). The mean age at surgery was 14 years (range from 1 year and 8 months to 19 years and 11 months). The site of pressure ulcer intervention was 20 pelvic and 20 ankle/foot. The overall patient rate of recurrence of surgical interventions on pressure sores was 50.00% (10/20). Pressure ulcer interventions presented at all lesion levels in the following proportions of patients: 2 of 16, sacral; 10 of 42, lower lumbar; 5 of 16, upper lumbar; and 3 of 10, thoracic (Table 1). These procedures were mainly performed after 12 years of age (75.00%) (Fig. 1). There was no statistical evidence of an association between the individuals operated on for ulcers and the level of neurological lesion (P = 0.055) (Table 1).
Nationality distribution of the data over time
As a result of the immigration of their patients from Africa to Portugal to be treated and followed at our center, the nationality distribution pattern of our population of patients with MMC changed over time. Until 2000, there were 53 patients from Portugal and 2 patients from PALOP countries. After 2000, there were 17 Portuguese patients and 10 from PALOP countries. The decrease in the number of Portuguese patients born after 2000 could be verified by applying a proportion test (P < 0.001). We observed an increase over time of patients born in PALOP nations after 2000. With a level of significance of 5%, this increase was statistically relevant (P = 0.0209).
Surgical interventions and level of lesion
There was an association between the number of patients submitted to neurosurgical interventions and the level of lesion. The observed P-value, 0.0069, was calculated using the χ2 test for the contingency table, crossing different types of neurosurgical interventions and levels of lesions. A small P-value (<0.0001) was observed using the previous χ2 test for the contingency table, crossing different types of orthopedic surgeries and lesion levels. We also tested the contingency table resulting from the concatenation of the neurosurgical patients, orthopedic patients, pressure ulcer intervention patients, and urinary intervention patients. The P-value for this larger contingency table (<0.0001) allows one to conclude that there was also an association between different types of surgeries in MMC patients and their lesion levels (Table 1).
Multiple surgeries during the same surgical period
In our institution, there were 41 patients with at least 2 surgeries performed on the same day. There were 77 cases of 2 surgical interventions on the same day and 6 cases of 3 surgeries on the same day. There were 23 cases of 2 neurosurgical interventions on the same day and 45 cases of 2 orthopedic surgeries, 2 cases of 1 neurosurgery and 1 orthopedic surgery, and 1 case of 1 ulcer surgery and 1 orthopedic surgery.
Complications
Post-operative complications
A 16-year-old female patient with upper lumbar MMC, shunted since birth with two revisions, a stable ACMII and an asymptomatic tethered cord was partially ambulatory until 8 years of age, when she definitively lost this function after a surgical intervention on her hip.
Discussion
This retrospective review describes the natural history of surgeries across several age groups in a pediatric cohort with MMC, from the SB center at Dona Estefânia Hospital. We intended to define some clinical orientations according to our experience and to review the literature. Our concern was not to record the technique of surgery, the efficacy of each surgical procedure or its functional result.
The elevated number of registered surgical interventions indicates the excess of morbidity in those patients and consequently the economic burden on the Portuguese health care system. There were fewer patients in the younger (aged 0–6 years) group than in the older group, with an increase in the proportion of patients from PALOP countries, which might have been due to a lower frequency of MMC in Portugal and the replacement of our population by those patients coming from PALOP countries.
In our cohort, most cases of MMC are repaired during the first 24–48 hours of life. Optimally, back lesions should be closed within 72 hours of birth. A delay can have some impact on vital and functional prognoses, with higher risks of shunt malfunction and CNS infection14–17 and worse neurogenic bladder prognosis.18 In some patients, mostly from PALOP countries, there was a delay in MMC repair due to the unavailability of this procedure in their countries. These data corroborate the findings of Watson et al.19 that delayed repair of spinal MMC has been encountered in environments where access to neurosurgery is limited or impaired. As in the study by Mirzai et al.,15 in our country there might be some problems with delays in referral, which might be due to a lack of understanding of the importance of early referral to a neurosurgical center. Our rate of shunts in patients with MMC was in agreement with the literature, in which the rate ranges from 42.6 to 95.5%,15,20–31 with most patients requiring shunt placement during the first year of life. The great variation in shunt rates can be explained by the different designs of studies, with some including only samples with fetal MMC repair,26,28 which appears to reduce the need for shunts, and other studies including only patients with postnatal repair. In addition, the criteria adopted for shunting could have been different among centers.21,25,32 In agreement with other studies, in this study cohort, most of the cases of hydrocephalus in MMC patients were treated with ventriculoperitoneal shunts.20,22,33 There has been some controversy regarding the time of first shunt placement. The main arguments against simultaneous MMC repair and shunt placement have been based on the compromised immune function of the newborn, the catabolic response with prolonged wound healing, CSF exposure to organisms from the open sac, transient bacteremia during surgical manipulation for back closure, the higher rate of back wound infection and shunt infection, unrecognized urinary tract infections, and longer operative times.14,32,33 However, some authors have proposed simultaneous repair of MMC and shunt insertion.30,34,35 Several studies have not found worse outcomes when comparing concurrent surgical treatment to sequential.20,23,24 In our data set, most of the patients underwent sequential treatment approaches for shunt insertion. Patients with higher neurological levels more frequently needed shunts, which is a finding already recorded in other studies.21,26,28,31,36 Our data show that shunt placement in MMC carried a high risk of revision. We confirmed that most shunt revisions occur during the first 6 months to 1 year of life. However, shunt failure can occur at any age during the growth process. As in the studies by Pollack et al.37 and Teo et al.,38 some of the patients in our cohort manifested recurrent symptoms or signs of brain stem dysfunction with the need for decompression. Although more typically associated with SB occulta, tethered cord syndrome has also been described in children and adolescents as a late complication following surgical repair of MMC.39,40 In our study, the mean age of untethering was 7 years old, in agreement with the cohort of Talamonti et al.14 Some authors have theorized that patients who develop symptoms of tethered cord most likely present during periods of growth, as the spinal canal lengthens.41,42 Most of our patients were submitted to tethered cord release during periods of growth. However, as observed in other studies,43 secondary tethering of the spinal cord can occur up to 15 years of age. It is now recognized that tethered cord syndrome recurs,12,41,44,45 which was observed in our study. The need for a neurosurgical procedure over time emphasizes the importance of close follow-up over life, involving neurological, orthopedic, and urological examinations, even in late youth, to achieve better functional and surveillance outcomes.46
The incidence of pathological lumbar kyphosis approaches 20%,47 and it is more common in patients with a thoracic level of neurological function.48 Scoliosis with MMC is a common condition, with a prevalence reported by Trivedi et al.49 of 52%. Scoliosis in patients with MMC can be present in patients younger than 6 years old,50 and new curves can continue to develop until the age of 15.49 In our survey, the distribution of spine surgery across neurological levels was not equal, and a higher proportion of patients with a thoracic level of involvement were submitted to an intervention on the spine compared to those with lower neurological levels; thus, a higher level of neurological involvement is a good predictor of greater need for spinal surgery over time. Wright, who performed an evidence-based review from 1950 to 2009 regarding the benefits of spine surgery, concluded that the benefits of spine surgery were uncertain with Grade I, based on inconsistent or insufficient evidence and providing no guidance.51 At this time, simple interventions, such as chair modifications, should be investigated as possible means for shifting the trunk to improve coronal balance and sitting function before surgical intervention.52
Hip dislocation or subluxation is a frequent condition in patients with SB, and it can affect up to 36% of children, depending on neurological level.53 However, some controversy exists over the criteria for the selection of patients with MMC for surgical interventions on the hip. According to Gabrieli et al., who analyzed the influence of unilateral hip instability on gait pattern in patients with low lumbar MMC, the absence of hip contracture accounted for gait symmetry and had no correlation with hip dislocation.54 In contrast, when hip contracture is significant, there is some degree of gait asymmetry. In this context, authors have argued that reduction of a dislocated hip is not necessary with a low lumbar level of neurological involvement.54 However, correction of contractures should be the treatment of choice to restore gait symmetry in these patients.54–58 Swaroop and Dias argue that for sacral patients who can walk without support, hip dislocation could cause an asymmetric gait, and because gluteus lurch is quite significant with a loss of fulcrum, they suggest that in such cases, surgical relocation of hip could be considered.3,57,59 However, this topic has not been addressed currently by any other studies.3,59 According to Wright, surgical treatment in children with a neurological level below L4 and with unilateral hip dislocation suffering a decline in walking ability might benefit from surgical treatment. However, whether surgery improves function in these patients cannot be determined from the literature.51 Other authors have debated whether patients with MMC should even be considered for extensive surgery to reduce hip dislocation.58–60 Wright concluded that the literature provided no support for the surgical treatment of children with dislocations and with lesions above L4 who have a low probability of walking in the community beyond adolescence.51 However, these children require a functional range of motion of the hip without contracture61 such that the patients can sit satisfactorily in a wheelchair, and use an orthosis for standing and walking.2 In our center, some patients with walking potential, after being submitted to hip interventions, had their gait deteriorate. Thus, orthopedic surgeries could potentially cause decreases in muscle strength. There have been patients with upper and lower lumbar levels of neurological involvement submitted to hip reduction; thus, some of these patients might have not been an ideal group of patients to submit to surgical procedures. Because our data show the evolution of surgical practice in our unit over nearly two decades, some of the criteria and methods employed previously might not be acceptable today, according to the highest quality evidence derived from clinical research.
In a patient with the potential for ambulation, the goal of the treatment of a foot deformity in SB is a plantigrade, supple, and braceable foot with maximally preserved range of motion. Even in non-ambulatory patients, treatment might be necessary if the foot deformity prevents the wearing of a shoe or positioning in a wheelchair.62 These functional aspects could explain the distribution of surgeries on feet in our cohort across all spinal levels. Because the development of foot deformities in patients with lower neurological levels can threaten ambulatory ability in some patients,7 the persistence of a similar absolute frequency of these surgeries at those spinal levels before and after 9 years of age might have been the results of attempts to conserve gait.
In our cohort of patients, there was a relationship between pathological fractures and orthopedic surgeries, which might have been due to postoperative orthopedic inactivity.10,63 In addition, there was a routine of performing several surgical interventions during the same surgical period, which might have decreased total postoperative inactivity and consequently lowered the risk of fractures.
In a study presented many decades ago, Hoffer et al.11 observed that all patients who achieved functional ambulation did so prior to the age of 9 years. Williams et al. reported the ceasing of ambulation of children with MMC and high-level paralysis between 6 and 9 years of age,64 much earlier than previously reported.65 Hoffer et al. observed that none of their patients with thoracic levels of involvement had any sort of long-term functional ambulation despite vigorous therapy. Some patients achieved non-functional ambulation but only after long periods of rehabilitation in the hospital.11 It is usually impractical to continue non-functional ambulation after the age of 9 years.11 These concepts could explain, in part, the prevalence in our cohort of orthopedic surgeries on the hip and foot in thoracic and upper levels of neurological involvement in patients younger than 9 and the progressive decrease in those surgeries after 9 years of age, at which point the surgeon would have faced an irreversible loss of ambulatory ability and have focused his or her attention only on patients with lower neurological levels. One important issue is whether non-functional ambulation should be maintained or incentivized, as well as which surgeries in each clinical picture positively influence ambulation.
In our cohort, synchronous bladder reconstruction and an ACE procedure were performed in 1 patient; the literature suggests that this combined technique is effective.66 There have been doubts about the risk of renal transplantation when the patient has an abnormal lower urinary tract. According to a review by Müller, although patients with MMC are more prone to complications, renal transplantation for end-stage renal disease is the optimal treatment option in all age groups67; however, this topic remains understudied.
Pressure sores are an important issue in any population with sensory deficits, and patients with MMC can have major skin problems.2,68
Limitations
Some methodological concerns with the design of this study should be raised. Its retrospective nature caused difficulties in data interpretation. Surgical interventions could have been underreported. Following shunt infections, we decided to include in our count of shunt revisions not only the definitive shunt procedures but also temporary external ventricular drainage, which might have overestimated the number of shunt revisions and limited comparison with other studies. The reason for this decision was related to our knowledge of the date of a shunt procedure for some patients but the lack of information about the cause or the surgical technique used. Sometimes, we had references to the occurrence of surgery on the hip, but it was not possible to determine whether a reduction of the hip and/or a release of hip contracture was performed. Regarding tethered cord release, we considered in our count both symptomatic and prophylactic untethering for scoliotic fusion. Because some of the surgeries were performed in other institutions, and because we did not have universal access to descriptions of the techniques for spinal surgery, we might have lost some procedures of prophylactic untethering. The sample size of our study was small, making it difficult to identify minor subgroup differences regarding how neurological motor level influenced surgical intervention profile. Another relevant issue is the use for each patient of the latest neurological level in medical evaluation, not considering the already known dynamic properties of neurological lesion level over time.69,70 Moreover, clinical grading of muscle strength and assignment of neurosegmental level are subjective in nature,71 and the reliability and validity of any classification system should be established.72 Review of the literature is difficult because patients have been grouped according to different systems among the studies to classify the neurological level.72–75 Research into MMC has been based mainly on small convenience samples, with no comparison groups. With few exceptions, the available studies on surgical interventions have evaluated the technical outcomes of surgery and have not undertaken postoperative evaluation of QOL.76 Surgeries in children with SB do not have widely established research-based guidelines, and the decision to operate results from the experience of the surgeon, from subjective assessment of function, from the medical literature available for each period and, recently in the orthopedic system, from quantitative methods, such as gait analysis,3 that are unavailable at our center, which could account for some of the variability in the criteria for surgery at different centers. Thus, the role of routine surgery for patients with SB requires a re-evaluation. This study should be regarded as an initial exploration of the possible evolution of each patient, according to the neurological level of motor involvement, and it should not be used as a guide, because the course of disease from individual to individual is globally unpredictable. Rather, the results should be viewed as the attitudes toward surgical practice in Portugal over the last two decades, and the indications for surgery might have changed over time due to new medical evidence. Moreover, the decision to operate beyond the neurological level of involvement depends on other variables, such as the achievement of a better functional outcome.
Despite these limitations, this study has some strengths: the clinical examination of each patient was undertaken regularly by the same two consultant physicians, thereby avoiding interobserver variability in the clinical examinations. The benefits of our study include a potential positive impact on the management of these injuries, which could be valuable if the study is seen as the experience of a center that treats these patients.
Conclusion
This study provides insight about the complexity of this condition with multiple surgeries among patients with MMC with morbidity throughout late youth. The lack of definitive evidence has not helped to resolve some of the controversial issues regarding the use of surgical interventions in this group of patients and could cause some asymmetry among different centers with different methodologies of treatment.
Comment
Figures 1 to 4 show the distribution of surgical interventions over time. Of particular note is the intentional omission of interval 0–1 in Figures 1 and 3 due to the significantly higher frequency of surgical interventions in these intervals, which perturbs the analysis of differences in later intervals. In Figure 2, due to the agglomeration of MMC closures during the first year of life, with the representation of an elevated number of surgeries that could have disturbed the figure analysis, we decided to remove this item. Due to the small number of surgeries performed, we removed the fractures item from Figure 4.
Acknowledgment
We gratefully acknowledge the contributions of the statistician Dr Rui Deus in the statistical analysis.
Disclaimer statements
Contributors All authors contributed to this work.
Conflicts of interest None.
Ethics approval After obtaining the Ethics Committee approval from our hospital, written informed consent was obtained from all the patients, with parents accepting participation on behalf of their children.
Funding None.
References
- 1.Pruitt LJ. Living with spina bífida: a historical perspective. Pediatr 2012;130(2):181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbar M, Bresch B, Seyler TM, Wenz W, Bruckner T, Abel R, et al. . Management of orthopaedic sequelae of congenital spinal disorders. J Bone Joint Surg Am 2009;91(Suppl. 6):87–100. [DOI] [PubMed] [Google Scholar]
- 3.Swaroop VT, Dias L. Orthopedic management of spina bifida. Part I: hip, knee, and rotational deformities. J Child Orthop 2009;3(6):441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton DB, Brock JW, Joseph DB. Urologic management of spina bifida. Dev Disabil Res Rev 2010;16(1):88–95. [DOI] [PubMed] [Google Scholar]
- 5.Thomson JD, Segal LS. Orthopedic management of spina bifida. Dev Disabil Res Rev 2010;16(1):96–103. [DOI] [PubMed] [Google Scholar]
- 6.Medical Research Council Scale. Aids to examination of the peripheral nervous system. Memorandum no. 45 London: Her Majesty's Stationery Office; 1976. [Google Scholar]
- 7.Asher M, Olson J. Factors affecting the ambulatory status of patients with spina bifida cystica. J Bone Joint Surg Am 1983;65(3):350–6. [PubMed] [Google Scholar]
- 8.Seitzberg A, Lind M, Biering-Sørensen F. Ambulation in adults with myelomeningocele. Is it possible to predict the level of ambulation in early life? Childs Nerv Syst 2008;24(2):231–7. [DOI] [PubMed] [Google Scholar]
- 9.Danzer E, Gerdes M, Bebbington MW, Sutton LN, Melchionni J, Adzick NS, et al. . Lower extremity neuromotor function and short-term ambulatory potential following in utero myelomeningocele surgery. Fetal Diagn Ther 2009;25(1):47–53. [DOI] [PubMed] [Google Scholar]
- 10.Marreiros H, Monteiro L, Loff C, Calado E. Fractures in children and adolescents with Spina Bifida – experience of a Portuguese tertiary care hospital. Dev Med Child Neurol 2010;52(8):754–9. [DOI] [PubMed] [Google Scholar]
- 11.Hoffer M, Feiwell E, Perry J, Bonwitt C. Functional ambulation in patients with myelomeningocele. J Bone Joint Surg Am 1973;55(1):137–48. [PubMed] [Google Scholar]
- 12.Swank M, Dias L. Myelomeningocele: a review of the orthopaedic aspects of 206 patients treated from birth with no selection criteria. Dev Med Child Neurol 1992;34(12):1047–52. [DOI] [PubMed] [Google Scholar]
- 13.Harris MB, Banta JV. Cost of skin care in the myelomeningocele population. J Pediatr Orthop 1990;10(3):355–61. [DOI] [PubMed] [Google Scholar]
- 14.Talamonti G, D'Aliberti G, Colice M. Myelomeningocele: long-term neurosurgical treatment and follow-up in 202 patients. J Neurosurg 2007;107(5 Suppl.):368–86. [DOI] [PubMed] [Google Scholar]
- 15.Mirzai H, Ersahin Y, Mutluer S, Kayahan A. Outcome of patients with myelomeningocele: the Ege University experience. Childs Nerv Syst 1998;14(3):120–3. [DOI] [PubMed] [Google Scholar]
- 16.Bowman RM, Mclone DG. Neurosurgical management of spina bifida: research issues. Dev Disabil Res Rev 2010;16(1):82–7. [DOI] [PubMed] [Google Scholar]
- 17.McLone DG. Care of the neonate with a myelomeningocele. Neurosurg Clin N Am 1998;9(1):111. [PubMed] [Google Scholar]
- 18.Tarcan T, Onol FF, Ilker Y. The timing of primary neurosurgical repair significantly affects neurogenic bladder prognosis in children with myelomeningocele. J Urol 2006;176(3):1161–5. [DOI] [PubMed] [Google Scholar]
- 19.Watson JC, Tye G, Ward JD. Delayed repair of myelomingoceles. World Neurosurg 2014;81(2):428–30. [DOI] [PubMed] [Google Scholar]
- 20.Caldarelli M, Di Rocco C, La Marca F. Shunt complications in the first postoperative year in children with myelomeningocele. Childs Nerv Syst 1996;12(2):748–56. [DOI] [PubMed] [Google Scholar]
- 21.Chakraborty A, Crimmins D, Hayward R, Thompson D. Toward reducing shunt placement rates in patients with myelomeningocele. J Neurisurg Pediatrics 2008;1(5):361–5. [DOI] [PubMed] [Google Scholar]
- 22.Bowman RM, Mclone DG, Grant JA, Tomita T. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg 2001;34(3):114–20. [DOI] [PubMed] [Google Scholar]
- 23.Miller P, Pollack I, Pang D, Albright L. Comparison of simultaneous versus delayed ventriculoperitoneal shunt insertion in children undergoing myelomeningocele repair. J Child Neurol 1996;11(5):370–2. [DOI] [PubMed] [Google Scholar]
- 24.Parent AD, McMillan T. Contemporaneous shunting with repair of myelomeningocele. Pediatr Neurosurg 1995;22(3):132–6. [DOI] [PubMed] [Google Scholar]
- 25.Bruner JP, Tulipan N, Paschall RL, Boehm FH, Walsh WF, Silva SR, et al. . Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA 1999;282(19):1873–4. [DOI] [PubMed] [Google Scholar]
- 26.Johnson MP, Sutton LN, Rintoul N, Crombleholme TM, Flake AW, Howel LJ, et al. . Fetal myelomeningocele repair: short term clinical outcomes. Am J Obstet Gynecol 2003;189(2):482–7. [DOI] [PubMed] [Google Scholar]
- 27.Steinbok P, Irvine B, Cochrane DD, Irwin BJ. Long-term outcome and complications of children born with meningomyelocele. Childs Nerv Sys 1992;8(2):92–6. [DOI] [PubMed] [Google Scholar]
- 28.Tulipan N, Sutton LN, Bruner JP, Cohen BM, Johnson M, Adzick NS. The effect of intrauterine myelomeningocele repair on the incidence of shunt-dependent hydrocephalus. Pediatr Neurosurg 2003;38(1):27–33. [DOI] [PubMed] [Google Scholar]
- 29.Hunt GM, Oakeshott P, Kerry S. Link between the CSF shunt and achievement in adults with spina bifida. J Neurol Neurosurg Psychiatry 1999;67(5):591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chadduck WM, Reeding DL. Experience with simultaneous ventriculo-peritonal shunt placement and myelomeningocele repair. J Pediatr Surg 1988;23(10):913–16. [DOI] [PubMed] [Google Scholar]
- 31.Rintoul NE, Sutton LN, Hubbard AM, Cohen B, Melchionni J, Pasquariello PS, et al. . A new look at myelomeningoceles: functional level, vertebral level, shunting, and the implications for fetal intervention. Pediatrics 2002;109(3):409–13. [DOI] [PubMed] [Google Scholar]
- 32.Tamburrini G, Frassanito P, Iakovaki K, Pignotti F, Rendeli C, Murolo D, et al. . Myelomeningocele: the management of the associated hydrocephalus. Childs Nerv Syst 2013;29(9):1569–79. [DOI] [PubMed] [Google Scholar]
- 33. Oktem IS, Menku A, Ozdemir A. When should ventriculoperitoneal shunt placement be performed in cases with myelomeningocele and hydrocephalus? Turk Neurosurg 2008;18(4);387–91. [PubMed] [Google Scholar]
- 34.Machado HR, Santos de Oliveira R. Simultaneous repair of myelomeningocele and shunt insertion. Childs Nerv Syst 2004;20(2):107–9. [DOI] [PubMed] [Google Scholar]
- 35.Epstein N, Rosenthal E, Zito AD, Osipoff MJ. Shunt placement and myelomeningocele repair: simultaneous vs sequential shunting. Review of 12 cases. Child Nerv Syst 1985;1(3):145–7. [DOI] [PubMed] [Google Scholar]
- 36.Iborra J, Pagès E, Cuxart A. Neurological abnormalities, major orthopaedic deformities and ambulation analysis in a myelomeningocele population in Catalonia (Spain). Spinal Cord 1999;37(5):351–7. [DOI] [PubMed] [Google Scholar]
- 37.Pollack IF, Kinnunen D, Albright AL. The effect of early craniocervical decompression on functional outcome in neonates and young infants with myelodysplasia and symptomatic Chiari II malformations: results from a prospective series. Neurosurgery 1996;38(4):703–10. [PubMed] [Google Scholar]
- 38.Teo C, Parker EC, Aureli S, Boop FA. The Chiari II malformations: a surgical series. Pediatr Neurosurg 1997;27(5):223–9. [DOI] [PubMed] [Google Scholar]
- 39.Caldarelli M, Di Rocco C, Colosimo C Jr, Fariello G, Di Gennaro M. Surgical treatment of late neurological deterioration in children with myelodysplasia. Acta Neurochir 1995;137(3–4):199–206. [DOI] [PubMed] [Google Scholar]
- 40.Just M, Schwarz M, Emert JA, Higer HP, Voth D, Pfannenstiel P. Magnetic ressonance imaging of dysraphic myelodysplasia. Findings in 56 children and adolescents with post-repair meningomyelocele. Childs Nerv Syst 1987;4(3):35–9. [DOI] [PubMed] [Google Scholar]
- 41.Al-Holou WN, Muraszko KM, Garton HJ, Buchman SR, Maher CO. The outcome of tethered cord release in secondary and multiple repeat tethered cord syndrome. J Neurosurg Pediatrics 2009;4(1):28–36. [DOI] [PubMed] [Google Scholar]
- 42.Dias MS. Neurosurgical management of myelomeningocele (Spina Bifida). Pediatr Rev 2005;26(2):50–9. [DOI] [PubMed] [Google Scholar]
- 43.Tarcan T, Onol FF, Ilker Y, Simsek F, Ozek M. Does surgical release of secondary spinal cord tethering improve the prognosis of neurogenic bladder in children with myelomeningocele? J Urol 2006;176(4 Pt 1):1601–6. [DOI] [PubMed] [Google Scholar]
- 44.Maher CO, Goumnerova L, Madsen JR, Proctor M, Scott RM. Outcome following multiple repeated spinal cord untethering operations. J Neurosurg 2007;106(6 Suppl.):434–8. [DOI] [PubMed] [Google Scholar]
- 45.Reigel DH, Tchernoukha K, Bazmi B, Kortyna R, Rotenstein D. Change in spinal curvature following release of tethered spinal cord associated with spina bifida. Pediatr Neurosurg 1994;20(1):30–42. [DOI] [PubMed] [Google Scholar]
- 46.Hudgins RJ, Gilreath CL. Tethered spinal cord following repair of myelomeningocele. Neurosurg Focus 2004;16(2):E7. [DOI] [PubMed] [Google Scholar]
- 47.Carstens C, Koch H, Brocal DR, Niethard FU. Development of pathological lumbar kyphosis in myelomeningocele. J Bone Joint Surg Br 1996;78(6):945–50. [DOI] [PubMed] [Google Scholar]
- 48.Glard Y, Launay F, Viehweger E, Hamel A, Jouve J, Bollini G. Neurological classification in myelomeningocele as a spine deformity predictor. J Pediatr Orthop B 2007;16(4):287–92. [DOI] [PubMed] [Google Scholar]
- 49.Trivedi J, Thomson JD, Slakey CJB, Banta JV, Jones PW. Clinical and radiographic predictors of scoliosis in patients with myelomeningocele. J Bone Joint Surg Am 2002;84-A(8):1389–94. [DOI] [PubMed] [Google Scholar]
- 50.Samuelsson L, Eklöf O. Scoliosis in myelomeningocele. Acta Orthop Surg 1988;59:122–7. [PubMed] [Google Scholar]
- 51.Wright JG. Hip and spine surgery is of questionable value in spina bifida. An evidence-based review. Clin Orthop Relat Res 2011;469(5):1258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mercado E, Alman B, Wright JG. Does spinal fusion influence quality of life in neuromuscular scoliosis? Spine (Phila Pa 1976) 2007;32(19 Suppl.):S120–5. [DOI] [PubMed] [Google Scholar]
- 53.Broughton NS, Menelaus MB, Cole WG, Shurtleff DB. The natural history of hip deformity in myelomenigocele. J Bone Joint Surg Br 1993;75(5):760–3. [DOI] [PubMed] [Google Scholar]
- 54.Gabrieli AP, Vankoski MS, Dias LS. Gait analysis in low lumbar myeloeningocele patients with unilateral hip dislocation or subluxation. J Pediatr Othop 2003;23(3):330–6. [PubMed] [Google Scholar]
- 55.Feiwell E. Surgery of the hip in myelomeningocele as related to adult goals. Clin Orthop 1980;(148):87–93. [PubMed] [Google Scholar]
- 56.Sherk HH, Uppal GS, Lane G, Melchionni J. Treatment versus non-treatment of hip dislocations in ambulatory patients with myelomeningocele. Dev Med Child Neurol 1991;33(6):491–4. [DOI] [PubMed] [Google Scholar]
- 57.Dias L. Orthopaedic care in spina bifida: past, present, and future. Dev Med Child Neurol 2004;46(9):579. [DOI] [PubMed] [Google Scholar]
- 58.Correl J, Gabler C. The effect of soft tissue release of the hips on walking in myelomeningocele. J Pediatr Orthop B 2000;9(3):148–53. [DOI] [PubMed] [Google Scholar]
- 59.Dias L. Hip dislocation in spina bifida: when is surgery required and what type of surgery required and what type of surgery should be performed? Ortop Traumatol Rehabil 2011;13(2):101–3. [PubMed] [Google Scholar]
- 60.Feiwell E, Sakai D, Blatt T. The effect of hip reduction on function in patients with myelomeningocele. Potential gains and hazards of surgical treatment. J Bone Joint Surg Am 1978;60(2):169–73. [PubMed] [Google Scholar]
- 61.Menelaus MB. Progress in the management of the paralytic hip in myelomeningocele. Orthop Clin North Am 1980;11(1):17–30. [PubMed] [Google Scholar]
- 62.Swaroop VT, Luciano D. Orthopaedic management of spina bifida – part II: foot and ankle deformities. J Child Orthop 2011;5(6):403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marreiros H, Loff C, Calado E. Osteoporosis in paediatric patients with spina bifida. J Spinal Cord Med 2012;35(1):9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams EN, Broughton NS, Menelaus MB. Age-related walking in children with spina bifida. Dev Med Child Neurol 1991;41(7):446–9. [PubMed] [Google Scholar]
- 65.McDonald CM, Shurtleff DB, Menelaus MB. Modifications to the traditional description of neurosegmental innervation in myelomeningocele. Dev Med Child Neurol 1991;33(6):473–81. [DOI] [PubMed] [Google Scholar]
- 66.Roberts JP, Moon S, Malone PS. Treatment of neuropathic urinary and faecal incontinence with synchronous bladder reconstruction and the antegrade continence enema procedure. Br J Urol 1995;75(3):386–9. [DOI] [PubMed] [Google Scholar]
- 67.Müller T, Arbeiter K, Aufricht C. Renal function in meningomyelocele: risk factors, chronic renal failure, renal replacement therapy and transplantation. Curr Opin Urol 2002;12(6):479–84. [DOI] [PubMed] [Google Scholar]
- 68.Okamoto GA, Lamers JV, Shurtleff DB. Skin breakdown in patients with myelomeningocele in patients with myelomeningocele. Arch Phys Med Rehabil 1983;64(1):20–3. [PubMed] [Google Scholar]
- 69.Spindel MR, Bauer SB, Dyro FM, Krarup C, Khoshbin S, Winston KR, et al. . The changing neurourologic lesion in myelodysplasia. JAMA 1987;258(12):1630–3. [PubMed] [Google Scholar]
- 70.Begeer JH, Meihuizen de Regt MJ, HogenEsch I, Ter Weeme CA, Mooij JJ, Vencken LM. Progressive neurological deficit in children with spina bifida aperta. Z Kinderchir 1986;41(Suppl. 1):13–5. [DOI] [PubMed] [Google Scholar]
- 71.McDonald CM, Jaffe KM, Shurtleff DB. Assessment of muscle strength in children with myelomeningocele: accuracy and stability of measurement over time. Arch Phys Med Rehabil 1986;67(12):855–61. [PubMed] [Google Scholar]
- 72.Park KB, Park HW, Joo SY, Kim HW. Surgical treatment of calcaneal deformity in a select group of patients with myelomeningocele. J Bone Joint Surg Am 2008;90(10):2149–59. [DOI] [PubMed] [Google Scholar]
- 73.Bartonek A, Saraste H. Factors influencing ambulation in myelomeningocele: a cross-sectional study. Dev Med Child Neurol 2001;43(4):253–60. [DOI] [PubMed] [Google Scholar]
- 74.Noonan KJ. Myelomeningocele: calcaneal deformity. In: Morrissy RT, Weinstein SL, (eds.) Lovell and Winter's pediatric orthopaedics. 6th ed Philadelphia: Lippincott Williams and Wilkins; 2006. vol. 1, p. 631. [Google Scholar]
- 75.Bartonek A, Saraste H, Knutson LM. Comparison of different systems to classify the neurological level of lesion in patients with myelomeningocele. Dev Med Child Neurol 1999;41(12):796–805. [DOI] [PubMed] [Google Scholar]
- 76.Schoenmakers MA, Gulmans VA, Gooskens RH, Pruijs JE, Helders PJ. Spinal fusion in children with spina bifida: influence on ambulance level and function abilities. Eur Spine J 2005;14(4):415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]