Abstract
Detailed family history is a critical element of cancer risk assessment. The relative importance of pancreatic cancer (PC) in a close family member, particularly in hereditary breast-ovarian syndrome (HBOS), is not clearly defined. We use a case-control design to investigate the importance of a family history of PC to cancer risk assessment. Case and control families were identified from the University of Chicago Cancer Risk database (1994–2005). Pedigrees were analyzed for personal and familial clinical cancer data. Cases included all new subjects (probands) reporting a close relative (first or second degree) with PC. Controls included the probands enrolled in the database immediately prior to and subsequent to each case (i.e. two controls for each case). From 1,231 pedigrees, 103 PC were reported by the proband in 87 unique families. Many probands reported multiple or early-onset PCs: one third (28/87) of case families met criteria for a familial PC syndrome [≥2 first-degree relatives with PC (n=10) or PC diagnosed ≤50 (n=18)]. Of these families, the majority (75%) concurrently met criteria suggestive of hereditary breast-ovarian syndrome (HBOS). Because of a family history consistent with HBOS, at least one individual from each of 29 case and 55 control families underwent genetic testing for BRCA1/2. Among case families, 19 of 29 (66%) had a BRCA1/2 mutation compared with 16 of 55 (29%) controls. A significant association between family history of PC and a BRCA1/2 mutation was seen (OR 3.78, 1.32–10.9). This point estimate was strengthened but less precise in the non-Ashkenazi Jewish subset of tested families (OR 6.03, 1.68–22.14). In a high-risk population, a family history of PC, though infrequently reported, is nonetheless clinically meaningful. In risk assessment for HBOS, identifying a family history of PC should strongly raise the suspicion of an unrecognized BRCA1/2 mutation.
Keywords: Risk assessment, Pancreatic cancer, HBOS, BRCA1/2
Introduction
The querying of family history is a critical element of cancer risk assessment. Through pedigree analysis, the ages, genders, and relationships of family members may be taken into account when gauging risk (Palomares et al. 2005). Consequently, limited family information or small family size may lead to underestimation of heritable cancer risks (Weitzel et al. 2007).
Breast cancer is the second most commonly diagnosed malignancy in the USA, annually affecting nearly 200,000 American women, many of whom will enjoy long-term survival. Pancreatic cancer (PC), in contradistinction, is rare: 37,170 Americans were diagnosed with PC last year, and few will survive more than one year (Jemal et al. 2007). Women referred for hereditary breast-ovarian syndrome (HBOS) and BRCA1/2 testing comprise the predominant population in high-risk cancer clinics (Epplein et al. 2005; Hopwood et al. 2003). While the compilation of family history of breast and ovarian cancer is crucial in an HBOS evaluation, the importance of a family history of PC to cancer risk assessment is less clear. Pancreatic cancer is a manifestation of a number of cancer syndromes, including HBOS (anonymous, 1999; Jagelman et al. 1988; Lynch et al. 2001; Thompson et al. 2002). For known carriers of BRCA1/2 mutations, the relative risk of PC is elevated [BRCA1: RR 2.26 (1.26–4.06); BRCA2: RR 3.51 (1.87–6.58)] though absolute risks are small (anonymous, 1999; Thompson et al. 2002). Several tools designed to quantify an individual’s risk of carrying a BRCA1/2 mutation incorporate a family history of PC, though BRCAPRO, one of the best-known and most widely used, does not (Berry et al. 2002; Couch et al. 1997; Evans et al. 2004).
Using a case-control design, we investigate the importance of a family history of PC to cancer risk assessment. Specifically, our interest is to quantify the relative significance of a close family member with PC to the risk assessment of a proband presenting to a high-risk cancer clinic for evaluation. In addition to describing our clinical experience, we explore the association of a family history of PC with other known risk criteria.
Materials and Methods
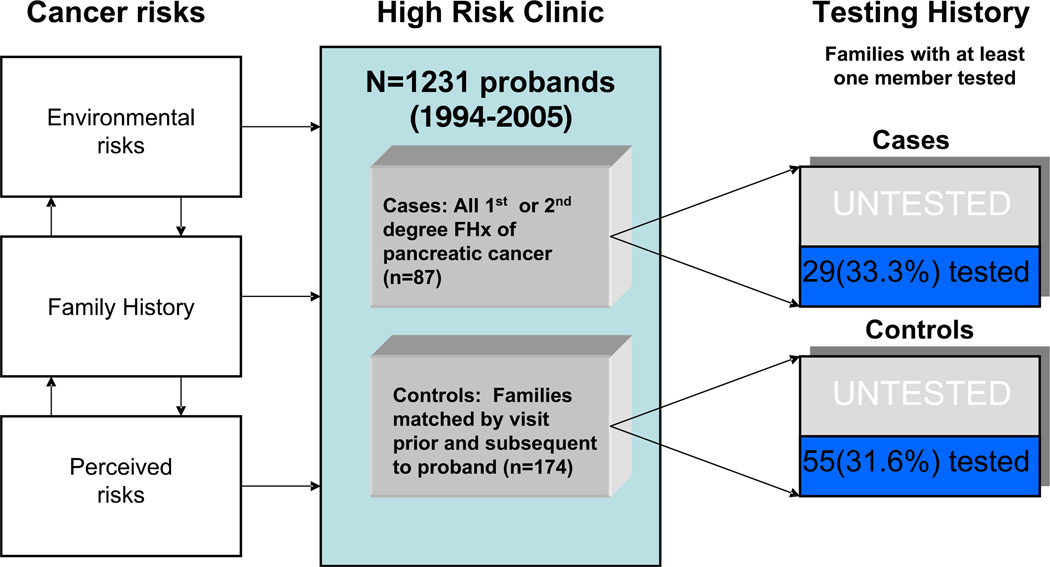
The study design is outlined in Fig. 1. Cases and controls were identified from a database of high-risk families maintained by the University of Chicago Cancer Risk Clinic. Case families included all families where the proband reported a close relative (first- or second-degree) with PC. Those families with PC in a relative more distant than the second-degree were excluded from this analysis (n=3), as were those families in whom the proband presented with a personal history of PC but no other family history of PC (n=2). Control families (two for each case) were identified as the prior and subsequent new patients enrolled in the database at the time of the initial case enrollment. In the event that an adjacent family was already identified as a separate case or control, the next consecutive family was chosen until a unique control was found.
Fig. 1.
Study Design.
Pedigrees were analyzed for all cancers, ethnicity (self-reported), familial relationship of cancers, family member ages, ages of cancer diagnoses, and known personal or familial mutations in cancer predisposition genes. Pancreatic cancer and other malignancy history were confirmed by medical record and/or death certificate when available (44% confirmed). Pedigrees meeting HBOS criteria had a BRCAPRO calculation performed for the proband (Cancer-Gene, version 3.3.1, 1998–2001).
Based on provider recommendation and patient preference, genetic testing was performed on a subset of families in the study cohort. The presence of a familial mutation in a cancer predisposition gene (APC, BRCA1/2, MLH1/MSH2/MSH6) was determined by clinical genetic testing in a CLIA-approved laboratory, or by proband report and medical record confirmation if the family member was not tested through the University of Chicago. Genetic testing results were ascertained retrospectively and were not influenced by the objectives of the current study. The decision to offer genetic testing is complex and is determined on an individual patient basis, but relies strongly on patient interest, risk modeling (i.e. BRCAPRO model risk calculation), provider judgment, and expert guidelines [i.e. Cancer Genetics Studies Consortium (Burke et al. 1997); US Preventive Services Task Force guidelines (Nelson et al. 2005)].
HBOS is defined here as families with (1) one first-degree relative (FDR) <30 years of age ± a second-degree relative (SDR) of any age with breast cancer, (2) two FDR if one <50 years of age or both are <60 years of age with breast cancer, (3) one FDR and one SDR, one with bilateral breast cancer, (4) two SDR with breast cancer, both maternal or paternal, with a sum of ages <80 years of age, (5) one FDR with breast cancer and one FDR with ovarian cancer <70 years of age, (6) two FDR with ovarian cancer, or with a known BRCA1 or BRCA2 mutation (White and Olopade 2000). Familial colorectal cancer (CRC) families were defined as those fulfilling the revised Bethesda guidelines [persons meeting the Amsterdam criteria (CRC <50 years, CRC in at least two generations, histologically verified CRC in three or more relatives, one of whom is a FDR or the other), persons with HNPCC/Lynch syndrome tumors (endometrial, ovarian, gastric, hepatobiliary, small bowel, transitional cell carcinoma of the renal pelvis), persons with CRC and a FDR with CRC and/or a HNPCC/Lynch syndrome tumor or colonic adenoma (one cancer must be <45 years, adenoma must be <40 years), persons with CRC or endometrial cancer <45 years, persons with a right-sided undifferentiated or any signet-ring cell type CRC<45 years, persons with colorectal adenomas <40 years] or those with a known familial mismatch repair or APC gene mutation (Umar et al. 2004; Vasen et al. 1999). Familial PC families were defined as those containing at least two FDRs or at least one early-onset PC (<50 years; Rulyak and Brentnall 2001).
Mean and median values for all continuous variables were calculated, and mean values compared by t test (two-sided) where appropriate. Associations between binary variables were tested using the χ2 test. Univariate analyses of categorical variables were performed by logistic regression analysis. A χ2 test of homogeneity of odds ratios across categories (homogeneity) as well as a test of the linear trend of the log odds across categories (trend of odds) is also reported for associations between binary and categorical variables. The significance of all tests of association is reported at a 95% confidence level (α=0.05). All statistical analyses were performed using STATA 9SE (San Mateo, CA).
Results
Population Characteristics
From a database of 1,231 families presenting to the University of Chicago High-Risk Clinic over an 11-year period (1994–2005), we identified 103 PC within 87 families containing 1,763 family members in total (see Table 1). The majority of case families (73/87, 84%) had only one relative with PC, while 12 families contained two PC and two families contained three PC. The mean age of PC diagnosis was 60.7 years (range 32–90), and was not significantly different among families containing one, two, or three PC. More PC was reported in maternal aunts, cousins and grandparents (51%) than paternal (39%). PC among both maternal and paternal relatives (5%) or in siblings (5%) was rare.
Table 1.
Pancreatic Cancers (n=103)
| Case families | n (%) | p |
|---|---|---|
| Total pancreatic cancers | 103 | |
| Total families | 87 (100) | |
| Number of pancreatic cancers per family | ||
| One | 73 (84) | |
| Two | 12 (14) | |
| Three | 2 (2) | |
| Families meeting FPC criteriaa | 28 (32) | |
| FPC also meeting HBOS criteria | 21 (75) | |
| FPC also meeting FAP/HNPCC-Lynch criteria | 6 (21) | |
| FPC also meeting HBOS and FAP/HNPCC-Lynch | 2 (7) | |
| Relationship of pancreatic cancers to proband | ||
| Paternal lineage | 34 (39) | 0.26 |
| Maternal lineage | 45 (51) | |
| Both maternal and paternal lineage | 4 (5) | |
| Sibling or descendent | 4 (5) | |
| Age at diagnosis of pancreatic cancer | mean (years) | |
| All case pancreatic cancers | 62.8 | |
| Families with one pancreatic cancer (n=72) | 63.1 | 0.62 |
| Families with two pancreatic cancers (n=11) | 60.6 | |
| Families with three pancreatic cancers (n=2) | 63.0 |
Familial pancreatic cancer (FPC) criteria includes two or more first-degree relatives with pancreatic cancer or one individual with pancreatic cancer diagnosed under the age of 50.
Pedigree characteristics were well-balanced between cases and controls (Table 2). Families were similar in proband age, proband cancer history, pedigree size (all first and second degree relatives), ethnicity, and cancer syndrome criteria fulfilled. Mean pedigree size was 22 persons/ family (22.0 cases, 22.5 controls, p=0.68). Nearly one third (28/87, 32%) of case families met criteria consistent with a familial pancreatic cancer (FPC) syndrome because of two or more FDRs with PC (n=10) or a diagnosis of PC in an individual ≤50 years (n=18). Twenty-one of these families concurrently met criteria for HBOS (21/28, 75%), and six (21%) met criteria for an FAP and/or Lynch syndrome. Two families (7%) fulfilled criteria for all three syndromes (FPC, HBOS, and HNPCC/Lynch syndrome).
Table 2.
Characteristics of Case and Control Families (n=261)
| Cases | Controls | p | |
|---|---|---|---|
| Probands and families | |||
| Proband | n=87 | n=174 | |
| Age (yrs) | 47.1 (26–77) | 44.4 (19–81) | |
| Female, n (%) | 80 (92.0) | 156 (90.0) | |
| Age at cancer diagnosis (years)a | 42.9 (21–77) | 43.1 (14–75) | |
| Family size, n | 22.0 (8–48) | 22.5 (6–54) | 0.68 |
| Proband cancera | 0.32 | ||
| Breast | 32 (36.8) | 56 (32.2) | |
| Ovarian | 1 (1.2) | 4 (2.3) | |
| Prostate | 1 (1.2) | 1 (1.2) | |
| Colorectal | 2 (2.3) | 14 (8.0) | |
| Multiple | 0 (0.0) | 3 (1.7) | |
| Other | 7 (8.0) | 8 (4.6) | |
| None | 40 (46.0) | 88 (50.6) | |
| Ethnicity | 0.74 | ||
| Caucasian (referent) | 32 (36.8) | 53 (30.5) | |
| African-American | 13 (14.9) | 33 (19.0) | |
| Ashkenazi Jewish | 11 (12.6) | 21 (12.1) | |
| Mixed | 4 (2.3) | 13 (1.7) | |
| Unknown | 27 (31.0) | 54 (31.0) | |
| Familial cancer syndrome | 0.11 | ||
| HBOS (referent = all non-HBOS) | 58 (66.7) | 124 (71.3) | |
| Lynch/FAP | 13 (14.9) | 40 (23.0) | |
| Neither | 20 (23.0) | 25 (14.4) | |
| Genetic Testing | 0.78 | ||
| Family member undergoing genetic testing | |||
| No | 58 (66.7) | 119 (68.4) | |
| Yes | 29 (33.3) | 55 (31.6) | |
| BRCA familial mutation | 19 (65.5) | 16 (29.1) | 0.001 |
| BRCA1 deleterious mutation | 6 (20.7) | 7 (12.7) | |
| BRCA1 variant | 3 (10.3) | 1 (1.8) | |
| BRCA2 deleterious mutation | 9 (31.0) | 6 (10.9) | |
| BRCA2 variant | 1 (3.4) | 2 (3.6) | |
| Non-BRCA familial mutation | 2 (6.8) | 5 (9.1) | |
| MMR gene (MLH1/MSH2/MSH6) | 1 (3.4) | 3 (5.4) | |
| APC | 1 (3.4) | 2 (3.6) |
Only for those probands reporting a personal history of cancer
Pancreatic Cancer Frequency
To assess the disease burden of PC in this high-risk cohort, we compared the frequency of PC to that of the general US population using estimates from a large population-based tumor registry (SEER: Surveillance Epidemiology and End Results, <seer.cancer.gov>). If 1,231 families have on average 22 members per family, with each member contributing 40 person-years of follow-up, and an estimated incidence of 1 in 10,000 for PC, 110 PC would be expected. If fewer years of follow-up are attributed to each family member (35 person-years), the number of expected cancers is 97, while if a higher rate (1/9,000) is assumed this number increases to 123 expected cancers. Thus the incidence of PC among the high-risk families studied here is roughly what one might expect to see in an age-, sex-, and race-adjusted population of similar size.
Genetic Testing Results
The proportion of families with at least one member known to have undergone genetic testing for a hereditary cancer predisposition gene was similar in cases (33%) and controls (32%; p=0.78). Nineteen (66%) cases had a familial BRCA1/2 mutation compared to 16/55 (29%) control families (p=0.05). The majority of mutations identified were deleterious [BRCA1: cases (n=6), controls (n=7); BRCA2: cases (n=9), controls (n=6)]. A BRCAPRO estimate of the likelihood of carrying a BRCA1/2 mutation was calculated for all case and control families: mean estimates were 35.8% and 44.3%, respectively. Familial mutations in APC (one case, two controls), MSH2 (one case), MLH1 (one control) and MSH6 (one control) were also identified (see Table 2).
Associations with Familial Cancer History Characteristics
Associations of familial cancer history characteristics with a reported family history of PC are presented in Table 3. A family history of PC was associated with an increase in the total number of cancers reported per pedigree. No associations were observed between a family history of PC and any reported family history of female or male breast cancer. However, a significant association was seen between a history of ovarian cancer in a sibling or descendant and a family history of PC (OR 3.64, 1.14–12.6).
Table 3.
Univariate Associations of Pedigree-based Covariates with a Family History of Pancreatic Cancer for Individuals with a Family History of Pancreatic Cancer (Cases) Versus those without (Controls)
| Characteristic | OR (95% CI) | p |
|---|---|---|
| Proband | ||
| Age at presentation to high-risk clinic | ||
| <35 years | – | |
| 35–40 years | 0.99 (0.47–2.11) | |
| 41–49 years | 2.35 (1.15–4.83) | |
| >50 years | 1.36 (0.62–2.97) | |
| Homogeneity | 0.05 | |
| Trend of odds | 0.11 | |
| Age at cancer diagnosisa | ||
| <35 | – | |
| 35–40 | 1.38 (0.52–3.65) | |
| 41–49 | 0.85 (0.32–2.23) | |
| >50 | 0.75 (0.25–2.17) | |
| Homogeneity | 0.68 | |
| Trend of odds | 0.45 | |
| Sex | ||
| Female | 1.32 (0.50–3.89) | 0.55 |
| Ethnicity/Race | ||
| Caucasian | – | |
| African-American | 0.65 (0.30–1.42) | 0.28 |
| Ashkenazi Jewish | 0.87 (0.37–2.03) | 0.74 |
| Pedigree/Family | ||
| Syndrome | ||
| No syndrome | – | |
| HBOS | 0.62 (0.32–1.21) | 0.16 |
| FAP/HNPCC-Lynch | 0.40 (0.17–0.96) | 0.04 |
| Family size | ||
| 1st quartile | – | |
| 2nd quartile | 0.90 (0.42–1.93) | |
| 3rd quartile | 1.64 (0.74–3.62) | |
| 4th quartile | 1.03 (0.48–2.21) | |
| Homogeneity | 0.38 | |
| Trend of odds | 0.61 | |
| Total cancers reported | ||
| <3 | – | |
| 3–4 | 4.01 (1.13–14.3) | |
| 5–6 | 12.1 (3.44–42.2) | |
| 7+ | 12.5 (3.42–45.7) | |
| Homogeneity | 0.001 | |
| Trend of odds | 0.001 | |
| Cancer density | ||
| 1st quartile | – | |
| 2nd quartile | 3.02 (1.22–7.44) | |
| 3rd quartile | 3.79 (1.54–9.32) | |
| 4th quartile | 7.38 (3.05–17.87) | |
| Homogeneity | 0.001 | |
| Trend of odds | 0.001 | |
| Family history of breast cancerc | ||
| Maternal-side breast cancer | 1.00 (0.58–1.73) | 1.00 |
| Paternal-side breast cancer | 0.74 (0.33–1.58) | 0.41 |
| Sibling/descendent breast cancer | 1.46 (0.79–2.69) | 0.19 |
| Family history of early (<50) breast cancer | 0.79 (0.45–1.37) | 0.38 |
| Male breast cancer | 0.28 (0.01–2.22) | 0.20 |
| Family history of ovarian cancerb | ||
| Maternal-side ovarian cancer | 1.21 (0.53–2.68) | 0.61 |
| Paternal-side ovarian cancer | 1.35 (0.27–5.86) | 0.65 |
| Sibling/descendent ovarian cancer | 3.64 (1.14–12.6) | 0.01 |
| Family history of early (<50) ovarian cancer | 1.83 (0.73–4.48) | 0.14 |
| Familial mutation statusb,c | ||
| Any mutation in a 1st/2nd degree relative | 3.08 (1.1–8.84) | 0.02 |
| Mutation in APC/MLH1/MSH2/MSH6 | 0.80 (0.07–4.98) | 0.79 |
| Any mutation in BRCA1 or BRCA2d | 3.78 (1.32–10.9) | 0.005 |
| Deleterious mutation in a BRCA1 or BRCA2 | 3.46 (1.15–10.5) | 0.01 |
All controls are used in each univariate analysis
Only for those probands reporting a personal history of cancer
Pedigree covariates are binary (yes/no)
Calculations performed for sub-sample of tested individuals (n=84)
Variants of uncertain significance and deleterious mutations
In the subset of families in whom at least one member underwent genetic testing, the presence of a mutation in BRCA1/2 (variants of uncertain significance and deleterious mutations) was strongly associated with a family history of PC (OR 3.78, 1.32–10.9, p=0.005). This relationship was maintained when the association was restricted to deleterious mutations (OR 3.46, 1.15–10.5, p=0.01). The association was strengthened though less stable when only non-Ashkenazi Jewish individuals (55/84) were considered (OR 6.03, 1.68–22.14, p=0.003).
Discussion
This study was designed to investigate the importance of PC history in a clinical population referred for cancer risk assessment. In total, 87 families were identified, representing 7.1% of the families enrolled in our database over an 11-year span. Because the majority of individuals presenting to high-risk cancer clinics in the USA and abroad are referred for evaluation for HBOS and/or BRCA1/2 genetic testing (Epplein et al. 2005; Hopwood et al. 2003), we also specifically examined the contribution that a family history of PC adds to the risk assessment of an HBOS proband.
We find that the presence of PC in an HBOS family is significantly associated with an increased relative risk of a familial BRCA1/2 mutation, whether all mutations (including uncertain variants) or only deleterious mutations are considered. The clinical applicability of this information is its greatest strength: comparatively, probands meeting similar HBOS criteria and having similar BRCAPRO estimates will have a three- to four-fold increased risk of harboring a mutation if PC is present in a close relative, potentially influencing tailored genetic counseling (e.g. estimation of risk) and medical referral (e.g. for preventive measures). Previous work in this area, while delineating the connection between BRCA1/2 and PC, has focused on somewhat less practical aspects of this relationship. For example, estimates of PC risk for mutation carriers [BRCA1: RR 2.26 (1.26–4.06), BRCA2: RR 3.51 (1.87–6.58)] have been published by the Breast Cancer Linkage Consortium (anonymous, 1999; Thompson et al. 2002). Likewise, the risk of BRCA1/2 mutations in incident PC has also been explored: germline mutations have been identified in 7–27% of PC in several series (Couch et al. 2007; Lal et al. 2000; Murphy et al. 2002; Ozcelik et al. 1997). Yet, the absolute risk of PC for BRCA1/2 carriers remains quite small, and few individuals with a personal history of PC outside HBOS are counseled or tested for BRCA1/2. Thus, the clinical applicability of these findings is limited. When a subset of non-Ashkenazi individuals was considered, BRCA1/2 mutation risk was further magnified (OR 6.03, 1.68–22.14). If confirmed, this may also have clinical relevance, as risk assessment depends more heavily on family history in non-Ashkenazi subjects in whom the population prevalence of BRCA1/2 mutations (a key component in the estimation of mutation risk using BRCAPRO) is comparatively much lower (Berry et al. 2002).
The association of familial PC to ovarian cancer in a sibling or descendent merits further examination. Genotype-phenotype correlation studies have defined an ovarian cancer cluster region (OCCR) on BRCA2, but no region(s) clearly linked to PC (Lubinski et al. 2004; Thompson et al. 2002; van Asperen et al. 2005). Whether women from HBOS families exhibiting PC should seek early oophorectomy or aggressive ovarian screening is unknown. Both ovarian and PC risks are also increased in HNPCC/Lynch syndrome, but no coupling of these risks has been reported to date (Aarnio et al. 1999; Lindor et al. 2005). In efforts to further explore this question, our group has recently identified a significant association between ovarian cancer (first primary malignancy) and PC (second primary malignancy) using the SEER registry (unpublished data).
Here, a history of PC was more frequently reported on the maternal side of case families. This may be related to reporting bias secondary to the largely female (>90%) population seen in our clinic, as well as to uneven transmission of family history information from parent to child, with daughters being more likely to seek medical information from their mothers. We also found that probands reporting the strongest family histories (five or more cancers in close relatives) were most likely to have a family history of PC. It is conceivable that among kindred with cancer syndromes, PC incidence increases as the overall disease burden increases, perhaps reflective of the greater penetrance of the risk gene in question. However, a family information bias may also be occurring, as persons with more extensive knowledge of their family history have been found to report both a greater total number of family cancers as well a larger number of the less commonly reported cancers, particularly when they are part of cancer registries (Murff et al. 2004; Sackett 1979; Wacholder 2004; Ziogas and Anton-Culver 2003).
It is striking that nearly one-third of our case families concurrently met criteria for familial pancreatic cancer (FPC) and a second cancer syndrome. In part, this risk overlap may be explained by the FPC criteria themselves: the development of malignancies at a younger-than-normal age is a common feature of many hereditary cancer syndromes, although early-onset PC is not alone diagnostic of HBOS, FAP, or HNPCC/Lynch syndrome. Whether or not an individual who meets both HBOS and FPC criteria has a risk of PC above the increased risk imparted by a BRCA1/2 mutation is unknown. Families highly enriched in PC have been described in several cancer syndromes (Lynch et al. 2001, 2002). At the same time, this finding emphasizes the importance of recognizing the heterogeneity of disease presentation in a given cancer syndrome and the possible clinical implications of that variability (e.g. modified risk estimates or screening recommendations).
In this study, the selection of cases and controls was independent of testing status. However, to make our conclusions valid, one must also assume that there was no association between the likelihood of being tested and a family history of PC, as this could increase the chances of finding BRCA1/2 mutations among cases. Two observations support this assumption: first, the fraction of subjects undergoing genetic testing was identical in cases and controls [that is, testing rates in cases (33.3%) and controls (31.6%) were equivalent]. Had a family history of PC been considered as an independent criterion for testing, one would have expected a higher fraction of tested families among cases. Secondly, a BRCAPRO estimate performed on case and control probands showed that cases in fact had a slightly lower risk estimate than controls, and therefore should have had no greater likelihood of being offered testing. Although at least two current decision tools factor a history of PC into account in BRCA1/2 risk modeling, neither was readily available or used by our group at the time of clinical decision-making in this study (Couch et al. 1997; Evans et al. 2004). Nonetheless, it must be emphasized that the decisions surrounding referral and testing were not included in this analysis, and that families were not necessarily offered testing based solely on an elevated BRCAPRO estimation. The BRCAPRO estimates we present are only indicators suggestive of whether a case or control family would have been considered high-risk and might have warranted genetic testing.
Ultimately, multiple medical and surgical options are now available to reduce the risk of breast and ovarian cancer in BRCA1/2 carriers, yet no consensus exists on the management of PC risk (Rebbeck et al. 1999; Rebbeck et al. 2004). Further, little is still known about the genetic origins of FPC. Several groups are actively working to identify an FPC gene (Klein et al. 2002; Petersen et al. 2006), and one autosomal dominant candidate gene has been proposed (Pogue-Geile et al. 2006). Intensive screening/prevention modalities for PC remain unproven and should be limited to clinical trials. Nevertheless, two groups have recently shown that smokers from FPC families develop PC at significantly earlier ages than non-smokers (Klein et al. 2004; Rulyak et al. 2003). Considering the dismal prognosis of PC, even small gains made through smoking cessation may have an enormous impact on quality of life and survival in high-risk individuals.
Acknowledgements
Dr. Hall is a recipient of a Young Investigator Award from the American Society of Clinical Oncology and a Mentored Career Development Award from the American Cancer Society. Dr. Olopade is a recipient of grant funding from the Ralph and Marion Falk Medical Research Trust.
Contributor Information
Michael J. Hall, Email: mjh15@columbia.edu, Departments of Medicine and Epidemiology, Columbia University College of Physicians and Surgeons, Mailman School of Public Health, New York, NY 10032, USA; New York Presbyterian Hospital, New York, NY 10032, USA; Division of Medical Oncology, Columbia University Medical Center, 161 Fort Washington Avenue, Room 9-907, New York, NY 10032, USA.
James J. Dignam, Department of Health Studies, University of Chicago, Chicago, IL 60637, USA
Olufunmilayo I. Olopade, Center for Clinical Cancer Genetics, University of Chicago, Chicago, IL 60637, USA
References
- Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. International Journal of Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- anonymous. Cancer risks in BRCA2 mutation carriers. The Breast Cancer Linkage Consortium. Journal of the National Cancer Institute. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- Berry DA, Iversen ES, Jr, Gudbjartsson DF, Hiller EH, Garber JE, Peshkin BN. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. Journal of Clinical Oncology. 2002;20:2701–2712. doi: 10.1200/JCO.2002.05.121. [DOI] [PubMed] [Google Scholar]
- Burke W, Daly M, Garber J, Botkin J, Khan MJ, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer genetics studies consortium. JAMA. 1997;277:997–1003. [PubMed] [Google Scholar]
- Couch FJ, DeShano ML, Blackwood MA, Calzone K, Stopfer J, Campeau L, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. New England Journal of Medicine. 1997;336:1409–1415. doi: 10.1056/NEJM199705153362002. [DOI] [PubMed] [Google Scholar]
- Couch FJ, Johnson MR, Rabe KG, Brune K, de Andrade M, Goggins M, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiology, Biomarkers & Prevention. 2007;6:342–346. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- Epplein M, Koon KP, Ramsey SD, Potter JD. Genetic services for familial cancer patients: A follow-up survey of National Cancer Institute Cancer Centers. Journal of Clinical Oncology. 2005;23:4713–4718. doi: 10.1200/JCO.2005.00.133. [DOI] [PubMed] [Google Scholar]
- Evans DG, Eccles DM, Rahman N, Young K, Bulman M, Amir E, et al. A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO. Journal of Medical Genetics. 2004;41:474–480. doi: 10.1136/jmg.2003.017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood P, van Asperen CJ, Borreani G, Bourret P, Decruyenaere M, Dishon S, et al. Cancer genetic services provision: A comparison of Seven European Centres. Community Genetics. 2003;6:192–205. doi: 10.1159/000079381. [DOI] [PubMed] [Google Scholar]
- Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988;1:1149–1151. doi: 10.1016/s0140-6736(88)91962-9. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer Journal for Clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Klein AP, Beaty TH, Bailey-Wilson JE, Brune KA, Hruban RH, Petersen GM. Evidence for a major gene influencing risk of pancreatic cancer. Genetic Epidemiology. 2002;23:133–149. doi: 10.1002/gepi.1102. [DOI] [PubMed] [Google Scholar]
- Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Research. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- Lal G, Liu G, Schmocker B, Kaurah P, Ozcelik H, Narod SA, et al. Inherited predisposition to pancreatic adenocarcinoma: role of family history and germ-line p16, BRCA1, and BRCA2 mutations. Cancer Research. 2000;60:409–416. [PubMed] [Google Scholar]
- Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2005;27:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- Lubinski J, Phelan CM, Ghadirian P, Lynch HT, Garber J, Weber B, et al. Cancer variation associated with the position of the mutation in the BRCA2 gene. Familial Cancer. 2004;3:1–10. doi: 10.1023/B:FAME.0000026816.32400.45. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Brand RE, Deters CA, Shaw TG, Lynch JF. Hereditary pancreatic cancer. Pancreatology. 2001;1:466–471. doi: 10.1159/000055849. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Brand RE, Hogg D, Deters CA, Fusaro RM, Lynch JF, et al. Phenotypic variation in eight extended CDKN2A germline mutation familial atypical multiple mole melanoma-pancreatic carcinoma-prone families: The familial atypical multiple mole melanoma-pancreatic carcinoma syndrome. Cancer. 2002;94:84–96. doi: 10.1002/cncr.10159. [DOI] [PubMed] [Google Scholar]
- Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. JAMA. 2004;292:1480–1489. doi: 10.1001/jama.292.12.1480. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Brune KA, Griffin C, Sollenberger JE, Petersen GM, Bansal R, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: Deleterious BRCA2 mutations in 17% Cancer Research. 2002;62:3789–3793. [PubMed] [Google Scholar]
- Nelson HD, Huffman LH, Fu R, Harris EL. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: Systematic evidence review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2005;143:355–379. doi: 10.7326/0003-4819-143-5-200509060-00012. [DOI] [PubMed] [Google Scholar]
- Ozcelik H, Schmocker B, Di Nicola N, Shi XH, Langer B, Moore M, et al. Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nature Genetics. 1997;16:17–18. doi: 10.1038/ng0597-17. [DOI] [PubMed] [Google Scholar]
- Palomares MR, Paz B, Weitzel JN. Genetic cancer risk assessment in the newly diagnosed breast cancer patient is useful and possible in practice. Journal of Clinical Oncology. 2005;23:3165–3166. doi: 10.1200/JCO.2005.05.157. [DOI] [PubMed] [Google Scholar]
- Petersen GM, de Andrade M, Goggins M, Hruban RH, Bondy M, Korczak JF, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiology, Biomarkers & Prevention. 2006;15:704–710. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- Pogue-Geile KL, Chen R, Bronner MP, Crnogorac-Jurcevic T, Moyes KW, Dowen S, et al. Palladin mutation causes familial pancreatic cancer and suggests a new cancer mechanism. PLoS Med. 2006;3:e516. doi: 10.1371/journal.pmed.0030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van ’t Veer L, Garber JE, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: The PROSE Study Group. Journal of Clinical Oncology. 2004;22:1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- Rebbeck TR, Levin AM, Eisen A, Snyder C, Watson P, Cannon-Albright L, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. Journal of the National Cancer Institute. 1999;91:1475–1479. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- Rulyak SJ, Brentnall TA. Inherited pancreatic cancer: Surveillance and treatment strategies for affected families. Pancreatology. 2001;1:477–485. doi: 10.1159/000055851. [DOI] [PubMed] [Google Scholar]
- Rulyak SJ, Lowenfels AB, Maisonneuve P, Brentnall TA. Risk factors for the development of pancreatic cancer in familial pancreatic cancer kindreds. Gastroenterology. 2003;124:1292–1299. doi: 10.1016/s0016-5085(03)00272-5. [DOI] [PubMed] [Google Scholar]
- Sackett DL. Bias in analytic research. Journal of Chronic Diseases. 1979;32:51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]
- Thompson D, Easton DF Breast Cancer Linkage Consortium. Cancer incidence in BRCA1 mutation carriers. Journal of the National Cancer Institute. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. Journal of the National Cancer Institute. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, Vasen HF, et al. Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. Journal of Medical Genetics. 2005;42:711–719. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- Wacholder S. Bias in intervention studies that enroll patients from high-risk clinics. Journal of the National Cancer Institute. 2004;96:1204–1207. doi: 10.1093/jnci/djh229. [DOI] [PubMed] [Google Scholar]
- Weitzel JN, Lagos VI, Cullinane CA, Gambol PJ, Culver JO, Blazer KR, et al. Limited family structure and BRCA gene mutation status in single cases of breast cancer. JAMA. 2007;297:2587–2595. doi: 10.1001/jama.297.23.2587. [DOI] [PubMed] [Google Scholar]
- White M, Olopade OI. Cancer risk assessment: Toward a primary prevention of breast and ovarian cancer. Oncol Econ. 2000;1:40–45. [Google Scholar]
- Ziogas A, Anton-Culver H. Validation of family history data in cancer family registries. American Journal of Preventive Medicine. 2003;24:190–198. doi: 10.1016/s0749-3797(02)00593-7. [DOI] [PubMed] [Google Scholar]