Abstract
Background and Objective
Port-wine stain (PWS) birthmarks affect ~22 million people worldwide. After several treatment sessions, complete disappearance of the PWS occurs in only ~10% of treated patients. There is a need to develop a new strategy to improve the efficacy of each treatment session and the overall treatment outcome. The study objective was to determine how intraoperative measurements of blood flow correlate with treatment response assessed several weeks post treatment.
Study Design/Materials and Methods
We employed Laser Speckle Imaging (LSI) to measure intraoperative blood-flow dynamics. We collected data from 24 subjects undergoing laser therapy for facial PWS birthmarks. Photographs were taken before treatment and at a follow-up visit, and analyzed by two expert observers.
Results
Intraoperative LSI enables real-time monitoring of blood-flow dynamics in response to laser treatment and can inform clinicians on the need for focused re-treatment. The degree of PWS blanching achieved is positively correlated with the log-transformed acute blood-flow reduction (P =0.022).
Conclusion
LSI is a simple, intraoperative monitoring tool during laser therapy of PWS birthmarks. LSI provides a single value for blood flow that correlates well with the degree of blanching achieved with laser therapy. Lasers Surg. Med.
Keywords: pulsed dye laser, optical imaging, speckle contrast, laser Doppler, blood perfusion
INTRODUCTION
Vascular malformations known as port-wine stain (PWS) birthmarks affect approximately 22 million people worldwide (0.3% incidence rate) [1]. A PWS consists of abnormally large dermal blood vessels, resulting in visual manifestation of pink-to-purple lesions typically on the face or neck [2]. In some reports, they are referred to as capillary malformations [3,4]; in others as post-capillary venular malformations [5]. In contrast to hemangiomas, PWS birthmarks do not involute in a spontaneous fashion. Instead, they progress slowly over decades, developing nodules and tissue hypertrophy and darkening in color [3].
Researchers have proposed several hypotheses on causes of PWS birthmark development. Smoller and Rosen [6] proposed that PWS development is due in part to either compromised or absent neuronal regulation during development. Research groups have proposed that angiogenic signaling is abnormal in PWS skin [3,7]. Recently, Shirley et al. [4] proposed that a somatic mosaic activating mutation in the GNAQ gene is associated with PWS birthmarks.
PWS birthmarks are associated with both physical and psychological problems. For example, the psychosocial development of individuals with PWS birthmarks is adversely affected [8]. In addition, facial PWS lesions have been associated with increased incidence of glaucoma [9]. The progressive nature of PWS skin may be due to lack of neuronal regulation of blood vessel size and can result in a darker appearance, soft tissue hypertrophy, nodularity, and overall further disfigurement [1,10,11]. Approximately 70% of patients over 50 years of age with a PWS show hypertrophy [10,11]. Finally, development of nodules is a concern, as they are associated with an increased risk of spontaneous bleeding [12].
To take advantage of the selective photothermolysis principles outlined by Anderson and Parrish [13], medical laser technology has evolved towards use of longer wavelengths, from 577 nm [14] to 585 nm [15] and to 595 nm [16] and longer pulse durations [17]. To treat regions with nodular PWS, Izikson et al. [18] demonstrated the potential utility of the Alexandrite laser (755 nm), which is known to penetrate deeper into skin. With the substantial advances in medical laser technology, a reduction in size and degree of redness of treated PWS skin is achieved in ~90% of treated patients. However, after five to seven treatment sessions, complete disappearance of the PWS occur in only ~10% of treated patients [19,20]. Furthermore, due to the pain associated with the laser therapy, children may undergo general anesthesia, thus requiring an anesthesiologist and considerable addition in the cost associated with the treatment session.
Since the acute goal of laser therapy is photocoagulation of the blood vessels, a rational approach to improving PWS treatment efficacy is intraoperative assessment of skin perfusion. To assess blood flow in PWS vessels, only a few studies exist in the peer-reviewed literature. In general, either laser Doppler imaging [21] or Doppler optical coherence tomography [22] has been employed. In particular, with Doppler optical coherence tomography evaluation of PWS skin, Nelson et al. [22] acknowledge the potential of blood flow characterization as a means to monitor PWS skin during laser therapy and retreat if photocoagulation of targeted vessels has not occurred. To address the need for noninvasive blood flow characterization to provide surgical guidance to the treating clinician, we selected the method of Laser Speckle Imaging (LSI), which we have used in a number of preclinical [23,24] and clinical studies [25,26] involving monitoring of the microvascular response to targeted laser therapy. The purpose of this study was to determine the degree of correlation between intraoperative measurements of blood-flow dynamics and the treatment response assessed several weeks post treatment.
METHODS
Patients
From July 2010 to January 2012, we enrolled 24 subjects with PWS birthmarks. The subjects ranged in age from 8 to 64 years (mean ± standard deviation =29 ± 17 years). Prior to LSI monitoring, we informed subjects of the measurement protocol that was approved by the Institutional Review Board at University of California, Irvine. All subjects of legal age gave written informed consent, and legal guardians gave written informed consent for subjects under the age of 18.
Outcome Assessments
Study visits
Two of the co-authors (JSN, KMK) performed the treatments. They used either a Pulsed-Dye Laser (PDL, Vbeam, 595 nm, Candela Corp, Wayland, MA) or an Alexandrite laser (755 nm, Candela Corp, Wayland, MA) for all treatments. The laser type, laser parameters, and number of treatment passes, all are covariates in this study. For each subject, we used digital photography prior to each treatment session and LSI during laser surgery in the operating room.
Efficacy
Two of the co-authors (JSN, KMK) analyzed photographs to score the degree of blanching of the subjects enrolled in this study. With the OMNIA Imaging System (Canfield, Fairfield, NJ), we collected digital photographs prior to each laser treatment session, at preset imaging angles ranging from 0° to 90°. The evaluators independently assigned a blanching score to each pair of images. Blanching scores of 0% and 100% represented no discernible change in PWS appearance or complete blanching of the PWS, respectively. A negative blanching score indicated darkening of the PWS.
Laser Speckle Imaging
The second-generation clinical LSI system consisted of three main hardware components: a laser source, CCD camera, and computer [27]. We enabled control of the device using software written in the LabVIEW programming environment (Version 8.0, National Instruments, Austin, TX). The laser source was a continuous-wave HeNe laser light (λ =633 nm, 30 mW, Edmund Industrial Optics, Barrington, NJ). The CCD camera was a thermoelectrically-cooled Retiga 2000R (QImaging, Burnaby, BC, Canada) with a pixel resolution of 1600 × 1200 (1.92 megapixels, each pixel having dimensions of 7.4 x 7.4 μm). The Retiga 2000R had a maximum frame rate of ~8.3 fps at full resolution.
The computer (Alienware Aurora, Dell, Round Rock, TX) was equipped with a GTS240 Graphics Processing Unit (GPU) (NVIDIA, Santa Clara, CA). To achieve real-time processing of the captured raw speckle images, we used a LabVIEW-based code described previously [28] that exploits the parallel-processing power of the GPU. All code is available as freeware at http://choi.bli.uci.edu/software. With this processing, we achieved maps of blood flow that we call Speckle Flow Index (SFI) maps [29].
To perform LSI in an operating room, we also included design changes to accommodate flexible camera maneuverability and filter out operating room lighting. The Retiga 2000R was mounted on a custom modified Bogen tripod that allowed for ease of maneuverability. To eliminate problems associated with contamination of LSI images by the broad wavelength spectrum of the overhead lights in the operating room, we integrated a 633 nm laser line filter to the device.
Imaging Protocol
Image collection and analysis protocol
A laser speckle pattern created by passing the laser source (λ =633 nm) through a ground-glass diffuser, was projected onto the surface of the skin. Images were continuously obtained before, during, and immediately after laser surgery using the CCD camera at an exposure time of 10 ms. The exposure time of 10 ms was found to be suitable based on previous flow-phantom studies [29]. Acquisition and processing were carried out on the computer with the LabVIEW-based GPU real-time algorithm [28].
Clinical Visualization/Usage of the Images in the OR
During the entire procedure, the treating clinician had access to the SFI images. SFI values were displayed as a graphical flow map. Higher SFI values represent regions of higher blood flow, and lower SFI values represent regions of lower blood flow, which appear as darker regions within the SFI color map.
Statistical Analysis
To investigate the association between blanching score and change in SFI, data were analyzed using linear methods and Pearson correlations. Nonlinear associations were explored by using log-transformed values for change in SFI. In this study, we did not adjust for potential covariates, such as age, gender, number of prior treatments, and treatment parameters.
RESULTS
Subjects
We collected imaging data during laser therapy, from 24 subjects (13 male, 11 female) with facial PWS. Their age (mean ± standard deviation) was 29 ± 17 years, with a range of 8–64 years. Eight of the 24 subjects were minors. The majority (22/24) of subjects had a PWS only on one side of the face; the other two subjects had a bilateral facial PWS.
Intraoperative LSI During Laser Therapy
Intraoperative LSI allows monitoring of real-time changes in photocoagulation during laser surgery
Figure 1 represents typical SFI perfusion maps and images acquired during surgery. The PWS birthmark for the subject in Figure 1 appears as a bright green region on the SFI image, representing a region of higher blood flow than the surrounding skin. Post-treatment images show a reduction in blood flow within the laser-treated PWS region, as indicated by lower SFI values, graphically seen as a darker blue region. The initial pretreatment SFI value of 1,150 was reduced to 725 post-treatment, representing a 37% reduction in SFI.
Fig. 1.

Intraoperative LSI enables real-time monitoring of photocoagulation in response to laser therapy. (A,C) Webcam and (B,D) SFI images extracted from the real-time video feed, are shown (A, B) before and (C,D) immediately after treatment of a PWS birthmark located on the left temple of an adult male subject. With a single pass of 585 nm pulsed-dye laser irradiation (7.5 J/cm2, 10 mm spot size, 0.45 ms pulse duration), a 37% reduction in SFI was observed.
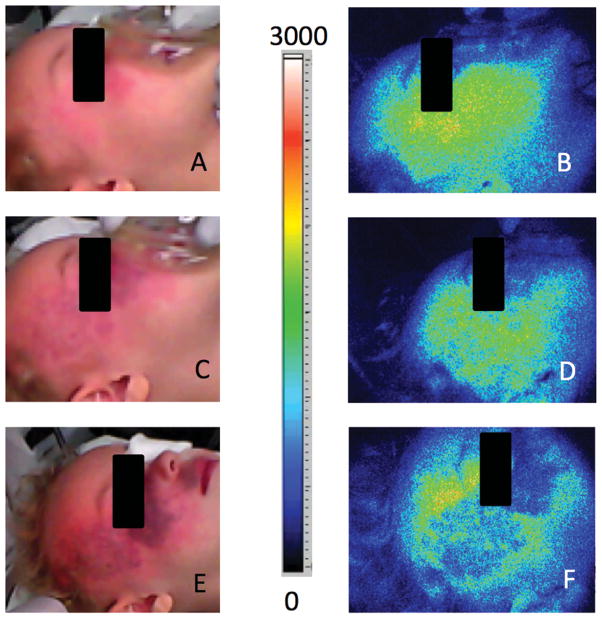
Intraoperative LSI enables real-time monitoring of photocoagulation of superficial vasculature achieved with different clinical laser systems
Figure 2 demonstrates the effects of different laser wavelengths on photocoagulation. Figure 2A represents the subject prior to laser treatment. Figure 2B is an image immediately after a single pass of a 755 nm alexandrite laser, a wavelength used to photocoagulate deeper regions of PWS birthmarks as described by Izikson et al. [18], with little observable change in superficial blood flow. The treating clinician then used the standard pulsed-dye laser to treat the PWS, and a marked reduction in PWS blood flow was observed as seen in Figure 2C. A rim-shaped region of higher blood flow surrounds the region of reduced blood flow, and most likely is representative of reactive hyperemia [25].
Fig. 2.
Intraoperative LSI enables real-time monitoring of photocoagulation of superficial vasculature achieved with different clinical laser systems. SFI images extracted from the real-time video feed, are shown (A) before laser treatment, (B) after a single pass of an alexandrite laser (60J/ cm2, 10 mm spot size, 3 ms pulse duration), and (C) after two passes of a pulsed-dye laser (Pass 1: 10J/ cm2, 10 mm spot size, 3 ms pulse duration; Pass 2: 6.5J/ cm2, 12 mm spot size, 0.45 ms pulse duration) treatment of a PWS birthmark located on the right cheek of a 40-year old female subject.
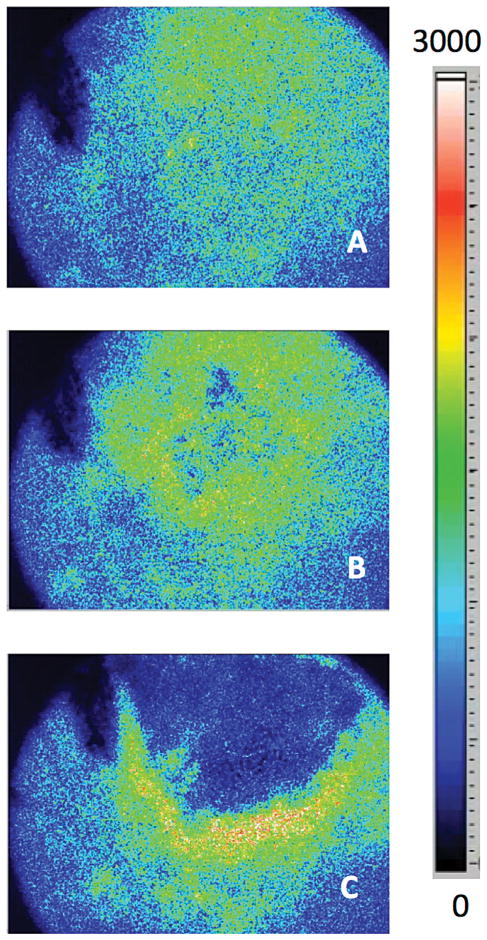
Intraoperative LSI potentially enables informed and immediate re-treatment of the port-wine stain
Figure 3 is an intraoperative example that demonstrates the potential role of LSI during laser surgery of PWS birthmarks. The clinician treated this subject with three separate passes of the PDL. After two consecutive passes, the clinician reviewed the appearance of the subject and decided to apply a third pass of PDL irradiation. With post-procedural review of the SFI images, we believe that the LSI data support the use of the third pass. The SFI images show minimal changes in SFI after the first two passes (Fig. 3D); with the third treatment pass, the clinician achieved a substantially higher degree of photocoagulation (Fig. 3F).
Fig. 3.
Intraoperative LSI data may inform clinicians with the need for immediate re-treatment of PWS skin. (A,C,E) Webcam and (B,D,F) SFI images extracted from the real-time video feed, are shown (A,B) before treatment, (C,D) after two consecutive passes (Pass 1: 7.0 J/cm2, 10 mm spot size, 3 ms pulse duration; Pass 2: 8.5 J/cm2, 10 mm spot size, 1.5 ms pulse duration), and (E,F) after a third pass (7J/ cm2, 10 mm spot size, 0.45 ms pulse duration) of a PWS birthmark located on the right temple and cheek of a 3-year old female subject.
Blanching of PWS
The degree of PWS blanching in response to laser surgery, increases with the magnitude of SFI reduction
To study the correlation between reduction in SFI and treatment outcome, we first determined the average change in SFI from specific regions of interest. The treating clinicians then scored color photographs taken prior to laser treatment and at a subsequent patient visit. In general, the degree of PWS blanching was correlated with the magnitude of reduction in SFI immediately following laser treatment (r =0.384, P =0.064) (Fig. 4). The data suggest a nonlinear relationship with the strongest correlation observed between degree of PWS blanching and the log-transformed value for SFI change (r =0.446, P =0.022).
Fig. 4.
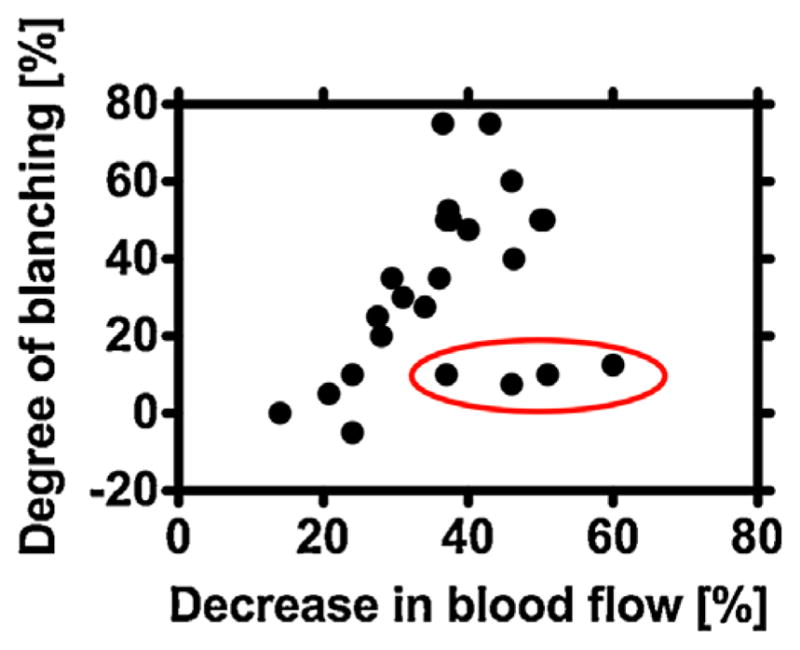
The degree of blanching of the PWS birthmarks increases with a decrease in SFI (n =24). We assessed degree of blanching with qualitative scoring of color photographs taken prior to laser treatment and at a follow-up visit by each subject. We quantified the decrease in SFI from the LSI data collected during laser treatment. Four of the 24 subjects (data points within red oval) experienced minimal blanching of their PWS birthmarks despite a relatively large decrease in SFI.
DISCUSSION
Our results collectively demonstrate the ability of the intraoperative LSI technology to monitor noninvasively and in real time, the dynamics of blood flow in response to laser treatment (Fig. 1); and the potential ability of LSI to inform the clinician to make immediate changes in treatment parameters (Fig. 2) or to re-treat immediately regions that still have appreciable blood flow (Fig. 3). Based on analysis of LSI data and color photographs taken from 24 subjects, we determined that the degree of PWS blanching in response to laser surgery, tends to increase with the magnitude of SFI reduction (Fig. 4).
Pulsed-dye laser therapy of PWS is a safe, effective method to achieve some degree of blanching [19,20,30]. However, in our experience, only ~10% of treated patients achieve complete blanching even after multiple treatment sessions. In several papers [22,31], we have demonstrated the possibility of using a priori knowledge of PWS anatomical features to inform clinicians on judicious selection of treatment parameters. This approach is confounded by the variation in PWS anatomy within a lesion [32] and the overall gap in the knowledge base on the acute and chronic biological response to photocoagulation of vasculature [5,7,23,24,33].
The overarching premise for using the PDL to treat PWS birthmarks, is selective photocoagulation of the enlarged vasculature and ultimately replacement of these abnormally large vessels with smaller ones. Hence, photocoagulation and presumably stoppage of blood flow, is a necessary first step towards achieving a desired degree of blanching. Thus, we focused specifically on developing and employing a method designed to assess blood flow changes associated with laser surgery. We previously studied the use of LSI to image subjects undergoing laser treatment of PWS birthmarks, with image collection performed before and approximately 40 minutes after treatment [25,26] and identified the common presence of a heterogeneous blood-flow pattern after treatment. Ren et al. [34] used LSI to measure blood flow of PWS and normal surrounding skin before Photodynamic Therapy (PDT) and at a follow-up visit scheduled three to six months after PDT. They reported a good correlation (r =0.73) between the measured change in blood flow and the degree of blanching achieved with PDT.
With evaluation of LSI data collected during laser surgery and color photographs taken at a follow-up visit, we identified that degree of blanching is positively correlated with acute measurements of the magnitude of reduction in SFI (Fig. 4). This result supports our approach of intraoperative LSI and the use of real-time feedback to enable immediate re-treatment of regions of persistent perfusion. Since treated areas routinely are irradiated with multiple passes of laser light [35,36], we do not expect that LSI-guided re-treatment will lead to an increase in post-operative complications. Nevertheless, the safety and efficacy associated with additional passes and the treatment parameters used for each of those passes, requires further evaluation. With additional clinical data, we anticipate identification of a target percent reduction in SFI associated with a good treatment outcome.
In previous publications [17,22,31], we advocated use of optical measurements to characterize quantitatively features of the PWS, such as depth of vasculature and characteristic size of PWS blood vessels. The purported benefit associated with such characterization is that, with accurate knowledge of these features, the clinician then is enabled to make an informed decision on selection of treatment parameters, such as optical wavelength and pulse duration. As an optimized selection of treatment parameters is expected to lead to maximum acute photocoagulation, the LSI methodology is amenable to study of the potential benefits of pre-treatment characterization of PWS skin on treatment outcome.
An unexpected finding was that the treated regions of four subjects had large reductions in SFI but minimal overall blanching (Fig. 4, data points in red oval). We currently do not offer an explanation, as the numbers are too few to draw any reasonable evidence-based hypotheses based on any specific characteristic. Further study is warranted.
Our study has limitations. As noted above, several covariates exist, including age, gender, baseline skin temperature, level of anesthesia, and treatment parameters - at this time, we made no attempt to control them. The treating clinicians (JSN and KMK) also assessed the photographs, which may affect the data shown in Figure 4; however, it is important to note that the clinicians did not have any advance information on the change in SFI resulting from treatment. Furthermore, we acknowledge that SFI depends not only on blood flow but also on local tissue optical properties [37], but blood flow appears to be the dominant contributor to SFI. Mazhar et al. [38] used spatial frequency domain imaging (SFDI) to measure an increase in absorption coefficient immediately following PDL treatment of PWS skin; such an increase would lead to a decrease in SFI. Research studies are underway to integrate SFDI with LSI, to mitigate effects of optical property dynamics on SFI measurements. The sample size of 24 subjects most likely does not enable strict definition of the shape of the positive correlation between blanching score and change in SFI, as the P values associated with linear, logarithmic, and square root transformations are 0.064, 0.022, and 0.037, respectively. Nevertheless, given the existence of these multiple covariates, we find that the significant correlation between blanching score and change in SFI to be quite remarkable, as the data suggest that measurements of acute blood flow changes alone are sufficient to inform the clinician on treatment progress during laser surgery. Future studies are planned in which specific covariates will be taken into account with the experimental design.
In conclusion, our results demonstrate the potential role of LSI as a simple, intrasurgical monitoring tool during laser therapy of PWS birthmarks. LSI provides a single number (SFI) that correlates well with the degree of blanching achieved with laser therapy. With further integration of LSI methodology into devices amenable for point-of-care diagnostics [39], we expect a marked increase in the role of LSI as a clinical tool with diagnostic value.
Acknowledgments
The authors thank Montana Compton for her assistance with the clinical measurements. Funding was provided in part by the Arnold and Mabel Beckman Foundation and the National Institutes of Health (Grant numbers AR047551, AR059244, P41 EB015890, R01 HD065536, and UL1 TR000148).
Contract grant sponsor: Arnold and Mabel Beckman Foundation; Contract grant sponsor: National Institutes of Health; Contract grant numbers: AR047551, AR059244, P41 EB015890, R01 HD065536, UL1 TR000148.
Abbreviations
- CCD
charge-coupled device
- GPU
graphics processing unit
- LSI
laser speckle imaging
- PDL
pulsed dye laser
- PWS
port wine stain
- SFI
Speckle Flow Index
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58(2):218–222. [PubMed] [Google Scholar]
- 2.Ch’ng S, Tan ST. Facial port-wine stains–Clinical stratification and risks of neuro-ocular involvement. Plast Reconstr Aesthet Surg. 2008;61(8):889–893. doi: 10.1016/j.bjps.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Revencu N, Boon LM, Mendola A, Cordisco MR, Dubois J, Clapuyt P, Hammer F, Amor DJ, Irvine AD, Baselga E, Dompmartin A, Syed S, Martin-Santiago A, Ades L, Collins F, Smith J, Sandaradura S, Barrio VR, Burrows PE, Blei F, Cozzolino M, Brunetti-Pierri N, Vicente A, Abramowicz M, Desir J, Vilain C, Chung WK, Wilson A, Gardiner CA, Dwight Y, Lord DJ, Fishman L, Cytrynbaum C, Chamlin S, Ghali F, Gilaberte Y, Joss S, del Boente MC, Leaute-Labreze C, Delrue MA, Bayliss S, Martorell L, Gonzalez-Ensenat MA, Mazereeuw-Hautier J, O’Donnell B, Bessis D, Pyeritz RE, Salhi A, Tan OT, Wargon O, Mulliken JB, Vikkula M. RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum Mutat. 2013;34(12):1632–1641. doi: 10.1002/humu.22431. [DOI] [PubMed] [Google Scholar]
- 4.Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, North PE, Marchuk DA, Comi AM, Pevsner J. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368(21):1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan W, Jia W, Sun V, Mihm MC, Jr, Nelson JS. Topical rapamycin suppresses the angiogenesis pathways induced by pulsed dye laser: Molecular mechanisms of inhibition of regeneration and revascularization of photocoagulated cutaneous blood vessels. Lasers Surg Med. 2012;44(10):796–804. doi: 10.1002/lsm.22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smoller BR, Rosen S. Port-wine stains. A disease of altered neural modulation of blood vessels? Arch Dermatol. 1986;122(2):177–179. doi: 10.1001/archderm.122.2.177. [DOI] [PubMed] [Google Scholar]
- 7.Laquer VT, Hevezi PA, Albrecht H, Chen TS, Zlotnik A, Kelly KM. Microarray analysis of port wine stains before and after pulsed dye laser treatment. Lasers Surg Med. 2013;45(2):67–75. doi: 10.1002/lsm.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troilius A, Wrangsjo B, Ljunggren B. Patients with port-wine stains and their psychosocial reactions after photothermolytic treatment. Dermatol Surg. 2000;26(3):190–196. doi: 10.1046/j.1524-4725.2000.09204.x. [DOI] [PubMed] [Google Scholar]
- 9.Hennedige AA, Quaba AA, Al-Nakib K. Sturge-Weber syndrome and dermatomal facial port-wine stains: Incidence, association with glaucoma, and pulsed tunable dye laser treatment effectiveness. Plast Reconstr Surg. 2008;121(4):1173–1180. doi: 10.1097/01.prs.0000304606.33897.71. [DOI] [PubMed] [Google Scholar]
- 10.Geronemus RG, Ashinoff R. The medical necessity of evaluation and treatment of port-wine stains. J Dermatol Surg Oncol. 1991;17(1):76–79. doi: 10.1111/j.1524-4725.1991.tb01597.x. [DOI] [PubMed] [Google Scholar]
- 11.van Drooge AM, Beek JF, van der Veen JP, van der Horst CM, Wolkerstorfer A. Hypertrophy in port-wine stains: Prevalence and patient characteristics in a large patient cohort. J Am Acad Dermatol. 2012;67(6):1214–1219. doi: 10.1016/j.jaad.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Brauer JA, Geronemus RG. Single-treatment resolution of vascular blebs within port wine stains using a novel 1,064-nm neodymium-doped yttrium aluminum garnet laser. Dermatol Surg. 2013;39(7):1113–1115. doi: 10.1111/dsu.12207. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RR, Parrish JA. Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220(4596):524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 14.Tan OT, Sherwood K, Gilchrest BA. Treatment of children with port-wine stains using the flashlamp-pulsed tunable dye laser. N Engl J Med. 1989;320(7):416–421. doi: 10.1056/NEJM198902163200702. [DOI] [PubMed] [Google Scholar]
- 15.Tan OT, Morrison P, Kurban AK. 585nm for the treatment of port-wine stains. Plast Reconstr Surg. 1990;86(6):1112–1117. doi: 10.1097/00006534-199012000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Chang CJ, Kelly KM, Van Gemert MJ, Nelson JS. Comparing the effectiveness of 585-nm vs 595-nm wavelength pulsed dye laser treatment of port wine stains in conjunction with cryogen spray cooling. Lasers Surg Med. 2002;31(5):352–358. doi: 10.1002/lsm.10102. [DOI] [PubMed] [Google Scholar]
- 17.Kimel S, Svaasand LO, Cao D, Hammer-Wilson MJ, Nelson JS. Vascular response to laser photothermolysis as a function of pulse duration, vessel type, and diameter: Implications for port wine stain laser therapy. Lasers Surg Med. 2002;30(2):160–169. doi: 10.1002/lsm.10016. [DOI] [PubMed] [Google Scholar]
- 18.Izikson L, Nelson JS, Anderson RR. Treatment of hypertrophic and resistant port wine stains with a 755nm laser: A case series of 20 patients. Lasers Surg Med. 2009;41(6):427–432. doi: 10.1002/lsm.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Horst CM, Koster PH, de Borgie CA, Bossuyt PM, van Gemert MJ. Effect of the timing of treatment of port-wine stains with the flash-lamp-pumped pulsed-dye laser. N Engl J Med. 1998;338(15):1028–1033. doi: 10.1056/NEJM199804093381504. [DOI] [PubMed] [Google Scholar]
- 20.Lanigan SW. Port-wine stains unresponsive to pulsed dye laser: Explanations and solutions. Br J Dermatol. 1998;139(2):173–177. doi: 10.1046/j.1365-2133.1998.02351.x. [DOI] [PubMed] [Google Scholar]
- 21.Troilius A, Wardell K, Bornmyr S, Nilsson GE, Ljunggren B. Evaluation of port wine stain perfusion by laser Doppler imaging and thermography before and after argon laser treatment. Acta Derm Venereol. 1992;72(1):6–10. [PubMed] [Google Scholar]
- 22.Nelson JS, Kelly KM, Zhao Y, Chen Z. Imaging blood flow in human port-wine stain in situ and in real time using optical Doppler tomography. Arch Dermatol. 2001;137(6):741–744. [PubMed] [Google Scholar]
- 23.Choi B, Jia W, Channual J, Kelly KM, Lotfi J. The importance of long-term monitoring to evaluate the microvascular response to light-based therapies. J Invest Dermatol. 2008;128(2):485–488. doi: 10.1038/sj.jid.5700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia W, Sun V, Tran N, Choi B, Liu SW, Mihm MC, Jr, Phung TL, Nelson JS. Long-term blood vessel removal with combined laser and topical rapamycin antiangiogenic therapy: Implications for effective port wine stain treatment. Lasers Surg Med. 2010;42(2):105–112. doi: 10.1002/lsm.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YC, Ringold TL, Nelson JS, Choi B. Noninvasive blood flow imaging for real-time feedback during laser therapy of port wine stain birthmarks. Lasers Surg Med. 2008;40(3):167–173. doi: 10.1002/lsm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YC, Tran N, Shumaker PR, Kelly K, Ross EV, Nelson JS, Choi B. Blood flow dynamics after laser therapy of port wine stain birthmarks. Lasers Surg Med. 2009;41(8):563–571. doi: 10.1002/lsm.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilar G, Choi B, Broekgaarden M, Yang O, Yang B, Ghasri P, Chen JK, Bezemer R, Nelson JS, van Drooge AM, Wolkerstorfer A, Kelly KM, Heger M. An overview of three promising mechanical, optical, and biochemical engineering approaches to improve selective photothermolysis of refractory port wine stains. Ann Biomed Eng. 2012;40(2):486–506. doi: 10.1007/s10439-011-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang O, Cuccia D, Choi B. Real-time blood flow visualization using the graphics processing unit. J Biomed Opt. 2011;16(1):016009. doi: 10.1117/1.3528610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi B, Ramirez-San-Juan JC, Lotfi J, Stuart Nelson J. Linear response range characterization and in vivo application of laser speckle imaging of blood flow dynamics. J Biomed Opt. 2006;11(4):041129. doi: 10.1117/1.2341196. [DOI] [PubMed] [Google Scholar]
- 30.Kauvar AN, Geronemus RG. Treatment of port-wine stains. N Engl J Med. 1998;339(9):635–636. doi: 10.1056/NEJM199808273390917. [DOI] [PubMed] [Google Scholar]
- 31.Choi B, Majaron B, Nelson JS. Computational model to evaluate port wine stain depth profiling using pulsed photo-thermal radiometry. J Biomed Opt. 2004;9(2):299–307. doi: 10.1117/1.1646173. [DOI] [PubMed] [Google Scholar]
- 32.Selim MM, Kelly KM, Nelson JS, Wendelschafer-Crabb G, Kennedy WR, Zelickson BD. Confocal microscopy study of nerves and blood vessels in untreated and treated port wine stains: Preliminary observations. Dermatol Surg. 2004;30(6):892–897. doi: 10.1111/j.1524-4725.2004.30259.x. [DOI] [PubMed] [Google Scholar]
- 33.Nelson JS, Jia W, Phung TL, Mihm MCJ. Observations on enhanced port wine stain blanching induced by combined pulsed dye laser and rapamycin administration. Lasers Surg Med. 2011;43(10):939–942. doi: 10.1002/lsm.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren J, Li P, Zhao H, Chen D, Zhen J, Wang Y, Gu Y. Assessment of tissue perfusion changes in port wine stains after vascular targeted photodynamic therapy: A short-term follow-up study. Lasers Med Sci. 2013 doi: 10.1007/s10103-013-1420-4. [DOI] [PubMed] [Google Scholar]
- 35.Tanghetti E, Sherr EA, Sierra R, Mirkov M. The effects of pulse dye laser double-pass treatment intervals on depth of vessel coagulation. Lasers Surg Med. 2006;38(1):16–21. doi: 10.1002/lsm.20273. [DOI] [PubMed] [Google Scholar]
- 36.Rajaratnam R, Laughlin SA, Dudley D. Pulsed dye laser double-pass treatment of patients with resistant capillary malformations. Lasers Med Sci. 2011;26(4):487–492. doi: 10.1007/s10103-011-0913-2. [DOI] [PubMed] [Google Scholar]
- 37.Mazhar A, Cuccia DJ, Rice TB, Carp SA, Durkin AJ, Boas DA, Choi B, Tromberg BJ. Laser speckle imaging in the spatial frequency domain. Biomed Opt Express. 2011;2(6):1553–1563. doi: 10.1364/BOE.2.001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazhar A, Sharif SA, Cuccia JD, Nelson JS, Kelly KM, Durkin AJ. Spatial frequency domain imaging of port wine stain biochemical composition in response to laser therapy: A pilot study. Lasers Surg Med. 2012;44(8):611–621. doi: 10.1002/lsm.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang O, Choi B. Laser speckle imaging using a consumer-grade color camera. Opt Lett. 2012;37(19):3957–3959. doi: 10.1364/OL.37.003957. [DOI] [PMC free article] [PubMed] [Google Scholar]