Abstract
Calcium (Ca2+) has an important role in nearly all types of cellular secretion, with a particularly novel role in the juxtaglomerular (JG) cells in the kidney. In JG cells, Ca2+ inhibits renin secretion, which is a major regulator of blood pressure and renal hemodynamics. However, whether alterations in afferent arteriolar (Af-Art) pressure change intracellular Ca2+ concentration ([Ca2+]i) in JG cells and whether [Ca2+]i comes from extracellular or intracellular sources remains unknown. We hypothesize that increases in perfusion pressure in the Af-Art result in elevations in [Ca2+]i in JG cells. We isolated and perfused Af-Art of C57BL6 mice and measured changes in [Ca2+]i in JG cells in response to perfusion pressure changes. The JG cells’ [Ca2+]i was 93.3 ± 2.2 nm at 60 mm Hg perfusion pressure and increased to 111.3 ± 13.4, 119.6 ± 7.3, 130.3 ± 2.9 and 140.8 ± 12.1 nm at 80, 100, 120 and 140 mm Hg, respectively. At 120 mm Hg, increases in [Ca2+]i were reduced in mice receiving the following treatments: (1) the mechanosensitive cation channel blocker, gadolinium (94.6 ± 7.5 nm); (2) L-type calcium channel blocker, nifedipine (105.8 ± 7.5 nm); and (3) calcium-free solution plus ethylene glycol tetraacetic acid (96.0 ± 5.8 nm). Meanwhile, the phospholipase C inhibitor, inositol triphosphate receptor inhibitor, T-type calcium channel blocker, N-type calcium channel blocker and Ca2+-ATPase inhibitor did not influence changes in [Ca2+]i in JG cells. In summary, JG cell [Ca2+]i rise as perfusion pressure increases; furthermore, the calcium comes from extracellular sources, specifically mechanosensitive cation channels and L-type calcium channels.
Keywords: angiotensin, juxtaglomerular apparatus, kidney, myogenic response, renin
INTRODUCTION
The renin–angiotensin system is one of the most important long-term blood pressure regulation systems. The rate-limiting enzyme in this hormonal cascade is renin. Renin is stored and secreted into circulation primarily from renal juxtaglomerular (JG) cells, which are the modified vascular smooth muscle cells of the renal afferent arteriole (Af-Art) adjacent to the glomerulus.
Renin secretion is stimulated by renal sympathetic nerve activity, low renal perfusion pressure, sodium depletion and inhibition of angiotensin II (Ang II) synthesis or action.1,2 A major control mechanism for renin secretion is the intrarenal baroreceptor. In the late 1950s, Tobian et al.3 first showed an inverse relationship of JG cell granularity to renal perfusion pressure, which was supported by subsequent experiments.4–7 In response to increases in perfusion pressure, Af-Arts constrict, in what is termed myogenic response8–11 accompanied by elevation of intracellular calcium concentrations ([Ca2+]i) in vascular smooth muscle cells.10–13 Unlike most secretory cells, renin secretion from the JG cell is inversely related to the [Ca2+]i.14 Calcium has been proposed as an important regulator in renin release. Lowering the concentration of calcium in incubation media, the use of calcium channel antagonists, and the use of calmodulin antagonists have been shown to increase renin release.15–24 Conversely, increasing [Ca2+]i by the infusion of a calcium ionophore inhibits renin release.25,26 However, it is unknown whether changes in perfusion pressure induce [Ca2+]i alterations in JG cells.
We used the isolated and perfused Af-Art and measured [Ca2+]i alterations in JG cells by changing the perfusion pressure of the Af-Art. We hypothesize that increases in perfusion pressure in the Af-Art result in elevations in [Ca2+]i in JG cells. The potential contributions of mechanosensitive cation channels, phospholipase C (PLC), inositol triphosphate (IP3)-induced release of intracellular calcium stores, membrane calcium channels, and T and L-type channels to [Ca2+]i alterations in JG cells were evaluated.
METHODS
Male mice of the C57BL6 strain weighing 22–25 g were used. Animals were fed with standard mouse chow and were allowed for free access to tap water. All procedures were approved by the Uppsala University and University of Mississippi Animal Care and Use Committee before any procedures being performed on animals.
Isolation and microperfusion of the mouse Af-Art
We used methods similar to those described previously to isolate and micro-perfuse the Af-Art.27–31 Briefly, the animals were killed by cervical dislocation, and the kidneys were removed and sliced along the corticomedullary axis. Slices were placed in ice-cold minimum essential medium containing 5% bovine serum albumin and were dissected under a stereomicroscope. A single superficial Af-Art and its intact glomerulus were microdissected. Using a micropipette, the microdissected complex was transferred to a temperature-regulated chamber mounted on an inverted microscope (Eclipse Ti, Nikon, Melville, NY, USA). The Af-Art was cannulated with an array of glass pipettes. Intraluminal pressure was measured according to Landis’ technique, using a fine pipette introduced into the Af-Art through the perfusion pipette. The Af-Art was perfused from the proximal end in an orthograde direction with minimum essential medium. The water in the bath was changed continuously at a rate of 1 ml min−1 with minimum essential medium. Microdissection and cannulation were completed within 60 min at 8 °C, after which the bath was gradually warmed to 37 °C for the rest of the experiment. Once the temperature was stable, a 30-min equilibration period was allotted before taking any measurements. The imaging system consisted of a microscope (Eclipse Ti, Nikon), a digital CCD camera (CoolSnap, Photometrics, Tucson, AZ, USA), a xenon light (LB-LS/30, Shutter Instruments, Novato, CA, USA) and an optical filter changer (Lambda 10–3, Shutter Instruments). Images were displayed and analyzed with NIS-Elements imaging software (Nikon).
Intracellular calcium ([Ca2+]i) measurement in JG cells
Once the Af-Art was perfused, calcium sensitive fluorescent dye fura-2 AM (3 µm in 0.1% dimethyl sulfoxide) was loaded from the lumen at 37 ± 1 °C for 30 min and then washed for 20 min. Intracellular dye was excited alternately at 340 and 380 nm and fluorescence emissions recorded at 510 nm. The JG cells were easily identified by their localization and morphology;32,33 these cells are specialized smooth muscle cells in the wall of the distal Af-Art and are distinguished by granulated cytoplasm. To measure changes of calcium concentration only in JG cells, we placed square-shaped regions of interest inside the cytoplasm of JG cells and measured mean intensity of fluorescence every 5 s for 2 min at each perfusion pressure. The [Ca2+]i was calculated from the following equation:34
where Kd is the dissociation constant of fura-2, R is the ratio of fluorescence at 380–340 nm, and Rmin and Rmax are the ratios for unbound and bound forms of the fura-2/Ca2+ complex in MD cells, respectively. Fo/Fs is the ratio of fluorescence at 380 nm in the absence of Ca2+ to saturating Ca2+ concentrations. We calibrated the system in situ.
Experimental protocols
After the 30-min equilibration period, the [Ca2+]i in the JG cells was measured for 2 min, as a basal measurement, when Af-Art was perfused at 60 mm Hg. Then, we increased the perfusion pressure to 80 mm Hg and measured the [Ca2+]i in the JG cells again for 2 min. After that, we returned the perfusion pressure to 60 mm Hg for 10 min and repeated the perfusion pressure manipulations from 80 to 140 mm Hg, measuring the [Ca2+]i in the JG cells. The relative perfusion pressure elevations used were from 60 to 80 mm Hg, from 60 to 100 mm Hg, from 60 to 120 mm Hg and from 60 to 140 mm Hg.
In the antagonist experiments, we only increased the perfusion pressure from 60 to 120 mm Hg. When the Af-Art was perfused at 60 mm Hg, the [Ca2+]i in the JG cells was measured for 2 min, as a basal measurement. Then, we increased the perfusion pressure to 120 mm Hg and measured the [Ca2+]i in the JG cells again for 2 min. After that, we returned the perfusion pressure to 60 mm Hg for 30 min, and one drug was added to the bath for each experiment. Then, we repeated the increases of perfusion pressure and [Ca2+]i measurement in the JG cells. The following drugs were used: (1) mechanosensitive cation channel blocker, adolinium (Gd3+) (30 µm); (2) PLC inhibitor, U-73122 (10 µM); (3) IP3 receptor inhibitor, 2-APB (100 µm); (4) calcium-free solution plus 5 µm ethylene glycol tetraacetic acid (EGTA) both in lumen and bath; (5) calcium ATPase inhibitor, thapsigargin (1 µm); (6) T-type calcium channel blocker, pimozide (10 µm); (7) N-type calcium channel blocker, ω-conotoxin GVIA (1 µm); and (8) L-type calcium channel blocker, nifedipine (25 µm).
Chemicals
Fura-2 AM was obtained from Invitrogen (Carlsbad, CA, USA). All other chemicals were obtained from Sigma (St Louis, MO, USA).
Statistics
Data were collected as repeated measures over time under different conditions. We tested only the effects of interest, using analysis of variance for repeated measures and a post hoc Fisher least significant difference test. The changes were considered significant if P<0.05. Data are presented as mean ± s.e.m.
RESULTS
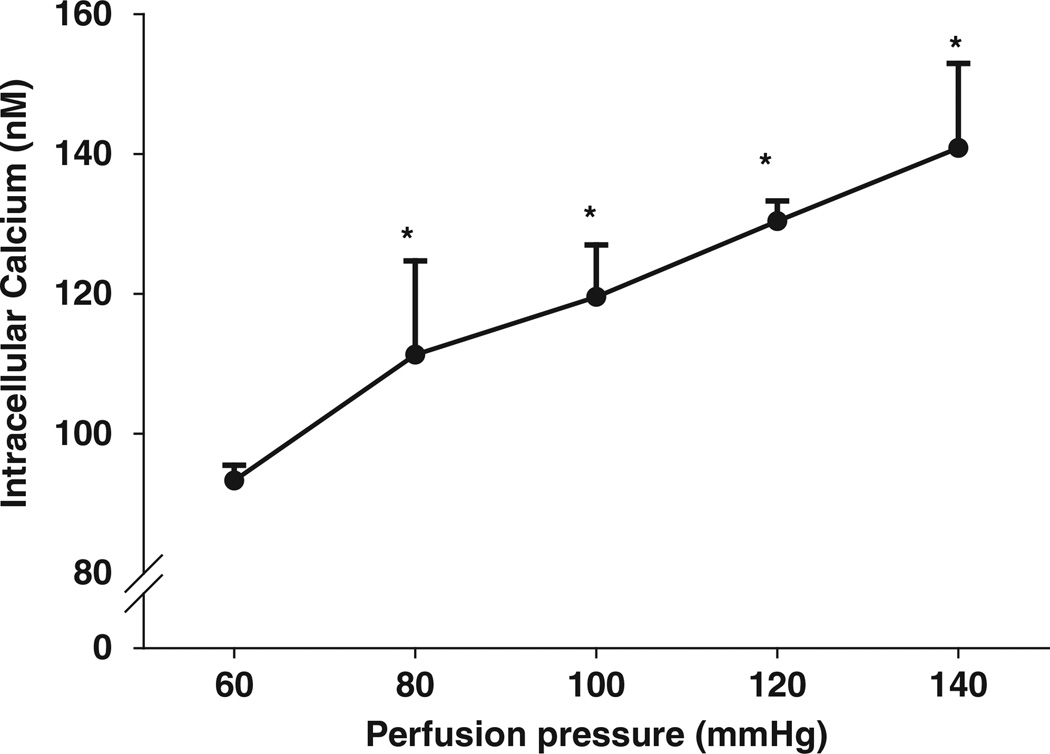
To measure [Ca2+]i in the Af-Art, we loaded calcium sensitive fluorescence dye fura-2 into the perfusate. JG cells were easily identified by their morphology and location in the perfused Af-Arts (Figure 1). When an Af-Art was perfused with 60 mm Hg, the basal [Ca2+]i in the JG cells was 93.3 ± 2.2 nm (n=45). When we increased perfusion pressure to 80, 100, 120 and 140 mm Hg, [Ca2+]i in JG cells were 111.3 ± 13.4 (n=6); 119.6 ± 7.3 (n=6); 130.3 ± 2.9 (n=45); and 140.8 ± 12.1 nm (n=6), respectively, with P<0.05 for all manipulations (Figures 1 and 2). Linear regression is Y=55.7 ± 0.623X (r2=0.474, P<0.0001).
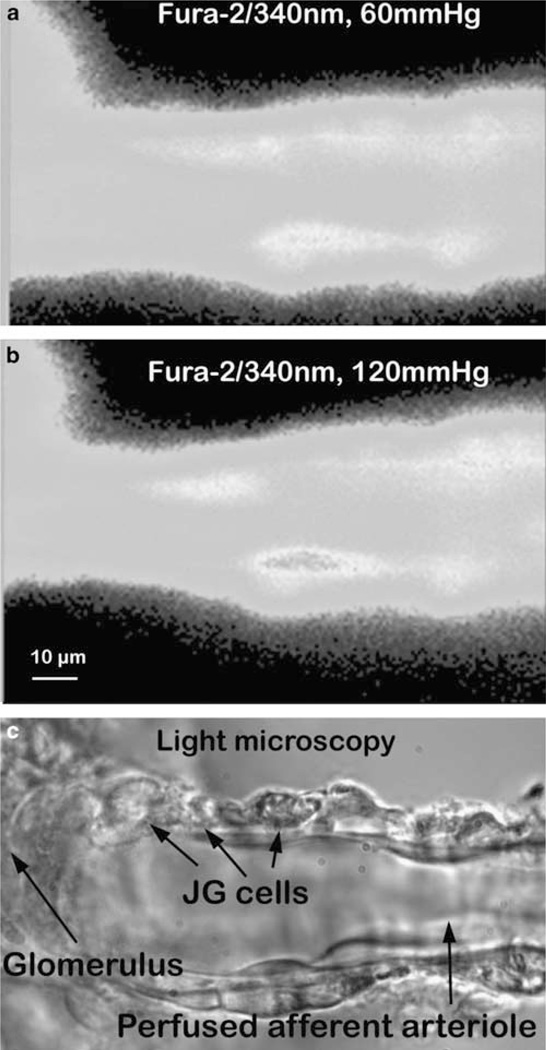
Figure 1.
The perfused afferent arteriole and juxtaglomerular (JG) cells. Fura-2 AM-loaded JG cells excited at 340 nm. Perfusion pressure was increased from 60 (a) to 120 mm Hg (b); intensity of fura-2 signals in JG cells increased, accompanied by vasoconstriction. (c) Image of light microscopy showing JG cells. A full color version of this figure is available at the Hypertension Research journal online.
Figure 2.
The changes in juxtaglomerular (JG) cell [Ca2+]i while the afferent arteriole pressure was increased from 60 to 80, 100, 120 and 140 mm Hg, *All with P<0.05 vs. 60 mm Hg. Linear regression is Y=55.7 ± 0.623X (r2=0.474, P<0.0001).
The [Ca2+]i in the JG cells was very sensitive to pressure changes from 60 to 80 mm Hg. When perfusion pressure was increased to 80 and 140 mm Hg, the [Ca2+]i in the JG cells responded in a linear manner. In the subsequent experiments, we used only one pressure change from 60 to 120 mm Hg. To demonstrate that the increases of pressure-induced [Ca2+]i in JG cells were reproducible, we performed time control experiments. When we increased the perfusion pressure of the Af-Art from 60 to 120 mm Hg, the [Ca2+]i in the JG cells increased from 95.5 ± 8.7 to 133.1 ± 9.2 nm. Then, we returned the perfusion pressure to 60 mm Hg for 30 min. When we increased the perfusion pressure of the Af-Art to 120 mm Hg again, the [Ca2+]i in the JG cells increased from 99.2 ± 6.5 to 129.1 ± 5.9 nm (n=4, P<0.01 60 vs. 120 mm Hg), indicating the pressure-induced [Ca2+]i changes in the JG cells were reproducible.
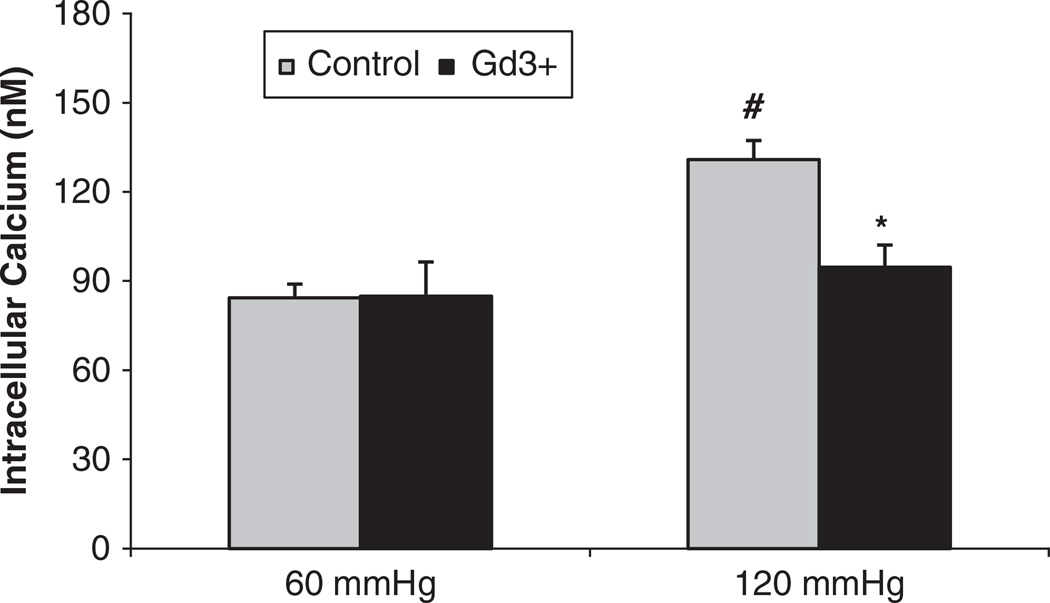
To determine whether the pressure-induced increases in [Ca2+]i in the JG cells were mediated by mechanosensitive cation channels, we used Gd3+, a mechanosensitive cation channel blocker, as shown in Figure 3. In control experiments, when we increased perfusion pressure of the Af-Art from 60 to 120 mm Hg, the [Ca2+]i in the JG cells increased from 84.3 ± 4.6 to 130.9 ± 11.5 nm (P<0.01). Then we returned the perfusion pressure to 60 mm Hg and added Gd3+ (30 µm) to the bath for 30 min. When we increased perfusion pressure of Af-Art to 120 mm Hg again in the presence of Gd3+, the [Ca2+]i in the JG cells increased from 84.8 ± 6.3 to 94.6 ± 7.5 nm (n=5, P<0.05) compared with the control group at the same pressure (Figure 3). These data indicate that mechanosensitive cation channels mediate pressure-induced changes of [Ca2+]i in JG cells.
Figure 3.
Juxtaglomerular (JG) cell [Ca2+]i changes induced by increases in afferent arteriolar pressure from 60 to 120 mm Hg with and without the mechanosensitive cation channel inhibitor gadolinium (Gd3+). The [Ca2+]i levels in the JG cells in the presence of Gd3+ (30 µm) were significantly decreased compared with that of the control group at 120 mm Hg. #P<0.01 vs 60 mm Hg; *P<0.05 vs. 120 mm Hg control, n=5.
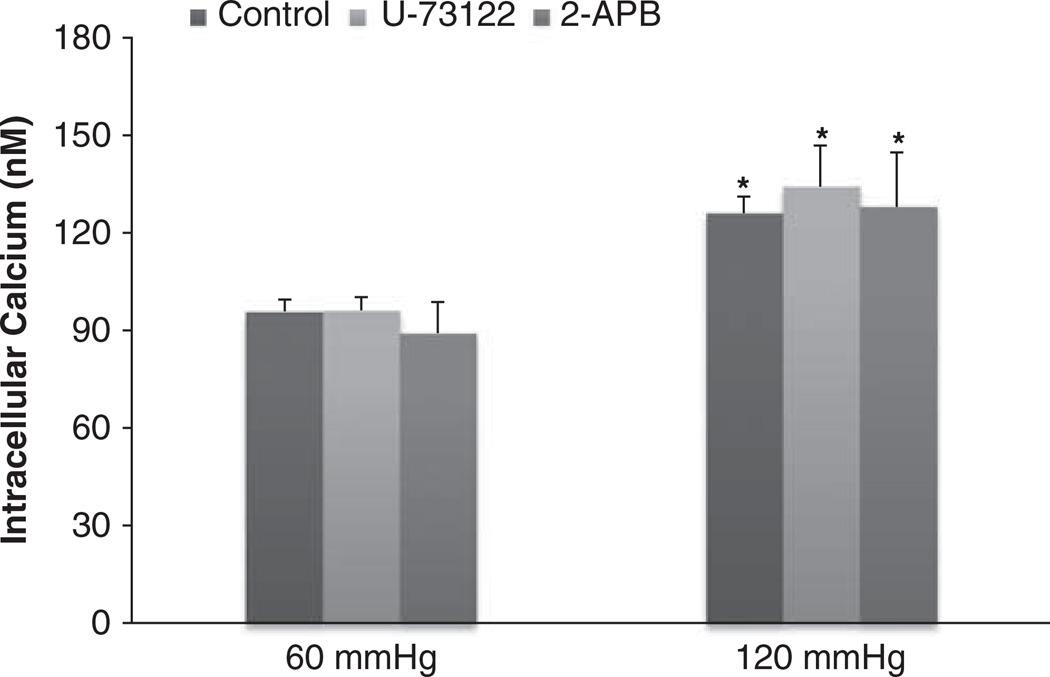
We next determined whether the pressure-induced increases of [Ca2+]i in the JG cells were due to the inhibition of PLC in the PLC–IP3 pathway, as shown in Figure 4.When we increased perfusion pressure of the Af-Art from 60 to 120 mm Hg, the [Ca2+]i in the JG cells increased from 98.2 ± 2.9 to 127.4 ± 8.1 nm (P<0.05). Then we returned the perfusion pressure to 60 mm Hg and added the PLC inhibitor U-73122 (10 µm) into the bath for 30 min. The [Ca2+]i in the JG cells was 96.13 ± 4.0 nm. When we increased perfusion pressure of the Af-Art to 120 mm Hg again in the presence of U-73122, the [Ca2+]i in the JG cells was 134.2 ± 12.6 nm (n=5, P=NS compared with control group). These data indicate that pressure-induced changes in the [Ca2+]i in the JG cells did not involve changes in PLC.
Figure 4.
Juxtaglomerular (JG) cell [Ca2+]i changes induced by increases in afferent arteriolar pressure from 60 to 120 mm Hg with and without the phospholipase C (PLC) or inositol triphosphate (IP3) inhibitors. The [Ca2+]i in JG cells in the presence of PLC inhibitor U-73122 (10 µm, n=5) or IP3 receptor inhibitor 2-APB (100 µm, n=3) was not significantly different from the control group at 120 mm Hg. *P<0.05 vs. respective 60 mm Hg controls.
To verify whether the pressure-induced increases of [Ca2+]i in the JG cells were from IP3 sensitive calcium stores, we used an IP3 receptor inhibitor. When we increased perfusion pressure of the Af-Art from 60 to 120 mm Hg, the [Ca2+]i in the JG cells increased from 92.2 ± 8.5 to 123.9 ± 5.6 nm (P<0.05). Then we returned the perfusion pressure to 60 mm Hg and added the IP3 receptor inhibitor 2-APB (100 µm) into the bath for 30 min. When we increased the perfusion pressure of the Af-Art to 120 mm Hg again in the presence of 2-APB, the [Ca2+]i in the JG cells increased from 89.1 ± 9.5 to 127.9 ± 16.7 nm (n=3, P=NS compared with the control group; Figure 4). These data indicate that pressure-induced changes in the [Ca2+]i in JG cells were not from IP3-induced release of intracellular calcium stores. Because there were no significant differences between the controls for U-73122 and 2-APB-treated groups, we combined the controls together in Figure 4.
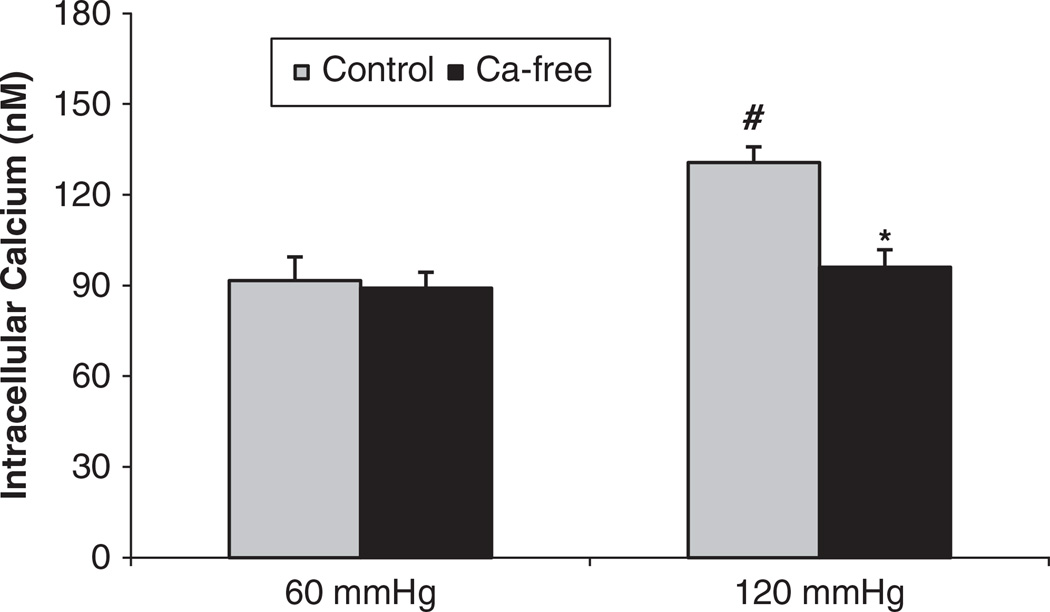
To measure whether the pressure-induced increases in the [Ca2+]i in the JG cells were via membrane calcium channels, we first repeated the experiment in a calcium-free solution. When we increased perfusion pressure of the Af-Art from 60 to 120 mm Hg, the [Ca2+]i in the JG cells increased from 91.6 ± 7.8 to 130.7 ± 5.3 nm (P<0.01). Then we returned perfusion pressure to 60 mm Hg and used a calcium-free solution plus 5 µm EGTA both in the lumen and the bath for 10 min. When we increased the perfusion pressure of the Af-Art to 120 mm Hg again, the [Ca2+]i in the JG cells increased to only 96.0 ± 5.8 nm (n=5, P<0.05 compared with the control group; Figure 5). These data indicate that pressure-induced changes of [Ca2+]i in JG cells was from extracellular sources via membrane channels.
Figure 5.
Juxtaglomerular (JG) cell [Ca2+]i changes induced by increases in afferent arteriolar pressure from 60 to 120 mm Hg with and without the calcium-free solution plus 5 µm EGTA. The [Ca2+]i in the JG cells in the presence of the calcium-free solution plus 5 µm EGTA was significantly decreased compared with that of the control group at 120 mm Hg. #P<0.01 vs. 60 mm Hg; *P<0.05 vs. 120 mm Hg control, n=5.
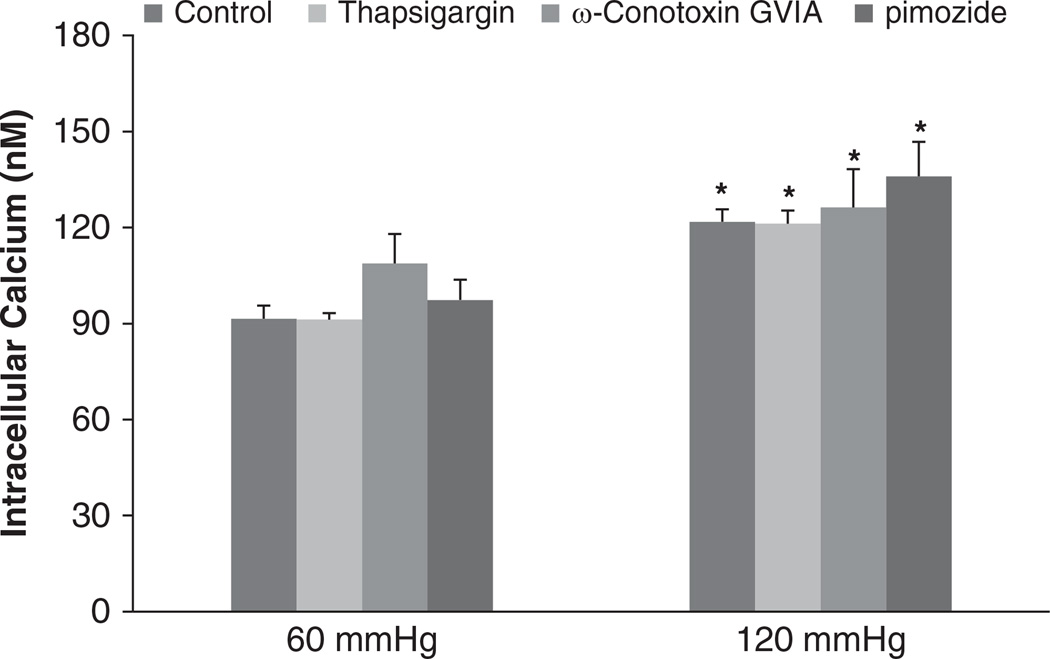
To determine whether the pressure-induced increases in the [Ca2+]i in the JG cells involved store-operated calcium influx, we used a Ca2+-ATPase inhibitor. When we increased the perfusion pressure of the Af-Art from 60 to 120 mm Hg, the [Ca2+]i in the JG cells increased from 84.2 ± 2.8 to 116.2 ± 1.4 nm (P<0.05). Then we returned the perfusion pressure to 60 mm Hg and added a calcium ATPase inhibitor, thapsigargin (1 µm), into the bath for 30 min. When we increased the perfusion pressure of the Af-Art to 120 mm Hg again in the presence of thapsigargin, the [Ca2+]i in the JG cells increased to 121.2 ± 4.1 nm (n=5, P=NS compared with the control group; Figure 6). These data indicated that the pressure-induced changes in the [Ca2+]i in the JG cells were not from store-operated calcium influx.
Figure 6.
Juxtaglomerular (JG) cell [Ca2+]i changes induced by increases in afferent arteriolar pressure from 60 to 120 mm Hg with and without the Ca2+-ATPase, T- or N-type inhibitors. The [Ca2+]i in the JG cells in the presence of Ca2+-ATPase inhibitor thapsigargin (1 µm, n=5), T-type calcium channel blocker pimozide (10 µm, n=4), or N-type calcium channel blocker pimozide ω-conotoxin GVIA (1 µm, n=5) were not significantly different from the control group at 120 mm Hg. *P<0.05 vs. respective 60 mm Hg controls.
We also studied whether the pressure-induced increases in [Ca2+]i in the JG cells were mediated by T-type calcium channels. In a control experiment, when we increased the perfusion pressure of the Af-Art from 60 to 120 mm Hg, the [Ca2+]i in the JG cells increased from 87.5 ± 5.9 to 128.4 ± 11.5 nm (P<0.05). Then we returned perfusion pressure to 60 mm Hg and added a T-type calcium channel blocker, pimozide (10 µm), to the bath for 30 min. When we increased the perfusion pressure of the Af-Art to 120 mm Hg again in the presence of pimozide, the [Ca2+]i in the JG cells increased from 97.3 ± 5.9 to 135.9 ± 22.0 nm (n=4, P=NS compared with the control group; Figure 6). These data indicate that the T-type channels did not cause pressure-induced changes in the [Ca2+]i in the JG cells.
To test whether N-type calcium channel causes pressure-induced increases of [Ca2+]i in JG cells, we measured [Ca2+]i levels during inhibition of this channel. When we increased the perfusion pressure of the Af-Art from 60 to 120 mm Hg, the [Ca2+]i in the JG cells increased from 102.8 ± 9.2 to 120.7 ± 2.6 nm (P<0.05). Then we returned the perfusion pressure to 60 mm Hg and added an N-type calcium channel antagonist, ω-conotoxin GVIA (1 µm), to the bath for 30 min. When we increased the perfusion pressure of the Af-Art to 120 mm Hg again in the presence of ω-conotoxin GVIA, the [Ca2+]i in the JG cells increased to 126.3 ± 11.9 nm (n=5, P=NS compared with the control group; Figure 6). These data indicate that the pressure-induced changes in the [Ca2+]i in the JG cells were not from N-type calcium channels. Because the controls for the above three groups were not significantly different, we combined the controls together in Figure 6.
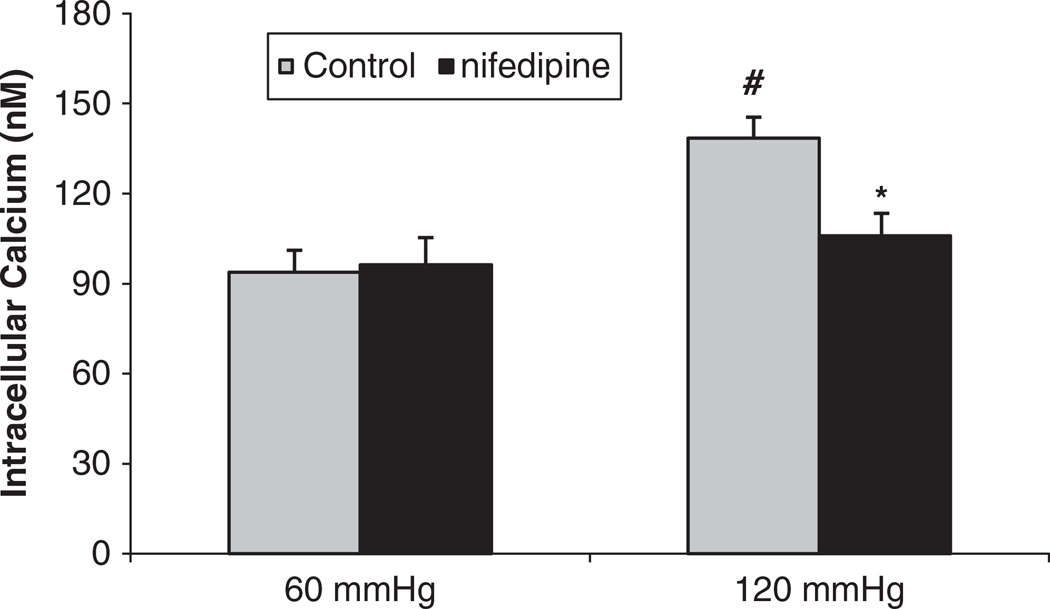
To determine whether the pressure-induced increases of [Ca2+]i in the JG cells were mediated by L-type calcium channels, we used nifedipine, an antagonist of L-type calcium channels. In a control experiment, when we increased the perfusion pressure of the Af-Art from 60 to 120 mm Hg, the [Ca2+]i in the JG cells increased from 93.8 ± 7.3 to 138.4 ± 9.1 nm (P<0.01). Then we returned the perfusion pressure to 60 mm Hg and added the L-type calcium channel blocker nifedipine (25 µm) to the bath for 30 min. When we increased the perfusion pressure of the Af-Art to 120 mm Hg again in the presence of nifedipine, the [Ca2+]i in the JG cells increased to only 105.8 ± 7.5 nm (n=5, P<0.05 compared with the control group; Figure 7). These data indicate that pressure-induced changes in [Ca2+]i entry into the JG cells were partially via L-type channels.
Figure 7.
Juxtaglomerular (JG) cell [Ca2+]i changes induced by increases in afferent arteriolar pressure from 60 to 120 mm Hg with and without the L-type calcium channel blocker nifedipine (25 µm). The [Ca2+]i in JG cells in the presence of nifedipine was significantly decreased compared with that of the control group at 120 mm Hg. #P<0.01 vs. 60 mm Hg; *P<0.05 vs. 120 mm Hg control, n=5.
DISCUSSION
Ca2+ supports exocytosis in most secretory cells, but has an inverse relationship in only JG cells and the parathyroid gland.14 In JG cells, Ca2+ inhibits renin secretion, which is a major regulator of blood pressure and renal hemodynamics. During increases in Af-Art pressure, a myogenic response and a decrease in renin secretion by JG cells occurs.10–13 However, whether alterations in Af-Art pressure affect [Ca2+]i in JG cells and whether Ca2+ comes from extracellular or intracellular sources remains unknown.
We have shown that increases in Af-Art pressure causes increases in [Ca2+]i in JG cells. As the Af-Art pressure was increased from 60 to 140 mm Hg in a stepwise manner, the [Ca2+]i increased in a linear manner. Therefore, [Ca2+]i changes in JG cells during alterations in Af-Art pressure are an excellent candidate for the regulation of renin release.
Other new information provided by this study includes the sources of the increase in [Ca2+]i during elevation of Af-Art pressure. In our first study, we blocked pressure-gated mechanosensitive Ca2+ channels with gadolinium, as shown in Figure 3. Gadolinium has previously been shown to block these mechanosensitive channels in Af-Arts.35 The ability of this drug to block Ca2+ entry during high pressures demonstrates the importance of mechanosensitive channels in the [Ca2+]i of JG cells.
During different stimulations, multiple mechanisms may be involved in alterations of [Ca2+]i in JG cells, for example, voltage-gated L-type and T-type calcium channels;16,36 endoplasmic reticulum intracellular calcium stores, including IP3 and ryanodine receptor sensitive stores;37–39 and store-operated calcium channels.39 Most research studying signaling of [Ca2+]i in JG cells are performed in cultured cells. To our knowledge, pressure-induced alterations in [Ca2+]i in JG cells in perfused Af-Arts have not been studied.
First, we determined whether the source of the [Ca2+]i was intracellular or extracellular. We found that the pressure-induced Ca2+ entry into JG cells was markedly attenuated by perfusing JG cells with a calcium-free solution plus EGTA. However, inhibition of PLC and IP3 receptors had no effect on [Ca2+]i. These data indicate that the calcium increases in [Ca2+]i in the JG cells were primarily from extracellular sources.
A couple of elegant studies have emphasized the importance of [Ca2+]i in the Af-Art myogenic response and renin release. Inscho et al.40 found that calcium released from intracellular calcium stores has an important role in pressure-mediated Af-Art constriction. Increasing perfusion to 130 and 160 mm Hg reduced the Af-Art diameter. The pressure-induced vasoconstriction was also significantly attenuated by Ca2+-ATPases or PLC inhibition. Schweda et al.39 reported that store-operated calcium influx is a powerful mechanism to inhibit renin secretion from JG cells. Renin secretion stimulated by isoproterenol was inhibited by endoplasmatic Ca2+-ATPases antagonists thapsigargin and cyclopiazonic acid, which appears contradictory to our results. We believe that the discrepancy in the Schweda data and the present experimental results is due to the different experimental models. The Schweda experiments were performed in isolated perfused kidneys. Increasing perfusion pressure inhibits reabsorbtion in the proximal tubule and raises flow rate and NaCl delivery to macula densa, which induces adenosine release. Adenosine or ATP acts on the Af-Art, inducing a tubuloglomerular feedback response. Either adenosine or ATP induces calcium mobilization by intracellular stores.41 In our preparation, we totally excluded confounding factors such as tubuloglomerular feedback (TGF), angiotensin II and the sympathetic nervous system. In addition, we specifically focused on JG cells.
Next, we measured which Ca2+ channel is involved in pressure-induced increases of [Ca2+]i in JG cells. Although we found no evidence that T channels have a role in JG cell Ca2+ entry, inhibition of L-type Ca2+ channels with nifedipine considerably decreased the [Ca2+]i in the JG cells. Therefore, a pattern has emerged indicating that the sources of increased JG cell Ca2+ levels under high pressure are predominately extracellular. The mechanosensitive channels may initiate Ca2+ entry, which could contribute to membrane depolarization and, in turn, could open the voltage sensitive L-type Ca2+ channels. It is not clear why the T-type channels did not have a role in this response, but perhaps their density in JG cells is low.
A single dose (10 mg) of nifedipine exerts a potent arteriolar vasodilating action, resulting in a prompt fall in mean arterial pressure in hypertensive patients.42 Intravenous or intrarenal administration of nifedipine lower arterial pressure,43,44 increase renal blood flow (RBF) and glomerular filtration rate (GFR) by a preferential afferent dilatory mechanism45 and induce renin release.46 However, in our experiment, nifedipine had no effect on [Ca2+]i in JG cells when the Af-Art was perfused at 60 mm Hg, which is in agreement with the data from isolated perfused kidney and arterioles. In the isolated perfused kidney, when administered under basal conditions, nifedipine had no effect on renal perfusate flow, GFR or the diameter of Af-Art.35,47–50 When the perfusion pressure increased above 120 mm Hg, calcium antagonists nifedipine or verapamil showed a vasodilator effect.48,51,52 In microdissected and perfused Af-Arts, stimulation with nifedipine or verapamil in mice53 and rats54 did not change the intracellular calcium concentration of the arterioles when they were perfused at 60 mm Hg, potentially due to the lack of intrinsic renal vascular tone.
Recently, N-type calcium channels have reportedly been important in renin activity. A clinical trial showed that an N-type calcium channel blocker reduced plasma renin activity compared with amlodipine in hypertensive patients.55 An animal experiment revealed that renin mRNA expression was higher in renal cortical tissue of spontaneously hypertensive rat (SHR) than in Wistar Kyoto rat (WKY) and was not affected by an N-type calcium channel blocker.56 Therefore, we measured pressure-induced changes in [Ca2+]i in JG cells in the presence of an N-type calcium channel antagonist, ω-conotoxin GVIA.57,58 Our data did not support the involvement of N-type channels in the pressure-induced [Ca2+]i of the JG cells. However, in the above two reports,56,55 the calcium channel blocker used was cilnidipine, which is an L/N-type calcium blocker. We cannot exclude the possibility that cilnidipine is more potent than ω-conotoxin GVIA for N-type channels in JG cells, if it really exists in JG cells. In addition, we are not sure if there exists any interaction between N- and L-type channels.
The increase in [Ca2+]i in JG cells during high pressure may inhibit renin release. This could be caused by decreases in adenylyl cylase, as has been shown by Grunberger et al.59 in isolated JG cells. They concluded that the calcium-induced suppression of renin release in isolated JG cells was mediated by inhibition of adenylyl cyclase activity. Later experiments by Ortiz-Capisano et al.60 showed that a decrease in the adenylyl cyclase V isoform specifically caused a decrease in renin release during high cytosolic calcium condition.
In summary, this study has shown that [Ca2+]i in JG cells increase in isolated perfused Af-Arts as perfusion pressure increases. The sources of this calcium are extracellular, specifically mechanosensitive cation channels and L-type Ca2+ channels. Increases in JG cell cytosolic calcium could be responsible for decreases in JG cell renin release.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health Grants RO1-HL086767 (RL) and by the Swedish Research Council (K2009-64X-03522-38-2) and the Swedish Heart and Lung Foundation (20090264) (AEGP).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 2.Wagner C, Kurtz A. Regulation of renal renin release. Curr Opin Nephrol Hypertens. 1998;7:437–441. doi: 10.1097/00041552-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Tobian L, Tomboulian A, Janecek J. The effect of high perfusion pressures on the granulation of juxtaglomerular cells in an isolated kidney. J Clin Invest. 1959;38:605–610. doi: 10.1172/JCI103838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaine EH, Davis JO, Prewitt RL. Evidence for a renal vascular receptor in control of renin secretion. Am J Physiol. 1971;220:1593–1597. doi: 10.1152/ajplegacy.1971.220.6.1593. [DOI] [PubMed] [Google Scholar]
- 5.Bock HA, Hermle M, Brunner FP, Thiel G. Pressure dependent modulation of renin release in isolated perfused glomeruli. Kidney Int. 1992;41:275–280. doi: 10.1038/ki.1992.39. [DOI] [PubMed] [Google Scholar]
- 6.Kirchheim H, Ehmke H, Persson P. Physiology of the renal baroreceptor mechanism of renin release and its role in congestive heart failure. Am J Cardiol. 1988;62:68E–71E. doi: 10.1016/s0002-9149(88)80015-8. [DOI] [PubMed] [Google Scholar]
- 7.Nobiling R, Münter K, Bührle CP, Hackenthal E. Influence of pulsatile perfusion upon renin release from the isolated perfused rat kidney. Pflugers Arch. 1990;415:713–717. doi: 10.1007/BF02584010. [DOI] [PubMed] [Google Scholar]
- 8.Casellas D, Moore LC. Autoregulation and tubuloglomerular feedback in juxtamedullary glomerular arterioles. Am J Physiol. 1990;258:F660–F669. doi: 10.1152/ajprenal.1990.258.3.F660. [DOI] [PubMed] [Google Scholar]
- 9.Holstein-Rathlou NH, Marsh DJ. Renal blood flow regulation and arterial pressure fluctuations: a case study in nonlinear dynamics. Physiol Rev. 1994;74:637–681. doi: 10.1152/physrev.1994.74.3.637. [DOI] [PubMed] [Google Scholar]
- 10.Takenaka T, Harrison-Bernard LM, Inscho EW, Carmines PK, Navar LG. Autoregulation of afferent arteriolar blood flow in juxtamedullary nephrons. Am J Physiol. 1994;267:F879–F887. doi: 10.1152/ajprenal.1994.267.5.F879. [DOI] [PubMed] [Google Scholar]
- 11.Yip KP, Marsh DJ. [Ca2+]i in rat afferent arteriole during constriction measured with confocal fluorescence microscopy. Am J Physiol. 1996;271(5 Part 2):F1004–F1011. doi: 10.1152/ajprenal.1996.271.5.F1004. [DOI] [PubMed] [Google Scholar]
- 12.Carmines PK, Mitchell KD, Navar LG. Effects of calcium antagonists on renal hemodynamics and glomerular function. Kidney Int Suppl. 1992;36:S43–S48. [PubMed] [Google Scholar]
- 13.Hayashi K, Epstein M, Loutzenhiser R. Pressure-induced vasoconstriction of renal microvessels in normotensive and hypertensive rats. Studies in the isolated perfused hydronephrotic kidney. Circ Res. 1989;65:1475–1484. doi: 10.1161/01.res.65.6.1475. [DOI] [PubMed] [Google Scholar]
- 14.Beierwaltes WH. The role of calcium in the regulation of renin secretion. Am J Physiol Renal Physiol. 2010;298:F1–F11. doi: 10.1152/ajprenal.00143.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumbach L, Skott O. Renin release from isolated rat glomeruli: seasonal variations and effects of D600 on the response to calcium deprivation. J Physiol. 1981;310:285–292. doi: 10.1113/jphysiol.1981.sp013549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Churchill PC. Effect of D-600 on inhibition of in vitro renin release in the rat by high extracellular potassium and angiotensin II. J Physiol (Lond) 1980;304:449–458. doi: 10.1113/jphysiol.1980.sp013335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Churchill PC. Calcium channel antagonists and renin release. Am J Nephrol. 1987;7:32–38. doi: 10.1159/000167540. [DOI] [PubMed] [Google Scholar]
- 18.Cohen AJ, Laurens P, Fray JCS. Suppression of renin secretion by insulin: dependence on extracellular calcium. Am J Physiol. 1983;245:E531–E534. doi: 10.1152/ajpendo.1983.245.6.E531. [DOI] [PubMed] [Google Scholar]
- 19.Ettienne EM, Fray JC. Influence of potassium, sodium, calcium, perfusion pressure, and isoprenaline on renin release induced by high concentrations of magnesium. J Physiol. 1979;292:373–380. doi: 10.1113/jphysiol.1979.sp012857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fray JC, Park CS. Influence of potassium, sodium, perfusion pressure, and isoprenaline on renin release induced by acute calcium deprivation. J Physiol. 1979;292:363–372. doi: 10.1113/jphysiol.1979.sp012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura M, Inagami T. Calmodulin antagonists stimulate renin release from isolated rat glomeruli. Endocrinology. 1983;112:1857–1859. doi: 10.1210/endo-112-5-1857. [DOI] [PubMed] [Google Scholar]
- 22.Park CS, Han DS, Fray JCS. Calcium in the control of renin secretion: Ca2+ influx as an inhibitory signal. Am J Physiol. 1981;240:F70–F74. doi: 10.1152/ajprenal.1981.240.1.F70. [DOI] [PubMed] [Google Scholar]
- 23.Scholz H, Hamann M, Gotz KH, Kurtz A. Role of calcium ions in the pressure control of renin secretion from the kidneys. Pflugers Arch. 1994;428:173–178. doi: 10.1007/BF00374855. [DOI] [PubMed] [Google Scholar]
- 24.van Dongen R, Peart WS. Calcium dependence of the inhibitory effect of angiotensin on renin secretion in the isolated perfused kidney of the rat. Br J Pharmacol. 1974;50:125–129. doi: 10.1111/j.1476-5381.1974.tb09599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumbach L, Leyssac PP. Studies on the mechanism of renin release from isolated superfused rat glomeruli: effects of calcium, calcium ionophore and lanthanum. J Physiol. 1977;273:745–764. doi: 10.1113/jphysiol.1977.sp012121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churchill PC. Possible mechanism of the inhibitory effect of ouabain on renin secretion from rat renal cortical slices. J Physiol. 1979;294:123–134. doi: 10.1113/jphysiol.1979.sp012919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai EY, Fahling M, Ma Z, Kallskog O, Persson PB, Patzak A, Persson AE, Hultström M. Norepinephrine increases calcium sensitivity of mouse afferent arteriole, thereby enhancing angiotensin II-mediated vasoconstriction. Kidney Int. 2009;76:953–959. doi: 10.1038/ki.2009.261. [DOI] [PubMed] [Google Scholar]
- 28.Lai EY, Martinka P, Fahling M, Mrowka R, Steege A, Gericke A, Sendeski M, Persson PB, Persson AE, Patzak A. Adenosine restores angiotensin II-induced contractions by receptor-independent enhancement of calcium sensitivity in renal arterioles. Circ Res. 2006;99:1117–1124. doi: 10.1161/01.RES.0000249530.85542.d4. [DOI] [PubMed] [Google Scholar]
- 29.Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, Persson AE. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int. 2006;70:690–698. doi: 10.1038/sj.ki.5001650. [DOI] [PubMed] [Google Scholar]
- 30.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int. 2004;66:268–274. doi: 10.1111/j.1523-1755.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 31.Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int. 2004;66:1479–1485. doi: 10.1111/j.1523-1755.2004.00911.x. [DOI] [PubMed] [Google Scholar]
- 32.Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol. 2006;291:F473–F480. doi: 10.1152/ajprenal.00425.2005. [DOI] [PubMed] [Google Scholar]
- 33.Rosivall L, Mirzahosseini S, Toma I, Sipos A, Peti-Peterdi J. Fluid flow in the juxtaglomerular interstitium visualized in vivo. Am J Physiol Renal Physiol. 2006;291:F1241–F1247. doi: 10.1152/ajprenal.00203.2006. [DOI] [PubMed] [Google Scholar]
- 34.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 35.Takenaka T, Suzuki H, Okada H, Hayashi K, Kanno Y, Saruta T. Mechanosensitive cation channels mediate afferent arteriolar myogenic constriction in the isolated rat kidney. J Physiol. 1998;511(Part 1):245–253. doi: 10.1111/j.1469-7793.1998.245bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friis UG, Jorgensen F, Andreasen D, Jensen BL, Skott O. Molecular and functional identification of cyclic AMP-sensitive BKCa potassium channels (ZERO variant) and L-type voltage-dependent calcium channels in single rat juxtaglomerular cells. Circ Res. 2003;93:213–220. doi: 10.1161/01.RES.0000085041.70276.3D. [DOI] [PubMed] [Google Scholar]
- 37.Fellner SK, Arendshorst WJ. Ryanodine receptor and capacitative Ca2+ entry in fresh preglomerular vascular smooth muscle cells. Kidney Int. 2000;58:1686–1694. doi: 10.1046/j.1523-1755.2000.00329.x. [DOI] [PubMed] [Google Scholar]
- 38.Fellner SK, Arendshorst WJ. Angiotensin II Ca2+ signaling in rat afferent arterioles: stimulation of cyclic ADP ribose and IP3 pathways. Am J Physiol Renal Physiol. 2005;288:F785–F791. doi: 10.1152/ajprenal.00372.2004. [DOI] [PubMed] [Google Scholar]
- 39.Schweda F, Riegger GA, Kurtz A, Kramer BK. Store-operated calcium influx inhibits renin secretion. Am J Physiol Renal Physiol. 2000;279:F170–F176. doi: 10.1152/ajprenal.2000.279.1.F170. [DOI] [PubMed] [Google Scholar]
- 40.Inscho EW, Cook AK, Mui V, Imig JD. Calcium mobilization contributes to pressure-mediated afferent arteriolar vasoconstriction. Hypertension. 1998;31:421–428. doi: 10.1161/01.hyp.31.1.421. [DOI] [PubMed] [Google Scholar]
- 41.Bell PD, Lapointe J-Y, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA. 2003;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olivari MT, Bartorelli C, Polese A, Fiorentini C, Moruzzi P, Guazzi MD. Treatment of hypertension with nifedipine, a calcium antagonistic agent. Circulation. 1979;59:1056–1062. doi: 10.1161/01.cir.59.5.1056. [DOI] [PubMed] [Google Scholar]
- 43.Nathan HJ, Laganiere S, Dube L, Foster B, McGilveray I, Harrison M, Bourke M, Cattran C, de LaSalle G, Robblee J. Intravenous nifedipine to treat hypertension after coronary artery revascularization surgery. A comparison with sodium nitroprusside. Anesth Analg. 1992;74:809–817. doi: 10.1213/00000539-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Terris S, Bourdillon PD, Cheng DT, Pitt B. Direct cardiac and peripheral vascular effects of intracoronary and intravenous nifedipine. Am J Cardiol. 1986;58:25–30. doi: 10.1016/0002-9149(86)90235-3. [DOI] [PubMed] [Google Scholar]
- 45.Heller J, Horacek V. The effect of two different calcium antagonists on the glomerular haemodynamics in the dog. Pflugers Arch. 1990;415:751–755. doi: 10.1007/BF02584016. [DOI] [PubMed] [Google Scholar]
- 46.Imagawa J, Kurosawa H, Satoh S. Effects of nifedipine on renin release and renal function in anesthetized dogs. J Cardiovasc Pharmacol. 1986;8:636–640. doi: 10.1097/00005344-198605000-00029. [DOI] [PubMed] [Google Scholar]
- 47.Loutzenhiser R, Epstein M, Horton C, Sonke P. Reversal of renal and smooth muscle actions of the thromboxane mimetic U-44069 by diltiazem. Am J Physiol. 1986;250:F619–F626. doi: 10.1152/ajprenal.1986.250.4.F619. [DOI] [PubMed] [Google Scholar]
- 48.Loutzenhiser R, Horton C, Epstein M. Effects of diltiazem and manganese renal hemodynamics: studies in the isolated perfused rat kidney. Nephron. 1985;39:382–388. doi: 10.1159/000183410. [DOI] [PubMed] [Google Scholar]
- 49.Marre M, Misumi J, Raemsch KD, Corvol P, Menard J. Diuretic and natriuretic effects of nifedipine on isolated perfused rat kidneys. J Pharmacol Exp Ther. 1982;223:263–270. [PubMed] [Google Scholar]
- 50.Steele TH, Challoner-Hue L. Renal interactions between norepinephrine and calcium antagonists. Kidney Int. 1984;26:719–724. doi: 10.1038/ki.1984.207. [DOI] [PubMed] [Google Scholar]
- 51.Cauvin C, Lukeman S, Cameron J, Hwang O, van BC. Differences in norepinephrine activation and diltiazem inhibition of calcium channels in isolated rabbit aorta and mesenteric resistance vessels. Circ Res. 1985;56:822–828. doi: 10.1161/01.res.56.6.822. [DOI] [PubMed] [Google Scholar]
- 52.Ono H, Kokubun H, Hashimoto K. Abolition by calcium antagonists of the autoregulation of renal blood flow. Naunyn Schmiedebergs Arch Pharmacol. 1974;285:201–207. doi: 10.1007/BF00498990. [DOI] [PubMed] [Google Scholar]
- 53.Hansen PB, Friis UG, Uhrenholt TR, Briggs J, Schnermann J. Intracellular signalling pathways in the vasoconstrictor response of mouse afferent arterioles to adenosine. Acta Physiol (Oxf) 2007;191:89–97. doi: 10.1111/j.1748-1716.2007.01724.x. [DOI] [PubMed] [Google Scholar]
- 54.Salomonsson M, Arendshorst WJ. Calcium recruitment in renal vasculature: NE effects on blood flow and cytosolic calcium concentration. Am J Physiol. 1999;276(5 Part 2):F700–F710. doi: 10.1152/ajprenal.1999.276.5.F700. [DOI] [PubMed] [Google Scholar]
- 55.Konoshita T, Makino Y, Kimura T, Fujii M, Wakahara S, Arakawa K, Inoki I, Nakamura H, Miyamori I. A new-generation N/L-type calcium channel blocker leads to less activation of the renin-angiotensin system compared with conventional L type calcium channel blocker. J Hypertens. 2010;28:2156–2160. doi: 10.1097/HJH.0b013e32833d01dd. [DOI] [PubMed] [Google Scholar]
- 56.Fan YY, Kohno M, Nakano D, Ohsaki H, Kobori H, Suwarni D, Ohashi N, Hitomi H, Asanuma K, Noma T, Tomino Y, Fujita T, Nishiyama A. Cilnidipine suppresses podocyte injury and proteinuria in metabolic syndrome rats: possible involvement of N-type calcium channel in podocyte. J Hypertens. 2010;28:1034–1043. doi: 10.1097/hjh.0b013e328336ade3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olivera BM, Miljanich GP, Ramachandran J, Adams ME. Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- 58.Won YJ, Ono F, Ikeda SR. Identification and modulation of voltage-gated Ca2+ currents in zebrafish Rohon-Beard neurons. J Neurophysiol. 2011;105:442–453. doi: 10.1152/jn.00625.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grunberger C, Obermayer B, Klar J, Kurtz A, Schweda F. The calcium paradoxon of renin release: calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res. 2006;99:1197–1206. doi: 10.1161/01.RES.0000251057.35537.d3. [DOI] [PubMed] [Google Scholar]
- 60.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased intracellular calcium stimulates renin release via calcium-inhibitable adenylyl cyclase. Hypertension. 2007;49:162–169. doi: 10.1161/01.HYP.0000250708.04205.d4. [DOI] [PubMed] [Google Scholar]