Abstract
BACKGROUND
The combination of fludarabine, cyclophosphamide, and rituximab (FCR) has produced improved response rates and a prolonged survival in patients with chronic lymphocytic leukemia (CLL). However, its therapeutic power is counterbalanced by significant hematologic toxicity. Persistent and new-onset cytopenia after the completion of FCR raise concern about disease recurrence, the development of therapy-related myeloid malignancies (TRMM), and infections.
METHODS
A total of 207 patients with CLL who achieved complete response, complete response with incomplete bone marrow recovery, or nodular partial remission were analyzed after frontline FCR therapy.
RESULTS
Three months after the completion of therapy, 35% of patients had developed grade 2 to 4 cytopenia (according to Common Terminology Criteria for Adverse Events [version 4.0]). Factors found to be associated with cytopenia at 3 months after therapy were older age, advanced Rai stage disease, and lower baseline blood counts. Moreover, patients with cytopenia were less likely to have completed 6 courses of therapy with FCR. At 6 months and 9 months after therapy, the prevalence of grade 2 to 4 cytopenia was 24% and 12%, respectively. No differences in progression-free survival and overall survival were noted between cytopenic and noncytopenic patients or between patients with persistent and new-onset cytopenia. The prevalence of TRMM was 2.3% and did not differ significantly between cytopenic and noncytopenic patients or between those with persistent and new-onset disease. Late infections were more common in patients who were cytopenic at 9 months (38%) and were mostly bacterial (67%).
CONCLUSIONS
Cytopenia after the completion of therapy is a common complication of frontline FCR that improves over time, particularly for new-onset cases. The presence of persistent cytopenia (lasting up to 9 months after the completion of therapy) should not raise concern about CLL recurrence of the development of TRMM, but should encourage surveillance for bacterial infections for an additional 9 months.
Keywords: chronic lymphocytic leukemia, combination of fludarabine, cyclophosphamide, and rituximab, cytopenia, prognosis
INTRODUCTION
The combination of fludarabine, cyclophosphamide, and rituximab (FCR) has changed the clinical course of patients with chronic lymphocytic leukemia (CLL). A complete response (CR) rate of 44% to 72% and an overall response rate of 90% to 95% have been reported in the frontline setting, with significant survival improvement noted in comparison with previously used regimens.1–4 Eradication of minimal residual disease was achieved in up to 49% of patients, with further implications for outcome.5
However, this gain in response quality and duration has been associated with hematologic toxicity. Myelosuppression that persists at the completion of therapy has defined a new response class of CR with persistent cytopenia (CR with incomplete bone marrow recovery [CRi]).6 Approximately 34% to 52% of FCR cycles were complicated by grade 3 or 4 neutropenia in the 2 large frontline studies published to date, and neutropenia was significantly more frequent with FCR therapy than with the FC regimen in the German randomized trial.1,2 Major infections were reported in 2.6% of cycles1 and in 25% of patients.2 A small percentage of cycles were complicated by anemia (5%) and thrombocytopenia (5%–7%). Analogous results were reported for previously treated patients.7–9 Hematologic toxicity was mostly drug related, with infrequent autoimmune events reported.10
Although there are substantial data regarding early hematologic toxicity, to the best of our knowledge persistent cytopenia after FCR has been poorly investigated. In a large study evaluating FCR, 19% of patients (most of whom had advanced Rai stage disease) had cytopenia for 3 months after the completion of therapy, and 28% developed later-onset cytopenia.3 A higher prevalence (31%) was reported in a retrospective analysis including both untreated patients and those with recurrent-refractory CLL.11 Persistent and new-onset cytopenia after FCR have traditionally raised concern of an increased risk of infection, CLL recurrence, and the development of therapy-related myeloid malignancies (TRMM).12–18 For the latter, a risk of 2.8% at 6 years was reported in what to our knowledge is the most recent update of the original FCR study.3
Given the unresolved questions surrounding this subject, we conducted a post hoc/retrospective analysis of patients prospectively enrolled in our original phase 2 study of frontline FCR and who developed persistent or new-onset cytopenia after treatment.
MATERIALS AND METHODS
Case Selection
The current study was a post hoc/retrospective analysis of 300 patients with CLL who were prospectively enrolled in a phase 2 study of frontline FCR at The University of Texas MD Anderson Cancer Center in Houston between 1999 and 2003. The clinic and laboratory features were confirmed by review of the medical records. Laboratory testing, including conventional cytogenetic analysis and evaluation of the somatic mutation status of the immunoglobulin heavy chain variable region (IGHV) and expression of CD38 and ZAP70 (zeta-chain-associated protein kinase 70), were performed as previously described.19–21 Fluorescence in situ hybridization analysis was not routinely available in the study institution at the time of accrual and therefore was not included in the current analysis.
FCR therapy was administered according to the previously described schedule.1 The National Cancer Institute-sponsored Working Group criteria were used to initiate therapy and to classify treatment response.22 At the time of the completion of therapy, antiviral prophylaxis was indicated for at least 1 year, but was left to the discretion of the local physician. The use of growth factors during treatment and after its completion was also at the discretion of the local physician.
To limit the influence of residual CLL on observed cases of cytopenia, only patients achieving CR, CRi, or nodular partial remission (nPR) 2 months after the completion of therapy were included in the analysis (269 patients). A complete blood count was available for 207 patients at 3 months (±1 month) after day 3 of the last cycle of therapy, in 191 patients at 6 months (±1 month), and in 198 patients at 9 months (±1 month). Cytopenia was defined as grade 2 to 4 hematologic toxicity according to Common Terminology Criteria for Adverse Events (version 4.0): an absolute neutrophil count < 1.5 × 109/L, hemoglobin < 10 g/dL, and a platelet count < 75 × 109/L. Cytopenia that lasted from the time of the 3-month evaluation was defined as persistent, whereas cytopenia arising at 6 months or 9 months and not described at the time of the previous evaluation was defined as new-onset cytopenia.
All patients provided written informed consent.
Statistical Analysis
Progression-free survival (PFS) and overall survival (OS) were calculated from the date of treatment to the date of disease recurrence and death, respectively. Survival distributions were calculated using the Kaplan-Meier method, and univariate comparisons were made using the log-rank test. Categorical and continuous variables were compared using the chi-square or Fisher exact tests or the Mann-Whitney U test, as appropriate. All P values were 2-sided and considered significant at < .05.
RESULTS
Cytopenia at 3 Months and Comparison With Noncytopenic Patients
A total of 300 patients with CLL received frontline FCR at the study institution between 1999 and 2003. Of these, 269 patients (90%) achieved a CR, CRi, or nPR 2 months after the completion of therapy. A complete blood count at 3 months after the completion of therapy was available for 207 of these individuals (77%).
Seventy-two of the 207 evaluable patients (35%) were found to have grade 2 to 4 cytopenia at 3 months; 32 patients (15%) had grade 2 cytopenia and 40 patients (19%) had grade 3 to 4 cytopenia. Baseline and treatment characteristics are shown in Table 1, along with a comparison with the characteristics of patients who were not cytopenic at 3 months. Factors associated with the presence of cytopenia at 3 months were older age (P =.02), advanced Rai stage disease (P =.01), and baseline neutropenia (P =.04), anemia (P =.04), and thrombocytopenia (P =.001). Baseline renal function did not appear to differ significantly between the 2 groups (P =.63). Other factors not found to be associated with the presence of cytopenia included sex, absolute lymphocyte count, hepatosplenomegaly, number of lymph node sites, β-2 microglobulin levels, conventional cytogenetic analysis, IGHV mutational status, and CD38 and ZAP70 expression. Patients who were cytopenic at 3 months were less likely to have completed 6 courses of therapy (P =.001).
TABLE 1.
Baseline Characteristics and Comparison of Patients With Cytopenia Versus Those Without Cytopenia After Treatment With FCR
| Characteristic | Cytopenia N = 72 | No Cytopenia N = 135 | Pa |
|---|---|---|---|
| No. of males | 47 (65%) | 95 (70%) | .5 |
| Age (range), y | 59 (17–82) | 56 (35–78) | .02 |
| Rai stage III-IV, no. | 32 (44%) | 35 (26%) | .01 |
| WBC (range), K/μL | 60 (2–408) | 77 (4–620) | .37 |
| ALC (range), K/μL | 51 (1–391) | 66 (2–558) | .55 |
| ANC (range), K/μL | 7 (1–50) | 9 (1–62) | .04 |
| Hemoglobin (range), g/dL | 12.1 (7.7–16.2) | 12.7 (7.1–18.7) | .04 |
| Platelets (range), K/μL | 127 (8–367) | 166 (44–406) | .001 |
| Creatinine (range), mg/dL | 1.1 (0.7–2) | 1 (0.6–1.8) | .63 |
| Liver BCM (range), cm | 0 (0–9) | 0 (0–8) | .61 |
| Spleen BCM (range), cm | 0 (0–20) | 0 (0–19) | .95 |
| No. of LN sites, (range) | 3 (0–3) | 3 (0–3) | .25 |
| B2M (range), mg/L | 3.6 (1.8–12.7) | 3.6 (1.6–16.4) | .29 |
| CBA, no. | |||
| del13q | 0/35 (0%) | 2/73 (3%) | 1 |
| +12 | 4/35 (11%) | 13/73 (18%) | .57 |
| Diploid | 23/35 (66%) | 47/73 (64%) | 1 |
| del11q | 4/35 (11%) | 7/73 (9%) | .74 |
| del17p | 0/35 (0%) | 0/73 (0%) | 1 |
| CBA, no. | |||
| Diploid | 23/35 (66%) | 47/73 (64%) | 1 |
| Complex | 6/35 (16%) | 9/73 (12%) | .56 |
| Othersb | 6/35 (16%) | 17/73 (27%) | .62 |
| Mutated IGHV, no. | 21/45 (47%) | 30/87 (34%) | .17 |
| ZAP70+, no. | 13/21 (62%) | 30/59 (51%) | .38 |
| CD38 ≥30%, no. | 14/59 (24%) | 25/117 (21%) | .72 |
| 6 FCR cycles | 48/72 (67%) | 116/135 (86%) | .001 |
Abbreviations: +, positive; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; BCM, below the costal margin; B2M, beta-2 microglobulin; CBA, conventional banding analysis; FCR, combination of fludarabine, cyclophosphamide and rituximab; IGHV, immunoglobulin heavy chain variable region; LN, lymph nodes (sites were cervical, axillary, and inguinal); WBC, white blood cell count; ZAP70, zeta-chain–associated protein kinase 70.
Bold type indicates statistical significance.
del1, del3, del11, del13, del14, del20, t(11;14), +12, and +15.
Follow-Up at 6 Months and 9 Months
Follow-up complete blood count results were available in 191 patients at 6 months and in 198 patients at 9 months.
At 6 months, 45 patients (24%) had cytopenia: 20 patients (10%) had grade 2 cytopenia and 25 patients (13%) had grade 3 to 4 cytopenia. Cytopenia was persistent from the time of the previous evaluation (3 months) in 32 patients (71%) and of new onset in 13 patients (29%). Grade 3 to 4 cytopenia was observed in 20 of the 32 patients with persistent cytopenia (63%) and 5 of the 13 new-onset cases (38%); 11 of the 13 new-onset cases (85%) had a grade 1 cytopenia present at 3 months of follow-up.
At 9 months, cytopenia was reported in 24 patients (12%) and was grade 2 in 8 patients (4%) and grade 3 to 4 in 16 patients (8%). Cytopenia was persistent from the previous evaluation (6 months) in 22 patients (92%) and was of new onset in 2 patients (8%). Grade 3 to 4 cytopenia was reported in 16 of the 22 persistent cases (73%) and both of the new-onset cases. Both patients with new-onset cytopenia had grade 1 cytopenia at 6 months.
Progression-Free and Overall Survival
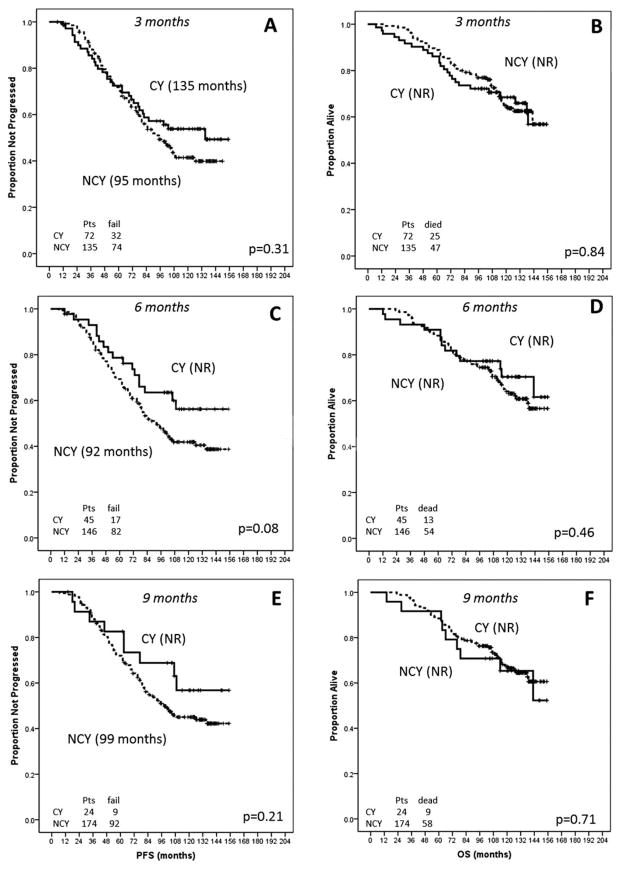
After a median follow-up of 114 months (range, 7–155 months), the median PFS for patients who were cytopenic at 3 months was 135 months and the OS had not been reached. No significant differences in PFS (P =.31) or OS (P =.8) were observed between patients with cytopenia at 3 months and those without (Fig. 1). No differences in PFS (P =.08) or OS (P =.46) were observed between patients who were cytopenic at 6 months versus those who were not (Fig. 1); among patients with cytopenia at 6 months, no statistically significant differences in PFS (P =.84) or OS (P =.86) were observed between persistent and new-onset cases. No differences in PFS (P =.21) and OS (P =.71) were reported between patients who were cytopenic at 9 months and noncytopenic patients (Fig. 1); among patients who were cytopenic at 9 months, no differences in PFS and OS were reported between persistent and new-onset cases (P value not significant [NS]). No significant differences in PFS and OS were observed when patients with cytopenia at any time were compared with patients without cytopenia (P =.19 and P =.98, respectively).
Figure 1.
Progression-free survival is shown according to the presence of cytopenia at (A) 3 months, (C) 6 months, and (E) 9 months. Overall survival (OS) is shown according to the presence of cytopenia at (B) 3 months, (D) 6 months, and (F) 9 months. CY indicates cytopenia; NCY, noncytopenia.
TRMM and Infections
During follow-up, TRMM were observed in 2 patients who were cytopenic at 3 months (2.8%) and in 2 patients who were cytopenic at 6 months (4%); no cases of TRMM were observed among patients who were cytopenic at 9 months. The prevalence did not differ significantly from that observed in patients who were not cytopenic at 3 months (5 patients; 3.7%) (P value NS), 6 months (4 patients; 3%) (P value NS), and 9 months (7 patients; 4%) (P value NS). Among patients who were cytopenic at 6 months and 9 months, no differences in the incidence of TRMM were observed between patients with persistent and those with new-onset disease (P value NS for both). In addition, no differences in the incidence of TRMM were reported when comparing patients who were cytopenic at any time with those who were not (P =.70).
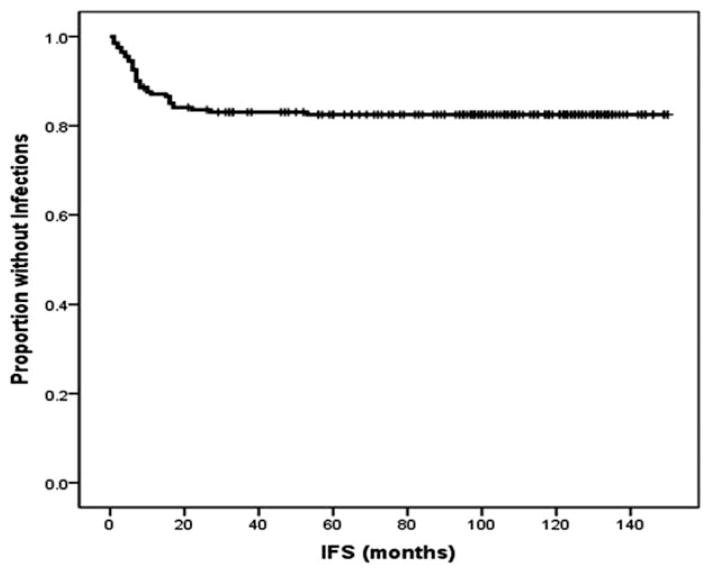
During the years of remission, major (grade 3 or 4) infections were observed in 15 patients who were cytopenic at 3 months (21%), 10 patients who were cytopenic at 6 months (22%), and 9 patients who were cytopenic at 9 months (38%). When compared with noncytopenic patients, the incidence of infections was significantly higher for patients who were cytopenic at 9 months (38% vs 14%; P =.009), but not for patients who were cytopenic at 3 months (22% vs 15%; P =.25) or 6 months (22% vs 16%; P =.38). Infections reported for patients who were cytopenic at 9 months were bacterial (6 patients), zoster reactivations (2 patients), and aspergillus pneumonia (1 patient) and 3 patients had grade 2 to 4 neutropenia at the time of infection. The distribution of infectious etiologies did not differ significantly when compared with patients who were not cytopenic at 9 months (25%) (P =.26). Among patients who were cytopenic at 6 months and 9 months, no differences in infection rates were observed between persistent and new-onset cases (P =.70 and P =.13, respectively). No differences in infection rates were reported when patients who were cytopenic at any time were compared with patients who were noncytopenic at any time (P =.13). Most of the major infections were reported within 18 months of the completion of therapy and a plateau was reached after 48 months (Fig. 2).
Figure 2.
Infection-free survival (IFS) is shown. IFS was defined as the time from the completion of therapy to the onset of major infections. The majority of infectious episodes happened within 18 months and a plateau was reached after 48 months.
DISCUSSION
Chemoimmunotherapy has changed the natural history of CLL, producing a high overall response rate and improved outcome. The predominant complication is myelosuppression, with neutropenia being the most commonly observed hematologic adverse event.1,2 It is interesting to note that the addition of rituximab, an anti-CD20 monoclonal antibody, to FC has significantly increased the rate of neutropenia2,11 through a mechanism that is currently not well understood.2,9,23,24
Although there are substantial data regarding hematologic toxicity observed during FCR treatment, to our knowledge few studies to date have analyzed features and outcomes associated with persistent or new-onset cytopenia. Such cytopenias traditionally raise concern about CLL recurrence, TRMM, and the risk of infections.
To minimize the role played by residual disease in cytopenia, only patients who achieved CR, CRi, or nPR 2 months after completing therapy with FCR were included in the current analysis. In the current study, 35% of evaluable patients had grade 2 to 4 cytopenia 3 months after the completion of therapy. Persistent cytopenia was more likely in older patients and in patients with lower baseline counts, thereby highlighting the importance of bone marrow reserve. There was no significant association noted between cytopenia and renal function, although a baseline creatinine level < 2 mg/dL was required for patients to be enrolled in the protocol. It is interesting to note that 40% to 60% of intravenous fludarabine is reportedly excreted through the renal filter and a creatinine clearance < 80 mL/minute significantly increases the risk of hematological toxicity.25 No association with cytogenetic abnormalities was observed. However, only conventional cytogenetic analysis was used in the current study, and therefore whether differences could be observed according to baseline fluorescence in situ hybridization abnormalities is not known.
The prevalence of cytopenia decreased from 35% at 3 months to 24% at 6 months and 12% at 9 months, with a relative decrease in new-onset cases from 29% to 8%. It is interesting to note that only 2 cases of new-onset cytopenia were observed at 9 months, making the latter a very rare finding. No differences in PFS were observed between patients with and those without cytopenia.
A potential correlation between FCR therapy and TRMM has been reported previously, with the majority of patients presenting with persistent cytopenia.15–18 However, no significant association between cytopenia (both persistent and new-onset) after FCR and the incidence of TRMM was observed in the current study. It is interesting to note that in the current study, the median time from therapy initiation to the development of TRMM was 42 months (range, 30 months–81 months). Thus, cytopenia arising later than our observation time could still raise concern for the development of TRMM.
In the most recent follow-up of the original FCR frontline study, persistent neutropenia was reported in 19% of patients and late major infections in 9%3(it is interesting to note that a threshold of 1 × 109/L was used to define neutropenia, whereas a higher threshold, 1.5 × 109/L, was used in the current study); slightly higher rates (31% and 10%, respectively) were observed in a retrospective analysis of both previously untreated patients and patients with recurrent CLL receiving FCR.11 In the current analysis, only cytopenia observed at 9 months after the completion of therapy was found to be associated with a higher risk of major infections, with no differences noted between persistent and new-onset cases. FCR treatment is also associated with prolonged lymphoid immunodeficiency,26 which makes it difficult to strongly correlate late infections with persistent neutropenia. However, myelosuppression persisting up to 9 months may encourage surveillance for bacterial infections and prolonged antiviral prophylaxis. It is interesting to note that no cases of Pneumocystis infections were observed in patients who were cytopenic at 9 months, despite the fact that these individuals were not receiving prophylaxis.
In conclusion, myelosuppression after the completion of therapy is a common complication of frontline FCR that improves over time, particularly for new-onset cases. The presence of persistent cytopenia (lasting up to 9 months after the completion of therapy) should not raise concern about CLL recurrence or the development of TRMM but should encourage surveillance for bacterial infections for an additional 9 months.
Acknowledgments
FUNDING SUPPORT
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 3.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gribben JG, O’Brien S. Update on therapy of chronic lymphocytic leukemia. J Clin Oncol. 2011;29:544–550. doi: 10.1200/JCO.2010.32.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30:980–988. doi: 10.1200/JCO.2011.36.9348. [DOI] [PubMed] [Google Scholar]
- 6.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wierda W, O’Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 8.Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117:3016–3024. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robak T, Dmoszynska A, Solal-Celigny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–1765. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 10.Borthakur G, O’Brien S, Wierda WG, et al. Immune anaemias in patients with chronic lymphocytic leukaemia treated with fludarabine, cyclophosphamide and rituximab–incidence and predictors. Br J Haematol. 2007;136:800–805. doi: 10.1111/j.1365-2141.2007.06513.x. [DOI] [PubMed] [Google Scholar]
- 11.Tam C, Seymour JF, Brown M, et al. Early and late infectious consequences of adding rituximab to fludarabine and cyclophosphamide in patients with indolent lymphoid malignancies. Haematologica. 2005;90:700–702. [PubMed] [Google Scholar]
- 12.Carney DA, Westerman DA, Tam CS, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010;24:2056–2062. doi: 10.1038/leu.2010.218. [DOI] [PubMed] [Google Scholar]
- 13.Tam CS, Seymour JF, Prince HM, et al. Treatment-related myelodysplasia following fludarabine combination chemotherapy. Haematologica. 2006;91:1546–1550. [PubMed] [Google Scholar]
- 14.McLaughlin P, Estey E, Glassman A, et al. Myelodysplasia and acute myeloid leukemia following therapy for indolent lymphoma with fludarabine, mitoxantrone, and dexamethasone (FND) plus rituximab and interferon alpha. Blood. 2005;105:4573–4575. doi: 10.1182/blood-2004-08-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Tang G, Medeiros LJ, et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/s-mall lymphocytic lymphoma. Mod Pathol. 2012;25:237–245. doi: 10.1038/modpathol.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki S, Nakamura F, Nannya Y, Nakagawa M, Ichikawa M, Kurokawa M. Early-onset therapy-related myelodysplastic syndrome originating from prolonged myelosuppression after fludarabine-based therapy. Intern Med. 2012;51:3427–3430. doi: 10.2169/internalmedicine.51.8310. [DOI] [PubMed] [Google Scholar]
- 17.Uppal GK, Leighton J, Da Costa D, Czulewicz A, Palazzo IE. Therapy related acute myeloid leukemia with t(10:16): a rare entity. Hematol Rep. 2011;3:e23. doi: 10.4081/hr.2011.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarid N, Eshel R, Rothman R, et al. Acute myeloid leukemia with 11q23/MLL rearrangement after ‘FCR’ regimen for chronic lymphocytic leukemia. Eur J Haematol. 2012;89:430–431. doi: 10.1111/ejh.12001. [DOI] [PubMed] [Google Scholar]
- 19.Shanafelt TD, Witzig TE, Fink SR, et al. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24:4634–4641. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 20.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 21.Dohner H, Stilgenbauer S, Fischer K, Bentz M, Lichter P. Cytogenetic and molecular cytogenetic analysis of B cell chronic lymphocytic leukemia: specific chromosome aberrations identify prognostic subgroups of patients and point to loci of candidate genes. Leukemia. 1997;11(suppl 2):S19–S24. [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 23.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 24.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 25.Martell RE, Peterson BL, Cohen HJ, et al. Analysis of age, estimated creatinine clearance and pretreatment hematologic parameters as predictors of fludarabine toxicity in patients treated for chronic lymphocytic leukemia: a CALGB coordinated intergroup study. Cancer Chemother Pharmacol. 2002;50:37–45. doi: 10.1007/s00280-002-0443-5. [DOI] [PubMed] [Google Scholar]
- 26.Ysebaert L, Gross E, Kuhlein E, et al. Immune recovery after fludarabine-cyclophosphamide-rituximab treatment in B-chronic lymphocytic leukemia: implication for maintenance immunotherapy. Leukemia. 2010;24:1310–1316. doi: 10.1038/leu.2010.89. [DOI] [PubMed] [Google Scholar]