Summary
Event-related fMRI was employed to characterize differences in brain activation between children ages 8–12 and adults, related to two forms of cognitive control: interference suppression and response inhibition. Children were more susceptible to interference and less able to inhibit inappropriate responses than were adults. Effective interference suppression in children was associated with prefrontal activation in the opposite hemisphere, as relative to adults. In contrast, effective response inhibition in children was associated activation of posterior, but not prefrontal, regions activated by adults. Children failed to activate a region in right ventrolateral prefrontal cortex that was recruited for both types of cognitive control by adults. Thus, children exhibited immature prefrontal activation that varied according to the type of cognitive control required.
Introduction
Cognitive control, or the ability to flexibly shape and constrain thoughts and actions in view of accomplishing internal goals, is essential for higher cognition. Two fundamental components of cognitive control are the ability to filter out irrelevant information in the environment (interference suppression) and the ability to inhibit inappropriate but prepotent response tendencies (response inhibition). Paradigms used to track the development of cognitive control (Dempster, 1992; Harnishfeger and Bjorkland, 1993) include tasks in which subjects must ignore irrelevant stimuli (Tipper et al., 1989; Ridderinkhof et al., 1997; Comalli et al., 1962; Lorsbach and Reimer, 1997) and inhibit prepotent response tendencies or strategies (Costantini and Hoving, 1973; Casey et al., 1997; Williams et al., 1999; Diamond, 1988; Luna et al., 2001). Cognitive control develops gradually over childhood, and improvements in control across childhood make an important contribution to higher cognitive function, as measured by tests of reasoning, problem-solving, and IQ (e.g., Dempster, 1992).
The development of cognitive control is thought to be related to the maturation of prefrontal cortex (PFC) (Goldman-Rakic, 1987; Diamond, 1988; Dempster, 1992). Prefrontal lesions in adults and nonhuman primates lead to impairments in cognitive control (Luria, 1966; Stuss and Benson, 1986; Miller and Cohen, 2001). PFC develops more slowly than other brain areas, reaching maturation only late in adolescence. Evidence for this delayed maturation is provided by measures of myelination (Yakovlev and Lecours, 1967; Pfefferbaum et al., 1993; Giedd et al., 1999), gray matter reduction (Jernigan et al., 1991; Pfefferbaum et al., 1993; Sowell et al., 2001), synaptogenesis (Huttenlocher, 1979), and resting metabolism (Chugani et al., 1987; for reviews, see Casey et al., 2000a; Gaillard et al., 2001; Diamond, 2002). PFC maturation may therefore be a limiting factor in the growth of cognitive control. There is, however, little direct evidence demonstrating a link between changes in prefrontal function and improvements in cognitive control across childhood (but see Casey et al., 1997; Luna et al., 2001). Functional brain imaging permits more direct examination of the functional maturation of neural circuitry underlying cognitive development.
The purpose of the present study was to use event-related functional MRI (fMRI) to characterize developmental changes in brain activation related to the performance of two different types of cognitive control. Children aged 8–12 and young adults performed a single task in the scanner that was designed to examine activation related to interference suppression and response inhibition. This task combined the Eriksen flanker (Eriksen and Eriksen, 1974) and go/no-go paradigms (Figure 1). In the flanker paradigm, subjects must respond on the basis of a central stimulus while ignoring flanking stimuli (flankers). Behavioral and brain imaging studies have shown that subjects involuntarily process the surrounding flankers despite their irrelevance for the task requirement of responding to the central target (Eriksen and Eriksen, 1974; Gratton et al., 1988; Botvinick et al., 1999; Hazeltine et al., 2000). Subjects are slower to respond to the central target when the flankers indicate a different response from the target than when they indicate the same response (Eriksen and Eriksen, 1974). In the go/no-go paradigm, subjects must withhold responding to an inappropriate stimulus, while responding to all other stimuli. Because the majority of trials require an active response (go), participants must inhibit a prepotent tendency to respond on all trials on the minority of no-go trials. The two tasks involve cognitive control because optimal performance requires either suppression of interfering information or inhibition of prepotent responses. In the present study, trials of different types were intermixed and were as similar to one another as possible, in order to facilitate the comparison of activations related to interference suppression and response inhibition. Both forms of cognitive control have been examined in brain-imaging studies of adults, but they have not been compared directly.
Figure 1. Trial Types Performed in the Scanner.
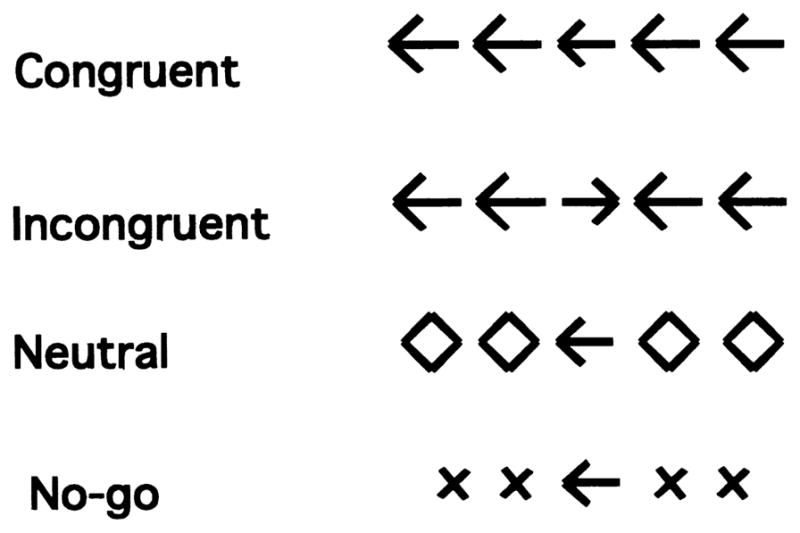
On each trial, subjects viewed an array of stimuli on the screen and responded by pressing the left button when the central arrow pointed to the left and by pressing the right button when it pointed to the right. On neutral trials, the flankers were not associated with a response. On congruent trials, they were associated with the same response as the target. On incongruent trials, they were associated with the opposite response from the target. Subjects were to refrain from pressing a button on no-go trials, when the flankers were ×’s.
Brain activation associated with interference suppression has not been examined in children on the flanker or any other task. In contrast, two brain-imaging studies have compared response inhibition between children and adults. One study employed a go/no-go paradigm (Casey et al., 1997), whereas another study examined the ability to make a saccadic eye movement in the opposite direction of a stimulus (Luna et al., 2001). These studies found that children recruited the same general network of cortical regions as adults, albeit to a greater or lesser degree. In the present study, we sought to determine whether this pattern of results holds for two types of cognitive control. Unlike previous developmental imaging studies, the present study both acquired whole-brain data and employed a performance-based analysis to identify brain regions correlated with task performance.
There are several challenges associated with using fMRI to examine developmental changes in brain activation. A number of changes that take place over childhood have the potential to lead to differences in fMRI activation between children and adults. These changes include the recruitment or maturation of neural circuitry underlying task performance, synaptic pruning, myelination, and changes in cognitive strategy. These changes reflect developmental processes of interest. However, there are also developmental changes that may lead to artifactual differences between groups, including differences in baseline glucose consumption and blood flow and amount of artifact due to motion, respiration, or cardiac activity (Gaillard et al., 2001; Casey et al., 2000b; Diamond, 2002). Each of these differences could affect the magnitude and/or extent of activation observed for a given subject, leading to problems interpreting group differences in activation. Additionally, the normalization of children’s brains to the adult template is expected to result in greater structural variability among children than adults (e.g., Muzik et al., 2000). Although techniques for normalizing children’s brains are not optimal at present, one study employing a similar normalization procedure to ours suggested that normalization did not result in artifacts for children aged 6 and above (Muzik et al., 2000).
In an effort to identify group differences in activation that result from differences in neural activity rather than being related to nonneural factors that could influence the fMRI signal, we approached the study in the following manner. First, a bite bar was used to restrict head motion, and average motion parameter estimates for children and adults were compared to ensure that there were no group differences in head motion. Second, we used multiple forms of convergent analyses. In our main analysis, we examined patterns of activation separately in children and adults to avoid the contribution of a number of factors that could lead to differences in the magnitude or extent of activation between children and adults. Critical differences were compared directly in region-of-interest (ROI) analyses, and overall patterns of activation were compared directly in a subsidiary analysis. Third, we identified brain regions for which level of activation was significantly correlated with performance. Changes in activation in these regions as a function of performance are likely to be meaningfully related to the development of cognitive control, rather than being artifactual. We examined correlations with performance rather than with age for two reasons: first, because we predicted that there would be substantial variability among children in terms of the age at which cognitive control reaches maturity; and second, because relationships between activation and age could potentially be due to systematic structural changes rather than functional ones.
An important issue in comparing activation between children and adults is that of differences in task performance. Experiencing difficulty with a task is likely to be associated with a number of psychological processes, including heightened error monitoring and attentional allocation, as well as frustration and physiological arousal. In order to avoid group differences in brain activation related to large differences in performance, a task was selected which both children and adults could perform with high accuracy. Further, an event-related design was employed, permitting the exclusion of error trials from analysis.
Results
Behavioral Testing in Children
The children performed above average on the standardized cognitive tests. Their scores were scaled relative to children their own age (vocabulary: 14.4 ± .6; word attack: 111.0 ± 2.3; word identification: 124.3 ± 5.3; coding: 13.7 ±.7; block design: 14.3 ±.9 [M ± SEM]). Average IQ (estimated from vocabulary and block design tests; see Spreen and Strauss, 1998) was 125 ± 4 (M ± SEM).
Performance in the Scanner
For incongruent, congruent, and neutral trials, pressing the wrong button or failing to respond were considered to be errors. For no-go trials, failures to withhold responding were considered to be errors. Accuracy was high for both adults and children (congruent: 99.9 ± 1%, 97.9 ± 1%; neutral: 99.9 ± 1%, 99.2 ± 4%; incongruent: 100%, 98.4%; no-go: 95.5 ± 9%, 89.7 ± 2%, for adults and children, respectively). Both groups made the majority of their errors on no-go trials (i.e., failing to withhold their response). Most errors in the other conditions consisted of incorrect responses rather than nonresponses. A 2 × 4 ANOVA was performed with group (children, adults) as a between-subjects factor and condition (congruent, incongruent, neutral, no-go) as a within-subjects factor. Adults made fewer errors than children (main effect of group [F(1,30) = 12.1; p = .0016]). Accuracy varied across conditions (main effect of condition [F(3,90) = 35.6; p < .0001]), and there was a group × condition interaction (F[3,90] = 4.1; p = .009). Planned contrasts revealed that children were less accurate than adults on incongruent (t[30] = 3.2; p = .003, two-tailed) and no-go (t[30] = 2.9; p = .007, two-tailed) trials. Group differences in accuracy tended toward significance for congruent (t[30] = 1.7; p. = 09, two-tailed) and neutral (t[30] = 1.6; p = .13, two-tailed) trials.
Outlier response times (RTs) greater than 2 SD from the mean for each subject were removed prior to analysis. One adult was excluded from the RT analysis and from the fMRI analyses of the interference suppression manipulation on the basis of RTs that were greater than 2 SD from the adult group mean for congruent, neutral, and incongruent trials. Average RTs were longer and more variable for children than adults (adults: 544 ± 12, 560 ± 13, 583 ± 12; children: 683 ± 27, 693 ± 30, 737 ± 29; M ± SEM for congruent, neutral, and incongruent trials, respectively). RTs for correct trials were submitted to a 2 × 3 ANOVA with group (children, adults) as a between-subjects factor and condition (congruent, neutral, incongruent) as a within-subjects factor. Adults responded more quickly than children (main effect of group [F(1,29) = 20.5; p < .0001]), and response times varied across conditions (main effect of condition [F(2,58) = 53.4; p < .0001]). The group × condition interaction tended toward significance (F[2,58] = 2.93; p = .06). Planned contrasts revealed that children were slower to respond than adults on all three conditions (t[29] > 4; p < .0005). Children exhibited a greater absolute interference effect than adults (average incongruent – neutral RT difference [in ms] for adults was 21.9 ± 5.9, for children was 44.4 ± 6.7; M ± SEM; t[29] = 2.5; p = .019). In order to account for baseline differences in response times between children and adults, the interference effect was expressed as a proportional increase in RTs for incongruent relative to neutral trials. Differences between children and adults in terms of proportional interference effects were marginally significant (t[29] = 1.6; p = .056, one-tailed).
A comparison of the performance of younger and older children (seven children aged 8–9 and nine children aged 11–12, respectively) revealed no differences on the indices of cognitive control. Younger children were slower to respond than older children on all conditions (F[1,4] = 5.6; p = .03, two-tailed), but did not exhibit greater susceptibility to interference than older children (RT difference for incongruent – neutral trials: t[14] = .84; p = .42, two-tailed). Accuracy did not significantly differ between younger and older children (F[1,14] = .51; p = .49, two-tailed). Because age was a poor predictor of performance across children, we did not examine differences in brain activation between younger and older children.
Brain Imaging Results
Multiple analyses were performed in an attempt to characterize the neural changes underlying the development of interference suppression and response inhibition. First, group contrasts were used to identify regions that were consistently engaged across children and across adults. These regions are likely to be important for task performance but may not covary with behavioral performance if there is little variability in their recruitment. Second, regression analyses were used to identify regions for which activation was correlated with task performance. These regions may not be identified in a group contrast because they are variably recruited across individuals. Third, two-sample t tests were performed on contrast images to confirm the presence of group differences in activation. Fourth, ROI analyses enabled the characterization of activation in one group within regions identified functionally from the other group. Fifth, group contrasts were computed separately for the better-performing and worse-performing children in order to determine whether better-performing children exhibited more adult-like patterns of activation. Sixth, correlations between task performance and independent measures of cognitive development were examined in order to shed light on possible strategies employed by children in the interference suppression manipulation. Finally, conjunction analyses were performed for the purpose of identifying regions commonly activated across tasks.
Interference suppression
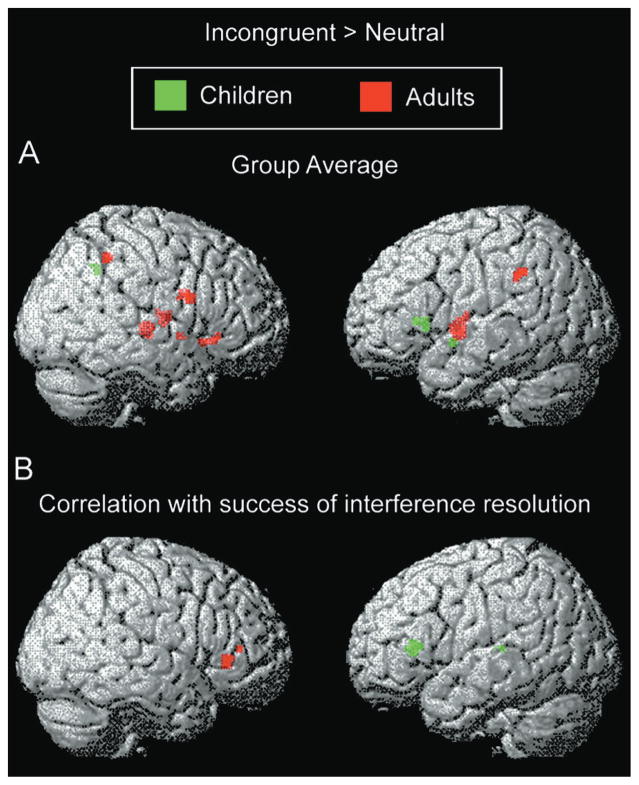
In adults, interference suppression (incongruent > neutral contrast) was associated with activation of right-lateralized ventrolateral PFC (Brodmann’s areas [BA] 44, 45, and 47) and insula (BA 13) and bilateral inferior parietal lobule (BA 40) and putamen (Table 1; Figure 2A). These regions, with the exception of parietal cortex, were significantly more active in adults than in children (Table 1). In children, activations included left-lateralized ventrolateral PFC (BA 45) and insula (BA 13) and right inferior parietal lobule (BA 40) (Table 1; Figure 2B). These regions were significantly more active in children than in adults (Table 1). ROI analyses revealed that the magnitude of activation of left and right ventrolateral PFC for children mirrored that of adults (children: .047 ± .012 in left PFC, .021 ± .022 in right PFC; adults: .025 ± .012 in left PFC; .042 ± .011 in right PFC; mean increase in parameter estimates ± SEM). Thus, children’s activation of left ventrolateral PFC was similar in magnitude and variability to adults’ activation of right ventrolateral PFC.
Table 1.
Activations for Incongruent versus Neutral Trials
| Region of Activation | B.A. | Talairach | Volumea | Z Score | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| Children: Group Contrast | ||||||
|
| ||||||
| Frontal | ||||||
| Inferior frontal | L45 | −38 | 20 | 4 | 152 | 3.41* |
| Anterior insula | L13 | −42 | 26 | 6 | local | 3.38* |
| Parietal | R40 | 26 | −52 | 38 | 96 | 3.55* |
| Temporal | ||||||
| Superior temporal | L38 | −44 | 2 | − 8 | 48 | 3.72* |
| Midbrain | R | 2 | −30 | −10 | 56 | 3.32 |
|
| ||||||
| Children: Positive Correlation between Activation and Success of Interference Suppression | ||||||
|
| ||||||
| Frontal | ||||||
| Insula | L13 | −26 | 30 | 8 | 424 | 4.12 |
| Pulvinar | L | −16 | −28 | 10 | 48 | 3.92 |
|
| ||||||
| Adults: Group Contrast | ||||||
|
| ||||||
| Frontal | ||||||
| Inferior frontal | R44 | 42 | 2 | 24 | 48 | 3.54 |
| R44/45 | 38 | 10 | 22 | 112 | 3.50* | |
| R47 | 36 | 24 | − 6 | 120 | 3.94* | |
| R44 | 52 | 6 | 22 | local | 3.46* | |
| Anterior insula | R13 | 34 | 16 | − 8 | local | 3.22* |
| Posterior insula | L13 | −32 | −2 | 0 | 65 | 4.02* |
| Parietal | ||||||
| Inferior parietal | R40 | 46 | −46 | 48 | 80 | 3.64 |
| L40 | −60 | −42 | 38 | 88 | 3.60 | |
| Putamen | R | 28 | −20 | 0 | 344 | 4.11* |
| R | 26 | −8 | 8 | 216 | 3.56* | |
| L | −30 | −8 | 10 | local | 3.35* | |
|
| ||||||
| Adults: Positive Correlation between Activation and Success of Interference Suppression | ||||||
|
| ||||||
| Frontal | ||||||
| Inferior frontal | R47/13 | 34 | 30 | 0 | 176 | 3.83 |
| Middle frontal | R46/10 | 38 | 40 | 10 | 40 | 3.43 |
Asterisk indicates clusters also identified by relevant two-sample t-test (children > adults or adults > children)
Volume reported in mm3.
Figure 2. Activation Related to Interference Suppression in Children and Adults.
(A) Group contrast and (B) regions exhibiting a positive correlation between activation and success of interference suppression.
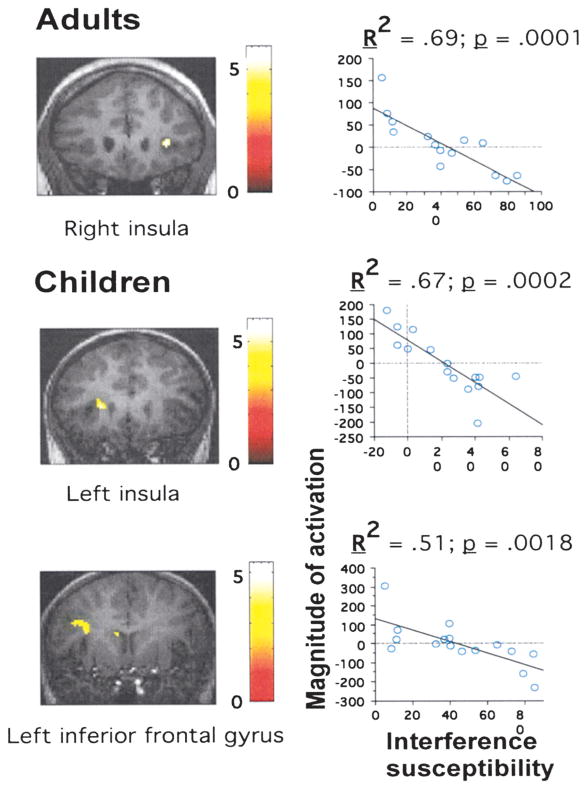
SPM99 regression analyses were used to identify regions for which level of activation across subjects in the incongruent > neutral contrast correlated significantly with efficiency of interference suppression, as measured by the amount of slowing of RTs for incongruent relative to neutral trials. In adults, these regions were right inferior frontal gyrus/anterior insula (BA 47/13) and an anterior portion of the right middle frontal gyrus (BA 10/46) (Table 1; Figure 2B). In children, these regions were left anterior insula, extending into the left caudate nucleus, and the left pulvinar nucleus of the thalamus (Table 1; Figure 2B). For all these regions, greater activation was associated with better performance (i.e., smaller interference-related slowing of RTs; Figure 3). At a more lenient threshold (p < .005 uncorrected for multiple comparisons), a large region in left inferior frontal gyrus (BA 44) exhibited a similar correlation with performance for children (Figure 3).
Figure 3. Brain-Behavior Correlations for Interference Suppression.
(A) Regions identified in regression analyses for adults or children, and (B) magnitude of activation (as measured by the fitted amplitude of response) plotted against interference susceptibility (in ms) across individuals.
The children were divided equally into groups consisting of the children who suppressed interference the best (interference effect: 5–39 ms) and the worst (40–85 ms). Although better-performing children exhibited similar interference effects to adults (M ± SEM: 23 ± 5 and 22 ± 6, respectively), both better and worse performers exhibited activation of left rather than right lateral PFC. Moreover, better performers exhibited more extensive activation of left PFC than worse performers. Activation of the inferior parietal lobule was observed bilaterally for better performers and only on the right side for worse performers.
Correlations between Behavioral Measures and Interference Susceptibility
There was a tendency for children who were less susceptible to interference to perform better on word attack (R = .45; p = .08), a measure of fluid verbal ability. There was no such correlation between interference susceptibility and either of two measures of crystallized verbal ability: vocabulary (R = .20; p = .47) or word identification (R = .03; p = .90). Interference susceptibility was also uncorrelated with coding, a measure of speed of processing (R = .06; p = .82), and block design (R = .08; p = .76), a measure of nonverbal intelligence. In contrast, overall RTs (averaged over congruent, neutral, and incongruent trials) were significantly correlated with coding (R = .65; p =.007).
Response Inhibition
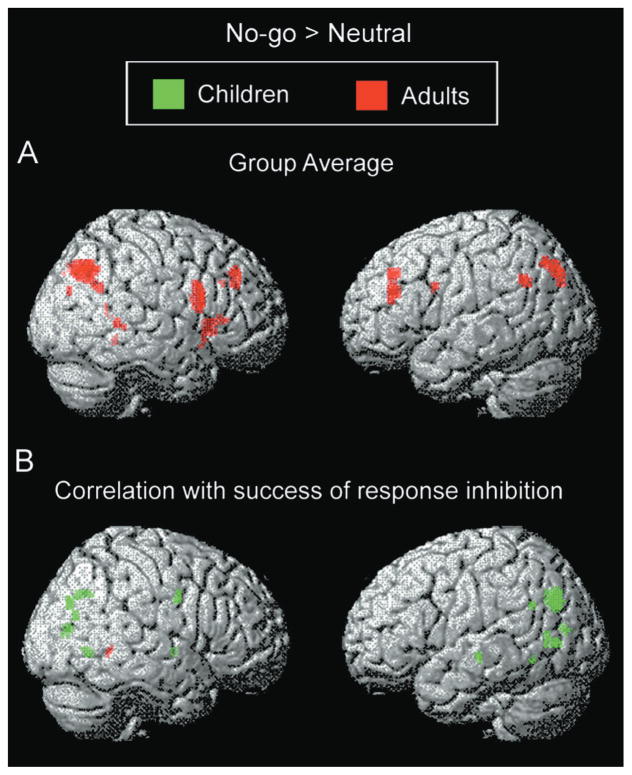
In adults, the response inhibition contrast (no-go > neutral) was associated with activation of a number of regions in PFC, including bilateral ventrolateral (R BA 44/45, L BA 44) and dorsolateral (BA 9/46) regions (Table 2; Figure 4A). This contrast additionally activated anterior and posterior cingulate cortices (BA 32, 30/23), left superior and inferior parietal lobules (BA 7, 39), bilateral precuneus (BA 19), right temporal lobe (BA 39, 21), and right cerebellum. Many of these regions were significantly more activated in adults than in children (Table 2). In children, no activation survived the statistical threshold of p < .001 uncorrected for multiple comparisons. At the more lenient threshold of p < .005 uncorrected, small foci in right inferior frontal gyrus (BA 44) and medial frontal gyrus (BA 6; Table 2) were significant.
Table 2.
Activations for No-Go versus Neutral Trials
| Region of Activation | B.A. | Talairach | Volume | Z Score | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| Children: Group Contrasta | ||||||
|
| ||||||
| Frontal | ||||||
| Inferior frontal | R44 | 38 | 0 | 30 | 104 | 2.96 |
| Medial frontal | R6 | −6 | 32 | 44 | 120 | 3.31* |
|
| ||||||
| Children: Positive Correlation between Activation and Success of Interference Suppression | ||||||
|
| ||||||
| Frontal | ||||||
| Premotor | R6 | 36 | −2 | 36 | 144 | 4.43 |
| Parietal | ||||||
| Angular gyrus | L39 | −42 | −56 | 30 | 48 | 3.32 |
| Precuneus | R7 | 14 | −68 | 36 | 288 | 3.81 |
| R19 | 24 | −76 | 34 | 144 | 3.38 | |
| Occipital | ||||||
| Cuneus/precuneus | L19 | −22 | −70 | 30 | 976 | 4.63 |
| R19 | 24 | −76 | 34 | 144 | 3.38 | |
| L17 | −18 | −78 | 12 | 64 | 3.25 | |
| R17 | 10 | −78 | 10 | 128 | 3.84 | |
| Lingual gyrus | L18 | −24 | −72 | 2 | 144 | 4.07 |
| R18 | 24 | −62 | −2 | 160 | 3.30 | |
| Fusiform gyrus | L19 | −32 | −56 | −8 | 40 | 3.26 |
| Temporal | ||||||
| Middle temporal | R39 | 34 | −70 | 22 | 72 | 3.62 |
| L21 | −52 | −20 | −6 | 64 | 3.57 | |
| L37 | −54 | −64 | 4 | 40 | 3.30 | |
| Lentiform Nucleus | ||||||
| Globus Pallidus | R | 18 | −6 | −2 | 48 | 3.43 |
|
| ||||||
| Adults: Group Contrast | ||||||
|
| ||||||
| Frontal | ||||||
| Inferior frontal | R44 | 48 | 12 | 18 | 824 | 3.76* |
| R45 | 48 | 28 | 6 | 48 | 3.49 | |
| L44 | −46 | 8 | 30 | 88 | 3.40* | |
| Middle frontal | L9/46 | −36 | 36 | 26 | 432 | 3.74* |
| L8/9 | −34 | 38 | 38 | 176 | 3.45 | |
| R8 | 32 | 36 | 40 | 360 | 3.44 | |
| Superior frontal | R9/46 | 28 | 36 | 30 | local | 3.33* |
| Limbic | ||||||
| Anterior cingulate | R32 | 20 | 28 | 32 | 40 | 3.42* |
| Posterior cingulate | R30/23 | 12 | −52 | 10 | 56 | 3.37* |
| Parietal | ||||||
| Precuneus | R19 | 34 | −66 | 42 | 1888 | 4.81 |
| L19 | −26 | −78 | 42 | 1024 | 4.74 | |
| Superior parietal | L7 | −34 | −70 | 46 | local | 3.62 |
| Angular gyrus | L39 | −52 | −56 | 34 | 144 | 3.80* |
| Temporal | ||||||
| Middle temporal | R21 | 56 | −46 | 4 | 88 | 3.33* |
| Superior temporal | R39 | 52 | −56 | 28 | local | 3.23* |
| Cerebellum | R | 20 | −46 | −8 | 56 | 3.83 |
|
| ||||||
| Adults: Positive Correlation between Activation and Success of Interference Suppression | ||||||
|
| ||||||
| Occipital | ||||||
| Lingual gyrus | R19 | 28 | −48 | −2 | 88 | 3.42 |
Asterisks indicate clusters also identified by relevant two-sample t-test (children > adults or adults > children)
At the more lenient threshold of p < .005 uncorrected.
Figure 4. Activation Related to Response Inhibition in Children and Adults.
(A) Group contrast and (B) regions exhibiting a positive correlation between activation and success of response inhibition.
SPM99 regression analyses were used to identify regions for which level of activation across subjects in the no-go > neutral contrast was correlated with effectiveness of response inhibition, as measured by the magnitude of the reduction in accuracy for no-go relative to neutral trials. For adults, no region was significantly correlated with success of response inhibition, with the exception of a small region in the left lingual gyrus (Table 2; Figure 4B). In contrast, a regression analysis with children identified a number of regions for which activation was correlated with success of response withholding (Table 2; Figure 4B). These regions included bilateral parietal cortex (R BA 7, L BA 39), right premotor cortex (BA 6), right globus pallidus, bilateral middle temporal gyrus (R BA 39, L BA 21, 37), and bilateral occipital cortex (BA 17, 18, 19). Within PFC, a region in right middle frontal gyrus displayed a weaker, but also positive, correlation between activation and performance (identified at p < .01; BA 9; [44, 14, 32]; z = 3.00; 144 mm3).
The children were divided equally into groups consisting of the children who inhibited no-go responses the best (errors of commission: 2%–8%) and the worst (errors of commission: 9%–26%). Better-performing children exhibited similar no-go error rates to adults (M ± SEM: 5 ± 1 and 5 ± 1, respectively). Worse performers activated left ventrolateral and bilateral dorsolateral PFC, and better performers activated bilateral inferior parietal lobule. Neither better nor worse performers activated right ventrolateral PFC, a region activated by adults.
Regions Commonly Activated by Interference Suppression and Response Inhibition
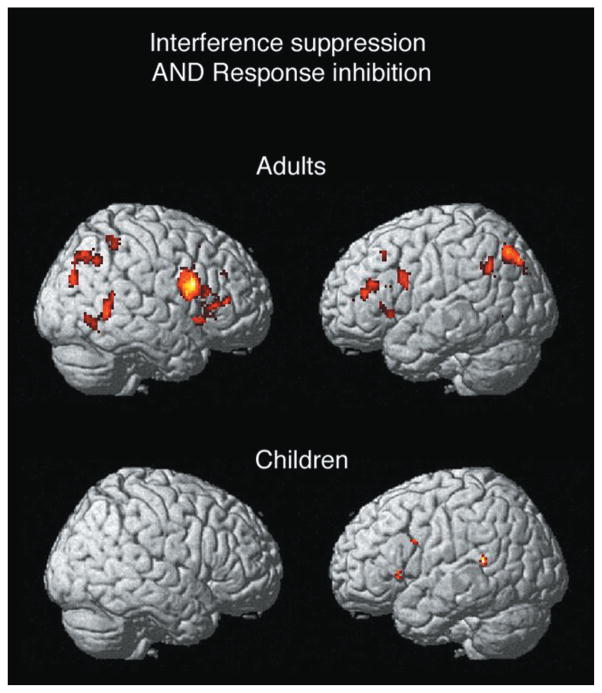
Conjunction analyses were performed separately for adults and children to identify regions commonly activated by interference suppression and response inhibition. In adults, the conjunction analysis was associated with a number of activations, including a large region in right inferior frontal gyrus (BA 44/45/47; [38, 10,24]; z = 5.43; 7744 mm3), as well as foci in left inferior and middle frontal gyri, bilateral superior and inferior parietal lobules and precentral gyri, and right-lateralized caudate, putamen, and temporo-occipital regions (Figure 5). In children, a few small foci were observed in medial frontal gyrus, left inferior frontal gyrus, right precentral gyrus, and temporal cortex (Figure 5). An ROI analysis confirmed that the large region in right ventrolateral PFC identified in adults was not significantly activated by either manipulation in children (incongruent versus neutral: t[15] = .92; no-go versus neutral: t[15] = .04).
Figure 5. Common Areas for Cognitive Control in Prefrontal Cortex.
(A) Region in inferior frontal gyrus activated by both interference suppression and response inhibition in adults.
(B) Magnitude of activation in this region across adults and children.
Discussion
Brain imaging revealed different patterns of immaturity in children aged 8–12 for two types of cognitive control. During interference suppression, children recruited different brain regions from adults. This developmental pattern, which has not previously been observed in a brain imaging study, suggests a shift in cognitive strategy between childhood and adulthood. In contrast, during response inhibition, children who performed the task well tended to recruit a subset of the adult response inhibition circuitry, suggesting that the functional circuitry recruited by adults is recruited to a greater extent over the course of childhood. Thus, these different types of cognitive control may have different developmental time courses. One commonality between the two tasks examined in the present study is the recruitment of right ventrolateral PFC by adults but not children for the purpose of cognitive control. In concert with the relevant neuropsychological literature, these findings suggest that immaturity in cognitive control is associated with an inability to recruit PFC regions in a similar manner to healthy young adults.
Behavioral Results
Consistent with previous studies, children were less able to withhold inappropriate responses than were adults (Costantini and Hoving, 1973; Casey et al., 1997; Williams et al., 1999) and were more susceptible to interference from the environment (Tipper et al., 1989; Ridderinkhof et al., 1997). Several aspects of the behavioral results deserve mention. First, because both children and adults exhibited high accuracy on all trial types, similar numbers of correct trials were submitted to the fMRI analysis for both groups. Furthermore, the event-related design allowed for examination of correct trials only, so that differences between children and adults cannot be attributed to brain activations associated with errors. Second, children exhibited fewer response inhibition failures than in other studies (Casey et al., 1997; Vaidya et al., 1998). This finding is likely to be related to the interleaving of no-go trials with flanker trials, as discussed below. Finally, the ability to identify for each manipulation a subgroup of children whose performance was similar to that of adults enabled us to examine group differences in activation that could not be explained by differences in performance.
Interference Suppression
This first brain-imaging study of interference suppression in children revealed an unexpected difference in lateralization of prefrontal activation between children and adults. Adults activated right ventrolateral PFC and insula, and greater activation of these regions was associated with greater ability to suppress interference. Children, in contrast, exhibited activation of and brain-behavior correlations for left, rather than right, ventrolateral PFC and insular cortex—regions of the brain implicated in language processing (Dronkers et al., 2000). This difference in lateralization cannot be attributed to a difference in performance between groups, as better-performing children—whose performance was similar to that of adults—failed to recruit right PFC and instead recruited left PFC more extensively than worse performers. Nor can this finding be attributed to differences in signal measurement between groups, because the left prefrontal activation in children was similar in magnitude and variability to the right prefrontal activation in adults.
The unexpected difference in laterality may be related to a difference in strategy between the two groups. Susceptibility to interference on the arrows task was significantly correlated among children with an independent measure of fluid verbal ability—word attack, which involves pronouncing novel nonwords—but not with either of two measures of crystallized verbal ability. Children may rely on their fluid verbal abilities to perform the novel task introduced to them in this experiment. These findings suggest that children may have adopted a verbal strategy during performance of a task that is not inherently verbal. A plausible strategy would be the recoding of the central arrow into a verbal label (“left” or “right”) for the purpose of limiting the distracters’ influence during the planning of the response. In any case, children and adults recruited homologous regions but in opposite hemispheres.
For both children and adults, the region of PFC most strongly correlated with the ability to suppress interference was the anterior insula, rather than the adjacent lateral surface of the inferior frontal gyrus. Insular activation is remarkably ubiquitous in imaging studies but rarely remarked upon. Foci in the more posterior part of the anterior insula, adjacent to the precentral gyrus, have been observed for studies related to verbal articulation (Dronkers, 1996; lesion study) or phonological processing (e.g., Paulesu et al., 1993). More anterior foci (adjacent to the inferior frontal gyrus; y = 8 to y = 30) have been observed for studies that required cognitive control (e.g., Garavan et al., 1999; Bunge et al., 2000, 2001; Dove et al., 2000; Rubia et al., 2001). Thus, the present findings, in accordance with many prior studies, indicate that anterior insula may play as important a role in cognitive control as the frontal and cingulate regions which have received much attention.
Response Inhibition
Adults exhibited little variability in terms of either performance or regions activated. This low level of variability resulted in robust activation of regions that have been identified in previous studies of response inhibition (Casey et al., 1997; Rubia et al., 2000) but resulted in weak brain-behavior correlations. In contrast, there was substantial variability among children in terms of performance and regions activated. This high level of variability resulted in a weak group contrast but robust brain-behavior correlations for children. The possibility that weaker group activations among children were related to greater anatomical variability is mitigated by the finding that children’s activations in the interference suppression contrast were equal in magnitude to those of adults. Rather, group differences in activation for the response interference contrast are likely to be task related.
Improvements in the ability to withhold inappropriate responses between the ages of 8 and 12 were associated with increased activation in a subset of the mostly posterior association areas consistently recruited by adults. Brain regions activated in adults which were also correlated with performance in children included bilateral precuneus, left angular gyrus, and right middle temporal gyrus, as well as right middle frontal gyrus (at a more liberal threshold). Group analyses of better- and worse-performing children supported the observation that activation of posterior association areas was a stronger determinant of performance in children than prefrontal regions: worse performers exhibited left ventrolateral and bilateral dorsolateral PFC activation, whereas better performers exhibited bilateral inferior parietal activation. The prefrontal activations observed in worse-performing children may be related to the use of strategies that are not central to the ability to withhold responses during performance of this particular task.
Unlike the present study, previous go/no-go studies have observed robust lateral prefrontal activation in children (Casey et al., 1997; Vaidya et al., 1998). This discrepancy may relate to important differences in task design and analysis between the present study and previous studies. Unlike the event-related design used in the present study, the previous studies used blocked designs in which subjects alternated between performing blocks in which they had to respond on every trial and blocks that included a certain proportion of no-go trials. In the blocked design studies, it was not possible to exclude error trials from the fMRI analysis or to rule out the possibility that subjects employed different strategies on go and no-go blocks. Either of these factors might lead to enhanced prefrontal activation. An additional difference between the present and previous studies relates to the prepotency of responding. In the previous studies, go trials far outnumbered no-go trials, and subjects always pressed the same button on the go trials. Thus, subjects developed a prepotent tendency to respond to each stimulus, which they had to override when the no-go stimulus appeared on the screen. In contrast, the present study employed an event-related design in which trials of different kinds were pseudorandomly interleaved. Subjects had to analyze each stimulus array as it appeared on the screen in order to determine whether they should press a left button, a right button, or withhold their response. Thus, subjects in our study are unlikely to have developed a strongly prepotent response tendency that needed to be overridden, because they could not plan a precise response (i.e., the plan to move a specific finger) until the stimulus appeared. Because PFC activation is more likely to be critical for task performance as the prepotency of the to-be-inhibited response increases, low prepotency of responding may explain the lack of robust prefrontal activation in children in the present study. Another difference from previous go/no-go studies (Casey et al., 1997) is that activation was not observed in orbitofrontal cortex during response withholding. Because of the large susceptibility artifacts at 3 T in tissues bordering the orbital cavities, there was substantial loss of signal in this region in our functional data set.
Regions Implicated across Inhibitory Tasks
In adults, the largest and most robust common activation across tasks was in right ventrolateral PFC, a region that has been activated across a number of studies involving tasks that require subjects to withhold or stop responding (Casey et al., 1997; Konishi et al., 1998; Rubia et al., 2001), suppress interference from irrelevant stimuli or stimulus dimensions (Hazeltine et al., 2000), or shift cognitive sets (Konishi et al., 1999). Together with the present finding that greater activation of the right inferior frontal gyrus is associated with less interference susceptibility, this group of findings suggests that this region plays an important role in suppressing interference between competing stimuli, response options, or strategies across a variety of cognitive tasks.
Whereas both adults and children activated left ventrolateral PFC across tasks, children failed to activate right ventrolateral PFC for either task (although a more dorsal region was weakly recruited by children during performance of no-go trials). Thus, children in this study failed to recruit the region that was most robustly activated by both tasks in adults. The differences in task performance between children and adults may be related to differences in the ability to effectively recruit this and other brain regions—including parietal cortices—for cognitive control.
Future Directions
This and other studies constitute only initial steps in the use of functional neuroimaging to enhance the study of human developmental cognitive neuroscience. These studies reveal direct relations between brain functions and cognitive abilities in children, a step forward from prior analyses that involved analogies to adult focal lesions or extrapolations from either animal research or postmortem brain measures. Nevertheless, future research can aim for more complete and precise functional analyses. The large differences in the present crosssectional study suggests that a major transformation occurs between ages 12 and 19, and imaging studies with adolescents in that age range, as well as longitudinal studies tracking neural and cognitive changes within individuals, may illuminate how that maturation unfolds. Methodological improvements, such as the creation of brain templates for children of all ages, will likely enhance the validity of the anatomical localization of activations. Such advances will enable further examination of how the development of the brain subserves the maturation of the mind.
Experimental Procedures
Subjects
Healthy right-handed volunteers were recruited from Stanford University and the community and were paid for their participation. Sixteen children (nine males; ages 8–12, M = 10) and sixteen adults (nine males; ages 19–33, M = 24) were included in the study. Three additional adults were excluded—two on the basis of poor normalization to the template brain and one on the basis of technical difficulties related to data acquisition.
Behavioral Testing
Children participated in a separate behavioral testing session (on average) 20 days before scanning and no more than 3 months prior to scanning. Children were administered a series of standardized tests to estimate IQ (vocabulary and block design [Wechsler, 1991; see Spreen and Strauss, 1998]), to estimate verbal ability and screen for reading disabilities (word attack, word identification [Woodcock, 1998]), and to index speed of processing (coding [Wechsler, 1991]).
Task
Subjects performed a modified flanker task in the MRI scanner (Eriksen and Eriksen, 1974; Eriksen and Schultz, 1979). On each trial, they viewed an array of five stimuli, including a central arrow and two stimuli on either side of it (flankers) (Figure 1). Using the index and middle fingers of their right hand, subjects pressed a left button if the central arrow pointed to the left and the right button if it pointed to the right. They were instructed to ignore the flankers on either side of the central arrow, and to respond as quickly yet as accurately as possible.
Each scan included four experimental conditions: congruent, incongruent, neutral, and no-go trials, as well as additional fixation trials. On congruent trials, the flankers were arrows pointing in the same direction as the target. On incongruent trials, the flankers were arrows pointing in the opposite direction of the target. On neutral trials, the flankers were diamonds, stimuli not associated with any response. On no-go trials, the flankers were ×’s, which indicated that subjects should withhold their response.
The trials followed a rapid event-related design with a 3 s intertrial interval. On each trial, the stimulus array was presented for 800 ms, followed by a blank screen (300 ms) and then a crosshair (1600 ms). The next trial began 300 ms later. On fixation trials, subjects viewed a crosshair for 2700 ms and a blank screen for 300 ms. The trial sequence was specified according to a stochastic design in SPM99, in which the probability of each condition varied sinusoidally between 0 and 1 over a 30 s period. Each condition had a probability function with a different phase; over time, all conditions occurred with equal probability. Subjects performed 46–58 trials of each condition (across subjects, an average of 51–52 trials per condition) in addition to 44 fixation trials over the course of two scans. The trial sequence was specified by one set of lists for half the subjects and another set of lists for the other half. The order of scans was counterbalanced within these groups. Following Ridderinkhof et al. (1997), the target and flanker stimuli subtended approximately .8° and 1° of visual angle, respectively, and the entire array of stimuli subtended approximately 6.5° horizontally and 1° vertically.
Testing Procedure
Subjects practiced 10–20 trials of the task prior to scanning. During scanning, subjects responded by pressing either of two buttons on a button box with the index and middle fingers of their right hand. Psyscope (Cohen et al., 1993) was used to generate stimuli and to collect responses. A magnet-compatible projector was used to display the stimuli on a screen near the subject’s head. Subjects viewed these stimuli using a mirror mounted on the head coil.
Data Acquisition
Whole-brain imaging data were acquired on a 3 T MRI Sigma LX Horizon Echospeed scanner (G.E. Medical Systems, 8.2.5 systems revision). T1-weighted flow-compensated spin-echo anatomical images (Minimum TR, 500 ms TE) were acquired in 16 contiguous 7 mm axial slices, parallel to the plane of the anterior commissure and the posterior commissure. Functional images were acquired for the same set of slices using a T2*-sensitive gradient echo spiral pulse sequence (Glover and Lai, 1998) (1000 ms TR, 30 ms TE, 60° flip angle, 24 cm field of view, 64 × 64 data acquisition matrix).
Data Analysis
SPM99 (Wellcome Department of Cognitive Neurology, UK) was used to process and analyze the functional data. Functional images were corrected for differences in slice acquisition time and motion-corrected. Estimated motion parameters computed by SPM99 were examined on a subject-by-subject basis to ensure that the amount of absolute motion did not exceed 2 mm (the dimensions of a normalized voxel). All subjects exhibited less than 1 mm of absolute motion over the course of the experiment. Children and adults did not differ in terms of estimated motion parameters (average movement in children: .188 mm; in adults; .189 mm; t[30] = .03; p = .98). Functional images were normalized to a standard template brain with SPM99 and interpolated to 2 × 2 × 2 mm voxels. Normalized images were spatially smoothed with a Gaussian filter (6 mm full width-half maximum) and temporally filtered (low-pass filter: 4 ms Gaussian; high-pass: SPM default calculated on the basis of trial frequency). Single subjects’ data were analyzed with a fixed effects model within the framework of the General Linear Model (Friston et al., 1994). Only correct trials were submitted to statistical analysis. fMRI responses were modeled by a canonical hemodynamic response function and its temporal derivative, as described by Friston et al. (1998). Group data were analyzed using a random effects model (Holmes and Friston, 1998). For the group analyses, images were averaged to create one image of mean activation per contrast and subject. Each mean image was globally scaled to a mean signal intensity of 100.
In order to identify regions recruited across subjects for cognitive control, one-sample t tests were performed on the average images. To identify regions for which level of activation across subjects was correlated with performance, simple regression analyses were performed on the average images. The group contrasts and regression analyses were performed separately for children and adults and employed a statistical threshold of p < .001, uncorrected for multiple comparisons, with an extent threshold of five contiguous voxels. To confirm the finding that several regions identified by the group contrasts were significantly more activated by one group than the other, two-sample t tests (p < .05 uncorrected) were performed on the children’s and adults’ contrast images. Clusters identified in the children’s and adults’ group contrasts that were also identified in the relevant two-sample t test (children > adults and adults > children, respectively; clusters no more than 2 mm in each dimension from maxima in group contrast) are indicated by asterisks in Tables 1 and 2. To examine whether better and worse performers exhibited different patterns of activation, children were divided equally into two groups—the eight best performers and the eight worst performers—for each of the two manipulations. Because of the lower number of subjects, a lower statistical threshold was used to examine activation for each of the two groups of eight children (p < .005 uncorrected for multiple comparisons; extent threshold, 5 contiguous voxels). Similar findings were obtained when the threshold was made even more lenient (p < .01 uncorrected).
Regions activated for both types of cognitive control were identified by conjunction analyses performed with the simple regression analysis tool in SPM99. This type of analysis identifies regions that exhibit a main effect of both manipulations and excludes regions for which activation differs significantly between the two. Conjunction analyses were performed separately for children and adults. A combined threshold of p < .001 uncorrected for multiple comparisons was used for the conjunction analyses, corresponding to p < .01 for each contrast. ROI analyses were performed by computing the mean parameter estimate of activation for functionally defined clusters for each condition and subject.
Acknowledgments
The authors thank Rebecca Ray, Gayle Deutsch, and Kalina Christoff for their assistance. The authors were supported by grant 61426 from National Institutes of Mental Health (J.D.E.G.) and a fellowship from the Baxter Foundation (S.A.B.).
References
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Klingberg T, Jacobsen RB, Gabrieli JDE. A resource model of the neural basis of executive working memory. Proc Natl Acad Sci USA. 2000;97:3573–3578. doi: 10.1073/pnas.050583797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, et al. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000a;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci USA. 2000b;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Cohen J, MacWhinney B, Flatt M, Provost J. Psyscope: an interactive graphical system for designing and controlling experiments in the Psychology laboratory using Macintosh computers. Behavior Research Methods, Instrumentation, and Computers. 1993;25:257–271. [Google Scholar]
- Comalli PE, Waoner S, Werner H. Interference effects of Stroop color-word test in children, adulthood and aging. J Genet Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- Costantini AF, Hoving KL. The relationship of cognitive and motor response inhibition to age and IQ. J Genet Psychol. 1973;123:309–319. doi: 10.1080/00221325.1973.10532690. [DOI] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Dev Rev. 1992;12:45–75. [Google Scholar]
- Diamond A. Abilities and neural mechanisms underlying AB performance. Child Dev. 1988;59:523–527. [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy and biochemistry. In: Knight SA, editor. The Frontal Lobes. London: Oxford University Press; 2002. in press. [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Redfern BB, Knight RT. The neural architecture of language disorders. In: Gazzaniga M, editor. The New Cognitive Neurosciences. 2. Cambridge, MA: MIT Press; 2000. pp. 949–958. [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum Brain Mapp. 1994;1:153–171. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Grandin CB, Xu B. Developmental aspects of pediatric fMRI: considerations for image acquisition, analysis, and interpretation. Neuroimage. 2001;13:239–249. doi: 10.1006/nimg.2000.0681. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, editor. Handbook of Physiology, Volume V. New York: Oxford University Press; 1987. pp. 373–417. [Google Scholar]
- Gratton G, Coles MG, Sirevaag EJ, Eriksen CW, Donchin E. Pre- and poststimulus activation of response channels: a psychophysiological analysis. J Exp Psychol Hum Percept Perform. 1988;14:331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Harnishfeger KK, Bjorkland DF. The ontogeny of inhibitory mechanisms: a renewed approach to cognitive development. In: Howe ML, Pasnak R, editors. Emerging Themes in Cognitive Development. Vol. 1. New York: Springer-Verlag; 1993. pp. 28–49. [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JDE. Neural activation during response competition. J Cogn Neurosci. 2000;12:118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage: Abstracts of the 4th International Conference on Functional Mapping of the Human Brain; 1998. p. S754. [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, part I: localization of age-related changes. Biol Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Lorsbach TC, Reimer JS. Developmental changes in the inhibition of previously relevant information. J Exp Child Psychol. 1997;64:317–342. doi: 10.1006/jecp.1996.2350. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luria AR. Higher Cortical Functions in Man. New York: Basic Books; 1966. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative MRI study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1993;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van der Molen MW, Band GPH. Sources of interference from irrelevant information: a developmental study. J Exp Child Psychol. 1997;65:315–341. doi: 10.1006/jecp.1997.2367. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests—Administration, Norms and Commentary. 2. New York: Oxford University Press; 1998. [Google Scholar]
- Stuss DT, Benson DF. The Frontal Lobes. New York: Raven; 1986. [Google Scholar]
- Tipper SP, Bourque TA, Anderson SH, Brehaut JC. Mechanisms of attention: a developmental study. J Exp Child Psychol. 1989;48:353–378. doi: 10.1016/0022-0965(89)90047-7. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JDE. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Woodcock RW. Woodcock Reading Mastery. Circle Pines: American Guidance Service; 1998. [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowsky A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]