Abstract
New neurons are generated in the hippocampus each day and their survival is greatly enhanced through effortful learning (Shors, 2014). The numbers of cells produced can be increased by physical exercise (van Praag et al., 1999). These findings inspired us to develop a clinical intervention for humans known as Mental and Physical Training, or MAP Training. Each session consists of 30 min of mental training with focused attention meditation (20 minutes sitting and 10 minutes walking). Meditation is an effortful training practice that involves learning about the transient nature of thoughts and thought patterns, and acquiring skills to recognize them without necessarily attaching meaning and/or emotions to them. The mental training component is followed by physical training with 30 min of aerobic exercise performed at moderate intensity. During this component, participants learn choreographed dance routines while engaging in aerobic exercise. In a pilot “proof-of-concept” study, we provided supervised MAP Training (2 sessions per week for 8 weeks) to a group of young mothers in the local community who were recently homeless, most of them having previously suffered from physical and sexual abuse, addiction, and depression. Preliminary data suggest that MAP Training improves dependent measures of aerobic fitness (as assessed by maximal rate of oxygen consumed) as well as decreases symptoms of depression and anxiety. Similar changes were not observed in a group of recently homeless women who did not participate in MAP Training. It is not currently possible to determine whether new neurons in the human brain increase in number as a result of MAP Training. Rather these preliminary results of MAP Training illustrate how neuroscientific research can be translated into novel clinical interventions that benefit human health and wellness.
Introduction
Tens of thousands of new neurons are produced each day in a “normal” adult brain. Many of these cells are produced in the hippocampus, a brain region necessary for various types of learning (Figure 1A). The cells are produced in a part of the hippocampus known as the dentate gyrus, whose primary neuronal phenotype is the granule neuron (Figure 1B). Once the new cells differentiate into granule neurons (Figure 1C), they produce axons and dendrites and eventually express action potentials, as they become incorporated into the rest of the brain (van Praag et al., 2002). In animal studies, it has been determined that newly-generated cells in the dentate gyrus are especially responsive to environmental conditions that humans often experience (Figure 2; Shors et al., 2011). For example, stressful life experiences significantly decrease cell production as does daily drinking of moderate amounts of alcohol, an amount comparable to the legal driving limit (Anderson et al., 2012). However, not all life experiences decrease cell production in animal models. Most notably, aerobic exercise greatly increases the number of cells that are made. Animals that are given the opportunity to run on a daily basis can produce nearly twice as many new cells as sedentary controls (van Praag et al., 1999).
Figure 1.
The hippocampus (A; Timm stain; Shors 2014) produces thousands of new neurons each day (B). The new cells (shown labeled with BrdU in brown) reside in the granular layer of the dentate gyrus (B; unpublished photomicrograph). If they survive and many do in response to learning, most differentiate and mature as granule neurons (double labeled with BrdU in red and Tuj1 in green; Leuner et al., 2004).
Figure 2.
Numbers of new neurons in the rodent hippocampus decrease after stressful life experience (Kozorovitskiy and Gould, 2004), opiate use (Eisch et al., 2000), sleep deprivation (Hairston et al., 2005) and alcohol consumption (Nixon and Crews, 2002) (A), while numbers increase with sexual activity (Leuner et al., 2010), environmental enrichment (Kempermann et al. 1997), silence (Kirste et al., 2014) and physical exercise (van Praag et al., 1999; Fabel et al., 2009) (B). Fewer new cells were produced in response to moderate amounts of alcohol. New cells are shown labeled with BrdU (brown) in both males and females (Anderson et al., C) while intense exercise training produced more new neurons. New neurons (brown) are shown labeled with doublecortin.
Neurogenesis and Effortful Learning
The newly-generated cells in the hippocampus do not necessarily survive. Indeed, more than half of them die and do so within several weeks of being born, often before they have fully differentiated into neurons (Anderson et al., 2011). Nevertheless, a great number of these newly-generated cells can be “rescued” from death if animals engage in an effortful learning experience. In 1999, Drs. Shors and Gould reported that new neurons in the rat hippocampus are rescued from death by new learning and may even be involved in learning (Gould et al., 1999; Shors et al., 2001). Since then, it has been determined that not all types of training keep new neurons alive. Rather training tasks that are difficult to learn are the most effective (Waddell and Shors, 2008; Waddell et al., 2010; Curlik and Shors 2011). Moreover, learning itself is critical. Animals that learn well retain more cells than animals that are trained but don’t learn as well (Dalla et al., 2007). Within animals, those that learn but require more trials of training to learn, retain more of the new cells (Curlik et al., 2011; Waddell and Shors, 2008). Overall, these data indicate that learning keeps new neurons alive, provided that the training experience is both effortful and successful.
Learning a new physical skill can also increase the survival of new neurons in the hippocampus (Curlik et al., 2013; Figure 3). During one such task, a laboratory rodent learns to balance on a rod as it rotates faster and faster during one trial of training. Over trials, the animals learn to remain on the rod, despite the increase in speed. Learning this type of motor skill might be comparable to mastering a new sport or learning a new dance routine in humans (Sherwood and Jeffery, 2000). Training with the physical motor skill increased the number of surviving neurons in the adult hippocampus. Consistent with previous studies, only animals that learned the new skill retained the new cells. Animals that didn’t learn retained only as many new cells as those that were not trained. Also consistent with previous findings, we determined that the task conditions must be sufficiently difficult to learn in order to rescue the new cells from death. For example, training on a relatively easy version of the task, during which the rod rotates slowly and consistently throughout a trial does not increase the number of surviving cells. Finally, individual differences in performance were once again important. Animals that learned well retained the most new neurons. Together these various studies indicate that learning can rescue new neurons from death but the experience must be new and effortful -- and learning itself must occur. These effects on cell survival were not attributable to exercise, per se. Animals that exercised during the same time period did not retain more cells than animals that did not exercise.
Figure 3.
During physical skill training, rodents learn to remain on a rod that is rotating faster and faster during each trial (A). They learn to remain on the rod longer over trials of training (B). Those that are trained retain more newly-generated cells than those that are not trained (Curlik et al., 2013). Those that learn best retain more cells than those that do not learn well (D).
To summarize, behaviors such as aerobic exercise increase the production of new neurons in the adult hippocampus while effortful and successful learning keeps a significant number of those cells alive (Curlik and Shors 2012). As noted, once the new cells are rescued from death, they remain in the hippocampus for months at least, by which time they have acquired the characteristics of mature neurons, including the ability to produce action potentials (Leuner et al., 2004; van Praag et al., 2002). Because humans also generate new neurons throughout life (Eriksson et al., 1998; Manganas et al., 2007; Spalding et al., 2013), it is assumed that aerobic exercise increases their numbers as it does in rodents and indirect evidence supports this premise. For example, exercise training on a treadmill increased blood flow to the human dentate gyrus (Pereria et al., 2007) and similar exercise regimens have increased hippocampal volume (Erickson et al., 2011). With respect to learning, it is assumed that effortful training procedures which engage skill learning would, in turn, keep a great number of newly-generated cells alive in the hippocampus, although this particular assumption cannot be tested in humans at this point in time. Based on these laboratory studies and assumptions, we developed a clinical intervention that combines mental and physical training, referred to as MAP Training.
The MAP Training Intervention
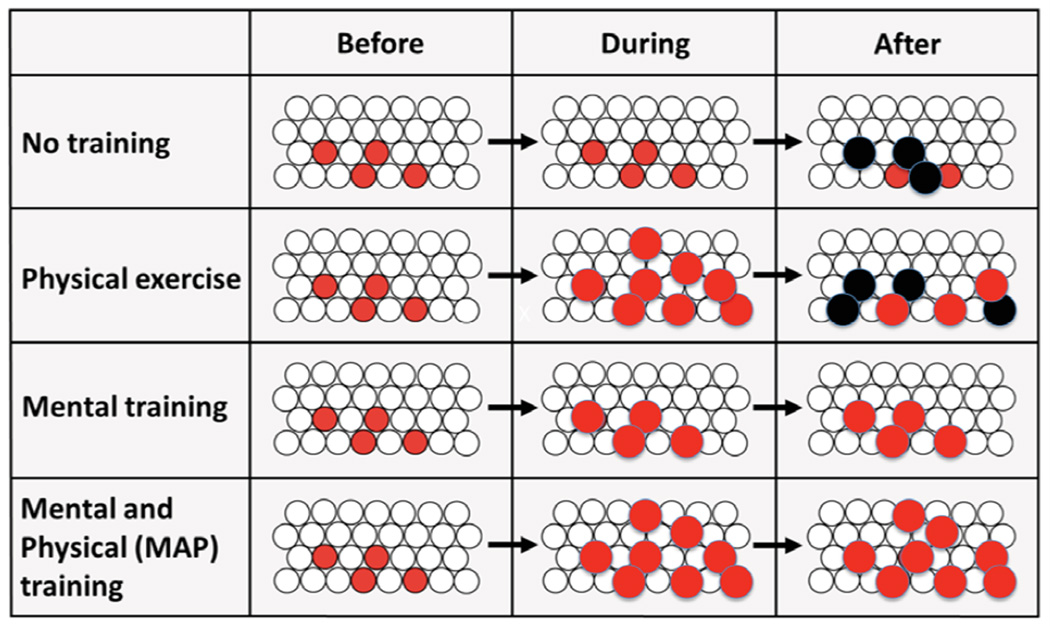
MAP Training is a clinical and practical intervention, which was inspired by research connecting neurogenesis with learning and exercise. This intervention is based on animal laboratory studies demonstrating that physical exercise increases the number of cells produced while mental training keeps the cells alive (Figure 4; Curlik and Shors, 2012; van Praag et al., 1999). We propose that the combination of the two activities is better than either one alone, again based on evidence that new neurons are kept alive by learning once they are produced. For the intervention, we chose activities that humans can readily engage in and yet are known to enhance mental and physical health (Herring et al., 2012; Heyward, 1998; Hofmann et al., 2010; Kabat-Zinn, 1990; Davidson and McEwen, 2012; Chiesa and Serretti, 2010; van Vugt et al., 2012). Our goal was to translate the neurogenesis/ learning data into a clinical intervention that can be easily disseminated and practiced by everyone in our community. Therefore, we did not choose eyeblink conditioning or spatial maze learning because those training tasks would not be especially easy to administer in humans and they would not necessarily provide “new” learning experiences across repeated training sessions (Nokia et al., 2012). But more importantly, we wanted to provide the participants with an opportunity to learn new skills that once acquired, they could continue to use in their everyday lives to maintain increases in mental and physical health outcomes.
Figure 4.
MAP Training in humans was translated from laboratory studies about neurogenesis in the hippocampus. With no training, many new cells die in the dentate gyrus of the hippocampus. With physical exercise, more are produced, but some still die. With mental training, most cells survive as long as the process is effortful and learning occurs. Theorectically, a combination of physical exercise with mental training which is sustained over time should increase the number of new neurons that ultimately reside in the adult brain (adapted from Curlik and Shors, 2012).
MAP Training Methods
For the mental training component, participants engaged in the practice of focused attention meditation. We chose this particular meditation procedure, because it is challenging to learn and practice. (Recall that new neurons in animal models are rescued from death by tasks that are challenging to learn; Waddell et al., 2010; Curlik and Shors, 2010; Shors 2009, 2014). During the initial session, participants were instructed to sit on a Zafu cushion in an upright position with legs crossed and hands folded together in the middle of the lap. They were instructed to breathe slowly and “watch” their breath, counting each one as the final air is expelled during exhalation. As thoughts arise, participants new to meditation typically lose count of the breath. At this point, they were told to let go of the thought(s) without judgment and return to counting each breath, beginning again with the number one. With practice, a person learns how difficult it is to pay attention to the breath. As a consequence, he or she learns how busy the mind is and how transient thoughts are, which may interfere with concentration. With practice, one also learns to let go of these recurring thoughts about the past as well as worrisome thoughts projecting into the future. Focused attention meditation is an effortful learning experience, during which one learns to be present in this moment while being less reactive to ongoing thoughts and emotions.
After 20 min of sitting meditation, participants were instructed to stand to begin 10 min of walking meditation. Walking meditation is similar in principle to sitting meditation. However, instead of focusing on the breath, individuals focus on the movement of the feet. In our program, we adopted a very slow walking practice. Individuals gathered in a circle, walking slowly with hands folded behind their back. They were instructed to keep their attention in each foot as the weight transferred from one foot to the next, from the heel to the ball of the foot to the toe. As a person walks, once again, thoughts tend to arise and interfere with concentration. And once again, participants were instructed to bring their focus of attention back to the feeling of their feet. With practice, one learns to redirect the focus of attention back to the body. Walking meditation is an effortful training experience during which individuals learn to be present in this moment even while moving forward.
The mental training component was immediately followed by the physical component of MAP training, during which individuals engaged in 30 minutes of directed aerobic exercise. It is well established that aerobic exercise promotes mental and physical health (Dishman et al., 2006; Arena et al., 2010; Charansonney, 2011; Herring et al., 2012), and as noted, it increases the number of new neurons produced in the hippocampus of laboratory animals (van Praag et al., 1999; Nokia et al., 2014). For the study presented here, participants exercised in a group while learning choreographed dance routines similar in style to the widely popular dance programs Zumba and Jazzercise. Before beginning, all participants were provided a pedometer to tally the number of steps accrued during the session as well as a heart rate monitor (Polar Electro, Finland) to assess heart rate throughout the session and monitor exercise intensity. Each session began with a short warm-up (3 min) followed by 25 min of aerobic-based dance exercise training at a moderate intensity, followed by a 2–3 min cool down period. During each session, participants were taught new sequences of physical motor skills set to music of popular songs. This component of MAP Training has been especially well received by the women, presumably because it elevates mood and social interaction while decreasing rumination (Sherwood and Jefferey, 2000; Nolen-Hoeksema, 1991; 2012).
Preliminary Data from Underserved Individuals in the Community
The MAP Training intervention has produced positive results in three study populations so far. In the present review, we present preliminary data from one population. The participants in this study were all young women (18–36 years; mean 25±5) who were recently homeless, having suffered months and in some cases years of poverty and trauma, as well as addictive behaviors. Nearly all of the women reported being sexually and/or physically abused either as children or young adults. They were rescued from the streets and given housing and food in a residential center, where they live with their children. We provided MAP Training in a large conference room at their residence. The sessions occurred twice a week for 8 weeks. The participants had neither meditated nor engaged in aerobic exercise prior to MAP Training. Therefore, we propose that the 16 sessions of MAP Training resulted in consistent and effortful learning experiences over time, at least as can be assessed through verbal communication and observation. In this review, we present preliminary data from a group of women (n=8) who completed all 8 weeks of supervised MAP Training, as well as a group (n=6) of previously homeless women who resided at the center but did not participate in MAP Training. Instead of MAP Training, the treatment-as-usual (TAU) group participated in group activities which did not include aerobic exercise or focused attention meditation.
Physical Health Outcomes
Before MAP Training, participants were informed about the study and provided written informed consent that was approved by the Institutional Review Board at Rutgers University. On a separate day, participants were transported from the residence to the Exercise Psychophysiology Laboratory at Rutgers University for baseline testing. After MAP Training or an equivalent amount of time at the center without MAP Training (i.e., treatment-as-usual controls), participants again returned to the laboratory for post-testing. During the baseline and post-intervention assessments, the Mini International Neuropsychiatric Interview (M.I.N.I.) and a series of mental and physical health outcomes were administered, including tasks of attention, hippocampal-dependent learning, event-related brain potentials (ERPs) through EEG, heart rate variability, maximal oxygen consumption, and psychological outcomes. In this review, we present preliminary data for several of these health outcomes (Figure 5).
Figure 5.
In a preliminary “proof-of-concept” study, MAP Training was provided twice a week for 8 weeks to underserved women in our community who were recently homeless (n=8). Maximal rate of oxygen consumed (V02 peak) was assessed during a stress test before and after MAP Training. After 16 sessions of training, participants increased their aerobic fitness, as indicated by an increase in oxygen consumption (A). Before training, participants expressed symptoms of depression and anxiety as assessed with Beck Inventories. After MAP Training, symptoms of depression and anxiety were significantly reduced (B). A group of recently homeless women receiving treatment as usual (TAU) but who did not participate or complete 16 sessions of MAP Training (n=6) did not express significant changes in VO2, BDI or BAI.
The first physical outcome measure reported here is VO2 peak, which is the maximum rate of oxygen consumption during incremental exercise and is widely considered the gold standard measurement of aerobic physical fitness. Specifically, cardiorespiratory fitness (VO2 peak) was assessed through a submaximal exercise test using a motor-driven treadmill and a modified Bruce protocol (Bruce et al., 1975). The speed of the treadmill was initially set at 1.7 mph and was increased to 2.5, 3.4, 4.2, 5.0, and 5.5 mph at 3-min intervals throughout the test. The treadmill incline was initially at a 10% grade and was subsequently increased by 2% every 3 min until participants reached 85% of age-predicted maximal HR (220 bpm - age in years), at which time the test was terminated. A Polar heart rate (HR) monitor (Polar Electro, Finland) was used to measure HR throughout the test. Oxygen consumption was measured through indirect calorimetry using a Parvo MedicsTrueOne 2400 Metabolic Measurement Cart (Parvo Medics, Inc., Sandy, UT) averaged over 15-s intervals. VO2 peak (mL· kg−1·min−1) was established from direct expired gas exchange data from the metabolic system and was predicted by extrapolating the linear regression between HR and measured oxygen consumption measured up to the agepredicted maximal HR (ACSM, 2013). A 3–5 min cool-down period was then performed at preferred walking speed and 0% grade to ensure participants returned to near baseline cardiovascular values.
After MAP Training, maximal oxygen consumption (VO2 peak) increased from numbers in ranges considered fair to good (30–35 mL· kg−1·min−1) into those considered excellent and even superior (>40 mL· kg−1·min−1) for women of this age group (Figure 5A). These data are consistent with their performance during MAP Training. During each session of MAP Training, participants wore heart rate monitors in order to evaluate maximum, minimum and average heart rates during each component of MAP Training. During meditation, the average heart rate was 77 beats/min, and this increased to an average of 137 beats/min with an average maximum of 167 beats/min during exercise. Participants also wore pedometers to monitor the number of steps that were taken during each session. The average number of steps per training session exceeded 2,330, with a range between 1006–3791 steps.
For a comparison group, we also tested a group of women (n=6) who were recently homelessness and received treatment as usual (TAU) at the center but did not participate in MAP Training. The mean VO2 peak for the TAU group was 34.68 ±3.47 mL· kg−1·min−1 during the pretesting baseline period and 36.43 ±2.93 mL· kg−1·min−1 after 8 weeks of living at the center with no significant change across time (p>0.05). Analysis of variance revealed a significant interaction between MAP Training and time between VO2 peak assessments, F(1,10)=11.66, p<0.01. Only the group that completed MAP Training expressed an increase in oxygen consumption (p<0.05). Therefore, participation in the MAP Training program, and not their new living conditions, significantly increased aerobic fitness. This was expected because the participants did not regularly participate in aerobic exercise and VO2 would not be expected to increase in response to changes in improvements in food, shelter, medicine, etc. These data suggest the physical training component of MAP Training enhanced the cardiovascular fitness or endurance capacity of our participants. Of relevance, increases in VO2 peak have been associated with an increased volume of the hippocampus in humans (Erickson et al., 2009). Most importantly, VO2 peak is one of the best predictors of all-cause mortality and cardiovascular disease (Kodama et al., 2009).
Mental Health Outcomes
After 16 sessions of MAP Training (twice a week for eight weeks), participants expressed significant changes in mental health outcomes. Before training, participants reported higher symptoms of depression consistent with a diagnosis for major depressive disorder (MDD), as assessed with the Beck Depression Inventory (BDI-II; Beck, Steer, & Carbin, 1988). After 8 weeks of MAP Training, mean BDI scores decreased significantly. As shown in Figure 5B, BDI scores after MAP Training were less than half what they were before MAP Training. Before training, the participants also expressed higher symptoms of anxiety as detected with the Beck Anxiety Inventory (Beck, Epstein, & Brown, 1988). Following MAP Training, BAI scores were significantly reduced, suggesting that participants were experiencing fewer symptoms of anxiety. The same mental health outcomes were assessed in a comparison group of previously homeless women who live at the center but who did not participate in the MAP Training program (n=6). No change in BDI [F(1,5)=0.14; p=0.72] or BAI [F(1,5)=1.21; p=0.32] scores were found over the course of 8 weeks in women living under similar conditions without participation in MAP Training. With respect to depression, there was a significant interaction between MAP Training and time of assessment, F(1,12)=7.61; p<0.05. As noted, only those who participated in the MAP Training intervention reported decreases in depression symptoms. With respect to anxiety, there was no interaction between the intervention and time between testing periods (pre to post assessment) because both groups (MAP Trained and TAU) reported a decrease in anxiety symptoms over time. This is not surprising because the participants in the center program also experience other changes in their lives that are known to enhance mental health, including other psychological programs and group therapies, as well as medical assistance and nutritious food and the comforts of heat and shelter. Therefore, we are not claiming that 8 week of MAP Training is responsible for all of the positive mental and physical health outcomes, but rather that it synergistically enhances the health outcomes that were observed, especially those related to depression. It is further noted that we have observed similar outcomes in a group of depressed adults and otherwise healthy controls. In these studies, we have also observed a significant decrease on BDI scores after MAP Training, even in the healthy control group (Alderman et al., 2014a,b).
Relevance to Neurogenesis and Learning
A recent study reported that female rodents that experience early life stressors produce fewer hippocampal neurons (Loi et al., 2014), while another study reported less complex arborization in cells that are produced under stressful conditions (Leslie et al., 2011). These findings in laboratory studies may have some potential relevance to the population of homeless women that we have been studying, because they have experienced a number of early life stressors. That said, it is important to note that we do not know whether the women began training with fewer new neurons nor do we know whether the positive outcomes of MAP Training are due to an increase in neurogenesis and/or the survival of new neurons in the hippocampus. The MAP Training intervention was “inspired” by neuroscientific data from laboratory experiments but further studies and analyses will be necessary in order to identify the mechanisms of action. This study represents the translational interface between basic scientific discoveries and clinical medicine application with the goal of improving public health.
Conclusion
Overall, preliminary findings presented here suggest that MAP Training enhances mental and physical health outcomes for young women who have experienced a number of traumatic events and stressors in their lives, including poverty, homelessness, abuse and addiction. We have also been providing the intervention to a large group of depressed adults as well as a group of healthy control participants, and have observed similarly positive results in mental and physical health outcomes (Alderman et al., 2014a,b). The MAP Training intervention is well accepted among participants, inexpensive to administer, and can be reasonably used in many clinical and community populations. Indeed, we anticipate that MAP training can be used to enhance mental and physical health in men and women from all walks of life -- from the college student struggling with depression to the housewife addicted to pain medications to the soldier coming home from war. In our opinion, the number of people that could benefit from MAP Training is limitless.
Highlights.
Physical exercise increases the number of new neurons in the adult hippocampus whereas effortful learning keeps them alive.
Mental and Physical (MAP) Training is a clinical intervention that combines aerobic exercise and meditation training.
MAP Training improves mental and physical health outcomes in humans suffering from stressful life events.
Neuroscientific research can inspire the development of novel interventions that enhance health in humans.
Acknowledgements
We thank the Center for Great Expectations (Somerset, NJ) for their assistance and support as well as the Soshimsa Zen Center for meditation training and guidance (Plainfield, NJ). We also thank Michelle Chang, Raelyn Loiselle, Megan Anderson, Dan Curlik, David Eddie, Preeti Tammineedi and Krishna Tobon for their important contributions.
Supported by NSF (IOS-0914386) to TJS and the Charles and Johanna Busch Memorial Fund at Rutgers, The State University of New Jersey to BLA and EAS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alderman BL, Olson RL, Bates ME, Selby EA, Buckman JF, Panza EA, Kranzler A, Eddie D, Shors TJ. Rumination in major depressive disorder is associated with impaired inhibitory control but preserved temporal dynamics of attention. NeuroImage:Clinical. 2014a In revision. [Google Scholar]
- Alderman BL, Olson RL, Bates ME, Selby EA, Shors TJ. Mental and physical (MAP) training reduces symptoms of depression while enhancing neuronal indices of cognitive control. 2014b In preparation. [Google Scholar]
- Anderson ML, Sisti H, Curlik DM, Shors TJ. Associative learning increases neurogenesis during a critical period. European Journal of Neurosci. 2011;33:175–181. doi: 10.1111/j.1460-9568.2010.07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M, Nokia M, Shors TJ. Moderate drinking? Alcohol consumption significantly decreases neurogenesis in the adult hippocampus. Neurosci. 2012;224C:202–209. doi: 10.1016/j.neuroscience.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena R, Myers J, Guazzi M. The future of aerobic exercise testing in clinical practice: is it the ultimate vital sign? Future Cardiol. 2010;6(3):325–342. doi: 10.2217/fca.10.21. 2010. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bruce RA, Pao YL, Ting N, Chiang BN, Li YB, Alexander ER, Beasley RP, Fisher LD, Chiang ST. Seven-year follow-up of cardiovascular study and maximal exercise of Chinese men. Circulation. 1975;51(5):890–901. doi: 10.1161/01.cir.51.5.890. 1975. [DOI] [PubMed] [Google Scholar]
- Charansonney OL. Physical activity and aging: a life-long story. Discov Med. 2011;12(64):177–185. [PubMed] [Google Scholar]
- Chiesa A, Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychol Med. 2010;40:1239–1252. doi: 10.1017/S0033291709991747. [DOI] [PubMed] [Google Scholar]
- Curlik DM, Maeng LY, Agarwal PR, Shors TJ. Physical skill training increases the number of surviving new cells in the adult hippocampus. PLoS One. 2013;8(2):e55850. doi: 10.1371/journal.pone.0055850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curlik DM, Shors TJ. Learning increases the survival of newborn neurons providing that learning is difficult to achieve and successful. Journal of Cognitive Neuroscience. 2011;23:2159–2170. doi: 10.1162/jocn.2010.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curlik DM, Shors TJ. Training the brain: Do mental and physical (MAP) training facilitate learning through the process of adult hippocampal neurogenesis? Neuropharmacology. 2013;64(1):506–514. doi: 10.1016/j.neuropharm.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Bangasser D, Edgecomb C, Shors TJ. Neurogenesis and learning: acquisition predicts how many new neurons survive after training. Neurobiology of Learning and Memory. 2007;88:143–148. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15:689. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of Exercise. Obesity. 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97(13):7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Nat Acad Sci USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci. 2009;3:50. doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, Heller HC. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94(6):4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- Herring MP, Puetz TW, O'Connor PJ, Dishman RK. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(2):101–111. doi: 10.1001/archinternmed.2011.696. [DOI] [PubMed] [Google Scholar]
- Heyward VH. Advance Fitness Assessment & Exercise Prescription. 3rd Edition. Dallas TX: The Cooper Institute for Aerobics Research; 1998. [Google Scholar]
- Hofmann SF, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78:169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living. New York: Delacorte Press; 1990. [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(1997):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kirste I, Nicola Z, Kronenberg G, Walker TL, Liu RC, Kempermann G. Is silence golden? Effects of auditory stimuli and their absence on adult hippocampal neurogenesis. Brain Struct Function. 2013 doi: 10.1007/s00429-013-0679-3. [EPub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci. 2004;24(30):6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AT, Akers KG, Krakowski AD, Stone SS, Sakaguchi M, Arruda-Carvalho M, Frankland PW. Impact of early adverse experience on complexity of adult-generated neurons. Transl Psychiatry. 2011 Aug 30;1:e35. doi: 10.1038/tp.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS One. 2010;5(7):e11597. doi: 10.1371/journal.pone.0011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozoroviskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. Journal of Neuroscience. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M, Koricka S, Lucassen PJ2, Joëls M. Age-and sex-dependent effects of early life stress on hippocampal neurogenesis. Front Endocrinol (Lausanne) 2014 Feb 20;5:13. doi: 10.3389/fendo.2014.00013. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83(5):1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nokia MS, Sisti HM, Choski M, Shors TJ. Learning to learn: Theta oscillations predict new learning which enhances related learning and neurogenesis. PLoS One. 2012;7:e31375. doi: 10.1371/journal.pone.0031375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Johansson PP, Lensu S, Koch LG, Britton SL, Kainulainen H. Effects of voluntary running and forced high intensity interval training on hippocampal adult neurogenesis and vascularization in the rat. 2014 (in preparation). [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Emotion regulation and psychopathology: the role of gender. Annu Rev Clin Psychol. 2012;8:161–187. doi: 10.1146/annurev-clinpsy-032511-143109. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NE, Jeffery RW. The behavioral determinants of exercise: Implications for physical activity interventions. Ann Rev Nutrition. 2000;20:21. doi: 10.1146/annurev.nutr.20.1.21. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Anderson ML, Curlik DM, Nokia MS. Use it or lose it: how neurogenesis keeps the brain fit for learning. Behavioral Brain Sciences. 2011;227(2):450–458. doi: 10.1016/j.bbr.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Neurogenesis is the adult hippocampus involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Saving new brain cells. Scientific American. 2009;300(3):46–54. doi: 10.1038/scientificamerican0309-46. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Effortful learning keeps new neurons alive. Current Directions in Psychological Sciences. 2014 In press. [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Vugt MK, Hitchcock P, Shahar B, Britton W. The effects of mindfulness-based cognitive therapy on affective memory recall dynamics in depression: a mechanistic model of rumination. Front Human Neurosci. 2012;6:257. doi: 10.3389/fnhum.2012.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Shors TJ. Neurogenesis, learning and associative strength. European Journal of Neuroscience. 2008;28:5290–5294. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Anderson ML, Shors TJ. Changing the rate and hippocampal dependence of trace eyeblink conditioning: slow learning enhances survival of new neurons. Neurobiology of Learning and Memory. 2010;95:159–165. doi: 10.1016/j.nlm.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]