Abstract
Heterotopic ossification, defined as the formation of bone in abnormal anatomic locations, can be clinically insignificant or devastating and debilitating, depending on the site and duration of new bone formation. There are many causes of heterotopic ossification (HO), including soft tissue trauma, central nervous system injury, vasculopathies, arthropathies, and inheritance. One of the least understood components of HO is the interaction of the peripheral nervous system with the induction of this process. Recent work has shown that, upon traumatic injury, a cascade of events termed neurogenic inflammation is initiated, which involves the release of neuropeptides, such as substance P and calcitonin gene related peptide. Release of these peptides ultimately leads to the recruitment of activated platelets, mast cells, and neutrophils to the injury site. These cells appear to be involved with both remodeling of the nerve, as well as potentially recruiting additional cells from the bone marrow to the injury site. Further, sensory neurons stimulated at the injury site relay local information to the brain, which can then redirect neuroendocrine signaling in the hypothalamus towards repair of the injured site. While numerous studies have highlighted the important role of nerve-derived signals, both central and peripheral, in the regulation of normal bone remodeling of the skeleton,1 this review focuses on the role of the local, peripheral nerves in the formation of heterotopic bone. We concentrate on the manner in which local changes in bone morphogenetic protein (BMP) expression contribute to a cascade of events within the peripheral nerves, both sensory and sympathetic, in the immediate area of HO formation.
Keywords: heterotopic ossification, peripheral nervous system, BMP2, neurogenic inflammation, sensory, sympathetic
I. Heterotopic Ossification
I.A. Stages of Heterotopic Ossification
Heterotopic ossification (HO) appears to form de novo within tissues, presumably through the recruitment of stem cells and progenitors, which then undergo all stages of endochondral bone formation. Much speculation has suggested that injury to the tissue, through trauma, may lead to the recruitment of stem and progenitor cells to the injury site. Upon arrival, these progenitors are then exposed to osteoinductive factors that direct their differentiation towards the chondro-osseous fate. The newly formed ectopic bone is similar to skeletal bone, possesses a bone marrow cavity, and can often fuse the normal skeleton.
Studies from mouse models of HO,2,3 where bone formation is induced through delivery of bone morphogenetic protein 2 (BMP2), show a series of changes within the soft tissues, including nerves, vessels, and muscle. One of the initial changes observed at the site of new bone formation is the appearance of brown adipocytes. These cells are capable of utilizing their uncoupled aerobic respiration to reduce localized oxygen tension and effectively pattern the newly forming cartilage condensations.4 These unique cells are also able to express angiogenic factors, such as VEGF-A and -D, which can enhance rapid, new vessel formation.5 This vascular ingrowth must occur for the transition of avascular cartilage to bone.6 Therefore, it is not surprising that a mechanism exists for regulating both local oxygen tension and vessel growth as a component of HO. Interestingly, just prior to chondrogenesis, expression of markers of endothelial adhesion (E-selectin, SDF-1, CXCR4, VCAM) and vascular remodeling are elevated, simultaneous to the appearance of proliferating inflammatory-like cells (CD68+, SMA+, SMMHC+, Lysozyme M+) within the tissues (personal communication7). These inflammatory-like cells lose these more primitive markers upon expression of the chondrocyte/Schwann cell marker Sox9, forming a sharply demarcated perichondral region delineated by the Sox 9 expression.7 Further, we find that these cells appear to be adjacent to the oxygen reducing brown adipose, and thus, form a three dimensional architecture where the brown adipose may be regulating chondrogenic differentiation through hypoxia.4,7 As the cartilage and bone form, they appear to surround and engulf the muscle tissues, with significant muscle hypertrophy, and death. It is unclear what governs the inflammatory response after induction of bone morphogenetic protein (BMP) signaling, but recent studies in using these mouse models suggest a regulatory role for peripheral nerve signaling. In these models, BMP2 appears to lead to neuroinflammation, which involves recruitment of mast cells and neutrophils, activation of platelets, and significant expansion of myeloid progenitors (Salisbury et al., in preparation), suggesting that peripheral nerve stimulation by BMP2 may be involved in the induction of HO.
I.B. Clinical Scenarios of HO
Although many have speculated that HO is a heterogeneous disorder stemming from a number of different causes, study of the literature, across the different fields, reveals striking similarities to animal models. One commonality appears to be the enhanced expression or release of BMP2, at a time when stem cells and progenitors are identified within the tissues. Clearly, traumatic injury to skeletal bone and muscle leads to the increased expression of BMPs at the injury site, and recent reports suggest not only a role for this protein in bone formation, but also a critical role in muscle regeneration and repair.8 Clever et al. showed that many components of the BMP signaling pathway were activated within 24 hours of injury, and played a critical role in myoblast progenitor expansion.8 It is unclear how BMPs can be involved in muscle stem cell expansion and repair, and still induce bone formation. One possibility is that the levels of BMP within the tissue may be a defining factor. Often, in cases of significant muscle injury, the adjacent bone is also disrupted, which could lead to the substantial release of BMPs within the local environment. Since BMP expression within the muscle must be turned of in order for the muscle progenitors to differentiate, perhaps lower levels of BMPs are required for limited times. When these higher levels of BMP are found, the results are shifted towards endochondral bone formation. Alternatively, there could be a secondary mechanism evoked, beyond the alteration in BMP expression. It is intriguing that in certain types of traumatic injury, such as myositis ossificans traumatica, which appears to result from muscle trauma, HO occurs without injury to the skeletal bone.9 Beiner et al.9 demonstrated that the inflammatory response evoked appears to rapidly destroy the muscle fibers, which are replaced with heterotopic bone. Clinically, it is unclear what the inductive components are that lead to HO, but the data suggests that BMP expression upon injury may play a key role.
One of the most common causes of heterotopic ossification in the general population is central nervous system injury. Heterotopic ossification is an especially challenging problem for spinal cord injury patients who have an elevated risk for HO. While such patients often lose all motor and sensory function below the level of the spinal cord injury, the distal nerves themselves remain viable and functional, although they no longer communicate with the brain. Indeed, neuronal activity in the lower extremity of spinal cord injured patients can be abnormally high, frequently causing spasticity.
Surprisingly, studies of HO in cardiovascular tissues have striking similarities to HO at other sites. Two primary sites within cardiac tissues appear to form bone: cardiac valves and within atherosclerotic plaques. Although the mechanisms that govern aortic valve (AV) degeneration are largely unknown, many of the pathways involved in embryonic formation of the valve appear to be disrupted in AV degeneration.10 Sucosky et al. recently demonstrated that shear stress within the valve, from alterations in blood flow, appeared to rapidly enhance BMP2/4 signaling. The authors further suggested that the increase in BMP2/4 signaling led to localized inflammation and degeneration of the valve tissue.11 Intriguingly, the valve contains peripheral nerves, which undergo neuroinflammatory remodeling during AV degeneration.10
Like the valves, shear stress and changes in hemodynamics have been suggested to be responsible for HO formation in atherosclerotic plaques.12 Several BMPs have been detected in atherosclerotic plaques, including BMP2.13 Elevation in BMP signaling through shear stress and a reduction in blood flow is thought to be responsible for early vascular inflammation.14,15 Interestingly, Yao et al. demonstrated that increased BMP signaling led to the elevation of the endothelial adhesion molecules CD68, E-selectin, VCAM and ICAM-1.14 They speculated that induction of BMP signaling in cardiac tissues induces monocyte infiltration through elevation in these endothelial adhesion molecules.14 Further, the authors speculate that the elevated BMP signaling could also lead to osteochondrogenic lineage reprogramming of smooth muscle cells.16 Intriguingly, the mechanisms evoked in cardiovascular HO are very similar to what is observed in the BMP2 mouse model, again suggesting that this disorder may follow a common mechanism, regardless of the location of onset.
One of the best examples of the direct correlation between heterotopic bone formation and enhanced BMP signaling is the genetic disease fibrodysplasia ossificans progressiva (FOP).17 Recently, Shore et al.18 identified an activating mutation in the activin receptor type 1, a bone morphogenetic protein type 1 receptor, in patients with FOP, presumably leading to the formation of HO in skeletal muscle, tendons, and ligaments. This activating mutation leads to BMP signaling. However, the receptor activity can still be enhanced upon addition of BMP protein,18 suggesting that there is a threshold level of BMP required for induction of bone formation. Interestingly, in patients that possess the mutation, even minor trauma to the muscle appears to rapidly induce HO, presumably by the rapid elevation in BMP expression in muscle after injury.8 Perhaps this trauma releases BMPs within the muscle itself,17 providing the small amount of additional stimulus to form the bone. Alternatively, Kitterman et al. showed the formation of HO along the needle track after childhood vaccinations in patients with FOP, suggesting that peripheral nerves, such as sensory neurons, may also contribute to induction of the bone formation.19 BMPs have been shown to be expressed in normal peripheral nerves regulating neuronal function, and BMP signaling appears activated upon peripheral nerve damage, suggesting that BMPs play a role in the peripheral nerve's response to injury.20
We have highlighted the most common areas for heterotopic ossification to occur. However, the risk of HO within the general population is fairly low, approximately 5%, suggesting that it is still a very rare event. This most likely contributes to our lack of mechanistic knowledge of the subject. However, recent statistics from the military suggest that as many as 60% of all military casualties21 are reported to have some form of HO. These numbers are staggering and have led researchers to question what is behind the significant increase in incidence. One possible reason is the type of injuries sustained in the military population. Approximately 60% to 70% of traumatic injuries are a direct result of blast or burn injuries associated with improvised explosive devices (IEDs), which can have dramatic effects on peripheral and central nervous system signaling, but can sometimes, paradoxically, leave the body's tissues with undetected or minimal damage.21 One commonality among these types of injuries appears to be trauma to the peripheral nervous system. Here we examine the potential link between the peripheral nervous system and induction of heterotopic bone formation.
II. Heterotopic Ossification and the Sensory Nervous System
II.A. TRPV1 Sensory Neurons and Heterotopic Bone
Little is known about sensory nerves and bone. Studies in our own laboratory suggest a functional role for these nerves in HO. Recent studies in mice lacking TRPV1 (transient receptor potential cation channel V1) sensory neurons have shown these mice develop significantly less heterotopic bone after induction with BMP2, as compared to the normal counterpart (Salisbury et al., in preparation). Dissection of this sensory pathway after BMP2 induction showed a significant elevation in both substance P (SP) and calcitonin gene-related peptide (CGRP), which was absent in mice lacking TRPV1 sensory neuron function.
The small diameter, afferent sensory fibers of the peripheral nervous system (PNS) are of major importance in the release of SP and CGRP, and subsequent inflammatory effects. Within the tissues, these nociceptive primary afferent neurons respond to noxious mechanical, thermal, or chemical stimuli, providing feedback on pain and temperature.22 Upon injury or inflammation, noxious stimuli activate these nociceptive, sensory fibers, which release neuropeptides both in the periphery, leading to neurogenic responses, and centrally to transmit the nociception to the central nervous system. The vanilloid (capsaicin) receptor TRPV1 is a nociceptive, ion channel located on sensory nerve endings that is activated by some of these noxious stimuli and involved in the mediation of pain sensation.23,24 Capsaicin, the compound in hot chili peppers which gives them “heat,” is one chemical stimuli that can activate TRPV1, causing the ion channel to open, leading to an influx of calcium and sodium ions into the sensory neuron and triggering depolarization of the neuron. At normal levels, capsaicin binding transmits the sensation of pain. However, high doses of capsaicin lead to a massive influx of ions, resulting in cell death of sensory neurons expressing TRPV1.
II.B. Neurogenic Inflammation and Heterotopic Bone
While TRPV1 activation sends afferent signals to the central nervous system for the communication of pain, it also leads to neurogenic inflammation by the release of SP and CGRP within the tissue.25 Indeed, TRPV1 is highly coexpressed with the substance P-positive and CGRP-positive neurons of the dorsal root ganglion.26 This neurogenic inflammatory process is mediated by the release of neuropeptides from sensory nerves, which in turn act on target cells in the periphery, such as mast cells, to produce inflammation22,27 (Fig. 1).
Figure 1.

Schematic representation of the tentative neuroinflammatory mechanism and its relationship to heterotopic ossification. BMP2 can induce recruitment of mast cells and nerve tissue remodeling, through activation of sensory neurons and release of Substance P (SP) and calcitonin gene-related peptide (CGRP).
Intriguingly, BMP has been shown to upregulate CGRP, as well as SP, expression in sensory neurons cultured from dorsal root ganglia,28 suggesting this molecule plays a role in producing these neuroinflammatory responses. Therefore, release of BMP2, such as during the induction of HO in soft tissue, initiates neurogenic inflammation within the local environment (Fig. 1). It is important to note that the small diameter, capsaicin-sensitive sensory neurons, which are critical in generation of neurogenic inflammation, are themselves activated upon injury and trauma, consequently augmenting the inflammatory response produced by the sensory nerves in scenarios of HO involving traumatic injury. The ability of BMP signaling to evoke this mechanism may, in part, explain why patients with an inherited form of HO, FOP, exhibit an increase in mast cell density within the lesional area of heterotopic bone, as compared to unaffected tissues.29
These pro-inflammatory neuropeptides bind to receptors expressed on mast cells, stimulating their activation and subsequent release of a variety of enzymes and inflammatory factors from intracellular granules within the mast cell, a process referred to as degranulation30,31 (Fig. 1). Upon degranulation, mast cells release a variety of mediators, including serine proteases, such as chymase and tryptase, histamines, and cathepsins, which are associated with many types of tissue remodeling.30 In addition, many sensory nerve terminals are lined with receptors for the various mast cell mediators, which, upon activation, can lead to further release of SP and CGRP, creating a positive feedback loop for the perpetuation of neurogenic inflammation.30 Studies in our BMP2-induced mouse model of HO support a role for mast cell degranulation in the progression of HO (Salisbury et al., in preparation). Mice treated with cromolyn, which is known to inhibit mast cell degranulation, prior to BMP2-induction, develop a significantly smaller heterotopic bone lesion than untreated animals (Salisbury et al., in preparation).
Mast cell proteases released upon degranulation are also linked to remodeling of the peripheral nerve.32 Upon injury to the nerve, Schwann cells associated with the nerve start to repair the damaged nerve sheath.33 This phenomenon holds greater significance when given the current findings that stem cells, which contribute to other tissues, are stored within the nerve sheath. Recently, Adameyko et al.34 demonstrated the presence of a primitive stem cell within the nerve that contributed to melanocytes within the skin. Additionally, in patients with the complex disease neurofibromatosis, cells cannot migrate from the nerve; therefore, they remain within the nerve sheath and form the characteristic nerve-associated tumors of the disease.35 These patients also display skeletal and skin abnormalities, including partial, early closure of the growth plate, bone loss, and café au lait spots within the skin. These phenotypes hint at a mechanism where stem cells for bone and melanocytes also reside in the nerve and become trapped in this disorder, leading to improper bone formation and skin pigmentation. Finally, studies in the developing sciatic nerve isolated from rats revealed three distinct stem cell populations within the nerve: one, a population of multipotent, self-renewing progenitors, presumably derived from the neural crest,36 which contribute to the generation of peripheral nerves;37 two, a population that appeared to generate Schwann (glial) cell precursors, which express glial fibrillary acidic protein (GFAP); three, a population of smooth-muscle like cells, which appeared to be absent from other nerve structures, but the authors speculate could contribute to more mesenchymal lineages. Interestingly, these cells were SMA+, SMMHC+ similar to the cells identified as tentative chondrocytes13,38–40 and the prechondrocytes we observed in our model of HO. All of these studies point to a pool of stem cells within the nerve, with the potential to contribute to the structures of bone, including chondrocytes and osteoblasts.
In addition to nerve remodeling, the mediators released by mast cells can elicit a variety of pro-inflammatory effects within the tissues. In concert, SP and CGRP, along with activating mast cells, can induce other immune cells, including monocytes, macrophages, lymphocytes, and platelets.22,41,42 Both neuropeptides are potent vasodilators.43 We have observed an elevation of platelets in the blood early after injection of BMP2-producing cells, and at later times, we have observed an elevation of neutrophils (Salisbury et al., in preparation). Platelets play a critical role in wound healing and hemostasis, as well as in repairing bone fracture.44 Induction of the sensory neuropeptides, whether by injury, BMP, or a combination of the two, modulates the local immune response, thus promoting the progression of HO.
II.C. Sensory Neuropeptides and Skeletal Bone
Intriguingly, capsaicin-sensitive sensory neurons and sensory neuropeptides have been implicated in the maintenance of the normal skeleton as well.45 Capsaicin-induced denervation of the sensory neurons results in a loss of trabecular bone volume, decreased osteoblast activity, and impaired bone formation. Additionally, there is evidence that CGRP plays a fundamental role in osteoclast formation and function. Several studies showed that CGRP inhibits the formation of osteoclasts and that capsaicin-induced denervation leads to impaired recruitment of osteoclast precursors.45 Both SP and CGRP have also been identified to promote osteogenesis in vitro.46,47 Consequently, these neuropeptides appear to have the potential to interact with some of the principal cells, osteoblasts and osteoclasts, involved in bone formation and remodeling of the normal skeleton. This may suggest a similar potential in the formation of heterotopic bone formation, although these mechanisms have not currently been examined.
Additional evidence for the role of the peripheral nervous system, in particular the sensory nerves, in de novo bone formation comes from a number of clinical observations and basic science studies on the healing of fractured bone. Several animal studies have shown that transection or denervation of the complete peripheral nerve leads to an impaired healing of fractures.48–50 While these studies examined the effects of combined motor, sensory, and autonomic denervation, a more recent study by Apel et al. further demonstrated that sensory denervation alone impairs fracture healing.51 Using a model of capsaicin-denervated animals, which impairs the CGRP- and SP-positive nerve fibers of the PNS, the authors showed that sensory denervated animals displayed a fracture callus that is significantly larger and less ossified, with reduced mechanical strength, compared to fractures in animals with intact sensory nerves. In line with these results, clinical studies have revealed that the levels of the sensory peptides, such as CGRP and substance P, are significantly increased in patients within 24 h of bone fracture.52 Following fracture of the rat tibia, studies have also shown a substantial increase in CGRP-expressing neurons that colocalize with new bone formation,53 and a significant increase in the number of SP-positive nerve fibers.54 In addition to fracture models, studies examining the repair of an experimental bone defect model in the rat tibia also demonstrated an increase in the number of nerve fibers expressing substance P and CGRP within the first few days following the defect, which returned to normal by 3 weeks.55 All of these observations suggest that peripheral nerves, particularly the sensory component, are closely involved in fracture healing and bone repair following injury. Further, the data supports a global mechanism for bone formation involving the sensory neurons and neuroinflammation. Neuroinflammation mediated by the sensory nerves can lead to not only vasodilation, extravasation, and the recruitment of potential progenitors, but also potential nerve remodeling and the release of progenitors that contribute to bone formation.
III. Heterotopic Ossification and the Sympathetic Nervous System
BMPs have been demonstrated, in vitro, to induce development of sympathetic neurons from neural crest cell cultures. Additionally, in vivo studies revealed that delivery of the BMP antagonist, noggin, to the chick embryo during the time of sympathetic neuron differentiation prevented expression of noradrenergic marker genes and generation of sympathetic nerves.56 More recent studies, using conditional knockout embryos, have further defined the mechanisms by which BMP signaling regulates sympathetic nervous system (SNS) development, including a role for BMP signaling in survival of SNS precursors and SNS differentiation and proliferation.57 Moreover, BMP2 has been shown to induce neurotransmitter and neuropeptide expression in rat neonatal sympathetic neurons.58 Given the defined and important role of BMP during these key developmental events, it would not be surprising to observe BMP involvement in regulating SNS function during heterotopic bone formation within the adult organism.
III.A. Sympathetic Nerve Regulation of HO
As mentioned, one of the earliest steps in our mouse model of HO is the biogenesis of brown fat, approximately two days following injection of BMP2-producing cells.4 These brown fat cells are critical for patterning of the local oxygen environment necessary for further cartilage and bone formation. While the exact mechanism by which BMP2 induces the rapid production and expansion of brown fat is currently under investigation, the induction of brown adipose tissue (BAT) has been shown to involve the SNS. Interestingly, heterotopic ossification in Misty Grey Lean mice, which lack functional brown adipose,59 led to enhanced bone formation.4 In these studies, the white adipose appeared to compensate for the loss of brown adipose, by utilization of its lipid to induce a hypoxic environment. Thus, the contribution of BAT in this model could be considered inhibitory, since we obtained a greater response in bone formation. However, the utilization of the white adipose, which is unable to uncouple, in this model, is at the expense of creating considerably reactive oxygen.60 Recently, the mutation in Misty Grey Lean mice was identified to be in a protein known as dock 7,61 which is known to be involved in axonal migration. This suggests a possible relationship between the potential nerve cell migration and expansion from the sensory neurons and the production of brown adipose through SNS stimulation.
Noradrenaline release from sympathetic neurons stimulates β3-adrenergic receptors abundantly expressed on brown fat cells, ultimately directing a number of proteins involved in the upregulation of a brown fat phenotype.62 In support of sympathetic regulation of BAT, administration of β3-adrenergic receptor agonists increases BAT in mice, dogs, and primates63; adult humans with enhanced noradrenaline release, due to rare tumors of the adrenal glands, also develop more abundant brown fat deposits. Therefore, the SNS likely has a role in controlling the induction of BAT during HO (Fig. 2).
Figure 2.
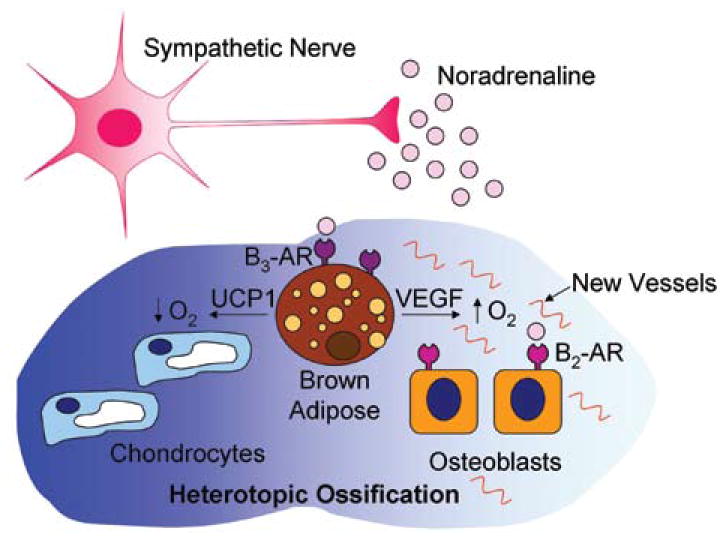
Schematic representation of the tentative interaction of the sympathetic nervous system and heterotopic ossification. Activation of the sympathetic nervous system, through sensory stimulation, leads to regulation of adipose, particularly the rapid appearance of brown adipose within the area of HO. The brown adipose appears to be critical to bone patterning and formation.
Interestingly, the production of BAT through sensory nerve stimulation during the initial stages of HO leads to further stimulation of sensory neurons within the local environment. Since sensory neurons, particularly the small diameter, afferent sensory fibers of the PNS, respond to thermal stimuli, heat produced by the brown adipose will continue to induce signaling and resultant neuroinflammation. Brown adipocytes, in addition to their ability to generate hypoxic stress within the tissue, are known for their function in heat generation, or thermogenesis.62 Brown adipocytes exclusively express UCP1 (uncoupling protein 1), which is capable of uncoupling the electron transport chain from the generation of ATP to the generation of heat.62 Therefore, an additional outcome of BAT activation is the release of heat within the local environment. Thus, the initial pulse of BMP ultimately sets in motion a cascade of neuronal signaling events that propagate and reinforce each other to lead to heterotopic bone formation.
Finally, one of the other factors released by mast cell degranulation is serotonin in lipid vesicles,64 although its function is unknown. It is conceivable that the serotonin released from mast cells leads to the stimulation of sympathetic neurons at the site of injury. Surprisingly, serotonin has been reported to have two opposing actions on bone remodeling. When released outside the hypothalamus, the hormone appears to inhibit bone formation, but when used as a neurotransmitter, it exerts positive effects on bone mass, by enhancing formation and limiting desorption.65
III.B. SNS Regulation of Osteoblasts
The SNS has also been linked to the regulation of orthotopic bone mass.66 Inhibitors of sympathetic signaling, such as the β-blocker propranolol, have been shown to increase bone mass in wild-type mice, and reduce bone loss in ovariectomized mice and rats.67 This sympathetic regulation of bone mass was further attributed to signaling mechanisms activated through β2-adrenergic receptors expressed on osteoblasts. While in the normal skeleton this sympathetic signaling mechanism appears to inhibit the formation of bone, the potential efect on heterotopic bone is currently unknown. However, these studies provide further evidence for an additional cell type involved in bone formation and potentially under the control of sympathetic signaling (Fig. 2).
The SNS may be regulating osteoblasts directly, or regulating progenitors of osteoblasts. We68 and others69 have shown that the hematopoietic stem cell (HSC) is the precursor for the osteoblast. Intriguingly, the SNS has been implicated in the recruitment and mobilization of HSCs.70,71 Sympathetic signaling has been demonstrated to regulate the release of stem cells from the bone marrow.72 Activation of β3-adrenergic receptors expressed on stromal cells within the bone marrow niche leads to the downregulation of Cxcl12, a chemokine critical for stem cell attraction within the marrow.70 Consequently, decreased expression of Cxcl12 within the bone marrow microenvironment encourages stem cell mobilization from the marrow to the peripheral circulation. Upon mobilization, these stem cells could then recruit to the area of new bone formation for further differentiation. This may suggest another pool of potential progenitor cells, in addition to the primitive stem cells within the local, peripheral nerves. It is also possible that the nerve-associated stem cells are progenitors to the HSC. Indeed, the large numbers of neural markers on HSCs has been noted before.73 Additionally, it has been previously reported that such neural stem cells can rescue lethally irradiated animals.74,75 Future studies aimed at further understanding and identification of these various progenitor sources, under the control of neuronal signals, will provide a new area for potential treatment and prevention of HO.
IV. Conclusions
As we have outlined, a number of recent studies are beginning to shed light on the role of the peripheral nerves in the production of HO. Sensory stimulation, by injury and BMP release, can evoke local, neuroinflammatory processes, which ultimately enable the recruitment of progenitors for chondroosseous differentiation. Neuroinflammation within the local environment may lead to the activation of the sympathetic nervous system, through the release of mast cell serotonin. Intriguingly, stimulation of the SNS then continues to trigger the sensory nervous system, through generation and thermogenesis of the brown adipose. Sensory neurons also transmit information regarding the local environment to the CNS and hypothalamus, potentially regulating both heterotopic bone formation and skeletal remodeling. This relationship is unclear, but, often, in clinical scenarios that favor HO, it appears to be at the expense of the adjacent skeletal bone, suggesting the production of HO is perhaps a response to replace the skeletal bone.
While heterotopic ossification is considered an aberrant process, its origins may stem from the critical need to maintain an intact skeleton for survival. In fact, it may be the peripheral nervous system that plays a key role in the surveillance required to preserve normal, functional bone. On one hand, the PNS may relay information to the CNS, to regulate the everyday remodeling of the normal skeleton, critical for maintaining homeostasis within the organism. This information may arise from mechanosensors on osteocytes, which provide additional signaling between the PNS and skeletal bone.76 However, when the body sustains a traumatic injury, and the normal environment becomes altered through trauma and BMP release, the sensory nerves may be the first in line to detect any damage to the bone itself. Once these nerves “sense” these alterations within the local environment, they may initiate a program of regeneration of the bone and soft tissues, over the normal, remodeling mechanisms. The sensory nerves signal to the CNS to override the remodeling program, to set in motion the mechanisms to rebuild de novo bone. In certain instances, this mechanism may generate new bone in incorrect places, and result in HO. Thus, knowledge of peripheral nerve regulation of HO may be translatable to other repair mechanisms and may provide invaluable insight into the body's ability to detect and regenerate those tissues most valuable for survival, including the bone.
Acknowledgments
We thank the following agencies for their support: Defense Advanced Research Projects Agency (W911NF-09–1-0040), Department of Defense (W81XWH-07–0281, W81XWH-07–1-0214, W81XWH-08–1-0489, W81XWH-07–1-025), the National Institute of Biomedical Imaging and Bioengineering (NIH RO1EB005173–01), the Kirschtein-National Research Service Award (T-32 HL092332–08), and the American Heart Association (AHA 10815339F).
References
- 1.Elefteriou F. Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci. 2005 Oct;62(19–20):2339–49. doi: 10.1007/s00018-005-5175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olmsted-Davis EA, Gugala Z, Gannon FH, Yotnda P, McAlhany RE, Lindsey RW, Davis AR. Use of a chimeric adenovirus vector enhances BMP2 production and bone formation. Hum Gene Ther. 2002 Jul 20;13(11):1337–47. doi: 10.1089/104303402760128568. [DOI] [PubMed] [Google Scholar]
- 3.Fouletier-Dilling CM, Gannon FH, Olmsted-Davis EA, Lazard Z, Heggeness MH, Shafer JA, Hipp JA, Davis AR. Efficient and rapid osteoinduction in an immune-competent host. Hum Gene Ther. 2007 Aug;18(8):733–45. doi: 10.1089/hum.2006.190. [DOI] [PubMed] [Google Scholar]
- 4.Olmsted-Davis E, Gannon FH, Ozen M, Ittmann MM, Gugala Z, Hipp JA, Moran KM, Fouletier-Dilling CM, Schumara-Martin S, Lindsey RW, Heggeness MH, Brenner MK, Davis AR. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007 Feb;170(2):620–32. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dilling CF, Wada AM, Lazard ZW, Salisbury EA, Gannon FH, Vadakkan TJ, Gao L, Hirschi K, Dickinson ME, Davis AR, Olmsted-Davis EA. Vessel formation is induced prior to the appearance of cartilage in BMP-2-mediated heterotopic ossification. J Bone Miner Res. 2010 May;25(5):1147–56. doi: 10.1359/jbmr.091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010 Aug 17;19(2):329–44. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shafer J, Davis AR, Gannon FH, Fouletier-Dilling CM, Lazard Z, Moran K, Gugala Z, Ozen M, Ittmann M, Heggeness MH, Olmsted-Davis E. Oxygen tension directs chondrogenic differentiation of myelo-monocytic progenitors during endochondral bone formation. Tissue Eng. 2007 Aug;13(8):2011–9. doi: 10.1089/ten.2006.0063. [DOI] [PubMed] [Google Scholar]
- 8.Clever JL, Sakai Y, Wang RA, Schneider DB. Inefficient skeletal muscle repair in inhibitor of differentiation knockout mice suggests a crucial role for BMP signaling during adult muscle regeneration. Am J Physiol Cell Physiol. 2010 May;298(5):C1087–99. doi: 10.1152/ajpcell.00388.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beiner JM, Jokl P. Muscle contusion injury and myositis ossificans traumatica. Clin Orthop Relat Res. 2002 Oct;(403 Suppl):S110–9. doi: 10.1097/00003086-200210001-00013. [DOI] [PubMed] [Google Scholar]
- 10.Flanagan TC, Pandit A. Living artificial heart valve alternatives: a review. Eur Cell Mater. 2003 Nov 20;6:28–45. doi: 10.22203/ecm.v006a04. discussion 45. [DOI] [PubMed] [Google Scholar]
- 11.Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009 Feb;29(2):254–60. doi: 10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y, Nowak S, Yochelis A, Garfinkel A, Bostrom KI. Matrix GLA protein, an inhibitory morphogen in pulmonary vascular development. J Biol Chem. 2007 Oct 12;282(41):30131–42. doi: 10.1074/jbc.M704297200. [DOI] [PubMed] [Google Scholar]
- 13.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009 Mar 27;104(6):733–41. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010 Aug 20;107(4):485–94. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005 Jul 22;97(2):105–14. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 16.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996 Aug 1;382(6590):448–52. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 17.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010 Sep;6(9):518–27. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006 May;38(5):525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 19.Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics. 2005 Nov;116(5):e654–61. doi: 10.1542/peds.2005-0469. [DOI] [PubMed] [Google Scholar]
- 20.Tsujii M, Akeda K, Iino T, Uchida A. Are BMPs involved in normal nerve and following transection?: a pilot study. Clin Orthop Relat Res. 2009 Dec;467(12):3183–9. doi: 10.1007/s11999-009-1009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009 May;91(5):1084–91. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 22.Schaible HG, Del Rosso A, Matucci-Cerinic M. Neurogenic aspects of inflammation. Rheum Dis Clin North Am. 2005 Feb;31(1):77–101. ix. doi: 10.1016/j.rdc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007 May;6(5):357–72. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 24.Khairatkar-Joshi N, Szallasi A. TRPV1 antagonists: the challenges for therapeutic targeting. Trends Mol Med. 2009 Jan;15(1):14–22. doi: 10.1016/j.molmed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Geppetti P, Holzer P, editors. Neurogenic inflammation. Boca Raton, FL: CRC Press; 1996. [Google Scholar]
- 26.Hwang SJ, Oh JM, Valtschanoff JG. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005 Jun 21;1047(2):261–6. doi: 10.1016/j.brainres.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 27.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002 Sep;302(3):839–45. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 28.Bucelli RC, Gonsiorek EA, Kim WY, Bruun D, Rabin RA, Higgins D, Lein PJ. Statins decrease expression of the proinflammatory neuropeptides calcitonin gene-related peptide and substance P in sensory neurons. J Pharmacol Exp Ther. 2008 Mar;324(3):1172–80. doi: 10.1124/jpet.107.132795. [DOI] [PubMed] [Google Scholar]
- 29.Gannon FH, Glaser D, Caron R, Tompson LD, Shore EM, Kaplan FS. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum Pathol. 2001 Aug;32(8):842–8. doi: 10.1053/hupa.2001.26464. [DOI] [PubMed] [Google Scholar]
- 30.Kleij HP, Bienenstock J. Significance of conversation between mast cells and nerves. Allergy Asthma Clin Immunol. 2005 Jun 15;1(2):65–80. doi: 10.1186/1710-1492-1-2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008 Mar;123(3):398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson D, Seeldrayers PA, Weiner HL. The role of mast cells in demyelination. 1. Myelin proteins are degraded by mast cell proteases and myelin basic protein and P2 can stimulate mast cell degranulation. Brain Res. 1988 Mar 15;444(1):195–8. doi: 10.1016/0006-8993(88)90929-8. [DOI] [PubMed] [Google Scholar]
- 33.Parrinello S, Napoli I, Ribeiro S, Digby PW, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010 Oct 1;143(1):145–55. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Muller T, Fritz N, Beljajeva A, Mochii M, Liste I, Usoskin D, Suter U, Birchmeier C, Ernfors P. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009 Oct 16;139(2):366–79. doi: 10.1016/j.cell.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 35.Starinsky-Elbaz S, Faigenbloom L, Friedman E, Stein R, Kloog Y. The pre-GAP-related domain of neurofibromin regulates cell migration through the LIM kinase/cofilin pathway. Mol Cell Neurosci. 2009 Dec;42(4):278–87. doi: 10.1016/j.mcn.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Shah AJ, Smogorzewska EM, Hannum C, Crooks GM. Flt3 ligand induces proliferation of quiescent human bone marrow CD34+CD38- cells and maintains progenitor cells in vitro. Blood. 1996 May 1;87(9):3563–70. [PubMed] [Google Scholar]
- 37.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999 Mar 5;96(5):737–49. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 38.El-Maadawy S, Kaartinen MT, Schinke T, Murshed M, Karsenty G, McKee MD. Cartilage formation and calcification in arteries of mice lacking matrix Gla protein. Connect Tissue Res. 2003;44(Suppl 1):272–8. [PubMed] [Google Scholar]
- 39.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997 Mar 6;386(6620):78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 40.Bobryshev YV. Transdifferentiation of smooth muscle cells into chondrocytes in atherosclerotic arteries in situ: implications for diffuse intimal calcification. J Pathol. 2005 Apr;205(5):641–50. doi: 10.1002/path.1743. [DOI] [PubMed] [Google Scholar]
- 41.Azzolina A, Bongiovanni A, Lampiasi N. Substance P induces TNF-alpha and IL-6 production through NF kappa B in peritoneal mast cells. Biochim Biophys Acta. 2003 Dec 7;1643(1–3):75–83. doi: 10.1016/j.bbamcr.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Graham GJ, Stevens JM, Page NM, Grant AD, Brain SD, Lowry PJ, Gibbins JM. Tachykinins regulate the function of platelets. Blood. 2004 Aug 15;104(4):1058–65. doi: 10.1182/blood-2003-11-3979. [DOI] [PubMed] [Google Scholar]
- 43.Wallengren J, Hakanson R. Effects of substance P, neurokinin A and calcitonin gene-related peptide in human skin and their involvement in sensory nerve-mediated responses. Eur J Pharmacol. 1987 Nov 10;143(2):267–73. doi: 10.1016/0014-2999(87)90542-5. [DOI] [PubMed] [Google Scholar]
- 44.Salari Sharif P, Abdollahi M. The role of platelets in bone remodeling. Inflamm Allergy Drug Targets. 2010 Jun 3; doi: 10.2174/187152810793938044. [DOI] [PubMed] [Google Scholar]
- 45.Offley SC, Guo TZ, Wei T, Clark JD, Vogel H, Lindsey DP, Jacobs CR, Yao W, Lane NE, Kingery WS. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J Bone Miner Res. 2005 Feb;20(2):257–67. doi: 10.1359/JBMR.041108. [DOI] [PubMed] [Google Scholar]
- 46.Shih C, Bernard GW. Neurogenic substance P stimulates osteogenesis in vitro. Peptides. 1997;18(2):323–6. doi: 10.1016/s0196-9781(96)00280-x. [DOI] [PubMed] [Google Scholar]
- 47.Shih C, Bernard GW. Calcitonin gene related peptide enhances bone colony development in vitro. Clin Orthop Relat Res. 1997 Jan;(334):335–44. [PubMed] [Google Scholar]
- 48.Aro H. Efect of nerve injury on fracture healing. Callus formation studied in the rat. Acta Orthop Scand. 1985 Jun;56(3):233–7. doi: 10.3109/17453678508993002. [DOI] [PubMed] [Google Scholar]
- 49.Aro H, Eerola E, Aho AJ. Development of nonunions in the rat fibula after removal of periosteal neural mechano-receptors. Clin Orthop Relat Res. 1985 Oct;(199):292–9. [PubMed] [Google Scholar]
- 50.Nordsletten L, Madsen JE, Almaas R, Rootwelt T, Halse J, Konttinen YT, Hukkanen M, Santavirta S. The neuronal regulation of fracture healing. Efects of sciatic nerve resection in rat tibia. Acta Orthop Scand. 1994 Jun;65(3):299–304. doi: 10.3109/17453679408995457. [DOI] [PubMed] [Google Scholar]
- 51.Apel PJ, Crane D, Northam CN, Callahan M, Smith TL, Teasdall RD. Efect of selective sensory denervation on fracture-healing: an experimental study of rats. J Bone Joint Surg Am. 2009 Dec;91(12):2886–95. doi: 10.2106/JBJS.H.01878. [DOI] [PubMed] [Google Scholar]
- 52.Onuoha GN. Circulating sensory peptide levels within 24 h of human bone fracture. Peptides. 2001 Jul;22(7):1107–10. doi: 10.1016/s0196-9781(01)00434-x. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Kreicbergs A, Bergstrom J, Stark A, Ahmed M. Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: A study in rat angulated tibia. J Orthop Res. 2007 Sep;25(9):1204–12. doi: 10.1002/jor.20406. [DOI] [PubMed] [Google Scholar]
- 54.Li J, Ahmed M, Bergstrom J, Ackermann P, Stark A, Kreicbergs A. Occurrence of substance P in bone repair under different load comparison of straight and angulated fracture in rat tibia. J Orthop Res. 2010 Dec;28(12):1643–50. doi: 10.1002/jor.21169. [DOI] [PubMed] [Google Scholar]
- 55.Aoki M, Tamai K, Saotome K. Substance P- and calcitonin gene-related peptide-immunofluorescent nerves in the repair of experimental bone defects. Int Orthop. 1994 Oct;18(5):317–24. doi: 10.1007/BF00180235. [DOI] [PubMed] [Google Scholar]
- 56.Schneider C, Wicht H, Enderich J, Wegner M, Rohrer H. Bone morphogenetic proteins are required in vivo for the generation of sympathetic neurons. Neuron. 1999 Dec;24(4):861–70. doi: 10.1016/s0896-6273(00)81033-8. [DOI] [PubMed] [Google Scholar]
- 57.Morikawa Y, Zehir A, Maska E, Deng C, Schneider MD, Mishina Y, Cserjesi P. BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways. Development. 2009 Nov;136(21):3575–84. doi: 10.1242/dev.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fann MJ, Patterson PH. Depolarization differentially regulates the effects of bone morphogenetic protein (BMP)-2, BMP-6, and activin A on sympathetic neuronal phenotype. J Neurochem. 1994 Dec;63(6):2074–9. doi: 10.1046/j.1471-4159.1994.63062074.x. [DOI] [PubMed] [Google Scholar]
- 59.Sviderskaya EV, Novak EK, Swank RT, Bennett DC. The murine misty mutation: phenotypic effects on melanocytes, platelets and brown fat. Genetics. 1998 Jan;148(1):381–90. doi: 10.1093/genetics/148.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes. 2010 Oct;17(5):446–52. doi: 10.1097/MED.0b013e32833c3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blasius AL, Brandl K, Crozat K, Xia Y, Khovananth K, Krebs P, Smart NG, Zampolli A, Ruggeri ZM, Beutler BA. Mice with mutations of Dock7 have generalized hypopigmentation and white-spotting but show normal neurological function. Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):2706–11. doi: 10.1073/pnas.0813208106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004 Jan;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 63.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000 Apr 6;404(6778):652–60. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 64.Ringvall M, Ronnberg E, Wernersson S, Duelli A, Henningsson F, Abrink M, Garcia-Faroldi G, Fajardo I, Pejler G. Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan. J Allergy Clin Immunol. 2008 Apr;121(4):1020–6. doi: 10.1016/j.jaci.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 65.Ducy P, Karsenty G. The two faces of serotonin in bone biology. J Cell Biol. 2010 Oct 4;191(1):7–13. doi: 10.1083/jcb.201006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takeda S, Karsenty G. Molecular bases of the sympathetic regulation of bone mass. Bone. 2008 May;42(5):837–40. doi: 10.1016/j.bone.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002 Nov 1;111(3):305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 68.Olmsted-Davis EA, Gugala Z, Camargo F, Gannon FH, Jackson K, Kienstra KA, Shine HD, Lindsey RW, Hirschi KK, Goodell MA, Brenner MK, Davis AR. Primitive adult hematopoietic stem cells can function as osteoblast precursors. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15877–82. doi: 10.1073/pnas.2632959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dominici M, Pritchard C, Garlits JE, Hofmann TJ, Persons DA, Horwitz EM. Hematopoietic cells and osteoblasts are derived from a common marrow progenitor after bone marrow transplantation. Proc Natl Acad Sci U S A. 2004 Aug 10;101(32):11761–6. doi: 10.1073/pnas.0404626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008 Mar 27;452(7186):442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 71.Mendez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010 Mar;1192(1):139–44. doi: 10.1111/j.1749-6632.2010.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Tomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006 Jan 27;124(2):407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 73.Goolsby J, Marty MC, Heletz D, Chiappelli J, Tashko G, Yarnell D, Fishman PS, Dhib-Jalbut S, Bever CT, Jr, Pessac B, Trisler D. Hematopoietic progenitors express neural genes. Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):14926–31. doi: 10.1073/pnas.2434383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999 Jan 22;283(5401):534–7. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 75.Shih CC, Weng Y, Mamelak A, LeBon T, Hu MC, Forman SJ. Identification of a candidate human neurohematopoietic stem-cell population. Blood. 2001 Oct 15;98(8):2412–22. doi: 10.1182/blood.v98.8.2412. [DOI] [PubMed] [Google Scholar]
- 76.Turner CH, Warden SJ, Bellido T, Plotkin LI, Kumar N, Jasiuk I, Danzig J, Robling AG. Mechanobiology of the skeleton. Sci Signal. 2009;2(68):pt3. doi: 10.1126/scisignal.268pt3. [DOI] [PMC free article] [PubMed] [Google Scholar]


