Abstract
Peripheral arterial disease (PAD) has not been as extensively investigated as other cardiovascular diseases. However, the available data suggest that nutrition-based treatment strategies have the potential to reduce the cost-economic burden of PAD substantially. Abdominal obesity is associated with PAD and prospective and cross-sectional studies have shown that a low dietary intake of folate and reduced synthesis of vitamin D are associated with an increased risk of PAD and severe walking impairment in patients who have the disease. However, dietary patterns that are associated with decreased cardiovascular risk might protect against PAD. A small number of clinical trials have provided evidence that increased intakes of niacin and insoluble fiber might be associated with decreased levels of LDL cholesterol and thrombogenic biomarkers, as well as increased serum levels of HDL cholesterol in patients with PAD. However, little evidence that antioxidants, vitamins B6 and B12, or essential fatty acid supplements improve clinical outcomes in these patients exists. Overall, data on the effects of nutrition, body composition, and nutritional supplementation on the risk, progression, and prognosis of PAD are scarce. Further research into these areas is required to allow the development of evidence-based nutritional guidelines for the prevention and treatment of the disease.
Introduction
Atherosclerosis, the primary cause of mortality in the USA,1 is recognized as a set of distinct clinical syndromes—coronary heart disease, ischemic stroke, and peripheral arterial disease (PAD)—that affects both individuals and communities. Although defined as atherosclerosis of the abdominal aorta and arteries outside the heart, the term PAD is most commonly used to describe arterial disease of the infrarenal aorta and the lower extremities.2 PAD is as common, but not as well known, as the other components of the cardiovascular disease burden, and is associated with an equal or higher risk of morbidity and fatal cardiovascular events, and a higher health economic cost, than coronary or stroke syndromes.3,4 In the US population, PAD affects 4–14% of individuals aged 50–80 years.5 Cardiovascular ischemic events (myocardial infarction and stroke) are frequent outcomes of PAD, and patients with the disease have significantly higher risks of leg amputation and premature mortality than the general population.5,6
PAD is associated with the presence of one or more risk factors, including smoking, diabetes mellitus, hypertension, obesity, or dyslipidemia, as well as a family history of PAD or other cardiovascular disease (Box 1).6,7 However, the body of research on risk factors for PAD is less robust than for other cardiovascular diseases, and PAD is persistently underdiagnosed and undertreated, in terms of both lifestyle interventions and pharmacological therapies. 6,8,9 Potential explanations for the underdiagnosis of PAD include a lack of knowledge on the part of physicians and lower investigational priority compared with other cardiovascular diseases. For example, only 25–50% of patients with PAD exhibit difficulty walking or leg symptoms, such as pain, cramping, or weakness, and the disease is frequently asymptomatic until the occurrence of a diagnosed ischemic event.1 In the USA, standards of clinical care present another barrier to the timely diagnosis and treatment of PAD. The majority of care providers either lack training in the measurement of ankle–brachial indices (ABI, the standard tool for PAD diagnosis), or report that time constraints limit their ability to conduct these simple tests.10 In addition, a profound lack of public awareness of PAD exists.6,8 New evidence-based clinical care guidelines for patients with PAD have been created to try to address these problems.11 However, these guidelines do not include dietary recommendations for the treatment or prevention of PAD. In this Review, we summarize the available data on the effect of nutrition and anthropometric factors on PAD incidence and risk, and discuss potential nutritional strategies for the prevention and treatment of the disease.
Box 1. Risk factors for peripheral arterial disease.
Lifestyle factors
Reduced physical activity
Tobacco use
Alcohol use
Body composition
Abdominal obesity
High BMI
High ratio of fat:lean body mass Nutrition
Insufficient micronutrient intake
Excessive calorific intake
Comorbidities
Hypertension
Insulin resistance
Dyslipidemia
Systemic inflammation
Heart disease
Nutritional factors and risk of PAD
Nutrition and dietary patterns might affect the etiology of PAD and cardiovascular disease in an analogous manner. In a number of prospective and cross-sectional studies (many nested within larger epidemiological studies), the associations between dietary intakes or body concentrations of various nutrients and the incidence and prevalence of PAD have been assessed, with varied, sometimes conflicting, results.12–33
Folate and vitamin B12
Impaired homocysteine regulation as a result of inadequate folic acid or vitamin B12 intake might increase the risk of PAD (Figure 1). Homocysteine, a crucial intermediate in the synthesis of cysteine from dietary methionine, cycles between conversion to the cysteine precursor cystathionine and remethylation back to methionine.34 The conversion to cystathionine is vitamin B6-dependent, whereas remethylation requires the cofactors folate and vitamin B12.34 Folate deficiency might contribute to a decreased ability to maintain homocysteine homeostasis and potentially lead to a toxic accumulation of unmetabolized homocysteine in the serum. The deficiency is associated with endothelial dysfunction, vasoconstriction, and inflammation.35,36 In studies in which folate intake and PAD incidence or prevalence were assessed, participants with lower folate intakes had a significantly higher prevalance of PAD than participants with higher intakes.24,27,33 A cross-sectional study of the US population with 7,203 participants, showed that the prevalence of PAD was 33% higher among individuals with the lowest folate intakes than those with the highest intakes.24 In addition, the average folate consumption of patients with PAD (338.0 ± 10.9 μg per day) was significantly lower than that of participants who did not have PAD (393.4 ± 2.83 μg per day; P <0.001).24 A retrospective analysis showed a significantly higher prevalence of folate deficiency in 204 patients with PAD and critical limb ischemia than in 429 patients with PAD and intermittent claudication (6.4% vs 2.9%, respectively; P <0.05).32 In studies in which the effect of vitamin B12 intake on the prevalence or incidence of PAD was assessed, no association was found.14,33 Whether patients with disordered gastrointestinal vitamin B12 reabsorption were excluded from these studies, or whether data were adjusted to account for their inclusion and prevent potential confounding of the results, is not clear.
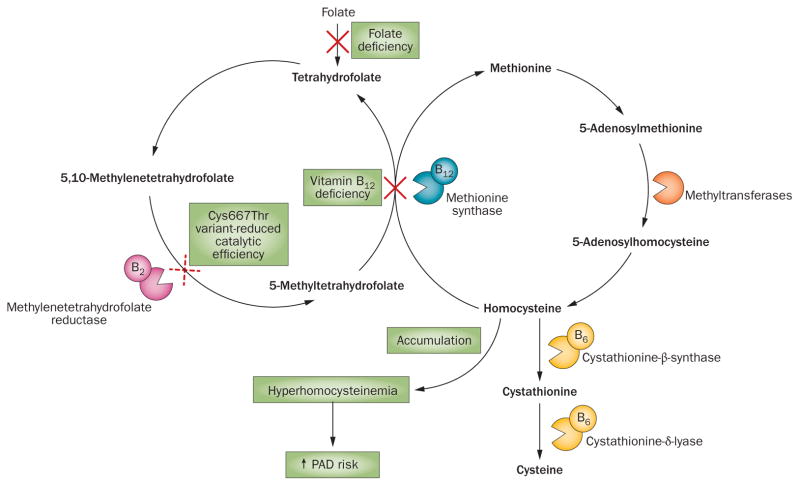
Figure 1.
Potential effects of vitamin B12 and folate deficiencies, and the Cys66Thr variant of the MTHFR gene on homocysteine regulation and PAD etiology. Vitamin B12 and the folate substrate 5-methyltetrahydrofolate are cofactors for methionine synthase, which catalyzes the conversion of homocysteine to methionine. Deficiencies in vitamin B12 or folate could, therefore, disrupt homocysteine homeostasis, leading to accumulation of homocysteine, hyperhomocysteinemia, and an increased risk of PAD. Methylenetetrahydrofolate reductase is required for the conversion of folate substrates. The Cys667Thr variant of the enzyme has reduced catalytic efficiency, so the presence of the variant might also lead to disruption of homocysteine homeostasis and possibly increase the risk of PAD. Abbreviations: B6, vitamin B6; B12, vitamin B12; PAD, peripheral arterial disease.
The role of single nucleotide polymorphisms (SNPs) in folate metabolism, homocysteine regulation, and PAD etiology has also been investigated. The Cys667Thr variant of the MTHFR gene, which encodes methylenetetrahydrofolate reductase, might cause an estimated 50% reduction in activity of the protein.37 Approximately 10% of the human population is homozygous for this point mutation, which decreases the body’s ability to utilize folate and regulate homocysteine.38,39 The presence of the variant might increase the risk of imbalanced homocysteine regulation and, therefore, possibly increase the risk of PAD (Figure 1).37,40 However, in three studies in which the frequency of the Cys667Thr variant was assessed in the general population, the serum homocysteine concentration was not measured, so whether the presence of the T allele resulted in increased serum homocysteine levels could not be determined.40–42 Although several genotype studies have been carried out, evidence of an increased frequency of the Cys667Thr variant among patients with PAD is limited.28,30,37,40,41,43–48 Six studies showed no significant difference in mutation prevalence between patients with PAD and healthy controls.37,40,42,43,45,46 However, two studies showed a higher prevalence of the MTHFR 667T allele or TT genotype in patients with PAD than in individuals without cardiovascular disease.41,44 The results of two meta-analysis were also conflicting. Zintzaras et al.49 and Khandanpour et al.44 analyzed data from seven and nine studies, respectively, with four studies overlapping. Zintzaras and colleagues concluded that no difference in MTHFR 667T allele distribution existed between patients with PAD and healthy controls.49 However, Khandanpour and colleagues reported that the TT genotype was associated with a higher risk of PAD than the CC genotype (OR 1.36, 95% CI 1.10–1.68, P = 0.005).49 Whether variants of the MTHFR genotype ultimately mediate the link between folate intake and PAD risk is, therefore, unclear.
Vitamin D
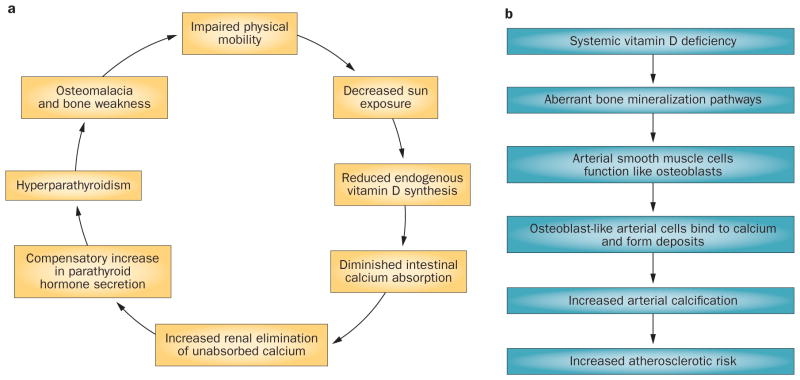
In the past decade, research has provided insight into the potential role of vitamin D deficiency in PAD. Low vitamin D status is associated with an increased incidence of various cardiovascular diseases, including heart failure, myocardial infarction, and hypertension, 50–52 and vitamin D might be of particular importance in PAD etiology. A mechanistic hypothesis posits that decreased sun exposure among patients with PAD as a result of impaired mobility could lead to a decline in vitamin D synthesis, which might cause a decrease in intestinal calcium absorption and increased renal calcium loss.53 A compensatory increase in parathyroid hormone secretion (to maintain serum calcium concentrations) might promote a self-perpetuating cycle in which hypovitaminosis D promotes hyperparathyroidism, which increases the risk of bone pain (osteomalacia), and further exacerbates patient immobility (Figure 2a).53
Figure 2.
Potential effects of vitamin D deficiency on the risk and progression of atherosclerosis and PAD. a | PAD-induced leg pain and discomfort can lead to decreased walking ability and an increase in sedentary, home-centered habits. This lifestyle, in turn, decreases the frequency and duration of sun exposure, which might lead to a self-reinforcing cycle in which vitamin D deficiency leads to abnormal calcium absorption, hyperparathyroidism, and even greater leg pain and discomfort. b | Vitamin D deficiency caused by inadequate sun exposure, dietary intake, or both might result in aberrant calcium metabolism and bone mineralization pathways. These irregularities might lead to alterations in the function of arterial smooth muscle cells and promote calcium deposition on arterial walls, arterial stiffness, and increased risk of atherosclerosis.
Hypovitaminosis D might also lead to arterial calcification, although the precise mechanism is not clear.54,55 Vitamin D has a regulatory role in bone mineralization and a deficiency might promote osteoblast-like function in arterial smooth muscle cells,55 resulting in calcium deposition in arterial walls and, therefore, contribute to atherosclerosis (Figure 2b).54 To date, no prospective studies or clinical trials of vitamin D supplementation in individuals with PAD have been performed. However, cross-sectional and case-controlled studies have shown that vitamin D deficiency is significantly associated with the prevalence and severity of the disease.17,22,26,29,53,55 In two analyses of National Health and Nutrition Examination Survey (NHANES) data, serum vitamin D concentrations were assessed in 8,35122 and 4,83926 participants, respectively. Both studies showed a significantly higher prevalence of PAD (7.7–8.1%) among participants with the lowest vitamin D levels.22,26 In another study that showed a significant association between calcium deposits and PAD, 6% of patients with PAD (n = 78) had arterial calcification compared with <1% of healthy controls (n = 74).55
Dietary interventions
Dietary patterns and risk of PAD
The findings discussed above suggest that increased intakes of folate and vitamin D might protect against incident PAD. However, a nutrient-specific approach is not a pragmatic basis on which to devise clinical guidelines or public health efforts, which aim to promote comprehensive changes in eating habits. Several researchers have, therefore, attempted to delineate dietary patterns that might be associated with a reduced risk of developing PAD. The majority of these studies relied on results from Food Frequency Questionnaires collected from nested cohorts within larger epidemiological surveys. In general, these questionnaires are not validated for the assessment of specific nutrient intakes, but they do provide a reliable indicator of general trends in eating habits within a population. In the USA, foods such as refined flour products are fortified with folate, therefore, individual folate intakes cannot be accurately assessed using questionaires, but they can be used to assess the general level of consumption of folate-containing foods. Overall, the studies showed that the prevalence of PAD was higher in participants who consumed diets rich in saturated fats19,21 or meat and meat products16 than in those with lower intakes of these foods. Conversely, participants who consumed greater amounts of foods that were rich in fiber (particularly insoluble cereal fiber),16,19,24 antioxidants (vitamins A, C, and E),12,24,25,31 and polyunsaturated fats12,19,21,24,25,31 had a significantly lower risk of PAD than participants who consumed less of these foods.
These findings are not surprising because studies of the effect of diet on the risk of cardiovascular disease have shown similar results.56 A Mediterranean-style diet has a similarly protective effect against cardiovascular outcomes in patients with PAD and patients with other cardiovascular diseases.57,58 A case-controlled assessment of Italian diabetics with or without PAD showed that patients who consumed diets that were rich in polyunsaturated fats and plant foods were less likely to have PAD than patients who consumed diets that were not as rich in these foods (OR 0.44, 95% CI 0.24–0.83, P = 0.043).15 However, the findings of other studies were more ambiguous. A Dutch epidemiological study of >4,000 participants showed significant sex differences in the associations between antioxidant-rich diets and PAD. Vitamin C seemed to protect against incident PAD only in women, whereas vitamin E intake was protective solely among men.23 The researchers hypothesized that this result might be attributable to sex-related differences in dietary patterns and specific nutrient intakes. In addition, two epidemiological studies of >40,000 participants who did not have cardiovascular disease or diabetes at the time of enrollment, showed no significant association between diets rich in fruits and vegetables and PAD incidence at 12-year follow-up.52,53 These findings might not negate those of other studies that reported protective effects of Mediterranean-style diets, but they do suggest that further investigation into the effect of dietary patterns on the incidence of PAD is warranted.
Treating symptoms and preventing progression
Compared with the number of general, observational studies of nutrition and PAD, the amount of research into the effects of dietary interventions in patients with PAD is small. Several studies have examined the effects of various nutrients on walking function, including pain-free walking distance and peak walking time, in patients with PAD.59–63 The effect of niacin supplementation, which might increase serum HDL-cholesterol concentrations and inhibit hepatic LDL-cholesterol synthesis,64,65 was assessed in a study of 385 patients with PAD and claudication. 60 Participants who received extended-release niacin plus statin treatment showed moderate increases in peak walking time from baseline (mean 37.8%).60 However, a similar increase from baseline (mean 38.6%) was shown in the control group who received a dietary recommendation and immediate-release niacin.60 In another study, 41 patients with PAD received an L-arginine-fortified nutrition bar.63 The researchers hypothesized that arginine would improve endothelial function in these patients.63 At 2-week follow-up, patients in the supplementation group reported a substantial reduction in leg pain and experienced an average increase of 66% in pain-free walking distance.63 However, when the effect of the arginine supplementation on intermittent claudication in 66 patients with PAD was analyzed in a 6-month, prospective, randomized trial, no improvement in symptoms was observed.66 The researchers suggested that the long-term supplementation might have activated counter-regulatory pathways leading to systemic arginine tolerance.66
Omega-3 essential fatty acids (EFAs) might have anti-inflammatory effects and could protect against endothelial damage and atherosclerosis.67 However, the majority of studies have shown no effect of omega-3 EFA supplementation on the symptoms or progression of PAD. In a 12-week trial, 89 patients with PAD who received eicosapentaenoic acid and docosahexaenoic acid supplements showed significant increases in walking distance and ABI (mean increases of 82 m and 0.18, respectively).62 However, a trial with a 2-year follow-up, which included 120 patients with PAD and intermittent claudication who were randomly assigned to receive either an EFA supplement (a combination of γ-linolenic and eicosapentaenoic acids) or a sunflower oil placebo, showed no change in physical function, ABI, or serum lipid concentrations from baseline in either group, and no difference in these outcomes between the supplementation and placebo groups.61 A meta-analysis of data from six trials of omega-3 supplementation, showed that the supplements had no discernible net impact on PAD outcomes, although the researchers did note a moderate increase in blood viscosity among individuals taking EFA capsules.68 These results are similar to those of previous systematic reviews and meta-analyses of the effect of polyunsaturated fat supplementation on the risks of coronary and cerebrovascular events and mortality.69–72 A small clinical trial of 32 patients also showed that omega-3 supplements did not improve the symptoms of PAD. No improvement in symptoms was observed from baseline in patients who received the supplements; nor was there a difference in symptoms between the supplementation and placebo groups at 3-month follow-up.73 However, a meta-analysis of 10 trials showed that omega-3 polyunsaturated fat supplementation significantly reduced measures of arterial stiffness (arterial compliance, cardio–ankle pulse wave velocity [PWV], and brachial–ankle PWV) in various cohorts, including healthy individuals, individuals with risk factors for cardiovascular disease, overweight individuals, and patients with type 2 diabetes or hypertension. 74 Omega-3 supplementation could have a similarly beneficial effect on arterial stiffness in patients with PAD, and future studies should include assessment of arterial factors, using measures such as carotid–femoral PWV. A 1-year trial of omega-3 supplementation in patients with PAD, which includes arterial sufficiency as an outcome measure, is ongoing.75
A supplementation trial, which included 60 men with peripheral vascular disease and intermittant claudication, utilized a more-comprehensive supplementation design than the majority of intervention studies with system-focused outcomes.59 Patients who were given a variety of EFAs, folate, and dietary counseling showed a 3.5-fold increase in walking distance and a 13% increase in ABI from baseline.
Finally, four studies showed no effect of nutritional supplements on overall progression of PAD.76–79 In three of these studies, folate79 or antioxidant supplementation (administered as a nutritional supplement or fruit juice)77,78 had no significant effect on inflammatory biomarkers (including fibrinogen,76,78 serum paraoxonase/arylesterase 1,77 and C-reactive protein)78,79 in patients with PAD. The fourth study, which included 80 patients with PAD, showed a significant decrease in fibrinogen levels (P <0.001) in response to niacin supplementation, but no effect of antioxidant supplementation on coagulation biomarkers apart from an increase in the concentration of proinflammatory von Willebrand factor (P = 0.04).76 The reasons for this increase are not clear.
Improving vascular function and prognosis
The third category of research in nutrition and PAD has focused on influencing the prognosis of patients who have the disease. A study designed to investigate the effects of folate and vitamin B6 supplementation on incident cardiovascular events in 273 patients with PAD showed a 24% decrease in the risk of these events in the supplement group compared with a placebo group.80 Additionally, patients in the supplement group who were hyperhomocysteinemic had a significantly lower risk of adverse outcomes than patients with normal homocysteine concentrations who received placebo, suggesting that folate and vitamin B6 might have protective effects that are independent of homocysteine regulation.80
Niacin inhibits hepatic LDL-cholesterol synthesis64,65 and might affect endothelial inflammatory mechanisms. 81–83 The effects of niacin supplementation on blood lipid profiles have been investigated in three studies.84–86 In these studies, patients with PAD who received niacin supplements showed significant increases (20–30%) in HDL-cholesterol levels from baseline.84–86 In the third study, which included 468 patients with PAD, the effect of niacin supplementation and glucose control on serum lipid profiles was assessed.84 Both diabetic and nondiabetic patients with PAD who took niacin showed significant decreases in blood triglyceride and LDL-cholesterol levels, as well as increases in HDL-cholesterol levels (all P <0.001). Patients in the supplementation group who had diabetes showed slight increases in blood glucose and glycated hemoglobin concentrations, but these changes were transient and returned to baseline levels with continued supplementation.84
The effect of nutrient supplementation on vascular function has been analyzed in three studies.87–89 In a cross-over study in which the effect of increasing the dose of flavonoid supplementation from 0 mg per day to 800 mg per day over 5 weeks on vascular function was assessed in 19 healthy men, significant, dose-dependent increases in arterial dilatation (≤2.3%) and decreases in arterial stiffness (mean change in stiffness index = −0.71 ± 0.29 m/s) from baseline were observed at 5 week follow-up.87 Another trial of 1,484 patients with PAD and intermittent claudication showed no effect of long-term (mean 3.7 years) vitamin E supplementation on risk of requiring vascular surgery.89 However, patients with PAD who took β-carotene showed a slight increase in the risk of requiring vascular surgery compared with patients in the placebo group (OR = 1.6).89 This increase in risk might have been caused by carotenoid uptake into arterial plaques, which could make them more prone to rupture.90 A clinical trial of selenium supplementation in 42 healthy men showed that the supplement significantly increased serum selenium concentrations from baseline, but had no effect on brachial blood flow or arterial diameter, suggesting that selenium supplementation might not improve vascular function in patients with PAD.88
Body composition and risk of PAD
Obesity is a well-established risk factor for cardiovascular disease,91,92 and the impact of body mass and body composition on the incidence and progression of cardiovascular disease is an area of active investigation. 93 Total body weight, estimated by BMI, is not an ideal predictor of overall atherosclerotic risk.94–96 An obese person might have a normal metabolic profile with no accompanying signs of cardiovascular disease, whereas an only slightly overweight person might experience severe cardiovascular disease with multiple comorbidities. 91 Analyses of body composition,97–101 defined by the quantity and locations of fatty tissue, have revealed that abdominal fat is a more-precise predictor of atherosclerosis risk than total weight.98,101 This finding is unsurprising because abdominal adipose mass is a metabolically active tissue that is capable of secreting hormones that influence serum lipid concentrations, glucose and insulin regulation, inflammatory pathways, and endothelial function.97,99,100 Waist circumference alone, or in combination with hip circumference, is commonly used as a measure of abdominal obesity in studies of cardiovascular disease.102 However, dual energy X-ray absorptiometry (DEXA) body density scans are a more-accurate method of assessing obesity than body measurements as they can be used to analyze precise percentages and distributions of fat, muscle, and bone mass. To date, 18 studies of the effects of obesity on the incidence and progression of PAD have been carried out.18,103–119 However, the methods used to assess body composition in these studies were not consistent. Eight studies used only BMI,18,103–109 and ten studies used BMI and abdominal adiposity.110–119 Only one study112 used DEXA body density scans rather than body circumference measurements to assess abdominal adiposity.110,111,113–119
All but one118 of five studies in which risk factors for PAD were investigated showed that participants who weighed more and had greater adiposity in their abdomens were significantly more likely to have PAD than those with lower weight and adiposity.107,110,114,117,118 A 9-year observational study of 1,453 overweight and 2,139 healthy-weight participants who did not have PAD or cardiovascular disease at the time of enrollment showed no significant association between total BMI and incident PAD.118 However, the presence of a cluster of metabolic variables, including increased waist circumference, impaired fasting glucose, increased blood pressure and serum triglyceride levels, and decreased serum HDL-cholesterol levels, was a significant predictor of low ABI scores.118
In nine studies in which the impact of body composition on various aspects of PAD progression and general prognosis, including inflammatory biomarkers, endothelial function, exercise tolerance, and overall survival, was assessed, abdominal obesity was a significant predictor of decreased ABI or adverse vascular events.103,104,106,108,109,111,113,115,116 All but three of the studies showed that greater total obesity (as measured by BMI) was associated with a greater frequency of adverse PAD-related outcomes.110,115,117 Of the eight studies in which only BMI was assessed, data from five studies showed that total obesity increased the risk of adverse outcomes, such as a more-rapid decline in peak walking distance109,111 or a cardiovascular event.113,116
Interestingly, data from two studies showed that increased BMI was significantly inversely associated with mortality in patients with PAD.104,108 In one study, which included 2,392 patients with PAD, overall mortality was 50% among patients of normal weight (BMI 20–30) compared with 31% in patients who were obese.104 The other study, which included 652 patients with PAD, showed an 18% decrease in the risk of all-cause mortality among patients with a high BMI compared with patients with a low BMI.108 Researchers have termed the phenomenon in which leaner body mass seems to be associated with a greater risk of atherosclerotic events the ‘obesity paradox’.120 In the context of PAD, the paradox has been attributed to confounding factors, such as high frequencies of smoking, chronic obstructive pulmonary disease, underweight status, and overall malnutrition, which are more common among lean individuals than heavier individuals. These factors are independent predictors of atherosclerosis and can contribute to PAD risk.104,108,120 An observational study of 5,419 US adults aged ≥65 years showed that obesity increased the risk of adverse cardiovascular events in patients with PAD who had never smoked (HR 1.32), but not in patients who had smoked.107 In addition, the underweight cohorts in the two studies that showed a significant inverse association between obesity and PAD-related mortality, contained a greater proportion of smokers and patients who were malnourished or had chronic obstructive pulmonary disease than the normal-weight cohorts.104,108 These characteristics of the study cohorts, in addition to the use of BMI as the sole parameter of obesity, might explain the inverse result.
Finally, in a study in which the effect of weight loss on vascular outcomes was investigated in 244 participants who were severely obese, but did not have PAD (including 67 participants who had lost ≥10% of their total body weight at 12-month follow-up), weight loss was significantly correlated with a decrease in resting arterial diameter (P = 0.001).105 This study is the only one to date in which the effects of changes in body composition on vascular outcomes have been prospectively assessed, and these findings set a precedent for expanding this avenue of research.
Ethnic disparities
Nutrition might be an important mediating factor in ethnic differences in PAD prevalence. African American populations have a significantly higher burden of PAD than their white counterparts,121–123 and this disparity is only partly explained by traditional risk factors such as a comparatively lower socioeconomic status and less access to, or use of, health-care facilities.122,124,125 A study in which possible reasons for the higher risk of PAD in African Americans were investigated showed that novel factors, such as an increased serum concentration of lipoprotein(a), which has a role in inflammation, might partially explain the disparity.126 However, even after adjustment for these factors, African American ethnicity was significantly associated with increased PAD risk.126
A possible nutritional explanation might be found in vitamin D. Evidence strongly suggests that there is a racial disparity in vitamin D deficiency in the USA. Patients with darker skin pigmentation, particularly African Americans, have significantly higher rates of vitamin D deficiency than their white counterparts.127 This difference might be a result of a differing ability to convert sun-derived precursor 7-dihydrocholesterol to pre-vitamin D3. Individuals with darker skin, and therefore higher cutaneous melanin concentrations, are less efficient in this conversion process than those with lighter skin.128,129 African Americans who live in northern, less sunny, latitudes are at an especially high risk of developing vitamin D deficiency. In two analyses of NHANES data collected between 2001 and 2004, racial differences in serum vitamin D concentrations and PAD risk were examined.22,29 One analysis of data from 8,351 participants showed that vitamin D deficiency increased the risk of cardiovascular disease in all ethnicities, but that African Americans had a higher prevalence of deficiency (97%) than white (68%) or Hispanic (88%) individuals.22 The other analysis, which included 3,763 participants, supported these conclusions and showed that after adjusting for all known PAD risk factors, hypovitaminosis D accounted for approximately one-third of excess PAD risk among African Americans.29
Conclusions
No established dietary recommendations exist for the prevention or treatment of PAD. However, several retrospective, cross-sectional studies have shown that Mediterranean-style diets that are low in saturated fats and rich in folate, vitamin D, insoluble fiber, polyunsaturated fats, and antioxidants might protect against incident PAD.12,15,16,19,21,31 The results of clinical trials in which the effects of dietary supplements on risk of PAD were assessed have been disappointing. However, supplement dosages and trial durations varied considerably, and baseline intakes and bodily concentrations of the nutrients of interest were not assessed; therefore, any a priori deficiencies were overlooked. Many of the studies used rigorous research methods and gave congruent results, but the relative paucity of research, and particularly of clinical trials, means that evidence-based nutritional guidelines for patients with PAD cannot currently be proposed. Further investigation is required to bridge the gap between the current body of evidence and real-world recommendations. A dual-pronged approach that combines epidemiological cohort studies and supplementation trials is necessary.
Future epidemiological studies should focus on total dietary patterns and food choices rather than specific nutrient intakes. Also, as food manufacturers fortify certain products with various nutrients, researchers should account for regional differences in fortification practices when observing various populations. Supplementation trials should improve upon the methodological limitations of previous studies. For example, studies of genetic risk factors for PAD should include assessment of pre-trial and post-trial body nutrient concentrations and the effect of patient genotypes on these concentrations. To date, no trials of vitamin D supplementation have been performed in patients with PAD, and future research in this area should control for baseline vitamin D status and ethnic and geographic influences on endogenous synthesis. Lastly, as BMI alone might not account for all sources of obesity-related PAD risk, researchers should utilize DEXA or other similar measurements of body composition, such as air-displacement plethysmography. Prospective clinical trials of weight loss interventions might be required to evaluate more precisely the causal relationship between obesity and PAD. Such interventions might be most effective if individuals with PAD are provided with ‘actionable knowledge’ of the impact of being overweight or obese on their disease. One-to-one counseling that emphasizes the benefits of consuming foods that are rich in micronutrients and is adapted to meet the needs of individual patients based on their weight, activity level, and cultural background might be more beneficial than general counseling on healthy eating.
The potential for nutritional research to improve PAD clinical practice should not be underestimated. Over $21 billion are spent annually on PAD treatment in the USA, with per-patient expenditures surpassing the costs of coronary artery disease by 23%.3 Endovascular therapies, such as stents and angioplasty, are standard treatments for PAD.4 However, repeated interventions are frequently required130 and often result in post-intervention complications, substantially increasing the cost of PAD treatment.131 In 2009, researchers attending a conference on the state of PAD research concluded that a general dearth of pathogenesis studies, a lack of prospective evidence from clinical trials, and an over-emphasis on late-stage treatment interventions create a clinical model in which PAD is underdiagnosed and undertreated.4 An expanded research effort is needed that might use nutritional prevention approaches to lower the PAD burden directly. Preliminary evidence suggests that therapies focused on nutrition and weight loss might be useful components of PAD treatment. Such nutritional interventions might lower the incidence of PAD, as well as ameliorate functional impairment and improve ischemic outcomes in patients with PAD. The expansion of research in these areas is, therefore, imperative.
Key points.
Folate and vitamin D deficiencies are associated with an increased risk of peripheral arterial disease (PAD) and increased walking impairment in patients with PAD
Diets that are rich in insoluble fiber, antioxidants, and polyunsaturated fats might protect against PAD; therefore, the existing nutritional recommendations for cardiovascular disease prevention could be applied to PAD
Further research into the effects of nutritional supplements on the incidence, symptoms, progression, and prognosis of PAD is required
Abdominal obesity is significantly associated with PAD
The high prevalence of PAD in African American populations might be attributable to high rates of vitamin D deficiency and high levels of novel risk factors such as lipoprotein(a)
Preliminary evidence suggests that nutritional interventions and weight loss might be useful components of PAD prevention or treatment strategies and could substantially reduce the burden of the disease
Review criteria.
A search for original articles published between January 1990 and April 2012 was performed in PubMed. The search terms used were “peripheral arterial disease”, “peripheral vascular disease”, “diet”, “dietary fiber”, “fat intake”, “B vitamins”, “folate”, “niacin”, “B12”, “vitamin D”, “vitamin C”, “vitamin E”, “β-carotene”, “dietary antioxidants”, “genetics”, “genotype”, “nutrigenetics”, “nutrigenomics”, “MTHFR”, “obesity”, “excess weight”, “body composition”, “abdominal obesity”, “ethnic differences”, “racial differences”, and “racial disparities”, both alone and in combination. All articles identified were full-text papers published in English. Only clinical and population studies were included.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
D. P. Brostow researched the data for the article, provided a substantial contribution to discussions of the content, and wrote the article. M. S. Kurzer made a substantial contribution to discussions of the content. All the authors reviewed and/or edited the manuscript before submission.
References
- 1.Rice TW, Lumsden AB. Optimal medical management of peripheral arterial disease. Vasc Endovascular Surg. 2006;40:312–327. doi: 10.1177/1538574406291835. [DOI] [PubMed] [Google Scholar]
- 2.Guidon M, McGee H. Exercise-based interventions and health-related quality of life in intermittent claudication: a 20-year (1989–2008) review. Eur J Cardiovasc Prev Rehabil. 2010;17:140–154. doi: 10.1097/HJR.0b013e3283377f08. [DOI] [PubMed] [Google Scholar]
- 3.Mahoney EM, et al. One-year costs in patients with a history of or at risk for atherothrombosis in the United States. Circ Cardiovasc Qual Outcomes. 2008;1:38–45. doi: 10.1161/CIRCOUTCOMES.108.775247. [DOI] [PubMed] [Google Scholar]
- 4.Norgren L, et al. The next 10 years in the management of peripheral artery disease: perspectives from the ‘PAD 2009’ Conference. Eur J Vasc Endovasc Surg. 2010;40:375–380. doi: 10.1016/j.ejvs.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 7.Syvänen K, Korhonen P, Partanen A, Aarnio P. Endothelial function in a cardiovascular risk population with borderline ankle-brachial index. Vasc Health Risk Manag. 2011;7:97–101. doi: 10.2147/VHRM.S17249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch AT, et al. Gaps in public knowledge of peripheral arterial disease: the first national PAD public awareness survey. Circulation. 2007;116:2086–2094. doi: 10.1161/CIRCULATIONAHA.107.725101. [DOI] [PubMed] [Google Scholar]
- 9.McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med. 1997;12:209–215. doi: 10.1046/j.1525-1497.1997.012004209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohler ER, 3rd, et al. Utility and barriers to performance of the ankle-brachial index in primary care practice. Vasc Med. 2004;4:253–260. doi: 10.1191/1358863x04vm559oa. [DOI] [PubMed] [Google Scholar]
- 11.Olin JW, et al. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral artery disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease) Vasc Med. 2010;15:481–512. doi: 10.1177/1358863X10390838. [DOI] [PubMed] [Google Scholar]
- 12.Antonelli-Incalzi R, et al. Association between nutrient intake and peripheral artery disease: results from the InCHIANTI study. Atherosclerosis. 2006;186:200–206. doi: 10.1016/j.atherosclerosis.2005.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleys J, et al. Serum selenium and peripheral arterial disease: results from the national health and nutrition examination survey, 2003–2004. Am J Epidemiol. 2009;169:996–1003. doi: 10.1093/aje/kwn414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunout D, et al. Low serum folate but normal homocysteine levels in patients with atherosclerotic vascular disease and matched healthy controls. Nutrition. 2000;16:434–438. doi: 10.1016/s0899-9007(00)00289-6. [DOI] [PubMed] [Google Scholar]
- 15.Ciccarone E, et al. A high-score Mediterranean dietary pattern is associated with a reduced risk of peripheral arterial disease in Italian patients with type 2 diabetes. J Thromb Haemost. 2003;1:1744–1752. doi: 10.1046/j.1538-7836.2003.00323.x. [DOI] [PubMed] [Google Scholar]
- 16.Donnan PT, Thomson M, Fowkes FG, Prescott RJ, Housley E. Diet as a risk factor for peripheral arterial disease in the general population: the Edinburgh Artery Study. Am J Clin Nutr. 1993;57:917–921. doi: 10.1093/ajcn/57.6.917. [DOI] [PubMed] [Google Scholar]
- 17.Fahrleitner-Pammer A, et al. Hypovitaminosis D, impaired bone turnover and low bone mass are common in patients with peripheral arterial disease. Osteoporos Int. 2005;16:319–324. doi: 10.1007/s00198-004-1693-3. [DOI] [PubMed] [Google Scholar]
- 18.Gaddipati VC, et al. The relationship of vitamin D status to cardiovascular risk factors and amputation risk in veterans with peripheral arterial disease. J Am Med Dir Assoc. 2011;12:58–61. doi: 10.1016/j.jamda.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Gimeno SG, et al. Fat and fiber consumption are associated with peripheral arterial disease in a cross-sectional study of a Japanese-Brazilian population. Circ J. 2008;72:44–50. doi: 10.1253/circj.72.44. [DOI] [PubMed] [Google Scholar]
- 20.Hung HC, et al. The association between fruit and vegetable consumption and peripheral arterial disease. Epidemiology. 2003;14:659–665. doi: 10.1097/01.ede.0000086882.59112.9d. [DOI] [PubMed] [Google Scholar]
- 21.Katsouyanni K, et al. Diet and peripheral arterial occlusive disease: the role of poly-, mono-, and saturated fatty acids. Am J Epidemiol. 1991;133:24–31. doi: 10.1093/oxfordjournals.aje.a115798. [DOI] [PubMed] [Google Scholar]
- 22.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am J Cardiol. 2008;102:1540–1544. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 23.Klipstein-Grobusch K, et al. Dietary antioxidants and peripheral arterial disease: the Rotterdam Study. Am J Epidemiol. 2001;154:145–149. doi: 10.1093/aje/154.2.145. [DOI] [PubMed] [Google Scholar]
- 24.Lane JS, et al. Nutrition impacts the prevalence of peripheral arterial disease in the United States. J Vasc Surg. 2008;48:897–904. doi: 10.1016/j.jvs.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Leng GC, et al. Plasma essential fatty acids, cigarette smoking, and dietary antioxidants in peripheral arterial disease. A population-based case-control study. Arterioscler Thromb. 1994;14:471–478. doi: 10.1161/01.atv.14.3.471. [DOI] [PubMed] [Google Scholar]
- 26.Melamed ML, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merchant AT, et al. A. Dietary fiber reduces peripheral arterial disease risk in men. J Nutr. 2003;133:3658–3663. doi: 10.1093/jn/133.11.3658. [DOI] [PubMed] [Google Scholar]
- 28.Rassoul F, et al. Plasma homocysteine and lipoprotein profile in patients with peripheral arterial occlusive disease. Angiology. 2000;51:189–196. doi: 10.1177/000331970005100302. [DOI] [PubMed] [Google Scholar]
- 29.Reis JP, Michos ED, von Muhlen D, Miller ER. 3rd Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease. Am J Clin Nutr. 2008;88:1469–1477. doi: 10.3945/ajcn.2008.26447. [DOI] [PubMed] [Google Scholar]
- 30.Sabino A, et al. Polymorphism in the methylenetetrahydrofolate reductase (C677T) gene and homocysteine levels: a comparison in Brazilian patients with coronary arterial disease, ischemic stroke and peripheral arterial obstructive disease. J Thromb Thrombolysis. 2009;27:82–87. doi: 10.1007/s11239-007-0172-z. [DOI] [PubMed] [Google Scholar]
- 31.Tornwall ME, et al. Prospective study of diet, lifestyle, and intermittent claudication in male smokers. Am J Epidemiol. 2000;151:892–901. doi: 10.1093/oxfordjournals.aje.a010293. [DOI] [PubMed] [Google Scholar]
- 32.Vega de Ceniga M, et al. Anaemia, iron and vitamin deficits in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2011;41:828–830. doi: 10.1016/j.ejvs.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Wilmink AB, et al. Dietary folate and vitamin B6 are independent predictors of peripheral arterial occlusive disease. J Vasc Surg. 2004;39:513–516. doi: 10.1016/j.jvs.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 34.Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75–81. doi: 10.1007/s10545-010-9177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Minno MN, Tremoli E, Coppola A, Lupoli R, Di Minno G. Homocysteine and arterial thrombosis: challenge and opportunity. Thromb Haemost. 2010;103:942–961. doi: 10.1160/TH09-06-0393. [DOI] [PubMed] [Google Scholar]
- 36.Dionisio N, Jardin I, Salido GM, Rosado JA. Homocysteine, intracellular signaling and thrombotic disorders. Curr Med Chem. 2010;17:3109–3119. doi: 10.2174/092986710791959783. [DOI] [PubMed] [Google Scholar]
- 37.Verhoeff BJ, Trip MD, Prins MH, Kastelein JJ, Reitsma PH. The effect of a common methylenetetrahydrofolate reductase mutation on levels of homocysteine, folate, vitamin B12 and on the risk of premature atherosclerosis. Atherosclerosis. 1998;141:161–166. doi: 10.1016/s0021-9150(98)00156-7. [DOI] [PubMed] [Google Scholar]
- 38.Guerzoni AR, et al. Methylenetetrahydrofolate reductase gene polymorphism and its association with coronary artery disease. Sao Paulo Med J. 2007;125:4–8. doi: 10.1590/S1516-31802007000100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fowkes FG, et al. Methylene tetrahydrofolate reductase (MTHFR) and nitric oxide synthase (ecNOS) genes and risks of peripheral arterial disease and coronary heart disease: Edinburgh Artery Study. Atherosclerosis. 2000;150:179–185. doi: 10.1016/s0021-9150(99)00366-4. [DOI] [PubMed] [Google Scholar]
- 41.Pollex RL, et al. Methylenetetrahydrofolate reductase polymorphism 677C>T is associated with peripheral arterial disease in type 2 diabetes. Cardiovasc Diabetol. 2005;4:17. doi: 10.1186/1475-2840-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones GT, Harris EL, Phillips LV, van Rij AM. The methylenetetrahydrofolate reductase C677T polymorphism does not associate with susceptibility to abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2005;30:137–142. doi: 10.1016/j.ejvs.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 43.Ciccarone E, et al. Homocysteine levels are associated with the severity of peripheral arterial disease in type 2 diabetic patients. J Thromb Haemost. 2003;1:2540–2547. doi: 10.1111/j.1538-7836.2003.00500.x. [DOI] [PubMed] [Google Scholar]
- 44.Khandanpour N, et al. Peripheral arterial disease and methylenetetrahydrofolate reductase (MTHFR) C677T mutations: a case-control study and meta-analysis. J Vasc Surg. 2009;49:711–718. doi: 10.1016/j.jvs.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Mueller T, et al. Factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations are not associated with chronic limb ischemia: the Linz Peripheral Arterial Disease (LIPAD) study. J Vasc Surg. 2005;41:808–815. doi: 10.1016/j.jvs.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 46.Santos ME, et al. Mutations in methylenetetrahydrofolate reductase and in cysthationine β synthase: is there a link to homocysteine levels in peripheral arterial disease? Mol Biol Rep. 2010;38:3361–3366. doi: 10.1007/s11033-010-0443-1. [DOI] [PubMed] [Google Scholar]
- 47.Sofi F, et al. Thrombophilic risk factors for symptomatic peripheral arterial disease. J Vasc Surg. 2005;41:255–260. doi: 10.1016/j.jvs.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Stricker H, Soldati G, Balmelli T, Mombelli G. Homocysteine, vitamins and gene mutations in peripheral arterial disease. Blood Coagul Fibrinolysis. 2001;12:469–475. doi: 10.1097/00001721-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Zintzaras E, Zdoukopoulos N. A field synopsis and meta-analysis of genetic association studies in peripheral arterial disease: The CUMAGAS-PAD database. Am J Epidemiol. 2009;170:1–11. doi: 10.1093/aje/kwp094. [DOI] [PubMed] [Google Scholar]
- 50.Forman JP, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 51.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang TJ, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fahrleitner A, et al. Vitamin D deficiency and secondary hyperparathyroidism are common complications in patients with peripheral arterial disease. J Gen Intern Med. 2002;17:663–669. doi: 10.1046/j.1525-1497.2002.11033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Canton C, et al. Vascular calcification and 25-hydroxyvitamin D levels in non-dialysis patients with chronic kidney disease stages 4 and 5. Nephrol Dial Transplant. 2011;26:2250–2256. doi: 10.1093/ndt/gfq650. [DOI] [PubMed] [Google Scholar]
- 55.Zagura M, et al. Aortic stiffness and vitamin D are independent markers of aortic calcification in patients with peripheral arterial disease and in healthy subjects. Eur J Vasc Endovasc Surg. 2011;42:689–695. doi: 10.1016/j.ejvs.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 56.Van Horn L, et al. The evidence for dietary prevention and treatment of cardiovascular disease. J Am Diet Assoc. 2008;108:287–331. doi: 10.1016/j.jada.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 57.de Lorgeril M, Salen P. Mediterranean diet in secondary prevention of CHD. Public Health Nutr. 2011;14:2333–2337. doi: 10.1017/S136898001100259X. [DOI] [PubMed] [Google Scholar]
- 58.Hardin-Fanning F. The effects of a Mediterranean-style dietary pattern on cardiovascular disease risk. Nurs Clin North Am. 2008;43:105–115. doi: 10.1016/j.cnur.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Carrero JJ, Lopez-Huertas E, Salmeron LM, Baro L, Ros E. Daily supplementation with (n-3) PUFAs, oleic acid, folic acid, and vitamins B6 and E increases pain-free walking distance and improves risk factors in men with peripheral vascular disease. J Nutr. 2005;135:1393–1399. doi: 10.1093/jn/135.6.1393. [DOI] [PubMed] [Google Scholar]
- 60.Hiatt WR, et al. Effect of niacin ER/lovastatin on claudication symptoms in patients with peripheral artery disease. Vasc Med. 2010;15:171–179. doi: 10.1177/1358863X09360579. [DOI] [PubMed] [Google Scholar]
- 61.Leng GC, et al. Randomized controlled trial of gamma-linolenic acid and eicosapentaenoic acid in peripheral arterial disease. Clin Nutr. 1998;17:265–271. doi: 10.1016/s0261-5614(98)80318-x. [DOI] [PubMed] [Google Scholar]
- 62.Madden J, et al. Fish oil induced increase in walking distance, but not ankle brachial pressure index, in peripheral arterial disease is dependent on both body mass index and inflammatory genotype. Prostaglandins Leukot Essent Fatty Acids. 2007;76:331–340. doi: 10.1016/j.plefa.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Maxwell AJ, Anderson BE, Cooke JP. Nutritional therapy for peripheral arterial disease: a double-blind, placebo-controlled, randomized trial of HeartBar. Vasc Med. 2000;5:11–19. doi: 10.1177/1358836X0000500103. [DOI] [PubMed] [Google Scholar]
- 64.Bays H, et al. Extended-release niacin/laropiprant lipid-altering consistency across patient subgroups. Int J Clin Pract. 2011;65:436–445. doi: 10.1111/j.1742-1241.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 65.Olsson AG. HDL and LDL as therapeutic targets for cardiovascular disease prevention: the possible role of niacin. Nutr Metab Cardiovasc Dis. 2010;20:553–557. doi: 10.1016/j.numecd.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 66.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116:188–195. doi: 10.1161/CIRCULATIONAHA.106.683656. [DOI] [PubMed] [Google Scholar]
- 67.Holy EW, et al. Dietary α-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler Thromb Vasc Biol. 2011;31:1772–1780. doi: 10.1161/ATVBAHA.111.226118. [DOI] [PubMed] [Google Scholar]
- 68.Sommerfield T, Price J, Hiatt WR. Omega-3 fatty acids for intermittent claudication. Cochrane Database of Systematic Reviews. (4) 2007:Art. No. CD003833. doi: 10.1002/14651858.CD003833.pub3. [DOI] [PubMed] [Google Scholar]
- 69.Egert S, Stehle P. Impact of n-3 fatty acids on endothelial function: results from human interventions studies. Curr Opin Clin Nutr Metab Care. 2011;14:121–131. doi: 10.1097/MCO.0b013e3283439622. [DOI] [PubMed] [Google Scholar]
- 70.Furenes EB, Seljeflot I, Solheim S, Hjerkinn EM, Arnesen H. Long-term influence of diet and/or omega-3 fatty acids on matrix metalloproteinase-9 and pregnancy-associated plasma protein-A in men at high risk of coronary heart disease. Scand J Clin Lab Invest. 2008;68:177–184. doi: 10.1080/00365510701663350. [DOI] [PubMed] [Google Scholar]
- 71.Hooper L, et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Systematic Reviews. 2004;(4):Art. No. CD003177. doi: 10.1002/14651858.CD003117.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lefevre M, Kris-Etherton PM, Zhao G, Tracy RP. Dietary fatty acids, hemostasis, and cardiovascular disease risk. J Am Diet Assoc. 2004;104:410–419. doi: 10.1016/j.jada.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Schiano V, et al. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr. 2008;27:241–247. doi: 10.1016/j.clnu.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 74.Pase MP, Grima NA, Sarris J. Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br J Nutr. 2011;106:974–980. doi: 10.1017/S0007114511002819. [DOI] [PubMed] [Google Scholar]
- 75.Leyva DR, et al. The effect of dietary flaxseed on improving symptoms of cardiovascular disease in patients with peripheral artery disease. Rationale and design of the FLAX-PAD randomized controlled trial. Contemp Clin Trials. 2011;32:724–730. doi: 10.1016/j.cct.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Chesney CM, et al. Effect of niacin, warfarin, and antioxidant therapy on coagulation parameters in patients with peripheral arterial disease in the Arterial Disease Multiple Intervention Trial (ADMIT) Am Heart J. 2000;140:631–636. doi: 10.1067/mhj.2000.109648. [DOI] [PubMed] [Google Scholar]
- 77.Dalgard C, et al. No influence of increased intake of orange and blackcurrant juices and dietary amounts of vitamin E on paraoxonase-1 activity in patients with peripheral arterial disease. Eur J Nutr. 2007;46:354–363. doi: 10.1007/s00394-007-0675-6. [DOI] [PubMed] [Google Scholar]
- 78.Dalgard C, et al. Supplementation with orange and blackcurrant juice, but not vitamin E, improves inflammatory markers in patients with peripheral arterial disease. Br J Nutr. 2009;101:263–269. doi: 10.1017/S0007114508995660. [DOI] [PubMed] [Google Scholar]
- 79.Khandanpour N, et al. Randomized clinical trial of folate supplementation in patients with peripheral arterial disease. Br J Surg. 2009;96:990–998. doi: 10.1002/bjs.6670. [DOI] [PubMed] [Google Scholar]
- 80.de Jong SC, et al. Normohomocysteinaemia and vitamin-treated hyperhomocysteinaemia are associated with similar risks of cardiovascular events in patients with premature peripheral arterial occlusive disease. A prospective cohort study. J Intern Med. 1999;246:87–96. doi: 10.1046/j.1365-2796.1999.00541.x. [DOI] [PubMed] [Google Scholar]
- 81.Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2009;202:68–75. doi: 10.1016/j.atherosclerosis.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 82.Ruparelia N, Digby JE, Choudhury RP. Effects of niacin on atherosclerosis and vascular function. Curr Opin Cardiol. 2010;26:66–70. doi: 10.1097/HCO.0b013e3283410c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu BJ, et al. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler Thromb Vasc Biol. 2010;30:968–975. doi: 10.1161/ATVBAHA.109.201129. [DOI] [PubMed] [Google Scholar]
- 84.Elam MB, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial Disease Multiple Intervention Trial. JAMA. 2000;284:1263–1270. doi: 10.1001/jama.284.10.1263. [DOI] [PubMed] [Google Scholar]
- 85.Garg R, et al. Effective and safe modification of multiple atherosclerotic risk factors in patients with peripheral arterial disease. Am Heart J. 2000;140:792–803. doi: 10.1067/mhj.2000.110090. [DOI] [PubMed] [Google Scholar]
- 86.Philipp CS, Cisar LA, Saidi P, Kostis JB. Effect of niacin supplementation on fibrinogen levels in patients with peripheral vascular disease. Am J Cardiol. 1998;82:697–699. doi: 10.1016/s0002-9149(98)00393-2. [DOI] [PubMed] [Google Scholar]
- 87.Grassi D, et al. Black tea consumption dose-dependently improves flow-mediated dilation in healthy males. J Hypertens. 2009;27:774–781. doi: 10.1097/HJH.0b013e328326066c. [DOI] [PubMed] [Google Scholar]
- 88.Hawkes WC, Laslett LJ. Selenium supplementation does not improve vascular responsiveness in healthy North American men. Am J Physiol Heart Circ Physiol. 2009;296:H256–H262. doi: 10.1152/ajpheart.00935.2008. [DOI] [PubMed] [Google Scholar]
- 89.Tornwall ME, et al. The effect of α-tocopherol and β-carotene supplementation on symptoms and progression of intermittent claudication in a controlled trial. Atherosclerosis. 1999;147:193–197. doi: 10.1016/s0021-9150(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 90.Mitchell DC, Prince MR, Frisoli JK, Smith RE, Wood RF. β-carotene uptake into atherosclerotic plaque: enhanced staining and preferential ablation with the pulsed dye laser. Lasers Surg Med. 1993;13:149–157. doi: 10.1002/lsm.1900130202. [DOI] [PubMed] [Google Scholar]
- 91.Giusti V. Management of obesity in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2007;34:576–582. doi: 10.1016/j.ejvs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Mangge H, et al. Inflammation, adiponectin, obesity and cardiovascular risk. Curr Med Chem. 2010;17:4511–4520. doi: 10.2174/092986710794183006. [DOI] [PubMed] [Google Scholar]
- 93.Canoy D. Coronary heart disease and body fat distribution. Curr Atheroscler Rep. 2010;12:125–133. doi: 10.1007/s11883-010-0092-9. [DOI] [PubMed] [Google Scholar]
- 94.Goodpaster BH, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 95.Sung KC, Ryu S, Reaven GM. Relationship between obesity and several cardiovascular disease risk factors in apparently healthy Korean individuals: comparison of body mass index and waist circumference. Metabolism. 2007;56:297–303. doi: 10.1016/j.metabol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 96.Wannamethee SG, Shaper AG, Morris RW, Whincup PH. Measures of adiposity in the identification of metabolic abnormalities in elderly men. Am J Clin Nutr. 2005;81:1313–1321. doi: 10.1093/ajcn/81.6.1313. [DOI] [PubMed] [Google Scholar]
- 97.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 98.Cabrera MA, et al. Metabolic syndrome, abdominal obesity, and cardiovascular risk in elderly women. Int J Cardiol. 2007;114:224–229. doi: 10.1016/j.ijcard.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 99.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 100.Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17:319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 101.Smith SC, Jr, Haslam D. Abdominal obesity, waist circumference and cardio-metabolic risk: awareness among primary care physicians, the general population and patients at risk-—the Shape of the Nations survey. Curr Med Res Opin. 2007;23:29–47. doi: 10.1185/030079906X159489. [DOI] [PubMed] [Google Scholar]
- 102.Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev. 2010;23:247–269. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- 103.Dias RM, et al. Obesity decreases time to claudication and delays post-exercise hemodynamic recovery in elderly peripheral arterial disease patients. Gerontology. 2009;55:21–26. doi: 10.1159/000155219. [DOI] [PubMed] [Google Scholar]
- 104.Galal W, et al. The obesity paradox in patients with peripheral arterial disease. Chest. 2008;134:925–930. doi: 10.1378/chest.08-0418. [DOI] [PubMed] [Google Scholar]
- 105.Hamburg NM, et al. Maladaptive enlargement of the brachial artery in severe obesity is reversed with weight loss. Vasc Med. 2010;15:215–222. doi: 10.1177/1358863X10362831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hooi JD, et al. Risk factors and cardiovascular diseases associated with asymptomatic peripheral arterial occlusive disease. The Limburg PAOD Study Peripheral Arterial Occlusive Disease. Scand J Prim Health Care. 1998;16:177–182. doi: 10.1080/028134398750003142. [DOI] [PubMed] [Google Scholar]
- 107.Ix JH, et al. Association of body mass index with peripheral arterial disease in older adults: the Cardiovascular Health Study. Am J Epidemiol. 2011;174:1036–1043. doi: 10.1093/aje/kwr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumakura H, et al. The influence of the obesity paradox and chronic kidney disease on long-term survival in a Japanese cohort with peripheral arterial disease. J Vasc Surg. 2010;52:110–117. doi: 10.1016/j.jvs.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 109.McDermott MM, et al. Obesity, weight change, and functional decline in peripheral arterial disease. J Vasc Surg. 2006;43:1198–1204. doi: 10.1016/j.jvs.2006.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fox CS, et al. Periaortic fat deposition is associated with peripheral arterial disease: the Framingham heart study. Circ Cardiovasc Imaging. 2010;3:515–519. doi: 10.1161/CIRCIMAGING.110.958884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gardner AW, Montgomery PS. The effect of metabolic syndrome components on exercise performance in patients with intermittent claudication. J Vasc Surg. 2008;47:1251–1258. doi: 10.1016/j.jvs.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gardner AW, Montgomery PS. Resting energy expenditure in patients with intermittent claudication and critical limb ischemia. J Vasc Surg. 2010;51:1436–1441. doi: 10.1016/j.jvs.2009.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giugliano G, et al. The prognostic impact of general and abdominal obesity in peripheral arterial disease. Int J Obes (Lond) 2010;34:280–286. doi: 10.1038/ijo.2009.244. [DOI] [PubMed] [Google Scholar]
- 114.Jakovljevic B, et al. Obesity and fat distribution as predictors of aortoiliac peripheral arterial disease in middle-aged men. Eur J Intern Med. 2011;22:84–88. doi: 10.1016/j.ejim.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 115.Lim PS, Hu CY, Wu MY, Wu TK, Chang HC. Plasma adiponectin is associated with ankle-brachial index in patients on haemodialysis. Nephrology (Carlton) 2007;12:546–552. doi: 10.1111/j.1440-1797.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 116.Makdisse M, et al. Prevalence and risk factors associated with peripheral arterial disease in the Hearts of Brazil Project. Arq Bras Cardiol. 2008;91:370–382. doi: 10.1590/s0066-782x2008001800008. [DOI] [PubMed] [Google Scholar]
- 117.Planas A, et al. Relationship of obesity distribution and peripheral arterial occlusive disease in elderly men. Int J Obes Relat Metab Disord. 2001;25:1068–1070. doi: 10.1038/sj.ijo.0801638. [DOI] [PubMed] [Google Scholar]
- 118.Skilton MR, et al. Metabolic health, obesity and 9-year incidence of peripheral arterial disease: the D.E.S.I.R. study. Atherosclerosis. 2011;216:471–476. doi: 10.1016/j.atherosclerosis.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 119.Golledge J, et al. Association of obesity and metabolic syndrome with the severity and outcome of intermittent claudication. J Vasc Surg. 2007;45:40–46. doi: 10.1016/j.jvs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 120.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 121.Saunders E, Ofili E. Epidemiology of atherothrombotic disease and the effectiveness and risks of antiplatelet therapy: race and ethnicity considerations. Cardiol Rev. 2008;16:82–88. doi: 10.1097/CRD.0b013e31815685fa. [DOI] [PubMed] [Google Scholar]
- 122.Ix JH, Allison MA, Denenberg JO, Cushman M, Criqui MH. Novel cardiovascular risk factors do not completely explain the higher prevalence of peripheral arterial disease among African Americans. The San Diego Population Study. J Am Coll Cardiol. 2008;51:2347–2354. doi: 10.1016/j.jacc.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 123.Allison MA, et al. Genetic ancestry and lower extremity peripheral artery disease in the Multi-Ethnic Study of Atherosclerosis. Vasc Med. 2010;15:351–359. doi: 10.1177/1358863X10375586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Allison MA, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;48:1190–1197. doi: 10.1016/j.jacc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 125.Criqui MH, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703–2707. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- 126.Khawaja FJ, et al. Association of novel risk factors with the ankle brachial index in African American and non-Hispanic white populations. Mayo Clin Proc. 2007;82:709–716. doi: 10.4065/82.6.709. [DOI] [PubMed] [Google Scholar]
- 127.Grant WB, Peiris AN. Possible role of serum 25-hydroxyvitamin D in black-white health disparities in the United States. J Am Med Dir Assoc. 2010;11:617–628. doi: 10.1016/j.jamda.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 128.Chen TC, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;319:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 130.Stoner MC, et al. Cost per day of patency: understanding the impact of patency and reintervention in a sustainable model of healthcare. J Vasc Surg. 2008;48:1489–1496. doi: 10.1016/j.jvs.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 131.Flu H, et al. Treatment for peripheral arterial obstructive disease: an appraisal of the economic outcome of complications. J Vasc Surg. 2008;48:368–376. doi: 10.1016/j.jvs.2008.03.029. [DOI] [PubMed] [Google Scholar]