Abstract
Background
Elective surgery can have long-term psychological sequelae, especially for patients who experience intraoperative awareness. However, risk factors other than awareness for symptoms of posttraumatic stress disorder (PTSD) after surgery are poorly defined, and practical screening methods have not been applied to a broad population of surgical patients.
Methods
The Psychological Sequelae of Surgery (Psych SOS) study was a prospective cohort study of patients previously enrolled in the United States and Canada in 3 trials for the prevention of intraoperative awareness. The 68 patients who experienced definite or possible awareness were matched, based on age, sex, surgery type, and awareness risk, with 418 patients who denied awareness. Participants completed the PTSD Checklist–Specific (PCL-S) and/or a modified Mini International Neuropsychiatric Interview telephone assessment to identify symptoms of PTSD and symptom complexes consistent with a PTSD diagnosis. We then used structural equation modeling to produce a composite PTSD score and examined potential risk factors.
Results
One hundred forty patients were unreachable; of those contacted, 303 (88%) participated a median of 2 years postoperatively. Forty-four of the 219 patients (20.1%) who completed the PCL-S exceeded the civilian screening cutoff score for PTSD symptoms resulting from their surgery (15 of 35 [43%] with awareness and 29 of 184 [16%] without). Nineteen patients (8.7%; 5 of 35 [14%] with awareness and 14 of 184 [7.6%] without) both exceeded the cutoff and endorsed a breadth of symptoms consistent with the DSM-IV diagnosis of PTSD attributable to their surgery. Factors independently associated with PTSD symptoms were poor social support, prior PTSD symptoms, prior mental health treatment, dissociation related to surgery, perceiving that one's life was threatened during surgery, and intraoperative awareness(all p ≤ 0.017). Perioperative dissociation was identified as a potential mediator for perioperative PTSD symptoms.
Conclusions
Events in the perioperative period can precipitate psychological symptoms consistent with subsyndromal and syndromal PTSD. We confirmed the high rate of postoperative PTSD in awareness patients, but also identified a significant rate in matched nonawareness controls. Screening surgical patients, especially those with potentially mediating risk factors such as intraoperative awareness or perioperative dissociation, for postoperative PTSD symptoms with the PCL-S is practical and could promote early referral, evaluation and treatment.
Introduction
More than 200 million major surgical procedures are performed each year worldwide, the majority with general anesthesia.1 Prospective studies using structured interviews have found that between 0.1 and 0.4% of all general anesthetics are complicated by unintended intraoperative awareness with explicit recall, hereafter referred to as awareness.2 Patients reporting awareness may experience ongoing psychological sequelae on a spectrum from subsyndromal to syndromal posttraumatic stress disorder (PTSD); small studies have suggested that between 0% and 71% develop PTSD.3-10 However, intraoperative awareness is likely to be only 1 of many perioperative experiences associated with posttraumatic stress spectrum disorder.11 Certain patient factors such as depressive symptoms and pain,12 as well as perioperative factors including intensive care unit (ICU) admission,13 mechanical ventilation,14 and in-hospital cardiac arrest,15 have been described as risk factors for healthcare-associated PTSD.
Many studies of healthcare-associated PTSD symptoms have used face-to-face interview techniques that, while valid, cannot feasibly be implemented for routine screening.4-7, 9, 10 Well-validated PTSD screening instruments have not been broadly applied to an elective surgical population. We previously conducted 3 large prospective trials of interventions to prevent awareness, enrolling more than 29,000 patients at 4 institutions.16-18 The assessment of psychological sequelae and/or PTSD was a prespecified secondary aim in all 3 trials and is the focus of the present Psychological Sequelae of Surgery (Psych SOS) study. The objectives of the Psych SOS study were 1) to apply practical screening instruments to detect symptoms of PTSD (e.g., subsyndromal PTSD) and symptom complexes consistent with a diagnosis of postoperative PTSD, and 2) to identify factors, such as intraoperative awareness, that are associated with increased risk of postoperative PTSD symptoms.
Methods
This multicenter study was approved by the Human Studies Committees at Washington University in St. Louis (USA), the University of Manitoba in Winnipeg (Canada), and the University of Michigan in Ann Arbor (USA). Verbal informed consent was obtained for each telephone interview; return of a completed study form implied consent for the written portion of the study.
The requirement for written informed consent was waived by the IRB. The study was not registered before patient enrollment. The parent studies (B-Unaware, BAG-RECALL, and MACS) were registered at clinicaltrials.gov as NCT00281489, NCT00682825, and NCT00689091, respectively. Evaluation of psychological sequelae was a secondary outcome of interest of these studies.
Study design
Psych SOS was designed as a matched double-cohort study. Patients who experienced definite or possible awareness were matched 1:4 with control patients who denied awareness from the same institution and trial. Other matching factors were sex, age (in study population quartiles: 18-50, 51-60, 61-70, and 71 years and older), ICU care after surgery, and surgical procedure (if possible) or service. Screening tools were used to identify patients with postoperative PTSD-complex symptoms (Supplemental Figure 1) and are described in detail below.
Patient population
Patients were drawn from participants in the B-Unaware trial (September 2005 to October 2006), BIS or Anesthetic Gas to Reduce Explicit Recall trial (BAG-RECALL) (May 2008 to May 2010), and the Michigan Awareness Control Study (MACS) (May 2008 to May 2010). Thus, inclusion criteria were based on those trials.16-18 In brief, the B-Unaware and BAG-RECALL trials recruited only patients who were considered to be at high risk of experiencing awareness and who were receiving potent volatile anesthetics. High-risk patients were defined in BAG-RECALL as those who had at least 1 of the following: preoperative long-term use of anticonvulsants, opiates, benzodiazepines, cocaine, or daily alcohol consumption; history of cardiac ejection fraction <40%, aortic stenosis, end-stage lung disease, marginal exercise tolerance, pulmonary hypertension, intraoperative awareness, difficult intubation, or anticipated difficult intubation; ASA physical status 4 or 5; or planned open-heart surgery. B-Unaware used fundamentally similar criteria. By contrast, MACS recruited patients at all risk levels receiving both inhaled and IV anesthetics. All 3 studies excluded non-English speakers and patients younger than 18 years. Postoperatively, all participants underwent structured modified Brice interviews to detect awareness.19 Patients from Washington University (BAG-RECALL, B-Unaware), the University of Manitoba (BAG-RECALL), and the University of Michigan (MACS) who experienced definite or possible awareness were matched with patients who denied awareness, as above. The decision to include patients with adjudicated definite or possible awareness was made a priori, as reflected in the study protocol submitted to the study site ethics committees. This decision was based on the possibility that any credible awareness experience could potentially cause psychological distress in a patient.
Assessment for psychological sequelae
Participants were contacted between August 2010 and December 2011. Two types of assessment were used: the PTSD Checklist – Specific (PCL-S),20 which was mailed to participants, and a modified version of the Mini International Neuropsychiatric Interview (MINI),21 which was administered by telephone. The modified version of the MINI is referred to herein as “mMINI.”
PCL-S
The PCL-S is a validated measure of PTSD symptoms after a defined incident (e.g., surgery).20 A copy of the PCL-S and an explanatory letter were mailed to study participants (Supplemental Document 1). Based on recommendations from the Department of Veterans' Affairs PCL-S scoring guide, the civilian cut-off score of 30 was selected to discriminate between patients with potentially clinically meaningful levels of PTSD symptoms and those without.22 We selected the cutoff score based on our goal to screen for, rather than diagnose, PTSD. We also evaluated the PCL-S responses of those with severity scores above the civilian cutoff to determine whether the participant endorsed a breadth of symptoms consistent with the Diagnostic and Statistical Manual Fourth Edition (DSM-IV) symptom criteria for diagnosis of PTSD (Supplemental Figure 1).
Telephone interview
The MINI was developed as a broad screening tool for many psychiatric diagnoses, according to the criteria described in the DSM-IV21 (Supplemental Figure 1). A psychologist with expertise in anxiety disorders expanded the questions in the PTSD section of the MINI by (a) including assessments for each symptom and (b) including severity scales for those symptoms for which it was possible to assess symptom severity. This brought a degree of dimensionality to the mMINI similar to that found in the PCL-S, which is appropriate when considering PTSD symptoms along a spectrum from mild to severe. We supplemented the MINI with an assessment of social support based upon the Multidimensional Perceived Social Support Scale,23 questions about mental health history, and the depression items from the 21-item version of the Depression Anxiety and Stress Scales.24 We also inquired about past PTSD symptoms attributed to the index surgery that had resolved before the interview, and past experiences with PTSD symptoms due to other events. Finally, 2 items to evaluate perioperative experiences of dissociation, i.e., a feeling that one is numbed, dazed, or dreaming during real events, were inserted. The telephone interview script is reproduced in Supplemental Document 2.
Interviewers from all 3 institutions received a standardized training regimen and were blinded to awareness status.
Statistical analysis
Descriptive and univariate statistics were performed using SPSS Statistics versions 19 and 20 (IBM Corporation, Somers, NY). Modeling of risk factors for the PTSD symptom complex using mMINI and PCL-S data was conducted using structural equation modeling with the software package Mplus (Version 7, Muthén&Muthén, Los Angeles, CA). For each participant, we estimated a composite PTSD factor corresponding to PTSD symptom severity using available information from the mMINI, PCL-S, or both. Self-report and interview responses to PTSD symptoms in the B, C, and D clusters (Supplemental Figure 1) were treated as ordered categorical variables and used to estimate the composite PTSD factor; missing data were estimated using available information.25 We then regressed the PTSD factor on predictors to determine what variables predicted PTSD symptom severity. This approach avoids the statistical and scientific problems associated with the use of a single measure of a construct, falsely dichotomizing dimensional scores,26 and inappropriately discarding cases with partially missing data. Indirect effects were assessed using bias-corrected bootstrapping with 5000 draws.27 A p value less than 0.05 was considered to indicate statistical significance.
Results
Patients
We attempted to recruit all 68 patients from the 3 institutions with definite or possible intraoperative awareness and an additional 418 who denied awareness. After exclusions for those who were deceased or unreachable, and those who refused participation, 49 patients with awareness and 254 without participated and contributed to the study (Figure 1) by completing the mMINI (n = 84), the PCL-S (n = 23), or both (n = 196). Because of the iterative process of matching within then on awareness cohort, more nonawareness patients were recruited than the planned 1:4 match. Nonawareness patients who completed 1 or both surveys were not excluded from analysis if it became apparent later that the awareness patient with whom they had been matched would not be participating in Psych SOS.
Figure 1.
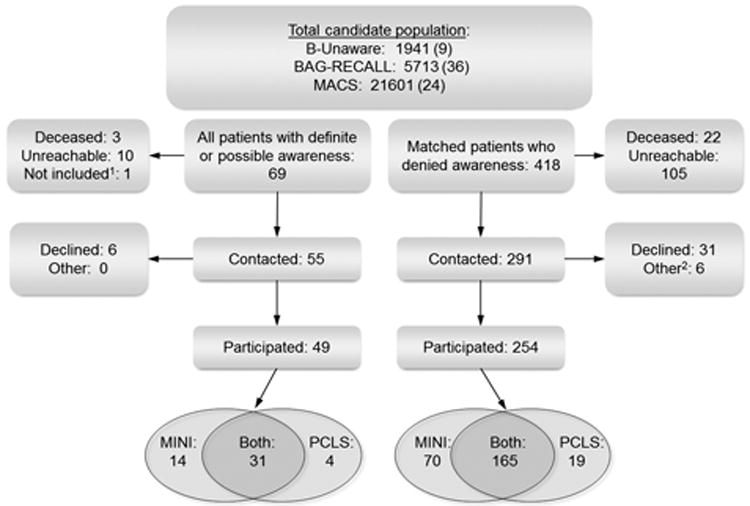
Participant flow diagram. 1, the single patient reporting awareness from the University of Chicago in the BAG-RECALL trial was not included in Psych SOS. 2, “other” includes patients who were unable to complete their initial screening tool because of hearing, speech, or reading difficulty or other neurologic disease.
Those who participated were not significantly different from those who did not, in terms of sex, whether or not they reported awareness, their study intervention, age at surgery, and the type of surgery they received. However, patients who participated were significantly more likely to have reported distress during their initial awareness experience (Table 1).28 Those who participated also had a significantly shorter time between their surgery and entry into the Psych SOS study, were more likely to have had an ICU stay, and were less likely to have been judged at high risk for awareness (Table 1).
Table 1.
Comparison of baseline data between participants and nonparticipants.
| Variable | Participated (% of participants) | Did not participate (% of nonparticipants) | P-value | |
|---|---|---|---|---|
| Male sex | 171 (56.4%) | 112 (61.2%) | 0.30a | |
| Age at surgery | 54.7 ± 15.0 | 54.5 ±14.4 | 0.88b | |
| Years surgery to contact (IQR) | 2 (2-3) | 2 (2-5) | 0.005c | |
| Trial source (publication year) | B-Unaware (2008) | 37 (12.2%) | 46 (25.1%) | <0.001a |
| BAG-RECALL (2011) | 107 (35.3%) | 67 (36.6%) | ||
| MACS (2012) | 159 (52.5%) | 70 (38.3%) | ||
| Type of surgery | Cardiothoracic | 103 (34.0%) | 62 (33.9%) | 0.12a |
| General | 61 (20.1%) | 48 (26.2%) | ||
| Gynecologic | 39 (12.9%) | 29 (15.8%) | ||
| Urologic | 23 (7.6%) | 11 (6.0%) | ||
| ENT or maxillofacial | 19 (6.3%) | 11 (6.0%) | ||
| Orthopedic | 26 (8.6%) | 6 (3.3%) | ||
| Otherd | 31 (10.2%) | 16 (8.7%) | ||
| Had ICU stay | 115 (38.0%) | 47 (25.6%) | 0.005a | |
| Study group | End-tidal anesthetic concentration monitoring | 143 (41.2%) | 91 (49.7%) | 0.092a |
| Bispectral index monitoring | 117 (38.6%) | 78 (42.6%) | ||
| No AWR monitor | 43 (14.2%) | 14 (7.7%) | ||
| High risk for AWR | 215 (71.0%) | 146 (79.8%) | 0.033a | |
| AWR | Definite | 24 (7.9%) | 7 (3.8%) | 0.14b |
| Possible | 25 (8.3%) | 12 (6.6%) | ||
| Denied | 254 (83.8%) | 164 (89.6%) | ||
| For only those patients who reported AWR: | ||||
| MACI Class | 1 | 20 (41%) | 5 (26%) | 0.63e |
| 2 | 15 (31%) | 6 (32%) | ||
| 3 | 6 (12%) | 4 (21%) | ||
| 4 or 5 | 8 (16%) | 4 (21%) | ||
| Distressing | No | 12 (24.5%) | 9 (47.4%) | 0.026a |
| Yes | 37 (75.5%) | 10 (52.6%) | ||
Percentages may not sum to 100% because of rounding.
chi-square test.
t-test.
Mann-Whitney U test.
“Other” includes surgical categories with less than 10 total patients, such as vascular surgery, neurosurgery, cardiac electrophysiology, and other miscellaneous procedure types.
Fisher's exact test.
AWR = intraoperative awareness with explicit recall, ENT = ear, nose and throat surgery, ICU=intensive care unit, MACI = Michigan Awareness Classification Instrument
Assessment for psychological sequelae and the PTSD symptom complex using the PCL-S
Of all patients who completed the PCL-S, 20.1% exceeded the civilian PCL-S cutoff score for the presence of significant PTSD symptoms attributed to their surgery and hospitalization. Twenty-nine of 184 patients (15.8%) who did not experience awareness and 15 of 35 (42.9%) who experienced definite or possible awareness screened positive (unadjusted absolute risk increase = 27.1%; 95% CI, 11.1% to 44.0%). Patients with a PCL-S total severity score higher than 30 were more likely to have reported definite or possible awareness (Table 2). Nineteen patients (8.7%; 14/184 who denied awareness and 5/35 with definite or possible awareness) had both a total severity score higher than 30 and reported symptoms on the PCL-S or during the mMINI interview from the B, C and D clusters consistent with presumptive DSM-IV criterion-based diagnosis of PTSD related to their surgery.
Table 2.
Univariate comparisons for patients with PCL-S scores higher than 30 versus those without.
| Variable | PCL-S < 30 n (%) | PCL-S ≥ 30 n (%) | P value |
|---|---|---|---|
| Age (median [IQR]) | 57 [46 – 67] | 53 [47 – 61] | 0.11a |
| Female gender | 80 (45.7%) | 19 (43.2%) | 0.76b |
| Underwent cardiac surgery | 47 (26.9%) | 6 (13.6%) | 0.067b |
| Stayed in ICU | 63 (36.0%) | 11 (25.0%) | 0.17b |
| Definite or possible AWR | 20 (11.4%) | 15 (34.1%) | <0.001b |
| For patients who reported AWR: | |||
| Nondistressing AWR experience | 18 (90.0%) | 12 (80.0%) | 0.40c |
Mann-Whitney U test.
chi square.
Fisher's exact test.
AWR = intraoperative awareness with recall, ICU = intensive care unit, IQR = interquartile range.
Modeling risk factors for the PTSD symptom complex using composite scores
Validity testing for confirmatory factor analysis
We first tested whether all the PTSD items regarding the B, C, and D clusters could be used to estimate a single PTSD factor by using a confirmatory factor analysis. A single factor fit the model well according to standard definitions, both locally and globally,29 indicating that both the self-report and interview essentially measure a single construct. Supporting this interpretation, when scaled scores were created from the same items, the interview and self-report severity scores correlated well (r = 0.77, p < 0.001).
Impact of intraoperative awareness and other hypothesized risk factors on PTSD symptom complex severity factor
Evaluation of independent relationships among the PTSD factor, awareness, and the additional predictors described in Table 3 excluded 25 participants who did not complete the interview and thus had incomplete data for 1 or more predictor. A sensitivity analysis excluding those predictors, so all participants could be included, yielded substantively identical results.
Table 3.
Predictors for severity of posttraumatic stress disorder (PTSD)-complex symptoms based on confirmatory factor analysis.
| Factor | Estimate ± standard error of the estimate | Standardized estimate | P value |
|---|---|---|---|
| Significant: | |||
| Past PTSD-complex symptoms | 0.42 ± 0.14 | 0.55 if yes | 0.003 |
| Perceived social support | -0.18 ± 0.07 | -0.20 per standard deviation of latent variable above zero | 0.006 |
| Prior mental health treatment | 0.19 ± 0.08 | 0.19 if yes | 0.017 |
| Intraoperative awareness (definite or possible) | 0.36 ± 0.15 | 0.47 if yes | 0.014 |
| Dissociation at the time of surgery | 0.44 ± 0.11 | 0.39 per standard deviation of latent variable above zero | <0.001 |
| Perceived degree of threat to life at the time of surgery | 0.16 ± 0.05 | 0.21 per standard deviation of latent variable above zero | 0.002 |
| Nonsignificant: | |||
| Age | -0.08 ± 0.07 | 0.21 | |
| Gender | 0.09 ± 0.12 | 0.49 | |
| Whether the patient received care in the ICU | 0.13 ± 0.18 | 0.40 | |
| Whether the patient underwent cardiac surgery | -0.31 ± 0.21 | 0.09 | |
Standardized estimate is the amount, in standard deviations, by which the value of the predictor is expected to change the severity of PTSD-complex symptoms. Latent variables were required when the predictor was derived from more than one question on the PCL-S and/or the mMINI.
For example, if a patient reports prior PTSD-complex symptoms (+0.55), a slightly low level of social support (1 standard deviation below the mean) (-1 * -0.20 = +0.20), a high degree (3 standard deviations above the mean)of dissociation at the time of surgery (3 * 0.44 = +1.31), denies prior mental health treatment, and had average perceived degree of threat to life during surgery, their PTSD-complex symptom severity is estimated to be 0.55 + 0.20 + 1.31 = 2.06, or 2 standard deviations above the mean severity for all people who underwent surgery.
ICU = intensive care unit
The significant independent predictors of the PTSD factor were dissociation related to surgery, perceiving that one's life was threatened during surgery, history of PTSD before surgery, low current social support, and a history of mental health treatment (Table 3). Awareness continued to be a significant predictor of PTSD-complex symptom severity with all other variables in the model. Age, sex, ICU care, and whether the patient underwent cardiac surgery were not independent predictors of PTSD symptoms attributed to the surgery itself in this sample.
Indirect effects
Tests for indirect effects provide a surrogate for a causal chain of events. There was a significant indirect effect for awareness on PTSD through dissociation (p < 0.01a). Furthermore, multiple regression demonstrated patients reporting awareness were more likely to uniquely endorse depersonalization and derealization (p = 0.04), as opposed to feeling numbed or dazed (p = 0.21), even after accounting for the overlap between the 2 dissociation items.
Discussion
Using practical screening tools, this study identified a striking percentage of postoperative patients with significant PTSD symptoms attributed to surgery and, through indirect analysis, demonstrated that the experience of dissociation in the perioperative period may be a mediating step to the development of PTSD symptoms after surgery (as has been shown for other trauma-related precipitants of PTSD).30 Although the cohort studied included patients with awareness who might be expected to be at risk for psychological sequelae, a large number of matched nonawareness controls also reported psychological symptoms attributed to surgery.
The presence of PTSD is regarded as dichotomous in terms of strict diagnostic criteria, but PTSD symptoms have a spectrum of severity, with even subsyndromal PTSD having major implications for patients' functionality and quality of life.31-35 The screening and analytic approaches used in Psych-SOS allowed for the fact that all participants in the study had the potential for some level of PTSD symptoms (whether very low, average or very high) attributable to their surgery that could be predicted within the model. The novel and practical approach to screening for postoperative PTSD in the current study yielded findings that are commensurate with previous studies of patients reporting intraoperative awareness (Table 4)3-10 and confirmed that awareness substantially increases the risk of PTSD symptoms. The findings are also consistent with a previous study identifying postoperative PTSD in 3 of 25 patients (12%) from a matched surgical cohort not experiencing awareness,5 and in 14 of 73 consecutive patients after elective lumbar spine arthrodesis, for whom awareness status was not assessed.36
Table 4.
Summary of studies evaluating psychological sequelae from intraoperative awareness.
| Study | Year | Recruitment method | Mean/median time (range) since awareness event | PTSD screening test or criteria used | Data collection method | Number of awareness patients studied (number with PTSD symptoms, %) |
|---|---|---|---|---|---|---|
| Schwender et al9 | 1998 | Advertising and referral | 9.6 (0.1-30) yr | No formal criteria used | Face-to-face | 45 (3, 7%) |
| Ranta et al7 | 1998 | Secondary outcome of a prospective awareness study | Within 2 weeks of surgery | SCID-NP and SCID-II (DSM-III-R criteria) | Face-to-face | 5a (0, 0%) |
| Domino et al3 | 1999 | Closed claims | Not reported | No formal criteria used | Claim file review | 61b (6, 10%) |
| Osterman et al6 | 2001 | Advertising and referral | 17.9 (0.25-38) yrs | CAPS (DSM-IV criteria) | Face-to-face | 16 (9, 56%) |
| Lennmarken et al4 | 2002 | Secondary outcome of a prospective awareness study | 27 (20-35) mos | DSM-IV (DSM-IV criteria) | Face-to-face | 9c (4, 44%) |
| Samuelsson et al8 | 2007 | Consecutive enrollment of surgical patients who reported previous awareness | 21 (0-66) yrs | No formal criteria used | Telephone | 46 (1, 2%)d |
| Leslie et al5 | 2010 | Secondary outcome of a prospective awareness study | 5.3 (4.3-5.7) yrs | CAPS | Face-to-face | 7e (5, 71%) |
| Laukkala et al10 | 2014 | Secondary outcome of a prospective awareness study | 17.2 (13.0-19.7) yrs | SCID-I (DSM-IV criteria) | Face-to-face | 9 (0, 0%) |
| Whitlock et al (present study) | 2014 | Secondary outcome of three prospective awareness studies | 2 (1-6) yrs | PCL-S | Mailed form completed at home | 35f (15, 43%) |
There were a total of 19 awareness patients, but only 5 had a completed psychiatric follow up.
This does not include patients in the study who experienced awake paralysis.
Nine other patients from the original study were lost to follow up (2), refused to participate (6), or died (1).
This patient had experienced episodes of “extreme mental stress” earlier in life.
Six other patients from the original study died.
Included definite and possible awareness patients; only patients responding to written screening tool included.
Abbreviations: SCID, structured clinical interview for the DSM-III-R or –IV (NP, non-patient version). CAPS, clinician-administered PTSD scale.
It is impractical to perform detailed psychological assessment of every patient who undergoes surgery. However, targeted screening could identify those at highest risk for subsequent referral to assessment or support services. In this study, we demonstrated 6 major risk factors for the PTSD symptom complex attributed to surgery (i.e., poor social support, history of PTSD symptoms, prior mental health treatment, dissociation related to surgery, perceiving that one's life was threatened during surgery, intraoperative awareness), that substantially overlap with known risk factors for PTSD after psychological trauma. These (and potentially other validated) risk factors could be applied to patients who have undergone surgery to identify a target population for further screening, progressing to formal assessment and intervention as needed (Figure 2). A similar step-wise care pathway has been shown to improve PTSD symptoms and remission rates in traumatic injury survivors.37
Figure 2.
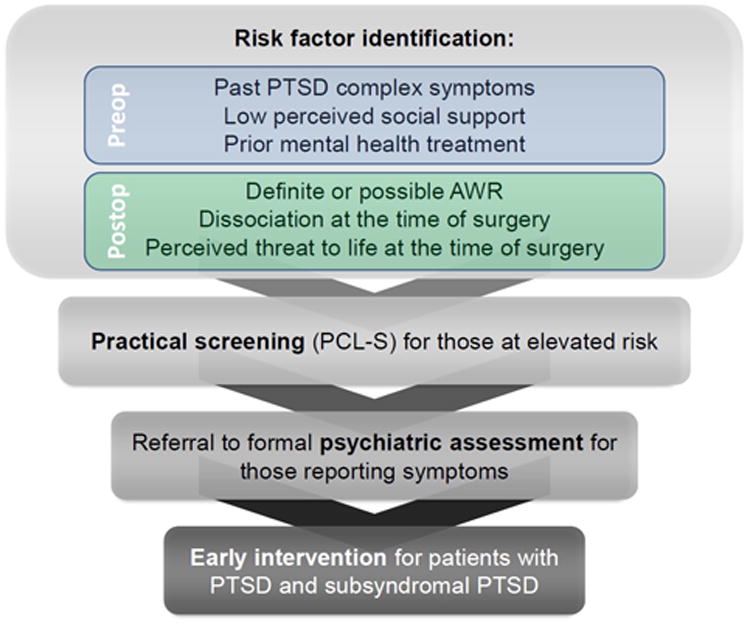
Proposed schematic for screening patients for persistent psychological sequelae following surgery.
The incidence of PTSD-related symptom complexes in Psych SOS was substantially higher than would be expected from a random sample of adults in the community. Using DSM-IV criteria, the National Comorbidity Survey Replication suggested that the lifetime prevalence of PTSD in United States adults is about 6.8%.38 The 12-month PTSD prevalence for adults was estimated at 3.5%.39 In contrast, 8.7% of the surgical patients in the cohort studied in Psych SOS (or 7.6% of those who did not report intraoperative awareness) had symptoms consistent with PTSD in response to a single event, surgery. PTSD symptoms and diagnoses, unlike other psychiatric diagnoses, are established in reference to a specific (traumatic) event, which in the case of Psych SOS was the index surgery. Indeed, a number of surgery-specific factors were independent predictors of PTSD symptomatology in the Psych SOS study. These data suggest that a surgery confers approximately the same risk of persistent PTSD symptoms as being mugged or witnessing a killing or serious injury.40 Although this risk appears surprisingly high, a recent study screened more than 500 stroke or transient ischemic attack survivors with the PCL-S and demonstrated that 18% of these patients had probable syndromal PTSD attributed to the event a mean of 2 years later.41
There are important limitations to consider when interpreting these results. First, we did not compare our screening methods against a “gold standard,” typically a face-to-face interview with a clinician experienced in the diagnosis of PTSD using DSM-IV criteria. Second, because no outcome data were assessed, we cannot be certain about inferences regarding the clinical importance of the PTSD symptoms we identified. However, even subsyndromal levels of PTSD symptoms significantly impair function, as discussed. Future work is needed to define the quality-of-life implications for persistent psychological distress many years after surgery. Third, the prevalence of PTSD symptoms in this study may not be generalizable to all surgical patients as this was a sample of surgical patients enriched for patients reporting intraoperative awareness and that was subject to the inclusion criteria of the parent studies. Furthermore, the awareness patients who participated in this study had a higher incidence of initial emotional distress than nonparticipants and thus might be expected to have a higher incidence of psychological sequelae attributed to awareness.8 Additionally, we were unable to compare patient-reported perioperative distress in control participants versus nonparticipants. Differential willingness of patients to participate in this study depending on perioperative (awareness or nonawareness) distress may have been a source of bias in our findings for which we were unable to adjust; 11% of those contacted declined to participate. Finally, no PCL-S score cutoff (above which a patient is considered to have a symptom complex consistent with PTSD) has been described for this population of postoperative patients 1-6 years after their index surgery. Therefore, further work to refine cutoff scores, taking into consideration the time elapsed since surgery, will be needed to optimize the screening. PTSD symptoms tend to wane with time, with median duration of about 60 months (although long-term longitudinal follow-up demonstrates symptoms fail to remit in approximately one-third of patients)42 and the distribution of PCL-S total severity scores if performed shortly after surgery may be different than the distribution we describe here. Variability in time-to-assessment may also underlie some of the differences in incidence rates reported in other peer-reviewed studies of PTSD after awareness (Table 3). Interestingly, 2 small studies of similar design to the present work found markedly different PTSD rates depending on time since the awareness event: a median of 5.3 years after surgery, 71% of the awareness patients studied had PTSD,5 compared with no patients a median of 17.2 years after surgery.10
One in 5 of our study population screened positive for PTSD-complex symptoms; 8.7% of the study population, including 7.6% of patients who did not experience awareness, reported lasting psychological symptoms attributed to surgery of the breadth and severity consistent with DSM-IV criteria for PTSD. This study provides evidence that there is a large population of surgical patients potentially at risk for postoperative PTSD. Our findings suggest that both perioperative physicians and general practitioners should be aware that persistent PTSD symptoms occur fairly frequently as a complication of surgery. However, this must be confirmed by further studies assessing reliability of the PCL-S screening tool and comparison with gold standard diagnostic techniques in the surgical population. Furthermore, surgical patients judged to be at risk for PTSD based on perioperative screening may benefit from a broad, multidisciplinary approach toward preoperative prevention and/or postoperative screening and treatment, as at-risk patients have in other settings.36,43 The data presented here provide a framework for risk identification and practical screening that could be broadly implemented in an effort to identify and appropriately refer these patients for formal psychiatric assessment and early intervention.
Supplementary Material
Supplemental Document 1: PTSD Checklist – Specific (PCL-S) and explanatory letter sent to participants.
Supplemental Document 2: Modified Mini International Neuropsychiatric Interview telephone script.
Supplemental Figure 1: Definition of posttraumatic stress disorder by DSM-IV criteria. All six lettered criteria (i.e., A-F) must be met for a diagnosis.
Acknowledgments
Funding: ELW was a predoctoral research scholar under grant number UL1 RR024992 and sub-award number TL1 RR024995 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. The B-Unaware trial was supported by a grant from the Barnes-Jewish Hospital Foundation, St. Louis, Missouri, to M. Avidan (Grant no. 6043-02). The BAG-RECALL trial was supported by a grant from the Foundation for Anesthesia Education and Research, Rochester, Minnesota, and the American Society of Anesthesiologists, Park Ridge, Illinois to M. Avidan (Grant no. CFM-08/15/2007). The Michigan Awareness Control Study was supported by the Cerebral Function Monitoring grant (to Dr. Mashour) from the Foundation for Anesthesia Education and Research, Rochester, Minnesota, and the American Society of Anesthesiologists, Park Ridge, Illinois and grant no. KL2 RR024987-01 (to Dr. Mashour) from the National Institutes of Health, Bethesda, Maryland. JS receives funding from the Manitoba Health Research Council Chair award.
Disclosures
Name: Elizabeth L. Whitlock, MD, MSc
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Elizabeth L. Whitlock has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Thomas L. Rodebaugh, PhD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Thomas L. Rodebaugh has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Afton L. Hassett, PsyD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Afton L. Hassett approved the final manuscript
Name: Amy M. Shanks, MS
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Amy M. Shanks approved the final manuscript
Name: Ellen Kolarik, MS
Contribution: This author helped conduct the study, analyze the data, and write the manuscript
Attestation: Ellen Kolarik approved the final manuscript
Name: Janet Houghtby, MS
Contribution: This author helped conduct the study, analyze the data, and write the manuscript
Attestation: Janet Houghtby approved the final manuscript
Name: Hannah M. West, Undergraduate Student
Contribution: This author helped conduct the study and write the manuscript
Attestation: Hannah M. West approved the final manuscript
Name: Beth A. Burnside, BA
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Beth A. Burnside approved the final manuscript
Name: Erik Shumaker, PhD
Contribution: This author helped conduct the study, analyze the data, and write the manuscript
Attestation: Erik Shumaker approved the final manuscript
Name: Alex Villafranca, MSc, PhD Candidate
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Alex Villafranca approved the final manuscript
Name: W. Alex Edwards, MD
Contribution: This author helped design the study, conduct the study, and write the manuscript
Attestation: W. Alex Edwards approved the final manuscript
Name: Cheri A Levinson, MA, PhD Candidate
Contribution: This author helped conduct the study, analyze the data, and write the manuscript
Attestation: Cheri A Levinson approved the final manuscript
Name: Julia K. Langer, MA, PhD Candidate
Contribution: This author helped conduct the study, analyze the data, and write the manuscript
Attestation: Julia K. Langer approved the final manuscript
Name: Katya C Fernandez, MA, PhD Candidate
Contribution: This author helped conduct the study and write the manuscript
Attestation: Katya C Fernandez approved the final manuscript
Name: Renee El-Gabalawy, MA, PhD Candidate
Contribution: This author helped conduct the study and write the manuscript
Attestation: Renee El-Gabalawy approved the final manuscript
Name: Elizabeth Y Zhou, MD
Contribution: This author helped conduct the study and write the manuscript
Attestation: Elizabeth Y Zhou approved the final manuscript
Name: JitenderSareen, MD, FRCPC
Contribution: This author helped design the study, conduct the study, and write the manuscript
Attestation: Jitender Sareen approved the final manuscript
Name: Eric Jacobsohn, MBChB, MHPE, FRCPC
Contribution: This author helped design the study, analyze the data, and write the manuscript
Attestation: Eric Jacobsohn approved the final manuscript
Name: George A Mashour, MD, PhD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: George A Mashour reviewed the analysis of the data and approved the final manuscript
Name: Michael S Avidan, MBBCh, FCASA
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Michael S Avidan reviewed the analysis of the data and approved the final manuscript
This manuscript was handled by: Sorin J. Brull, MD, FCARCSI (Hon)
Footnotes
The MPlus bias-corrected bootstrapped point estimate with 99% two-sided confidence interval for the effect of awareness (on PTSD) via dissociation is 0.211 [0.036-0.470]. The “p < 0.01” is reported based on the 99% confidence interval not including 0. These estimates do not have a clinical meaning (e.g., they cannot be translated into a standardized coefficient).
The authors declare no conflicts of interest.
Contributor Information
Elizabeth L. Whitlock, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri.
Thomas L. Rodebaugh, Department of Psychology, Washington University in St Louis, St. Louis, Missouri.
Afton L. Hassett, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, Michigan.
Amy M. Shanks, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, Michigan.
Ellen Kolarik, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, Michigan.
Janet Houghtby, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, Michigan.
Hannah M. West, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri.
Beth A. Burnside, Department of Anesthesiology, Washington University in St Louis, St. Louis, Missouri.
Erik Shumaker, Department of Psychology, Washington University in St Louis, St. Louis, Missouri.
Alex Villafranca, Department of Anesthesia and Perioperative Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
W. Alex Edwards, Department of Anesthesiology, Washington University in St Louis, St. Louis, Missouri.
Cheri A Levinson, Department of Psychology, Washington University in St Louis, St. Louis, Missouri.
Julia K. Langer, Department of Psychology, Washington University in St Louis, St. Louis, Missouri.
Katya C Fernandez, Department of Psychology, Washington University in St Louis, St. Louis, Missouri.
Renee El-Gabalawy, Department of Psychology and Community Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada.
Elizabeth Y Zhou, Department of Anesthesiology, Washington University in St Louis, St. Louis, Missouri.
Jitender Sareen, Department of Psychology and Community Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada.
Eric Jacobsohn, Department of Anesthesia and Perioperative Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
George A Mashour, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, Michigan.
Michael S Avidan, Department of Anesthesiology and Surgery, Washington University in St Louis, St. Louis, Missouri.
References
- 1.Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–44. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Sebel PS, Bowdle TA, Ghoneim MM, Rampil IJ, Padilla RE, Gan TJ, Domino KB. The incidence of awareness during anesthesia: a multicenter United States study. Anesth Analg. 2004;99:833–9. doi: 10.1213/01.ANE.0000130261.90896.6C. [DOI] [PubMed] [Google Scholar]
- 3.Domino KB, Posner KL, Caplan RA, Cheney FW. Awareness during anesthesia: a closed claims analysis. Anesthesiology. 1999;90:1053–61. doi: 10.1097/00000542-199904000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Lennmarken C, Bildfors K, Enlund G, Samuelsson P, Sandin R. Victims of awareness. Acta Anaesthesiol Scand. 2002;46:229–31. doi: 10.1034/j.1399-6576.2002.t01-1-460301.x. [DOI] [PubMed] [Google Scholar]
- 5.Leslie K, Chan MT, Myles PS, Forbes A, McCulloch TJ. Posttraumatic stress disorder in aware patients from the B-aware trial. Anesth Analg. 2010;110:823–8. doi: 10.1213/ANE.0b013e3181b8b6ca. [DOI] [PubMed] [Google Scholar]
- 6.Osterman JE, Hopper J, Heran WJ, Keane TM, van der Kolk BA. Awareness under anesthesia and the development of posttraumatic stress disorder. Gen Hosp Psychiatry. 2001;23:198–204. doi: 10.1016/s0163-8343(01)00142-6. [DOI] [PubMed] [Google Scholar]
- 7.Ranta SO, Laurila R, Saario J, Ali-Melkkila T, Hynynen M. Awareness with recall during general anesthesia: incidence and risk factors. Anesth Analg. 1998;86:1084–9. doi: 10.1097/00000539-199805000-00035. [DOI] [PubMed] [Google Scholar]
- 8.Samuelsson P, Brudin L, Sandin RH. Late psychological symptoms after awareness among consecutively included surgical patients. Anesthesiology. 2007;106:26–32. doi: 10.1097/00000542-200701000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Schwender D, Kunze-Kronawitter H, Dietrich P, Klasing S, Forst H, Madler C. Conscious awareness during general anaesthesia: patients' perceptions, emotions, cognition and reactions. Br J Anaesth. 1998;80:133–9. doi: 10.1093/bja/80.2.133. [DOI] [PubMed] [Google Scholar]
- 10.Laukkala T, Ranta S, Wennervirta J, Henriksson M, Suominen K, Hynynen M. Long-term psychosocial outcomes after intraoperative awareness with recall. Anesth Analg. 2014;119(1):86–92. doi: 10.1213/ANE.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 11.Moreau C, Zisook S. Rationale for a posttraumatic stress spectrum disorder. Psychiatr Clin North Am. 2002;25:775–90. doi: 10.1016/s0193-953x(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead DL, Perkins-Porras L, Strike PC, Steptoe A. Post-traumatic stress disorder in patients with cardiac disease: predicting vulnerability from emotional responses during admission for acute coronary syndromes. Heart. 2006;92:1225–9. doi: 10.1136/hrt.2005.070946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths J, Fortune G, Barber V, Young JD. The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med. 2007;33:1506–18. doi: 10.1007/s00134-007-0730-z. [DOI] [PubMed] [Google Scholar]
- 14.Jubran A, Lawm G, Duffner LA, Collins EG, Lanuza DM, Hoffman LA, Tobin MJ. Post-traumatic stress disorder after weaning from prolonged mechanical ventilation. Intensive Care Med. 2010;36:2030–7. doi: 10.1007/s00134-010-1972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Reilly SM, Grubb N, O'Carroll RE. Long-term emotional consequences of in-hospital cardiac arrest and myocardial infarction. Br J Clin Psychol. 2004;43:83–95. doi: 10.1348/014466504772812986. [DOI] [PubMed] [Google Scholar]
- 16.Avidan MS, Jacobsohn E, Glick D, Burnside BA, Zhang L, Villafranca A, Karl L, Kamal S, Torres B, O'Connor M, Evers AS, Gradwohl S, Lin N, Palanca BJ, Mashour GA. Prevention of intraoperative awareness in a high-risk surgical population. N Engl J Med. 2011;365:591–600. doi: 10.1056/NEJMoa1100403. [DOI] [PubMed] [Google Scholar]
- 17.Avidan MS, Zhang L, Burnside BA, Finkel KJ, Searleman AC, Selvidge JA, Saager L, Turner MS, Rao S, Bottros M, Hantler C, Jacobsohn E, Evers AS. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097–108. doi: 10.1056/NEJMoa0707361. [DOI] [PubMed] [Google Scholar]
- 18.Mashour GA, Shanks A, Tremper KK, Kheterpal S, Turner CR, Ramachandran SK, Picton P, Schueller C, Morris M, Vandervest JC, Lin N, Avidan MS. Prevention of intraoperative awareness with explicit recall in an unselected surgical population: a randomized comparative effectiveness trial. Anesthesiology. 2012;117:717–25. doi: 10.1097/ALN.0b013e31826904a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brice DD, Hetherington RR, Utting JE. A simple study of awareness and dreaming during anaesthesia. Br J Anaesth. 1970;42:535–42. doi: 10.1093/bja/42.6.535. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996;34:669–73. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. [PubMed] [Google Scholar]
- 22.US Department of Veterans Affairs National Center for PTSD. Using the PTSD Checklist (PCL) 2012 Jul; available at http://www.ptsd.va.gov/professional/pages/assessments/assessment-pdf/pcl-handout.pdf.
- 23.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. J Pers Assess. 1990;55:610–7. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 24.Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44:227–39. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 25.Muthén LK, Muthén BO. Mplus user's guide. Sixth. Los Angeles, CA: Muthén&Muthén; 1998-2010. [Google Scholar]
- 26.Ruscio AM, Ruscio J, Keane TM. The latent structure of posttraumatic stress disorder: A taxometric investigation of reactions to extreme stress. Journal of Abnormal Psychology. 2002;111:290–301. [PubMed] [Google Scholar]
- 27.Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76:408–420. [Google Scholar]
- 28.Mashour GA, Esaki RK, Tremper KK, Glick DB, O'Connor M, Avidan MS. A novel classification instrument for intraoperative awareness events. Anesth Analg. 2010;110:813–5. doi: 10.1213/ANE.0b013e3181b6267d. [DOI] [PubMed] [Google Scholar]
- 29.Hu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 30.Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 31.Grubaugh AL, Magruder KM, Waldrop AE, Elhai JD, Knapp RG, Frueh BC. Subthreshold PTSD in primary care: prevalence, psychiatric disorders, healthcare use, and functional status. J Nerv Ment Dis. 2005;193:658–64. doi: 10.1097/01.nmd.0000180740.02644.ab. [DOI] [PubMed] [Google Scholar]
- 32.Shelby RA, Golden-Kreutz DM, Andersen BL. PTSD diagnoses, subsyndromal symptoms, and comorbidities contribute to impairments for breast cancer survivors. J Trauma Stress. 2008;21:165–72. doi: 10.1002/jts.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornfield SL, Klaus J, McKay C, Helstrom A, Oslin D. Subsyndromal posttraumatic stress disorder symptomatology in primary care military veterans: treatment implications. Psychol Serv. 2012;9:383–9. doi: 10.1037/a0028082. [DOI] [PubMed] [Google Scholar]
- 34.Pietrzak RH, Goldstein MB, Malley JC, Johnson DC, Southwick SM. Subsyndromal posttraumatic stress disorder is associated with health and psychosocial difficulties in veterans of Operations Enduring Freedom and Iraqi Freedom. Depress Anxiety. 2009;26:739–44. doi: 10.1002/da.20574. [DOI] [PubMed] [Google Scholar]
- 35.Pietrzak RH, Schechter CB, Bromet EJ, Katz CL, Reissman DB, Ozbay F, Sharma V, Crane M, Harrison D, Herbert R, Levin SM, Luft BJ, Moline JM, Stellman JM, Udasin IG, Landrigan PJ, Southwick SM. The burden of full and subsyndromal posttraumatic stress disorder among police involved in the World Trade Center rescue and recovery effort. J Psychiatr Res. 2012;46:835–42. doi: 10.1016/j.jpsychires.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Deisseroth K, Hart RA. Symptoms of post-traumatic stress following elective lumbar spinal arthrodesis. Spine (Phila Pa 1976) 2012;37:1628–33. doi: 10.1097/BRS.0b013e318255e214. [DOI] [PubMed] [Google Scholar]
- 37.Zatzick D, Jurkovich G, Rivara FP, Russo J, Wagner A, Wang J, Dunn C, Lord SP, Petrie M, O'Connor SS, Katon W. A randomized stepped care intervention trial targeting posttraumatic stress disorder for surgically hospitalized injury survivors. Ann Surg. 2013;257:390–9. doi: 10.1097/SLA.0b013e31826bc313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 39.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55:626–32. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 41.Goldfinger JZ, Edmondson D, Kronish IM, Fei K, Balakrishnan R, Tuhrim S, Horowitz CR. Correlates of Post-traumatic Stress Disorder in Stroke Survivors. J Stroke Cerebrovasc Dis. 2014 May-Jun;23(5):1099–105. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995 Dec;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 43.Peris A, Bonizzoli M, Iozzelli D, Migliaccio ML, Zagli G, Bacchereti A, Debolini M, Vannini E, Solaro M, Balzi I, Bendoni E, Bacchi I, Trevisan M, Giovannini V, Belloni L. Early intra-intensive care unit psychological intervention promotes recovery from post traumatic stress disorders, anxiety and depression symptoms in critically ill patients. Crit Care. 2011;15:R41. doi: 10.1186/cc10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Document 1: PTSD Checklist – Specific (PCL-S) and explanatory letter sent to participants.
Supplemental Document 2: Modified Mini International Neuropsychiatric Interview telephone script.
Supplemental Figure 1: Definition of posttraumatic stress disorder by DSM-IV criteria. All six lettered criteria (i.e., A-F) must be met for a diagnosis.


