SUMMARY
Chronic itch is a prevalent and debilitating condition for which few effective therapies are available. We harnessed the natural variation across genetically distinct mouse strains to identify transcripts co-regulated with itch behavior. This survey led to the discovery of the serotonin receptor, HTR7, as a key mediator of serotonergic itch. Activation of HTR7 promoted opening of the ion channel TRPA1, which in turn triggered itch behaviors. In addition, acute itch triggered by serotonin or a selective serotonin reuptake inhibitor required both HTR7 and TRPA1. Aberrant serotonin signaling has long been linked to a variety of human chronic itch conditions, including atopic dermatitis. In a mouse model of atopic dermatitis, mice lacking HTR7 or TRPA1 displayed reduced scratching and skin lesion severity. These data highlight a role for HTR7 in acute and chronic itch, and suggest that HTR7 antagonists may be useful for treating a variety of pathological itch conditions.
INTRODUCTION
Chronic itch is a debilitating disorder that results from of a number of skin diseases such as atopic dermatitis, as well as systemic conditions including kidney failure, cirrhosis and some cancers. Although itch from allergy or bug bites is often treatable with antihistamines, most forms of chronic itch are resistant to antihistamine treatment (Mollanazar et al., 2015). Further, an estimated 10–20% of the population will suffer from chronic itch at some point in their lifetime (Matterne et al., 2011; Metz and Stander, 2010). Consequently, understanding the molecular basis of chronic itch is of significant clinical interest. Itch signals are transduced via a subset of primary afferent sensory neurons that innervate the skin. A number of studies have idenitified cells and receptors that transduce acute itch signals (Bautista et al., 2014; Garibyan et al., 2013; Mollanazar et al., 2015; Ross, 2011). However, the molecular mechanisms underlying the development, maintenance, and transmission of chronic itch signals remain largely unknown. Likewise, a number of unanswered questions about acute itch transduction persist, including the peripheral mechanisms by which itch is induced by the pruritogen serotonin.
Peripheral serotonin, or 5-hydroxytryptamine (5-HT), is a component of the “inflammatory soup” and has been identified as a potent inducer of itch (Bautista et al., 2014; Hoon, 2015) and pain (Bardin, 2011; Julius and Basbaum, 2001; Kayser et al., 2007; Zeitz et al., 2002). Indeed, in humans, aberrant 5-HT signaling in the skin is linked to itch in atopic dermatitis (Huang et al., 2004; Soga et al., 2007), as well as in other disorders including allergy (Liu et al., 2013; Lundeberg et al., 1999), uremia (Kerr et al., 1992), cholestasis (Schworer et al., 1995), and psoriasis (Nordlind et al., 2006). Although numerous studies have highlighted the importance of 5-HT in acute and chronic itch (Han et al., 2013; Huang et al., 2004; Imamachi et al., 2009; Liu et al., 2013; Mishra and Hoon, 2013), the 5-HT receptor subtypes that transduce serotonergic itch signals have remained enigmatic.
Here, we examined itch behaviors and sensory neuron gene expression across genetically distinct mouse strains. We observed a correlation between acute itch behavior and expression of the 5-HT receptor, HTR7. This first clue to the function of HTR7 in the periphery served as motivation for an extensive characterization of its role in serotonergic and chronic itch. We found that HTR7 is expressed in a subset of primary afferent sensory neurons that innervate the skin and promote acute itch but not pain behaviors. HTR7 is functionally coupled to the irritant receptor TRPA1, and together they trigger neuronal excitation and mediate acute serotonergic- and SSRI-evoked itch. We also established that HTR7 and TRPA1 are key mediators of chronic itch in a mouse model of atopic dermatitis. Our finding is the first demonstration of a 5-HT receptor that mediates both acute and chronic itch in the periphery.
RESULTS
Examining natural variation in itch behaviors and gene expression
Previous studies have shown that genetically distinct inbred mouse strains display variable somatosensory behaviors such as pain and itch (Green et al., 2006; Nair et al., 2011). We set out to identify transcripts that were co-regulated with itch behaviors across such mouse strains. We first surveyed the effects of a battery of pruritogens in BL6 and DBA mice (Figure 1A). As itch is mediated by both histamine-dependent and -independent itch circuits, we examined a number of pruritogens that target each pathway. Because we sought to compare responses to a single pruritogen across strains, rather than between pruritogens, we chose concentrations for each pruritogen that elicited reliable scratching behaviors in the literature (Green et al., 2006; Kuraishi et al., 1995; Kuraishi et al., 2000; Liu et al., 2012; Wilson et al., 2011; Wilson et al., 2013b). All histamine-independent compounds tested—the anti-malarial drug chloroquine, compound 48/80, and the endogenous pruritogens 5-HT, thymic stromal lymphopoietin (TSLP), and bovine adrenal medulla peptide 8–22 (BAM 8–22)—evoked more avid scratching in the DBA strain than in BL6 (Figure 1A). In contrast, histamine-evoked itch was more avid in the BL6 strain than in DBA (Figure 1A; Green et al., 2006).
Figure 1. A survey of mouse natural variation reveals Htr7 as a candidate itch transducer in DRG neurons.
(A) The mouse strains C57BL/6J and DBA/2J display differential scratching behavior to various pruritogens: TSLP, thymic stromal lymphopoietin (15 nM); 5-HT, 5-hydroxytryptamine (1 mM); BAM, bovine adrenal medulla peptide 8–22 (3.5 mM); CQ, chloroquine (40 mM); 48/80, compound 48/80 (4 mM); HIS, histamine (27 mM). Student’s t-test; *p < 0.05; **p < 0.01; n = 4–8/strain. Error bars represent SEM. At bottom, schematic representation of the BXD recombinant inbred strains from a cross between C57BL/6J and DBA/2J mouse strains. (B) Heatmap visualization of the 20 genes whose expression in dorsal root ganglia (DRG) neurons correlates most highly with itch behavior (time spent scratching, 30 min) across BXD mouse strains; each row reports normalized gene expression from one strain (white, low relative to gene median; dark blue, high) ordered based on CQ itch sensitivity (bottom). Black box highlights Htr7. See also Tables S1, and S2.
To ask if these parental itch phenotypes were heritable, we next assayed itch behaviors in a panel of inbred progeny strains from a cross between BL6 and DBA (Taylor et al., 1973) (BXD; Figure 1A). We were particularly interested in histamine-indepdent itch responses because most forms of chronic itch are histamine-independent. We chose to use the itch response to chloroquine as a representative of all histamine-independent pruritogens, for a number of reasons. First, chloroquine evokes histamine-independent itch in both mice and humans (Green et al., 2006; Liu et al., 2009). Second, the subpopulation of neurons that express the chloroquine receptor MrgprA3 mediate multiple forms of histamine-independent acute and chronic itch, and selective activation of these neurons triggers itch but not pain behaviors (Han et al., 2013). Finally, given that the DBA parental strain exhibited stronger itch behaviors to all non-histaminergic pruritogens tested (even those known to signal via distinct cellular pathways; Figure 1A), we hypothesized that this strain harbors one or more alleles that promote multiple forms of histamine-independent itch, for which chloroquine could serve as a proxy. We observed a wide range of itch responses in progeny strains from the BXD cross upon chloroquine injection (Figure 1B), far beyond the spread attributable to measurement error alone (broad-sense heritability = 0.65), reflecting a quantitative relationship between genetic background and this complex somatosensory behavior.
We considered that the DNA variation across strains that drove itch behaviors would also perturb expression of many genes, including some involved in distinct itch pathways. This expectation was based on the principle that a naturally occurring DNA sequence variant underlying a given phenotype (e.g., disease or behavior) often affects the expression of hundreds of genes of related but distinct function (Schadt, 2005; Cookson et al, 2009). Indeed, it is axiomatic that genes of many subtypes, though of broadly similar biological function, can be co-regulated transcriptionally. For example, nutrient restriction upregulates genes that are protective for starvation as well as those dispensable for this behavior but required for other related stress responses (Giaever and Nislow, 2014). We thus hypothesized that there could be transducers in any of a multitude of itch pathways among the transcripts co-regulated with chloroquine itch across mouse strains. To identify such transcripts, we transcriptionally profiled dorsal root ganglia (DRG), which contain the neurons responsible for detecting itch, touch and pain stimuli, in each BXD progeny strain. We identified 72 genes whose expression correlated positively across strains with itch behavior, and 37 negatively correlated with itch (|r| > 0.55; Figure 1B and Table S1); this set of transcriptional correlates of itch was enriched in a number of somatosensory and cell signaling categories, including 5-HT signaling (Table S2).
HTR7 is a candidate itch transducer
Among the top transcriptional correlates of chloroquine itch, we noted two 5-HT receptors, HTR7 and HTR1d. 5-HT has long been linked to chronic itch and pain (Bardin, 2011; Bautista et al., 2014; Hoon, 2015; Julius and Basbaum, 2001; Zeitz et al., 2002), and somatosensory neurons express ten distinct subtypes of 5-HT receptors (Bardin, 2011; Manteniotis et al., 2013) with the exact role of each subtype in somatosensation yet to be defined. We thus singled out for functional characterization Htr7, the receptor whose expression was most highly correlated with itch behavior (Figure 1B). Htr7 expression was 2-fold higher in the most sensitive progeny strain than in the least responsive progeny (Figure 2A), and 1.3-fold higher in the more itch-prone DBA parental strain than in BL6 (BL6 = 1031.67 ± 78.19 reads, DBA = 1305.00 ± 18.19 reads; p < 0.05; n = 3 mice/strain).
Figure 2. HTR7 is expressed in sensory neurons that innervate the skin.
(A) Htr7 expression in DRG neurons correlates strongly with CQ itch across BXD strains. Each point represents one BXD strain. (B) PCR amplification of Htr7 transcripts shows expression in mouse and human DRG. (C) Left, in situ hybridization of an Htr7 probe in mouse DRG. Scale bar, 100 μm. Middle, a higher magnification of the region encased by the yellow box in the left panel. Htr7-positive cell bodies are highlighted in yellow, Htr7-negative cell bodies in white. Scale bar, 50 μm. Right, Htr7 is expressed mostly in small diameter cells (n = 5 sections, 642 cells). (D) Immunostaining of hairy skin shows expression of HTR7 in a subset of TRPA1-positive sensory neuronal fibers. Left, Immunostaining of hairy skin with antibodies against HTR7 and TRPA1, counter stained with DAPI. Right, a higher magnification of region encased by the yellow box in the left panel. Arrowheads mark HTR7- and TRPA1-positive neuronal fibers, the arrow marks an HTR7-negative, TRPA1-positive neuronal fiber. Dotted line demarcates dermal-epidermal boundary based on DAPI staining. Scale bar, 10 μm. (E) Left, Retrogradely labeled cutaneous afferents with cholera toxin subunit B (CTB) were cultured and LP44 (100 μM) responses were assayed using calcium imaging. Scale bar, 100 μm. Right, LP44 promotes calcium influx in labeled cutaneous afferent 1, but not 2. (F) LP44 does not activate primary mouse keratinocytes; quantification of peak LP44-evoked Ca2+ responses in mouse primary keratinocytes to vehicle (VEH), LP44 and thapsigargin (THAP). One-way ANOVA, Tukey-Kramer post hoc test; ns, p ≥ 0.05; ***p < 0.001. Error bars represent SEM. See also Figure S1.
HTR7 is expressed and functional in sensory neurons that innervate the skin
Somatosensory ganglia contain an array of neurons that mediate itch, touch and pain. We expected that, if HTR7 played a role in itch transduction, we should observe expression in a subset of the small diameter DRG neurons that detect noxious stimuli and innervate the skin. In validation of our RNA-seq measurements, we detected Htr7 transcripts in human and mouse DRG with RT-PCR (Figure 2B). Using in situ hybridization, we detected the Htr7 transcript in 11.2% of DRG neurons, the majority of which were small-diameter neurons (average size = 17.7 μm; Figure 2C). Next, we probed hairy skin using antibodies against HTR7 and TRPA1, and found that HTR7 protein was expressed in 23.6 ± 6.9% of TRPA1-positive fibers that innervate the skin (Figure 2D). Although we also observed HTR7 immunoreactivity in the epidermis, such staining was non-specific, as it manifested in both wild-type and HTR7 knockout mice (Figure S1A). We next used ratiometric calcium imaging to investigate whether HTR7 was functional in cutaneous primary afferent sensory neurons. We first labeled cutaneous afferents via intradermal injection of the retrograde tracer cholera toxin-B-594 (CTB-594) and cultured dissociated sensory neurons 24 hours post-injection. Application of the HTR7-selective agonist, 4-[2-(Methylthio)phenyl]-N-(1,2,3,4-tetrahydro-1-naphthalenyl)-1-iperazinehexanamide hydrochloride (LP44), to cultured DRG neurons triggered a rise in intracellular calcium in 8.3 ± 0.76% of labeled cutaneous afferents (37.9± 5.2% of all neurons were labeled red), suggesting that HTR7 is functional in neurons that innervate the skin (Figure 2E).
The LP44-evoked neuronal response was dose-dependent with an EC50 at 85.7 ± 3.0 μM (Figure S1B). In contrast, LP44 failed to elicit a rise in intracellular calcium in primary mouse keratinocytes (Figure 2F), suggesting that HTR7 is not functional in keratinocytes. Overall, these data show that HTR7 is expressed and functional in a subset of small-diameter sensory neurons that innervate the skin and mediate itch and/or pain.
Previous studies have shown that neurons that transduce itch and pain signal via the TRPA1 and TRPV1 ion channels (Imamachi et al., 2009; Karai et al., 2004; Mishra and Hoon, 2013; Wilson et al., 2011; Wilson et al., 2013a). We found that all LP44-sensitive neurons were responsive to the TRPA1 agonist allyl isothiocyanate (AITC), and to the TRPV1 agonist capsaicin (Figure 3A–3C). LP44-positive neurons were also sensitive to other pruritogens, including 5-HT (100%), chloroquine (63.6%), histamine (27.3%), and BAM8-22 (13.6%), but not TSLP (Figure 3C). These data show that HTR7 is functionally expressed in a heterogeneous subpopulation of serotonergic neurons that may subserve distinct roles in itch or inflammation. LP44 also evoked membrane depolarization and action potential firing in DRG neurons (Figure 3D). Thus, HTR7 activation induces neuronal excitability in a subset of TRPV1- and TRPA1-positive neurons. To determine whether HTR7, TRPA1, and TRPV1 are required for LP44-evoked excitation, we evaluated responses in neurons isolated from mice lacking these receptors. LP44-evoked calcium responses were significantly attenuated in DRG neurons isolated from Htr7−/− and Trpa1−/− mice, but not in Trpv1−/− neurons (Figure 3E). Similar results were observed in trigeminal ganglia (TG; data not shown). These results suggest that HTR7 is functionally coupled to TRPA1, and that the two receptors work together to mediate LP44-evoked excitation in a subset of itch and/or pain neurons.
Figure 3. HTR7 signals through Gβγ, adenylate cyclase, and TRPA1 to promote neuronal excitation.
(A) Fura-2 loaded DRG neurons treated with LP44 (100 μM) and KCl (75 mM). Arrowhead depicts an LP44-responsive neuron. Pseudocolor bar represents Fura-2 ratio. (B) Representative trace shows a neuron that responds to LP44 (100 μM), 5-HT (100 μM), and allyl isothiocyanate (AITC; 100 μM). (C) Left, LP44-responsive neurons are all sensitive to 5-HT (100 μM), AITC (100 μM), and capsaicin (1 μM). Right, the majority of these neurons overlap with the population of chloroquine-sensitive neurons (64%), and smaller subsets overlap with chloroquine/BAM8-22-sensitive (6%) or histamine-sensitive neurons (27%). No overlap is observed in the CQ-insensitive, TSLP-positive population. (D) Current-clamp recording showing action potential firing evoked by LP44 (100 μM) in a representative DRG neuron. Inset, single action potential. (E) Percentage of calcium responders to LP44 (100 μM) is reduced in neurons from Htr7−/− and Trpa1−/− mice, but not Trpv1−/− mice, relative to wild-type (WT). (F) Percentage of calcium responders to LP44 (100 μM) is reduced in neurons pretreated with the adenylate cyclase blocker 2′,3′-dideoxyadenosine (DDA, 50 μM) or the Gβγ blocker gallein (GAL, 100 μM), but not the PLC blocker, U73122 (U7, 1 μM), relative to vehicle treatment (VEH). One-way ANOVA, Tukey-Kramer post hoc test; ns, p ≥ 0.05; **p < 0.01; n = 3 mice/genotype or treatment, n ≥ 200 cells/genotype or treatment. (G) An inward current is evoked by LP44 (100 μM) in HEK293 cells transfected with HTR7 and TRPA1 but not with HTR7 alone using voltage clamp recording. (H) Representative current-voltage trace of HEK293 cell transfected with HTR7 and TRPA1 in the absence (background, BKG) or presence of LP44 (100 μM) or AITC (100 μM). (I) HTR7 and TRPA1 are both required for LP44-evoked currents in transfected HEK cells; Quantification of LP44-induced peak currents (normalized to vehicle treatment) in HEK293 cells transfected as in panel (G). Each point represents one cell. Fisher’s exact test; *p < 0.05; n = 16 cells/transfection. (J) HTR7 and TRPA1 are both required for LP44-evoked calcium influx in transfected HEK cells; Quantification of peak LP44-evoked Ca2+ response in HEK293 cells transfected with pcDNA3 (CON), HTR7 and/or TRPA1. (K) HTR7 and TRPA1 are both required for 5-HT-evoked calcium influx in transfected HEK cells; Quantification of peak 5-HT-evoked (100 μM) Ca2+ response in HEK293 cells transfected with pcDNA3 (CON), HTR7 and/or TRPA1. One-way ANOVA, Tukey-Kramer post hoc test; ns, p ≥ 0.05; **p < 0.01; n = 3–5 transfections. Error bars represent SEM.
HTR7 activates TRPA1 via adenylate cyclase signaling
HTR7 is a Gs-coupled GPCR that activates adenylate cyclase (AC) via both Gα and Gβγ in a variety of cell types (Adham et al., 1998; Lovenberg et al., 1993; Tang and Gilman, 1991). Previous studies have also shown that both AC and Gβγ signaling activate the ion channel TRPA1 (Schmidt et al., 2009; Wilson et al., 2011). In sensory neurons, we found that inhibition of AC and Gβγ signaling with 2′,3′-dideoxyadenosine and gallein, respectively, attenuated LP44-evoked calcium signals, whereas U-73122, an inhibitor of PLC signaling, had no effect (Figure 3F). We next asked whether HTR7 could activate heterologous TRPA1 channels expressed in HEK293 cells. LP44-evoked currents were observed in HEK293 cells transfected with both hHTR7 and hTRPA1, but not in cells expressing hHTR7 alone (Figure 3G – 3I); AITC triggered a similar current of larger magnitude in cells expressing both hHTR7 and hTRPA1 (Figure 3G and 3H). Likewise, LP44 evoked significant increases in intracellular calcium in HEK293 cells expressing both HTR7 and TRPA1, but not in cells expressing either receptor alone (Figure 3J). HTR7 and TRPA1 together, but neither alone, also conferred 5-HT sensitivity to HEK293 cells (Figure 3K). Thus, HTR7 and TRPA1 receptors are functionally coupled and mediate response to both LP44 and 5-HT.
HTR7 activation causes itch but not pain
Somatosensory neurons are a diverse population that mediate acute and chronic itch and pain (Bautista et al., 2014; Ross, 2011). Likewise, 5-HT signaling in the somatosensory system has been linked to both itch and pain. Previous reports have investigated the role of HTR7 in pain behaviors with mixed results: several studies have found no role for HTR7 in promoting acute pain (Nascimento et al., 2011; Roberts et al., 2004), others found that systemic application of an HTR7 agonist had a modest attenuating effect on inflammatory pain (Brenchat et al., 2012; Brenchat et al., 2009), and most recently, injection of an HTR7 agonist into the anterior cingulate cortex attenuated mechanical hypersensitivity (Santello and Nevian, 2015). To investigate whether specific activation of peripheral HTR7 by LP44 triggered itch and/or pain behaviors, we used the mouse cheek model of itch, in which pruritic agents elicit scratching with the hindlimb, and nociceptive agents cause wiping with the forepaw (LaMotte et al., 2014). Injection of LP44 into the cheek of wild-type mice evoked scratching behaviors that were not observed following vehicle injection (Figure 4A). HTR7 is the sole mediator of LP44-evoked scratching as pharmacological inhibition (SB-269970; Figure 4A, gray) or genetic ablation (Figure 4B, red) of HTR7 significantly attenuated LP44-evoked scratching to levels similar to vehicle. We also observed significantly more scratching in the DBA mouse strain than in BL6 (Figure S1C), mirroring the higher Htr7 expression in DBA sensory ganglia. Given the requirement of TRPA1 for LP44-evoked signaling in neurons (Figure 3E), we hypothesized that TRPA1 would likewise be required for LP44-evoked behaviors. Indeed, animals lacking Trpa1, but not Trpv1, exhibited attenuated LP44-evoked scratching (Figure 4C and D). Echoing our finding of differences between DBA and BL6 mice, we observed some variation in LP44 response across distinct wild-type strains (compare wild-type mice in Figure 4B–D); analysis of any one mutant strain was thus carried out with respect to its isogenic wild-type. Htr7 and Trpa1 were required for LP44-evoked itch in both the cheek and neck itch models in line with the requirement of these receptors in LP44-evoked excitability in both TG and DRG neurons. Taken together, these data show that both HTR7 and TRPA1 are required for LP44-evoked itch behaviors.
Figure 4. HTR7 triggers robust acute itch behaviors, but not pain.
(A) LP44 (2 mM), but not vehicle (VEH; 20 μL, 2% DMSO in PBS) injection induces scratching behaviors in the cheek model of itch. This response is blocked by the pre-injection of HTR7 antagonist SB-269970 (SB, 5 mM). (B) Scratching evoked by LP44 injection is attenuated in Htr7−/− mice relative to Htr7+/+ wild-type mice (WT). (C) Scratching evoked by LP44 injection is attenuated in Trpa1−/− mice relative to Trpa1+/+ wild-type mice (WT). (D) LP44-evoked itch behavior is similar in Trpv1+/+ (WT) and Trpv1−/− mice. (E) TRPV1-positive neuronal ablation by resiniferatoxin (RTX) eliminates LP44-evoked scratching. (F) LP44-evoked itch behaviors are similar in mast cell deficient mice (KitW-sh) and wild-type control mice (WT). (G) LP44-evoked itch behaviors are similar in K14-Cre;Trpa1fl/fl mice and Trpa1fl/fl control mice. (H) LP44 (2 mM) does not evoke wiping behavior, while 1 mM 5-HT causes robust wiping. VEH, vehicle (I) Thermal hypersensitivity in mice injected with 5-HT (10 μM) and LP44 (2 mM). One-way ANOVA, Tukey-Kramer post hoc test; ns, p ≥ 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; n = 5–9 mice/genotype/behavior. Error bars represent SEM. See also Figure S1.
Does LP44 act on HTR7 and TRPA1 receptors that are expressed on sensory neurons, or on-non-neuronal cells in the skin to induce itch behaviors? We first asked if sensory are required for LP44-evoked itch. To address this question we used the TRPV1 agonist, resiniferatoxin (RTX), which when injected results in defunctionalization of TRPV1- and TRPV1/TRPA1-positive neurons (Karai et al., 2004; Mishra and Hoon, 2013; Wilson et al., 2013a). LP44- evoked scratching was significantly decreased in RTX-treated mice, to levels observed in vehicle-treated mice (Figure 4E). These findings show that LP44 induces itch via TRPV1+ sensory neurons, but do not reveal the site of transduction. Previous studies suggest that HTR7 and TRPA1 may be expressed in mast cells (Idzko et al., 2004; Oh et al., 2013), which release a variety of compounds that can activate sensory neurons and trigger itch. Thus, we next asked if HTR7-evoked itch is mast cell-dependent. We compared HTR7-evoked itch behaviors in a mouse strain lacking mast cells (KitW-sh) to wild-type controls (Figure 4F). LP44 triggered considerable scratching in both strains, suggesting that HTR7-mediated responses do not require mast cells, or pruritogens/cytokines released by mast cells, to trigger itch behaviors. TRPA1 has also been proposed to be expressed in keratinocytes; though whether it is expressed and functional in mouse keratinocytes remains to be determined. However, LP44-evoked itch behaviors in K14-Cre;Trpa1fl/fl mice and Trpa1fl/fl mice were indistinguishable (Figure 4G), indicating that TRPA1 in keratinocytes does not underlie this phenotype; these findings are consistent with our observation that keratinocytes exhibited no calcium response to LP44 (Figure 2F). Overall these data show that neither mast cells nor TRPA1-mediated signaling in keratinocytes are required for acute itch triggered by HTR7 activation, and suggest that HTR7 in sensory neurons may mediate LP44-evoked itch.
We next sought to establish whether activation of HTR7 could elicit pain. Unlike 5-HT, which has been shown to trigger both itch-evoked scratching and nocifensive wiping behaviors (Akiyama et al., 2010), we observed no wiping in LP44-injected animals (Figure 4H). Likewise, injection of LP44 into the hindpaw did not cause thermal hypersensitivity, in stark contrast to 5-HT injection, which induced a significant decrease in the thermal withdrawal latency (Figure 4I). Overall, these data make clear that acute activation of HTR7 by LP44 evokes itch but not pain behaviors.
HTR7 mediates serotonergic itch
We next set out to evaluate the role of HTR7 in serotonergic itch. Most studies measuring 5-HT–evoked itch and pain behaviors have used relatively high doses (1–94 mM; Akiyama et al., 2010; Kuraishi et al., 2000; Nojima and Carstens, 2003; Zeitz et al., 2002). Yet the elevated 5-HT levels in chronic itch patients and in mouse models have been measured to be in the micromolar range (Kerr et al., 1992; Liu et al., 2013; Lundeberg et al., 1999; Soga et al., 2007). To test for distinctions between these two 5-HT dose regimes, we evaluated the behavioral effects of each in mice. Upon injection of 1 mM 5-HT in wild-type animals, we observed both scratching and wiping behaviors (Figure 5A and 5B), consistent with previous reports (Akiyama et al., 2010; Moser and Giesler, 2014). By contrast, injection of 100 μM 5-HT in wild-type mice elicited robust scratching but little wiping (Figure 5A and 5B). Together, these data show that 5-HT has distinct physiological effects at different concentrations, and that 100 μM 5-HT preferentially activates itch pathways. HTR7 does not mediate behavioral responses to high 5-HT levels, as 1mM 5-HT evoked itch and pain behaviors in Htr7−/− mice indistinguishable from those of wild-type animals (Figure 5C, red). However, the itch-specific effects of 100μM 5-HT were mediated by HTR7: Htr7−/− animals exhibited a significant decrease in itch behavior, to levels on par with vehicle injection (Figure 5D, red versus white). Given that both HTR7 and TRPA1 were required for LP44-evoked signaling in neurons and HEK293 cells (Figure 3E and 3J–K), we hypothesized that TRPA1 would likewise be necessary for serotonergic itch. Indeed, Trpa1−/− animals displayed significantly decreased itch behaviors as compared to wild type littermates, in response to 100 μM 5-HT (Figure 5E, blue).
Figure 5. HTR7 is required for the detection of 5-HT dependent acute itch.
(A) 5-HT (100 μM and 1 mM) but not vehicle injection (VEH; 20 μL, PBS) causes robust scratching in the cheek model of itch. (B) Injection of 1 mM, but not 100 μM 5-HT evokes wiping behaviors. (C) Scratching behaviors evoked by 1 mM 5-HT are similar between Htr7+/+ (WT) and Htr7−/− mice. (D) Scratching behaviors evoked by 100 μM 5-HT are significantly attenuated in Htr7−/− versus Htr7+/+ (WT) mice. VEH; 20 μL, PBS. (E) Scratching behaviors evoked by 100 μM 5-HT are reduced in Trpa1−/− compared to Trpa1+/+ (WT) mice. (F) Skin 5-HT levels increase after injection with the selective serotonin reuptake inhibitor (SSRI), sertraline (100 μM). One-way ANOVA, ***p < 0.001 (G) Time course of cumulative scratching behavior to sertraline (100 μM) follows the time-dependent increase in skin 5-HT levels following sertraline injection, as shown in (F). (H) Htr7−/− and Trpa1−/− mice show significant decreases in scratching behaviors compared to wild-type (WT) mice following sertraline injection (100 μM). (I) Chloroquine (CQ, 40 mM), compound 48/80 (48/80, 4 mM), and histamine (HIS, 27 mM) injection evokes similar itch behaviors in both Htr7+/+ (WT) and Htr7−/− mice. (J) There is no difference in acute heat sensitivity between Htr7+/+ (WT) and Htr7−/− mice. (K) AITC application (10% in mineral oil) evokes similar nocifensive behaviors in Htr7+/+ (WT) and Htr7−/− mice. One-way ANOVA, Tukey-Kramer post hoc test; ns, p ≥ 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; n = 5–9 mice/genotype/behavior. Error bars represent SEM.
Previous studies have shown that SSRIs increase 5-HT levels (Blardi et al., 2002) and that elevated 5-HT levels promote itch sensations and scratching in humans (Cederberg et al., 2004; Rausl et al., 2013). Some studies have shown that SSRIs can alleviate certain forms of chronic itch and pain (Anjaneyulu and Chopra, 2004; Browning et al., 2003; Zylicz et al., 2003), while others have reported that SSRIs can induce severe itch and rash (Cederberg et al., 2004; Sannicandro et al., 2002; Warnock and Morris, 2002); FDA, 2014). We thus asked if intradermal injection of SSRIs increased local 5-HT levels to trigger itch behaviors in mice. Injection of the SSRI sertraline induced a rapid increase in skin 5-HT (Figure 5F) and induced itch-evoked scratching in wild-type animals (Figure 5G). Mirroring the results of 100μM 5-HT injection (Figure 5D and E), we observed a significant attenuation of SSRI-evoked scratching in Htr7−/− and Trpa1−/− mice as compared to wild type littermates (Figure 5H; no significant differences in behavior were observed between Htr7+/+ and Trpa1+/+ mice). Thus, HTR7 and TRPA1 together mediate some forms of serotonergic and SSRI-mediated itch.
We also examined the contribution of HTR7 to itch behaviors to a number of non-serotonergic pruritogens. Histamine, compound 48/80 and chloroquine all induced comparable itch behaviors in Htr7−/− and wild-type mice (Figure 5I). Htr7−/− mice also exhibited normal radiant heat- and AITC-evoked pain behaviors (Figure 5J and 5K). Overall, our findings make clear that HTR7 is a mediator of serotonergic itch, but not other forms of itch or pain.
HTR7 and TRPA1 are required for the development of chronic itch
Altered 5-HT signaling is associated with a variety of chronic itch conditions in humans, including atopic dermatitis (Huang et al., 2004; Soga et al., 2007). We next asked whether HTR7 plays a role in chronic itch in vivo, using a mouse model of atopic dermatitis. Previous studies have shown that topical treatment with a vitamin D analog, MC903, induces an atopic dermatitis-like phenotype that includes secretion of the atopic dermatitis cytokine, TSLP, immune cell infiltration, skin hyperplasia, and the development of eczematous lesions (Li et al., 2006). However, several additional hallmarks of human atopic dermatitis (AD), including high 5-HT levels in the skin and intense itch-evoked scratching, have yet to be quantified in this mouse model. To further validate this mouse model of human atopic dermatitis, we used a treatment course of 7 consecutive days of topical application of MC903 to the mouse cheek, and monitored itch behaviors for a total of 12 days; we also measured 5-HT levels and skin lesion severity immediately following the final treatment.
Consistent with previous reports (Li et al., 2006), we found that wild-type mice treated with MC903 developed eczematous-like lesions that worsened over time in redness (erythema), dryness (xerosis) and scabbing (excoriation; Figure 6A and 6B). As is true for human atopic dermatitis patients, we found that MC903-treated mice displayed high levels of 5-HT in eczematous skin (Figure 6C), concomitant with scratching behaviors that began on the third day of treatment and increased in intensity, even after the seven-day treatment period (Figure 6D). These data show for the first time that the itch behaviors and 5-HT levels associated with human atopic dermatitis are recapitulated in this atopic dermatitis model.
Figure 6. HTR7 is required for the development of chronic itch in a model of atopic dermatitis.
(A) Representative images of skin isolated from wild-type (WT), Htr7−/−, and Trpa1−/− mice treated topically with vehicle control (CON; 20 μl 100% ethanol) or the vitamin D analog, MC903 for the induction of atopic dermatitis (AD; 200 μM). (B) Atopic dermatitis lesion severity scores in mice treated with MC903 or vehicle (as described in panel A). (C) The AD model triggers and increases in the concentration of 5-HT treated skin isolated from WT, Htr7−/−, and Trpa1−/− mice. One-way ANOVA, Tukey-Kramer post hoc test; ns, p ≥ 0.05; *p < 0.05; **p < 0.01; ***p < 0.001; n = 5/genotype/treatment. (D) Scratching behavior during and after VITD treatment is attenuated in Htr7−/− and Trpa1−/− mice. ANOVA for multivariate linear models; ***p < 0.001; n = 10/genotype. Error bars represent SEM.
Htr7−/− mice treated with MC903 exhibited less severe lesions and scratched significantly less than the wild-type strain across the entire 12-day experiment (Figure 6B and 6D; red). Consistent with the requirement for TRPA1 in HTR7-evoked acute itch (Figure 3H), lesion severity and scratching were also significantly reduced in Trpa1−/− mice (Figure 6B and 6D; blue). No significant differences in itch behavior or lesion severity were observed between Htr7+/+ and Trpa1+/+ wild-type animals of each strain and thus we combined all data for comparison to knockout animals. Previous studies have implicated HTR7 in immune cell signaling (Idzko et al., 2004) which may contribute to 5-HT release in the skin; in the AD model, we found that 5-HT levels in eczematous skin isolated from Htr7−/− and Trpa1−/− mice were indistinguishable from those of wild-type mice (Figure 6C, red). Thus, HTR7 and TRPA1 are not required for the release of 5-HT in atopic dermatitis skin. Taken together, our data identify HTR7 and TRPA1 as key molecular determinants of atopic dermatitis (Figure 7).
Figure 7. Schematic diagram depicting the putative roles of HTR7 and TRPA1 signaling in acute and chronic itch.
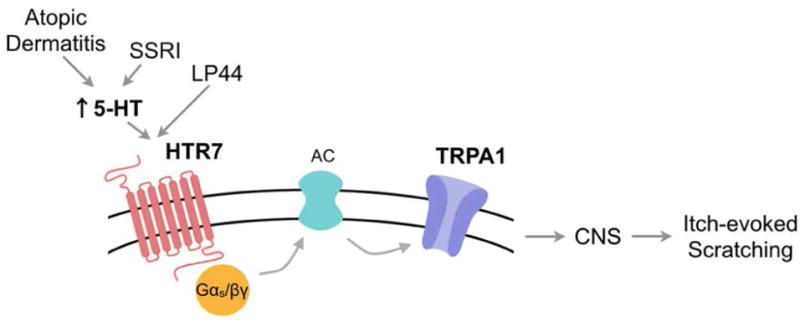
Model by which increased 5-HT levels in the skin, triggered by direct injection of 5-HT, SSRI administration or atopic dermatitis activates the 5-HT receptor, HTR7. HTR7 in turn signals to adenylate cyclase via Gα and Gβγ, to open TRPA1 ion channels, neuronal depolarization, action potential firing and ultimately trigger itch-evoked scratching.
DISCUSSION
In this work, we have taken advantage of natural variation in itch behavior in mice and gene expression to identify novel molecular determinants of itch in primary afferent sensory neurons. Many of the genes that we have found to be co-regulated with itch behavior have already been implicated in somatosensation, including Htr1d (Ahn and Basbaum, 2006; Tepper et al., 2002), Scn8a (Xie et al., 2013), and Ano1 (Cho et al., 2012). However, a number of novel genes were also identified, many of which may represent new candidate itch transducers. We focused on the 5-HT receptor, HTR7, and established its role as a key transducer of serotonergic acute and chronic itch.
In the periphery, 5-HT can act as an algogen to trigger acute pain or pain hypersensitivity (Bardin, 2011; Julius and Basbaum, 2001; Kayser et al., 2007; Zeitz et al., 2002), a pruritogen to trigger itch-evoked scratching, rash or wheal (Imamachi et al., 2009; Kalz and Fekete, 1960; Nojima and Carstens, 2003), or in some cases, both itch and pain (Akiyama et al., 2010; Moser and Giesler, 2014). A variety of 5-HT receptor subtypes are expressed in primary afferent sensory neurons, with HTR1-3 being the best studied. Pharmacological and genetic ablation studies have shown that HTR1a, HTR1b, HTR2a, HTR2b and HTR3a mediate some forms of acute and inflammatory pain transmission (Abbott et al., 1996; Kayser et al., 2007; Lin et al., 2011; Nojima and Carstens, 2003; Van Steenwinckel et al., 2008; Zeitz et al., 2002). Pharmacological studies have also implicated peripheral HTR2a, HTR2b and HTR3 receptors, and central HTR1a receptors in itch (Bonnet et al., 2008; Imamachi et al., 2009; Kyriakides et al., 1999; Liu et al., 2013; Zhao et al., 2014), but to date there has been no genetic evidence for any 5-HT receptor in transducing serotonergic itch signals in primary afferent neurons. We have used genetic and pharmacological tools to show that HTR7 plays a selective role in acute serotonergic and atopic dermatitis itch, but not pain. Our results augment the previous characterization of HTR7 in the CNS in circadian rhythm function (Glass et al., 2003; Lovenberg et al., 1993), central thermoregulation (Hedlund et al., 2003), neuroendocrine function (Jorgensen, 2007; Kim et al., 2013), migraine (Hedlund, 2009) and antinociception (Bardin, 2011; Santello and Nevian, 2015).
HTR7 mediates acute serotonergic itch
Our data implicate HTR7 as a key component of the 5-HT itch pathway. In designing methods to assay 5-HT-evoked itch, we found that different concentrations of 5-HT evoked distinct behaviors. Micromolar 5-HT concentrations triggered itch but not pain behaviors, while millimolar concentrations triggered both itch and pain. Most experimental analyses of 5-HT-evoked behavior have used millimolar concentrations (1–94 mM; Akiyama et al., 2010; Kuraishi et al., 2000; Nojima and Carstens, 2003; Zeitz et al., 2002), which are significantly higher than the 5-HT levels measured in chronic itch conditions (Kerr et al., 1992; Liu et al., 2013; Lundeberg et al., 1999; Soga et al., 2007) and thus may not target physiologically relevant sites and 5-HT receptor subtypes. Our results make clear that HTR7 is required for itch behaviors evoked by 5-HT at 100μM, but not for itch and pain at 1mM concentrations. These findings suggest that HTR7 may be preferentially activated by low 5-HT levels, which dovetail with studies showing that HTR7 has the highest affinity for 5-HT among HTR family members (Vanhoenacker et al., 2000). A role for HTR7 in only a subset of 5-HT-evoked behaviors under particular conditions, rather than all 5-HT responses, is not surprising given that many 5-HT receptors are expressed in sensory neurons (Bardin, 2011; Manteniotis et al., 2013) and that HTR7 is expressed in only ~35% of the 5-HT-positive neuronal population. The emerging picture is thus one of specific physiological responses to different doses of 5-HT that are mediated by distinct molecular and cellular mechanisms, with HTR7 mediating itch at 5-HT levels comparable to those measured in chronic itch skin.
HTR7 mediates acute SSRI-evoked itch
In analyzing the role of HTR7 in serotonergic itch, we also examined itch behaviors in response to injection of the SSRI sertraline in the cheek model of itch. SSRI induced an increase in local 5-HT levels and a concomitant increase in scratching behaviors in mice; HTR7 was required for SSRI-evoked itch behaviors. An estimated 2–4% of human patients report itch, rash and other related skin conditions as a side effect of taking SSRIs (Cederberg et al., 2004; Sannicandro et al., 2002); FDA, 2014). As HTR7 is also expressed in human primary afferent neurons (Figure 2B), it is tempting to speculate that antagonists may attenuate SSRI-evoked itch in humans. Paradoxically, SSRIs can also partially alleviate some forms of chronic itch (Anjaneyulu and Chopra, 2004; Browning et al., 2003; Zylicz et al., 2003). The mechanisms underlying this dual role for SSRIs in alleviating and causing itch in humans are unknown; it will be interesting to determine whether they involve different 5-HT signaling molecules between normal and chronic itch patients, or differential targeting of peripheral versus central targets (Browning et al., 2003).
HTR7 couples to the irritant receptor TRPA1
Previous studies showed that serotonergic itch is mediated by a subset of TRPV1-expressing neurons, though functional TRPV1 ion channels were dispensable for itch behaviors (Imamachi et al., 2009). Thus the receptors and downstream signaling pathways responsible for 5-HT itch transduction were unknown. Our results establish TRPA1 as the transduction channel required for HTR7-mediated acute serotonergic and SSRI-evoked itch behaviors, echoing the known role for TRPA1 in other forms of non-histaminergic itch (Cevikbas et al., 2014; Liu et al., 2013; Oh et al., 2013; Wilson et al., 2011; Wilson et al., 2013a; Wilson et al., 2013b). Furthermore, we found that HTR7 activation of TRPA1 required functional adenylate cyclase (Figure 3), consistent with previous studies showing that HTR7 is a Gαs-coupled GPCR that stimulates adenylate cyclase and cAMP production and that TRPA1 activity is regulated by AC and cAMP in sensory neurons (Schmidt et al., 2009). Interestingly, TRPA1 does not couple to all HTRs, as activation of HTR2 by α-methyl 5-HT triggers itch and pain behaviors independent of TRPA1 (Liu et al., 2013; Schmidt et al., 2009; Wilson et al., 2011). Overall, our data further highlight the importance of TRPA1 in mediating non-histaminergic itch.
Do HTR7 and TRPA1 receptors act in neurons to mediate itch behaviors?
HTR7 and TRPA1 expression have been reported in a variety of neurons in the central nervous system and non-neuronal cells in the skin (Bayer et al., 2007; Idzko et al., 2004; Kwan et al., 2009). Our study provides several lines of evidence that HTR7 in somatosensory neurons mediates itch behaviors. First, we found that HTR7 activation in mast cell-deficient mice evokes a normal itch response. Second, primary mouse and human keratinocytes do not express functional HTR7 receptors. Third, K14-Cre;Trpa1fl/fl mice and Trpa1fl/fl mice displayed similar HTR7-evoked itch responses. Fourth, neuronal ablation of TRPV1-expressing neurons resulted in a dramatic decrease in itch response evoked by HTR7 activation. Finally, HTR7 functions in the subset of the MrgprA3 population of neurons (Figure 2) that have proven to be indispensable in itch but not pain (Han et al., 2013). However, 5-HT is well known to play important roles in modulating itch and pain signal transmission at multiple levels in the CNS. Indeed, pharmacological activation of HTR7 in the forebrain has been recently shown to reduce neuropathic mechanical pain (Santello and Nevian, 2015). Also, 5-HT acts on a variety of immune and other non-neuronal cells to modulate itch and inflammation (Idzko et al., 2004; Soga et al., 2007). Thus, our findings using the global HTR7 knockout do not exclude a role for HTR7 outside primary afferent neurons. Future studies using tissue-specific knockout mice will determine the contributions of sensory neurons and other cell types to acute and chronic itch.
HTR7 and TRPA1 are both required for development of atopic dermatitis
In addition to our study of acute serotonergic itch, we also examined the role of HTR7 and TRPA1 in a mouse model of atopic dermatitis, motivated by the well-known links between 5-HT and itch in this condition (Huang et al., 2004; Soga et al., 2007). Our results revealed for first time that the MC903 induced mouse model of atopic dermatitis recapitulates the dysregulation of 5-HT in the skin, and the itch behaviors, characteristic of the human disorder (Huang et al., 2004). In addition, our mouse model is the first in which spontaneous itch behaviors manifest in the absence of a daily skin treatment: we observed itch behaviors that persisted five days after the last skin treatment, establishing this paradigm as a model of chronic rather than acute itch. We found that HTR7- and TRPA1-deficient mice both displayed less severe skin lesions and scratched significantly less than wild-type mice (Figure 6D). However, these mutant animals displayed elevated levels of 5-HT in atopic dermatitis skin, similar to levels observed in wild type (Figure 6C). Thus, HTR7 and TRPA1 are required for the detection, rather than the release of 5-HT under atopic dermatitis-like conditions.
When considered in light of previous studies, our data support a dual role for TRPA1 in atopic dermatitis. First, when co-expressed with the TSLP receptor, TRPA1 acts as a transducer of TSLP-evoked itch and inflammation (Wilson et al., 2013b). Second, when co-expressed with HTR7, TRPA1 acts as a transducer of 5-HT-evoked itch. We also highlight the importance of 5-HT signaling in the pathology of atopic dermatitis. Patients with severe atopic dermatitis eventually progress to develop asthma and allergic rhinitis, in a process known as the “atopic march” (Spergel and Paller, 2003). It is notable that elevated 5-HT levels are also linked to asthma (Bayer et al., 2007), and future studies will elucidate how TRPA1-expressing neuronal subtypes orchestrates the progression of atopic diseases.
Animals lacking Htr7 and Trpa1 still exhibited a residual degree of eczematous skin lesions and itch behaviors in the atopic dermatitis model. Thus an important question is the identity of the other molecular players required for the complete development and/or maintenance of atopic dermatitis. Such determinants likely include TSLP, given its upregulation in the human disease and the atopic dermatitis mouse model (Li et al., 2006), and its activity in sensory neurons that promotes itch behaviors (Wilson et al., 2013b). Interestingly, another 5-HT receptor co-regulated with itch, Htr1d (Figure 1B), has been previously implicated in inflammatory pain (Ahn and Basbaum, 2006; Tepper et al., 2002), and is also a compelling candidate mediator of atopic dermatitis and itch. Moreover, numerous inflammatory mediators are released in atopic dermatitis in addition to 5-HT and TSLP (Brandt and Sivaprasad, 2011) whose mechanisms have yet to be revealed.
Despite these complexities, our data clearly show that HTR7 and TRPA1 are key mediators of serotonergic acute and chronic itch. Aside from AD, a variety of human chronic itch disorders are linked to 5-HT. It is likely, therefore, that HTR7 antagonists may prove to be useful for the selective attenuation of itch that results from these pathological conditions.
EXPERIMENTAL PROCEDURES
RNA-seq analysis
We mapped RNA-seq reads from the DRG of each BXD strain to the DBA/2J and C57BL/6J using Tophat. HTSeq was used to sum the total read counts per gene. Read counts were normalized with the EDASeq package in R using the upper-quartile method. Gene ontology enrichment analysis was performed using the topGO package in R. Information regarding analyses packages is available in the supplemental methods. All expression data have been deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo).
Histology
Histology on skin and in situ hybridization on DRG was carried out as previously described (Gerhold et al., 2013).
Cell culture
Cell culture and transfection was carried out as previously described (Wilson et al., 2011). Cholera toxin subunit B, Alexa Fluor 594 conjugate (Life Technologies) was used for retrograde labeling of cutaneous afferent neurons.
Electrophysiology
Electrophysiology was performed as previously described (Wilson et al. 2013). Whole cell recordings in transfected cells were performed using the Port-a-patch system (Brueggemann et al., 2004) (Nanion Technologies).
Calcium imaging
Ca2+ imaging experiments were carried out as previously described (Wilson et al., 2011). Image analysis and statistics were performed using Igor Pro (WaveMetrics).
Behavioral studies
Htr7−/− mice were obtained from Jackson Laboratory, Trpv1−/− and Trpa1−/− mice were described previously (Bautista et al., 2006; Caterina et al., 2000), and K14-Cre;Trpa1fl/fl mice were provided by Cheryl Stucky. Behavioral experiments were performed as previously described (Wilson et al., 2011). All experiments were performed under the policies and recommendations of the International Association for the Study of Pain and approved by the University of California, Berkeley Animal Care and Use Committee.
Atopic Dermatitis Model
MC903 (R&D Systems) was applied to the mouse cheek once per day, for 7 days. Character of lesion scoring was adapted from previous studies (Hampton et al., 2012; Liu et al., 2013; Yun et al., 2011).
Statistical analysis
The following statistical tests were used where appropriate: Student’s t-test, one-way ANOVA, and ANOVA for multivariate linear models.
Supplementary Material
HIGHLIGHTS.
Activation of peripheral HTR7 receptors triggers itch but not pain behaviors
HTR7 promotes opening of the inflammatory ion channel TRPA1
Serotonin- and SSRI-evoked itch is mediated by HTR7 and TRPA1
HTR7 and TRPA1 are required for the development of atopic dermatitis in mice
Acknowledgments
We are grateful to Bautista lab members for critical comments on the manuscript. We thank E. Brock, J. Schwendinger-Schreck, C. Walsh, M. Reid, M. Hernandez for behavior data analyses and E. Lumpkin and K. Marsall for help with the CTB labelling protocol. This work was supported by National Institutes of Health grants AR059385 (D.M.B.), NS084812 (M.P.), N5040538 (C.L.S.), and NS077224 (R.B.B. and D.M.B.) and a National Science Foundation Fellowship (S.R.W.). This work used the Vincent J. Coates Genomics Sequencing Laboratory at UC Berkeley, supported by NIH S10 Instrumentation Grants S10RR029668 and S10RR027303. This work also used the Biological Imaging Facility, supported by NIH grant 1S10RR026866-01.
Footnotes
AUTHOR CONTRIBUTIONS
T.M. performed and analyzed gene expression experiments. S.P.M., L.M.B., J.F.W., and C.L.S. designed and implemented the behavioral studies. S.P.M and L.M.B. designed the histological studies. T.M., S.P.M., M.P., S.R.W., and D.M.B carried out the cellular imaging experiments. M.P., M.A.K., K.L., A.S.B.O performed electrophysiology experiments. T.M., S.P.M., L.M.B., R.B.B., and D.M.B analyzed data. T.M., S.P.M., R.B.B., and D.M.B. wrote the manuscript. R.B.B. and D.M.B. provided advice and guidance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott FV, Hong Y, Blier P. Activation of 5-HT2A receptors potentiates pain produced by inflammatory mediators. Neuropharmacology. 1996;35:99–110. doi: 10.1016/0028-3908(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Adham N, Zgombick JM, Bard J, Branchek TA. Functional characterization of the recombinant human 5-hydroxytryptamine7(a) receptor isoform coupled to adenylate cyclase stimulation. J Pharmacol Exp Ther. 1998;287:508–514. [PubMed] [Google Scholar]
- Ahn AH, Basbaum AI. Tissue injury regulates serotonin 1D receptor expression: implications for the control of migraine and inflammatory pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:8332–8338. doi: 10.1523/JNEUROSCI.1989-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. Journal of neurophysiology. 2010;104:2442–2450. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjaneyulu M, Chopra K. Fluoxetine attenuates thermal hyperalgesia through 5-HT1/2 receptors in streptozotocin-induced diabetic mice. European journal of pharmacology. 2004;497:285–292. doi: 10.1016/j.ejphar.2004.06.063. [DOI] [PubMed] [Google Scholar]
- Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol. 2011;22:390–404. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nature neuroscience. 2014;17:175–182. doi: 10.1038/nn.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer H, Muller T, Myrtek D, Sorichter S, Ziegenhagen M, Norgauer J, Zissel G, Idzko M. Serotoninergic receptors on human airway epithelial cells. American journal of respiratory cell and molecular biology. 2007;36:85–93. doi: 10.1165/rcmb.2006-0151OC. [DOI] [PubMed] [Google Scholar]
- Blardi P, De Lalla A, Leo A, Auteri A, Iapichino S, Di Muro A, Dell’Erba A, Castrogiovanni P. Serotonin and fluoxetine levels in plasma and platelets after fluoxetine treatment in depressive patients. Journal of clinical psychopharmacology. 2002;22:131–136. doi: 10.1097/00004714-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Bonnet MP, Marret E, Josserand J, Mercier FJ. Effect of prophylactic 5-HT3 receptor antagonists on pruritus induced by neuraxial opioids: a quantitative systematic review. British journal of anaesthesia. 2008;101:311–319. doi: 10.1093/bja/aen202. [DOI] [PubMed] [Google Scholar]
- Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. Journal of clinical & cellular immunology. 2011:2. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchat A, Rocasalbas M, Zamanillo D, Hamon M, Vela J Miguel, Romero L. Assessment of 5-HT7 Receptor Agonists Selectivity Using Nociceptive and Thermoregulation Tests in Knockout versus Wild-Type Mice. Advances in Pharmacological Sciences. 2012;2012:9. doi: 10.1155/2012/312041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchat A, Romero L, García M, Pujol M, Burgueño J, Torrens A, Hamon M, Baeyens JM, Buschmann H, Zamanillo D, Vela JM. 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain. 2009;141:239–247. doi: 10.1016/j.pain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Browning J, Combes B, Mayo MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. The American journal of gastroenterology. 2003;98:2736–2741. doi: 10.1111/j.1572-0241.2003.08662.x. [DOI] [PubMed] [Google Scholar]
- Brueggemann A, George M, Klau M, Beckler M, Steindl J, Behrends JC, Fertig N. Ion channel drug discovery and research: the automated Nano-Patch-Clamp technology. Curr Drug Discov Technol. 2004;1:91–96. doi: 10.2174/1570163043484833. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science (New York, NY) 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cederberg J, Knight S, Svenson S, Melhus H. Itch and skin rash from chocolate during fluoxetine and sertraline treatment: case report. BMC psychiatry. 2004;4:36. doi: 10.1186/1471-244X-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. The Journal of allergy and clinical immunology. 2014;133:448–460. doi: 10.1016/j.jaci.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, Back SK, Na HS, Harfe BD, Wang F, et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nature neuroscience. 2012;15:1015–1021. doi: 10.1038/nn.3111. [DOI] [PubMed] [Google Scholar]
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatologic Therapy. 2013;26:84–91. doi: 10.1111/dth.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold KA, Pellegrino M, Tsunozaki M, Morita T, Leitch DB, Tsuruda PR, Brem RB, Catania KC, Bautista DM. The star-nosed mole reveals clues to the molecular basis of mammalian touch. PloS one. 2013;8:e55001. doi: 10.1371/journal.pone.0055001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Nislow C. The yeast deletion collection: a decade of functional genomics. Genetics. 2014;197:451–465. doi: 10.1534/genetics.114.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Grossman GH, Farnbauch L, DiNardo L. Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7451–7460. doi: 10.1523/JNEUROSCI.23-20-07451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AD, Young KK, Lehto SG, Smith SB, Mogil JS. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain. 2006;124:50–58. doi: 10.1016/j.pain.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Hampton AL, Hish GA, Aslam MN, Rothman ED, Bergin IL, Patterson KA, Naik M, Paruchuri T, Varani J, Rush HG. Progression of ulcerative dermatitis lesions in C57BL/6Crl mice and the development of a scoring system for dermatitis lesions. JAALAS. 2012;51:586–593. [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, et al. A subpopulation of nociceptors specifically linked to itch. Nature neuroscience. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology. 2009;206:345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon MA. Molecular dissection of itch. Current opinion in neurobiology. 2015;34c:61–66. doi: 10.1016/j.conb.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li G, Xiang J, Yin D, Chi R. Immunohistochemical study of serotonin in lesions of chronic eczema. International Journal of Dermatology. 2004;43:723–726. doi: 10.1111/j.1365-4632.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- Idzko M, Panther E, Stratz C, Müller T, Bayer H, Zissel G, Dürk T, Sorichter S, Di Virgilio F, Geissler M, et al. The Serotoninergic Receptors of Human Dendritic Cells: Identification and Coupling to Cytokine Release. The Journal of Immunology. 2004;172:6011–6019. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen HS. Studies on the neuroendocrine role of serotonin. Danish medical bulletin. 2007;54:266–288. [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kalz F, Fekete Z. Studies on the mechanism of the white response and of the delayed blanch phenomenon in atopic subjects by means of Coomassie blue. The Journal of investigative dermatology. 1960;35:135–140. doi: 10.1038/jid.1960.97. [DOI] [PubMed] [Google Scholar]
- Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. The Journal of clinical investigation. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser V, Elfassi IE, Aubel B, Melfort M, Julius D, Gingrich JA, Hamon M, Bourgoin S. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A−/−, 5-HT1B−/−, 5-HT2A−/−, 5-HT3A−/− and 5-HTT−/− knockout male mice. Pain. 2007;130:235–248. doi: 10.1016/j.pain.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Kerr PG, Argiles A, Mion C. Whole Blood Serotonin Levels Are Markedly Elevated in Patients on Dialytic Therapy. American Journal of Nephrology. 1992;12:14–18. doi: 10.1159/000168411. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Bridle BW, Ghia JE, Wang H, Syed SN, Manocha MM, Rengasamy P, Shajib MS, Wan Y, Hedlund PB, Khan WI. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. Journal of immunology (Baltimore, Md: 1950) 2013;190:4795–4804. doi: 10.4049/jimmunol.1201887. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. European journal of pharmacology. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Yamaguchi T, Miyamoto T. Itch-scratch responses induced by opioids through central mu opioid receptors in mice. Journal of biomedical science. 2000;7:248–252. doi: 10.1007/BF02255473. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides K, Hussain SK, Hobbs GJ. Management of opioid-induced pruritus: a role for 5-HT3 antagonists? British journal of anaesthesia. 1999;82:439–441. doi: 10.1093/bja/82.3.439. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2014;15:19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:1–6. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Chang WJ, Lin CS, Huang CY, Wang HF, Sun WH. Serotonin receptor 5-HT2B mediates serotonin-induced mechanical hyperalgesia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:1410–1418. doi: 10.1523/JNEUROSCI.4682-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, Sui A, McKay MC, McAlexander MA, Herrick CA, Jordt SE. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB. 2013;27:3549–3563. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by beta-alanine. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, et al. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- Lundeberg L, Liang Y, Sundström E, Nordlind K, Verhofstad A, Lidén S, Johansson O. Serotonin in human allergic contact dermatitis. An immunohistochemical and high-performance liquid chromatographic study. Archives of dermatological research. 1999;291:269–274. doi: 10.1007/s004030050407. [DOI] [PubMed] [Google Scholar]
- Manteniotis S, Lehmann R, Flegel C, Vogel F, Hofreuter A, Schreiner BS, Altmuller J, Becker C, Schobel N, Hatt H, Gisselmann G. Comprehensive RNA-Seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in Trigeminal ganglia. PloS one. 2013;8:e79523. doi: 10.1371/journal.pone.0079523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matterne U, Apfelbacher CJ, Loerbroks A, Schwarzer T, Buttner M, Ofenloch R, Diepgen TL, Weisshaar E. Prevalence, correlates and characteristics of chronic pruritus: a population-based cross-sectional study. Acta dermato-venereologica. 2011;91:674–679. doi: 10.2340/00015555-1159. [DOI] [PubMed] [Google Scholar]
- Metz M, Stander S. Chronic pruritus--pathogenesis, clinical aspects and treatment. Journal of the European Academy of Dermatology and Venereology: JEADV. 2010;24:1249–1260. doi: 10.1111/j.1468-3083.2010.03850.x. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The Cells and Circuitry for Itch Responses in Mice. Science (New York, NY) 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollanazar NK, Smith PK, Yosipovitch G. Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out? Clinical reviews in allergy & immunology. 2015 doi: 10.1007/s12016-015-8488-5. [DOI] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ., Jr Itch elicited by intradermal injection of serotonin, intracisternal injection of morphine, and their synergistic interactions in rats. Neuroscience. 2014;274:119–127. doi: 10.1016/j.neuroscience.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair HK, Hain H, Quock RM, Philip VM, Chesler EJ, Belknap JK, Lariviere WR. Genomic loci and candidate genes underlying inflammatory nociception. Pain. 2011;152:599–606. doi: 10.1016/j.pain.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento EB, Jr, Seniuk JG, Godin AM, Ferreira WC, Dutra MB, Oliveira AC, Bastos LF, Fiebich BL, Coelho MM. Peripheral 5-HT1B and 5-HT2A receptors mediate the nociceptive response induced by 5-hydroxytryptamine in mice. Pharmacology, biochemistry, and behavior. 2011;99:598–603. doi: 10.1016/j.pbb.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Nojima H, Carstens E. 5-Hydroxytryptamine (5-HT)2 Receptor Involvement in Acute 5-HT-Evoked Scratching but Not in Allergic Pruritus Induced by Dinitrofluorobenzene in Rats. Journal of Pharmacology and Experimental Therapeutics. 2003;306:245–252. doi: 10.1124/jpet.103.049239. [DOI] [PubMed] [Google Scholar]
- Nordlind K, Thorslund K, Lonne-Rahm S, Mohabbati S, Berki T, Morales M, Azmitia EC. Expression of serotonergic receptors in psoriatic skin. Archives of dermatological research. 2006;298:99–106. doi: 10.1007/s00403-006-0652-6. [DOI] [PubMed] [Google Scholar]
- Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z, Zheng T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. Journal of immunology (Baltimore, Md: 1950) 2013;191:5371–5382. doi: 10.4049/jimmunol.1300300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausl A, Nordlind K, Wahlgren CF. Pruritic and vascular responses induced by serotonin in patients with atopic dermatitis and in healthy controls. Acta dermato-venereologica. 2013;93:277–280. doi: 10.2340/00015555-1473. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Krucker T, Levy CL, Slanina Ka, Sutcliffe JG, Hedlund PB. Mice lacking 5-HT7 receptors show specific impairments in contextual learning. European Journal of Neuroscience. 2004;19:1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Current opinion in neurobiology. 2011;21:880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Sannicandro TJ, Farrar MC, Markowitz JS. Selective serotonin reuptake inhibitor-induced rash: case report and review of the literature. Pharmacotherapy. 2002;22:516–518. doi: 10.1592/phco.22.7.516.33666. [DOI] [PubMed] [Google Scholar]
- Santello M, Nevian T. Dysfunction of cortical dendritic integration in neuropathic pain reversed by serotoninergic neuromodulation. Neuron. 2015;86:233–246. doi: 10.1016/j.neuron.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer H, Hartmann H, Ramadori G. Relief of cholestatic pruritus by a novel class of drugs: 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists: effectiveness of ondansetron. Pain. 1995;61:33–37. doi: 10.1016/0304-3959(94)00145-5. [DOI] [PubMed] [Google Scholar]
- Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. The Journal of investigative dermatology. 2007;127:1947–1955. doi: 10.1038/sj.jid.5700824. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. The Journal of allergy and clinical immunology. 2003;112:S118–127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Gilman AG. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science (New York, NY) 1991;254:1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Taylor BA, Heiniger HJ, Meier H. Genetic analysis of resistance to cadmium-induced testicular damage in mice. Proc Soc Exp Biol Med. 1973;143:629–633. doi: 10.3181/00379727-143-37380. [DOI] [PubMed] [Google Scholar]
- Tepper SJ, Rapoport AM, Sheftell FD. Mechanisms of action of the 5-HT1B/1D receptor agonists. Archives of neurology. 2002;59:1084–1088. doi: 10.1001/archneur.59.7.1084. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration/FDA Adverse Event Reporting System. FAERS Quarterly Data Files. Washington DC: 2009–2014. [Google Scholar]
- Van Steenwinckel J, Brisorgueil MJ, Fischer J, Verge D, Gingrich JA, Bourgoin S, Hamon M, Bernard R, Conrath M. Role of spinal serotonin 5-HT2A receptor in 2′,3′-dideoxycytidine-induced neuropathic pain in the rat and the mouse. Pain. 2008;137:66–80. doi: 10.1016/j.pain.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Vanhoenacker P, Haegeman G, Leysen JE. 5-HT7 receptors: current knowledge and future prospects. Trends in pharmacological sciences. 2000;21:70–77. doi: 10.1016/s0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]
- Warnock JK, Morris DW. Adverse cutaneous reactions to antidepressants. American journal of clinical dermatology. 2002;3:329–339. doi: 10.2165/00128071-200203050-00005. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nature neuroscience. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, Bautista DM. The ion channel TRPA1 is required for chronic itch. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013a;33:9283–9294. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013b;155:285–295. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Strong JA, Ye L, Mao JX, Zhang JM. Knockdown of sodium channel NaV1.6 blocks mechanical pain and abnormal bursting activity of afferent neurons in inflamed sensory ganglia. Pain. 2013;154:1170–1180. doi: 10.1016/j.pain.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JW, Seo JA, Jeong YS, Bae IH, Jang WH, Lee J, Kim SY, Shin SS, Woo BY, Lee KW, et al. TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. Journal of dermatological science. 2011;62:8–15. doi: 10.1016/j.jdermsci.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Liu XY, Jeffry J, Karunarathne WK, Li JL, Munanairi A, Zhou XY, Li H, Sun YG, Wan L, et al. Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron. 2014;84:821–834. doi: 10.1016/j.neuron.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz Z, Krajnik M, Sorge AA, Costantini M. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. Journal of pain and symptom management. 2003;26:1105–1112. doi: 10.1016/j.jpainsymman.2003.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








