Abstract
Background
African American (AA) men experience higher rates of prostate cancer (PCa) and vitamin D (vitD) deficiency than white men. VitD is promoted for PCa prevention, but there is conflicting data on the association between vitD and PCa. We examined the association between serum vitD and dietary quercetin and their interaction with PCa risk in AA men.
Methods
Participants included 90 AA men with PCa undergoing treatment at Howard University Hospital (HUH) and 62 controls participating in HUH’s free PCa screening program. We measured serum 25-hydroxy vitD [25(OH)D] and used the 98.2 item Block Brief 2000 Food Frequency Questionnaires to measure dietary intake of quercetin and other nutrients. Case and control groups were compared using two-sample t test for continuous risk factors and Fisher exact test for categorical factors. Associations between risk factors and PCa risk were examined via age-adjusted logistic regression models.
Results
Interaction effects of dietary quercetin and serum vitD on PCa status were observed. AA men (age 40–70) with normal levels of serum vitD (> 30 ng/ml) had a 71% lower risk of PCa compared to AA men with vitD deficiency (OR=0.29, 95% CI: 0.08–1.03; p=0.055). In individuals with vitD deficiency, increased dietary quercetin showed a tendency toward lower risk of PCa (OR=0.91, 95% CI: 0.82–1.00; p=0.054, age-adjusted) while men with normal vitD were at elevated risk (OR=1.23, 95% CI: 1.04–1.45).
Conclusions
These findings suggest that AA men who are at a higher risk of PCa may benefit more from vitD intake, and supplementation with dietary quercetin may increase the risk of PCa in AA men with normal vitD levels. Further studies with larger populations are needed to better understand the impact of the interaction between sera vitD levels and supplementation with quercetin on PCa in AA men.
Keywords: Vitamin D, Quercetin, Prostate cancer, African American
Introduction
With more than 238,000 cases of prostate cancer being diagnosed each year in the United States [1], American men are increasingly looking to dietary supplements to reduce their risk of developing prostate cancer and to delay progression after diagnosis [2, 3]. The increased use of dietary supplements for prostate cancer is occurring despite data showing consumption of some supplements actively promoted for anti-prostate cancer activity actually increased the risk of prostate cancer [4]. Rigorous research on the effectiveness of dietary supplements is essential for practitioners to provide authoritative answers, targeted to individual patients, regarding which supplements are safe and effective.
Vitamin D supplementation has been promoted for prostate cancer prevention based in part on a 2007 Harvard University study of nearly 15,000 men initially free of prostate cancer. Men whose plasma levels of vitamin D were below (versus above) the median had a significantly increased risk of developing aggressive prostate cancer (OR = 2.1, 95%CI: 1.2–3.4) [5]. A 2014 study of the association between vitamin D and prostate biopsy outcomes in 667 men found that vitamin D deficiency was associated with higher Gleason grade and tumor stage in both European-American and African American men and with increased odds of prostate cancer diagnosis on biopsy [6]. The findings of an association between vitamin D levels and aggressive prostate cancer were confirmed in a 2012 study from the United Kingdom that showed that lower 25-hydroxy vitamin D [25(OH)D] concentrations were associated with more aggressive cancers, but found no evidence of a link between vitamin D levels and overall prostate cancer risk [7]. The finding of no association between vitamin D levels and overall prostate cancer risk is consistent with a retrospective study of 479 prostate cancer patients with age-matched controls that showed no causal relationship between vitamin D levels and risk of prostate cancer [8], and a population-based cohort study of 1,476 prostate cancer patients that found no evidence that serum vitamin D levels measured after diagnosis affect prostate cancer prognosis [9]. Another study matching 1,000 prostate cancer patients with 1,000 controls found men with higher levels of vitamin D have an increased risk of prostate cancer [10]. Faced with such conflicting data, the National Cancer Institute does not recommend “for or against the use of vitamin D supplements to reduce the risk” of prostate cancer [11].
The majority of these studies did not, however, look at the association between vitamin D and prostate cancer risk in African American men. African American men have a significantly higher incidence of aggressive prostate cancer and significantly lower levels of vitamin D than white men [12, 13]. The normal range for vitamin D levels is 30–74 ng/ml [14], but an analysis of 194 African American men found that 61% had 25(OH) D levels < 15ng/ml, and only two of the participants had levels > 30ng/ml [15]. These lower levels of vitamin D are partly attributable to higher levels of melanin in the skin of African American men, which reduces the skin’s ability to produce vitamin D [16]. Higher levels of aggressive prostate cancer in African American men were found in a recently published study of 70,345 men with early-stage prostate cancer diagnosed between 2004 and 2008. African-American men were 1.84 times more likely to develop high-risk prostate cancer (P <0.01) compared with white men [7].
Men concerned about the risk of prostate cancer frequently supplement their diet with combinations of vitamins, minerals, and fruit/seed extracts, and more than 25% consume three or more supplements. Nearly 1 in 5 men at high risk of prostate cancer use fruit and seed extracts either alone or in combination with vitamins [2]. Quercetin is a component of fruits and seeds being actively studied as an anti-proliferative agent [9]. In vitro and in vivo mice studies, using prostate cancer cell lines, have found that quercetin provides chemoprotection, generates apoptosis, and increases antioxidant enzymes [17, 18]. An in vitro study reported that quercetin regulates insulin-like growth factor signaling and induces apoptosis in androgen-independent PC-3 prostate cancer cells [19]. A study of quercetin in mice injected with PC-3 cells reported that quercetin reverses epidermal growth factor-induced epithelial-to-mesenchymal transition, and may, therefore, prevent or delay prostate cancer metastases [20]. Further, quercetin supplementation was found to enhance the chemopreventive effects of green tea in prostate cancer cells in mice [21]. In addition, quercetin was shown to improve chronic prostatitis/chronic pelvic pain in a significant proportion of men [22].
Because African American men have a higher incidence of aggressive prostate cancer, and because aggressive prostate cancer is associated with lower levels of vitamin D, we hypothesize that vitamin D deficient (25(OH) D levels < 30ng/ml) African American patients have a higher risk of prostate cancer compared with African American men with normal levels of vitamin D when adjusted for age and quercetin levels. We examined the interaction between vitamin D and quercetin levels.
Methods
Patient Selection
Between 2005 and 2008, urologists at Howard University Hospital (HUH) recruited 91 African American men (cases) over the age of 40 who were diagnosed with adenocarcinoma of the prostate, and who had PSA > 2.5 ng/ml and a positive digital rectal exam. Men currently undergoing chemotherapy, radiation therapy, or androgen deprivation therapy were excluded. In addition, 91 African American men were recruited as controls from among men participating in HUH’s free Men Take Prostate Cancer Screening Program [23, 24]. Eligible controls had no diagnosis of prostate cancer, PSA < 2.5 ng/ml, negative DRE, no family history of prostate cancer among first-degree relatives, and no relationship to cases. They were matched with the cases by age based on a ±5 year window. After dropping observations because of missing data, cases were significantly older than controls despite the matching. Hence, we did not use matching as an analysis criterion. Missing data on PSA, levels of vitamin D, and/or quercetin reduced the number of cases to 90 and the number of controls to 62.
Serum 25-OH Vitamin D Assay
Vitamin D levels in blood were determined using an assay for 25-OH D, widely considered the most reliable measure of overall vitamin D status [25]. A 25-hydroxy vitamin D Enzyme Immunoassay kit from Immunodiagnostic Systems Ltd. (ADS Ltd, AZ) was used according to the manufacturer’s instructions and as previously described [5]. A level of 30 ng/ml was used as the threshold for vitamin D deficiency because of a growing consensus that vitamin D levels below 30 ng/ml raise the risk of bone loss and bone fracture in men [26, 27].
Dietary Quercetin
Dietary quercetin levels were determined using the 98.2 item Block Brief 2000 Food Frequency Questionnaires (FFQ) with a food list designed to cover greater than 90% of the average intake of over 30 nutrients in whites, African-Americans, and Hispanic Americans [28]. The Block FFQ was validated and used to assess dietary intake in an African-American population [29]. The completed FFQs were sent to Block Dietary Systems in Berkeley, CA for analysis.
Statistics
The primary goal for this study was to explore the risk factors associated with prostate cancer. The main risk factors in this paper included vitamin D level and dietary intake of quercetin. Patient characteristics included age at diagnosis; and nutrition measurements from food, including selenium, omega 3, lycopene, fatty acids (trans, saturated, polyunsaturated, and monosaturated), folate, glutathione, thiamine, isoflavinols, vitamin D, and fruit servings. Dietary supplement nutrients included quercetin, selenium, folic acid, omega 3, omega 6, vitamin D, and vitamin E. Vitamin D, vitamin E, selenium, and folic acid dietary supplements were in concordance in most individuals (pairwise concordances with agreement >=92%), as were supplements of omega 3 and omega 6 (96% agreement). Thus, composite outcomes on these supplements were created to avoid model collinearity issues. For each risk factor, descriptive statistics were summarized with mean, standard deviation (SD), median, and range for continuous outcomes, and the frequency for categorical outcomes by case and control groups was calculated. Comparisons between case and control groups were tested by two sample t tests for continuous risk factors, and by the Fisher exact test for categorical factors. Associations between risk factors and prostate cancer were examined via logistic regression models with age adjustment. Odds ratios and the corresponding 95% confidence interval (CI) were reported. An interaction effect between vitamin D level and dietary quercetin was examined in this case-control study. Multivariable analysis was initiated including potential risk factors with p-value less than 0.10 in univariate analysis and interaction effects between vitamin D level and dietary quercetin. Backward stepwise selection retained the variables with p-value less than 0.10. In addition, the interaction effect of vitamin D level and dietary quercetin on prostate cancer was evaluated by dichotomizing the dietary quercetin at its median value (5.8 mg). Supplementation with omega 3 or 6 was excluded from multivariable analysis due to the sparse numbers of patients in the case group who took omega 3 or 6 supplements. P-values less than 0.05 were considered as significant. Statistical software R3.0.2 was used in the analysis.
Results
Baseline characteristics and dietary behaviors are summarized for cases and controls in Table 1. Cases were significantly older than controls (p<0.001). Vitamin D deficiency was similar in cases (63%) and controls (67%, p =0.51). No significant differences were seen between cases and controls in dietary intake of vitamin D, quercetin, and 17 other nutrients in food. In addition, cases and controls did not differ significantly in patients who took any dietary supplements of selenium, folic acid, or vitamins E or D versus patients who did not take any dietary supplements of selenium, folic acid, and vitamins E and D composite (p=0.41).
Table 1.
Summary nutrient statistics and univariate analysis results with and without age adjustment
| Risk Factors | Overall (n=152) | Control (n=62) | Case (n=90) | p-value* | OR**(95% CI) | p-value** |
|---|---|---|---|---|---|---|
| Patients characteristics | ||||||
|
| ||||||
| Mean age (sd) | 64.14 (10.93) | 58.23(10.33) | 68.22(9.40) | <0.0001 | 0.11 (1.07,1.15) | <0.0001 |
| Median age (range) | 65 (40–88) | 56 (40–88) | 68.5 (44–87) | |||
|
| ||||||
| Serum Vitamin D level | n(%) | n(%) | n(%) | |||
| Vitamin D deficiency (≤30ng/ml) | 99(65.13) | 42(67.74) | 57(63.33) | 0.51 | 1 | |
| Vitamin D normal (>30 ng/ml) | 51(33.55) | 18(29.03) | 33(36.67) | 1.53(0.70,3.45) | 0.29 | |
|
| ||||||
| Dietary-Nutrition | Mean(SD) | Mean(SD) | Mean(SD) | |||
|
| ||||||
| Quercetin (mg) | 7.19(5.54) | 7.58(5.83) | 6.92(5.34) | 0.48 | 0.99(0.93,1.06) | 0.88 |
|
| ||||||
| Selenium (mcg) | 104.32(64.59) | 104.7(72.75) | 104.06(58.74) | 0.95 | 1.00(1.00,1.01) | 0.18 |
|
| ||||||
| Omega 3(gms) | 1.86(1.21) | 1.89(1.37) | 1.83(1.1) | 0.80 | 1.19(0.88,1.63) | 0.26 |
|
| ||||||
| Lycopene (mcg) | 4000.8(3578.92) | 3925.41 (3168.27.74) | 4052.74 (3852.91) | 0.82 | 1.00(1.00,1.01) | 0.62 |
|
| ||||||
| Total fat (gms) | 80.23(52.98) | 83.05(5.82) | 78.3(42.23) | 0.62 | 1.00(1.00,1.01) | 0.40 |
|
| ||||||
| Saturated fat (gms) | 24.05(17.81) | 25.27(22.23) | 23.20(14.06) | 0.52 | 1.01(0.99,1.03) | 0.50 |
|
| ||||||
| Polyunsaturated fat (gms) | 18.49(11.2) | 18.86(13.31) | 18.20(9.55) | 0.75 | 1.02(0.99,1.05) | 0.30 |
|
| ||||||
| Monounsaturated fatty acids (gms) | 31.13(20.61) | 32.19(25.88) | 30.40(16.13) | 0.63 | 1.01(0.99,1.03) | 0.42 |
|
| ||||||
| % of Kcal from fat | 35.95(7.11) | 35.90(7.73) | 35.98(6.69) | 0.95 | 1.00(0.95,1.05) | 0.93 |
|
| ||||||
| Trans fats, total (gms) | 2.66(2.24) | 2.78(2.82) | 2.57(1.74) | 0.59 | 1.07(0.91,1.26) | 0.44 |
|
| ||||||
| Food folate (mcg) | 290.77 (162.15) | 295.06(157.05) | 287.81(166.38) | 0.79 | 1.00(1.00,1.00) | 0.42 |
|
| ||||||
| Folic acid (mcg) | 153.13(133.1) | 150.18(139.27) | 155.17(129.44) | 0.82 | 1.00(1.00,1.00) | 0.38 |
|
| ||||||
| Growth hormone stimulation test (mg) | 40.82(26.44) | 43.36(31.66) | 39.07.06(22.17) | 0.36 | 1.00(0.99,1.02) | 0.69 |
|
| ||||||
| Growth hormone secretagogue receptor (mg) | 27.11(17.58) | 28.71(20.79) | 26.01(15.00) | 0.38 | 1.00(0.98,1.03) | 0.68 |
|
| ||||||
| Thiamine (mg) | 1.63(0.92) | 1.61(0.99) | 1.64(0.88) | 0.88 | 1.27(0.86,1.92) | 0.23 |
|
| ||||||
| Isoflavones (mg) | 2.73(6.9) | 2.48(6.62) | 2.91(7.11) | 0.71 | 1.02(0.97,1.10) | 0.40 |
|
| ||||||
| Vitamin D (IU) | 146.65(115.43) | 153.26(119.53) | 142.09(112.96) | 0.56 | 1.00(1.00,1.00) | 0.61 |
|
| ||||||
| Fruit servings (servings/day) | 1.39(0.9) | 1.44(0.92) | 1.36(0.90) | 0.57 | 0.92(0.61,1.37) | 0.68 |
|
| ||||||
| Fruit (total including juice) (cup) | 1.21(0.84) | 1.22(0.74) | 1.21(0.91) | 0.90 | 1.18(0.77,1.84) | 0.44 |
|
| ||||||
| Dietary – Supplement | n(%) | n(%) | n(%) | |||
|
| ||||||
| Selenium, folic acid or Vitamin D or E - No | 76(50) | 28(45.16) | 48(53.33) | 0.41 | 1 | |
| Selenium, folic acid or Vitamin D or E - Yes | 76(50) | 34(54.84) | 42(46.67) | 0.56(0.26,1.16) | 0.12 | |
|
| ||||||
| Omega 3 or 6 - No | 135(88.82) | 48(77.42) | 87(96.67) | 3e-04 | 1 | |
| Omega 3 or 6 - Yes | 17(11.18) | 14(22.48) | 3(3.33) | 0.10(0.02,0.35) | 0.0012 | |
p-values indicated the testing results for differences between case and control by t-test in continuous outcomes, or a Fisher exact test in categorical outcomes.
OR(95%CI): odds ratio and 95% confidence interval, and p-values were results from logistic regression models with age adjusted (not shown).
SD, standard deviation. Plus-minus values are means ± SD.
In age-adjusted results, neither serum vitamin D status nor dietary intake of quercetin were risk factors for prostate cancer (OR=1.53, CI:0.70–3.45; p=0.29 and OR=0.99, CI:0.93–1.06; p=0.88 respectively) (Table 1). In fact, no prostate cancer risk factors, beyond age, were identified in the dietary nutrition or supplements used, except for use of omega 3 or 6 supplements. Individuals who took omega 3 or 6 supplements had lower risk of prostate cancer (OR=0.10,95% CI: 0.02–0.35; p=0.0012) compared with subjects who did not take omega 3 or 6 supplements.
Prostate cancer versus serum vitamin D and dietary quercetin consumption
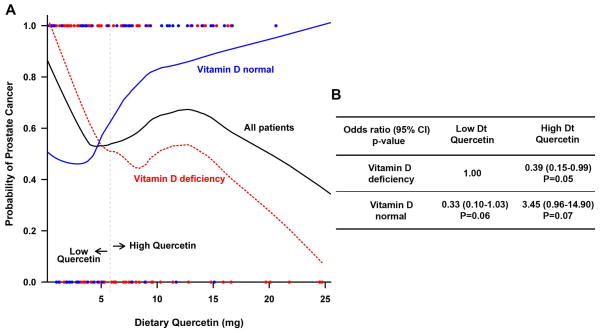
A complex relationship was detected between dietary quercetin consumption and prostate cancer risk with interaction effects of serum vitamin D status (Figure 1A). The risk of prostate cancer was negatively correlated to the dietary consumption of quercetin when serum vitamin D deficiency was taken into account and positively correlated among those with normal vitamin D levels. Thus, our final model results shown in Table 2 includes the interaction term of dietary quercetin and serum vitamin D status as well as age, dietary quercetin, and serum vitamin D status. When examining dietary quercetin at its median value (5.8 mg), all cases and controls were categorized as four groups based on individuals’ serum vitamin D status and amount of dietary quercetin. Figure 1B presents this dichotomization and interaction effect of serum vitamin D status and dietary quercetin on risk of prostate cancer with age adjustment.
Figure 1. Interaction effect between vitamin D deficiency and dietary quercetin in predicting risk of prostate cancer.
A) Dietary quercetin was treated as a continuous variable. Lowess smooth curves were fitted and graphed by serum vitamin D deficiency (≤30ng/ml) and normal vitamin D (>30ng/ml) group. B) Multivariable analysis with dichotomized dietary quercetin (low vs high, cut at median 5.8 mg) by vitamin D status. Odds ratios (ORs) were age-adjusted.
Table 2.
Final multivariable model predicting prostate cancer based on logistic regression
| Comparisons | Adjusted | OR (95% CI) | P-value |
|---|---|---|---|
| Serum Vitamin D (normal vs deficiency) | Dietary Quercetin + Age | 0.29 (0.08–1.03) | 0.055 |
| Dietary quercetin (per 1 increment) | Serum Vitamin D Deficiency +Age | 0.91 (0.82–1.00) | 0.054 |
| Dietary quercetin (per 1 increment) | Serum Vitamin D Normal + Age | 1.23 (1.04–1.45) | 0.015 |
| Age (per 1 increment) | Serum Vitamin D + Dietary Quercetin | 1.11 (1.06–1.15) | <0.0001 |
In this study, considering African American men with normal levels of serum vitamin D (> 30 ng/ml) only, a 71% lower risk of prostate cancer compared to men with vitamin D deficiency when controlling for age and dietary intake of quercetin as continuous variables (OR=0.29,95% CI: 0.08–1.03; p =0.055, Table 2) was observed. The significance of the interaction of serum vitamin D status and dietary quercetin (p=0.002) indicates that the association of prostate cancer risk with quercetin intake differs between vitamin D deficient African American men and vitamin D normal African American men. In addition, the magnitude of the OR in the interaction term (OR=1.23, 95%CI: 1.04–1.45) (between dietary quercetin and serum vitamin D level) compared to the OR of dietary quercetin alone (0.91) reflect an inverse relationship between dietary quercetin and prostate cancer risk in vitamin D deficient patients versus vitamin D normal patients. For the 65–68% (Table 1) of patients who had serum vitamin D deficiency, the probability of prostate cancer fell as dietary quercetin consumption increased. For this serum vitamin D deficient group of African American men, an increase of 1 mg of dietary quercetin was associated with a 9% decrease in risk of prostate cancer (OR=0.91,95% CI: 0.82–1.00; p = 0.054, age-adjusted, Table 2). The categorical finding that higher dietary quercetin consumption (≥5.8 mg) is associated with lower risk of prostate cancer in individuals with serum vitamin D deficiency, is more clearly shown in Figure 1B which dichotomizes quercetin consumption at its median value (OR=0.39, 95% CI: 0.15–0.99, p=0.05, Figure 1B). In contrast, in the 32–35% of patients with normal serum vitamin D levels, prostate cancer probability was higher in the high quercetin consumption group. This association comparing high (≥5.8 mg) vs low (<5.8 mg) intake of dietary quercetin patients was of borderline statistical significance (Figure 1B). For the serum vitamin D normal group however, a 1 mg increase in dietary quercetin consumption was associated with a statistically significant 23% increase in the risk of prostate cancer (OR=1.23,95% CI: 1.04–1.45, p=0.015, age-adjusted, Table 2).
Omega 3 or 6 supplementation was found to be significantly associated with protection from prostate cancer in age-adjusted logistic regression. However, because few individuals in the case group took omega 3 or 6 supplements, we excluded the use of omega 3 or 6 supplements from our final multivariable model.
Discussion
To our knowledge, this is the first study to explore the interaction of serum vitamin D level and dietary quercetin intake in predicting the risk of prostate cancer in African American men and in any male population. Our findings of no univariate relationship between serum vitamin D levels and prostate cancer risk in African American men were consistent with findings from similar studies in the general population [8, 9]. However, our study found that African American men whose serum vitamin D levels were deficient (≤30 ng/ml) had a higher risk of prostate cancer when adjusting for age and dietary quercetin levels. The differences were substantial; men whose vitamin D was in the normal range had a 79% lower risk of prostate cancer than men with vitamin D deficiency. These findings suggest that African American men who are at a higher risk of prostate cancer than the general population may benefit more than white men from vitamin D supplementation. We also found that 65%-67% of African American men had vitamin D deficiency, confirming earlier findings showing higher levels of vitamin D deficiency in African Americans than in whites [13].
The results showing an interaction between supplementation with the dietary flavonoid quercetin and serum vitamin D levels raised questions about when quercetin supplementation should be recommended for preventing prostate cancer. Although preclinical studies have shown a protective effect of dietary flavonoids against prostate cancer [17–21], and two case-control studies showed a weak protective effect of dietary flavonoids against prostate cancer [30, 31], our results show that quercetin supplementation is not associated with reduced prostate cancer risk in African American men overall. However, in men with vitamin D deficiency, quercetin supplementation was chemopreventive, while in men with normal levels of vitamin D, quercetin supplementation was associated with an increased risk of prostate cancer. This finding of increased cancer risk in African American men with normal vitamin D levels, although surprising, may or may not be a statistical anomaly. One preclinical study in rats found that quercetin exacerbated estrogen-induced breast tumors [32].
Although our data showing a protective effect of quercetin supplementation in vitamin D deficient African American men might lead to the conclusion that these men should use quercetin supplements while their vitamin D levels are deficient, larger studies are needed before reaching such a conclusion. Moreover, the interaction between dietary quercetin intake and vitamin D levels may be confounded by the propensity of men to take supplements and modify their diet to include more fruits after they have been diagnosed with cancer. To exclude this problem, intake of dietary supplements should be measured prior to diagnosis. Further, vitamin D levels vary by season. For the European population studied by Li [5], median levels of 25(OH)D were 24 ng/ml in the winter and spring, and 32 ng/ml in the summer and fall. Thus, the timing of the measurement of vitamin D could introduce variability into the analysis that alters the results.
Numerous previous studies have failed to find a relationship between vitamin D levels and overall prostate cancer risk in the general population [9, 10, 16]. However, three studies found evidence of a relationship between vitamin D deficiency and aggressive prostate cancer in the general population [5–7]. Li also noted an association between the incidence of prostate cancer and the interaction between low levels of 25(OH)D and the vitamin D receptor (VDR) Fok1 FF, Ff, and ff genotypes. Patients with low 25(OH)D and the ff genotype (compared with FF and Ff genotypes and higher vitamin D levels) faced increased risk of total (OR=1.9, 95% CI: 1.1–3.3) and aggressive prostate cancer (OR=2.5, 95% CI: 1.1–5.8). In men whose plasma 25(OH)D levels exceeded the median, the ff genotype was not associated with increased risk; men with the ff genotype and a high plasma 25(OH)D level (above versus below the median), faced significantly (60%-70%) lower risks of total and aggressive prostate cancer [5]. Li’s study included men primarily (94%) of Northern European decent and found a large incidence of insufficient levels of vitamin D (51%–77%) in this population.
Our finding suggests that African American men who are deficient in serum vitamin D may have reduced risk of prostate cancer with increased consumption of quercetin. However, African American men with adequate levels of serum vitamin D showed increased prostate cancer with quercetin consumption. In sum, provocative findings of our study, along with the retrospective design, small sample size, and lack of age-matched controls support the need for a larger, prospective, and randomized study of the relationship between vitamin D and prostate cancer, taking into account genotype and potential interactions with quercetin and/or omega 3 and 6 in African American men.
Acknowledgments
These data were generated in Dr. Kanaan’s laboratory supported by US Army Medical Research and Materiel Command (USAMRMC) [DAMD17-03-1-0069]. Support was also provided by the Cancer Center Support Grant at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973).
Footnotes
Disclosure: None of the authors have existing conflicts.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Uzzo RG, Brown JG, Horwitz EM, Hanlon A, Mazzoni S, Konski A, Greenberg RE, Pollack A, Kolenko V, Watkins-Bruner D. Prevalence and patterns of self-initiated nutritional supplementation in men at high risk of prostate cancer. BJU Int. 2004;93:955–960. doi: 10.1111/j.1464-410X.2004.04759.x. [DOI] [PubMed] [Google Scholar]
- 3.Nam RK, Fleshner N, Rakovitch E, Klotz L, Trachtenberg J, Choo R, Morton G, Danjoux C. Prevalence and patterns of the use of complementary therapies among prostate cancer patients: an epidemiological analysis. J Urol. 1999;161:1521–1524. [PubMed] [Google Scholar]
- 4.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Stampfer MJ, Hollis JB, Mucci LA, Gaziano JM, Hunter D, Giovannucci EL, Ma J. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy AB, Nyame Y, Martin IK, Catalona WJ, Hollowell CM, Nadler RB, Kozlowski JM, Perry KT, Kajdacsy-Balla A, Kittles R. Vitamin D deficiency predicts prostate biopsy outcomes. Clin Cancer Res. 2014;20:2289–2299. doi: 10.1158/1078-0432.CCR-13-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, Neal DE, Lane JA, Martin RM. Associations of circulating 25-hydroxyvitamin D with prostate cancer diagnosis, stage and grade. Int J Cancer. 2012;131:1187–1196. doi: 10.1002/ijc.27327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaturu S, Zdunek S, Youngberg B. Vitamin d levels in subjects with prostate cancer compared to age-matched controls. Prostate Cancer. 2012;2012:524206. doi: 10.1155/2012/524206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt SK, Kolb S, Fu R, Horst R, Feng Z, Stanford JL. Circulating levels of 25-hydroxyvitamin D and prostate cancer prognosis. Cancer Epidemiol. 2013;37:666–670. doi: 10.1016/j.canep.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albanes D, Mondul AM, Yu K, Parisi D, Horst RL, Virtamo J, Weinstein SJ. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Biomarkers Prev. 2011;20:1850–1860. doi: 10.1158/1055-9965.EPI-11-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.http://www.cancer.gov/cancertopics/factsheet/prevention/vitamin-D
- 12.Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate. 2005;62:243–252. doi: 10.1002/pros.20052. [DOI] [PubMed] [Google Scholar]
- 13.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 14.Topiwala S. 25-hydroxy vitamin d test. Medline Plus. 2013 Dec 27; [Google Scholar]
- 15.Tseng M, Giri V, Bruner DW, Giovannucci E. Prevalence and correlates of vitamin D status in African American men. BMC Public Health. 2009;9:191. doi: 10.1186/1471-2458-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert R, Martin RM, Beynon R, Harris R, Savovic J, Zuccolo L, Bekkering GE, Fraser WD, Sterne JA, Metcalfe C. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control. 2011;22:319–340. doi: 10.1007/s10552-010-9706-3. [DOI] [PubMed] [Google Scholar]
- 17.Sharmila G, Bhat FA, Arunkumar R, Elumalai P, Raja Singh P, Senthilkumar K, Arunakaran J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin Nutr. 2013 doi: 10.1016/j.clnu.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Ward AB. The effects of quercetin on prostate cancer. Cancer Research. 2014;74(19 suppl):Abstract 2128. [Google Scholar]
- 19.Senthilkumar K, Elumalai P, Arunkumar R, Banudevi S, Gunadharini ND, Sharmila G, Selvakumar K, Arunakaran J. Quercetin regulates insulin like growth factor signaling and induces intrinsic and extrinsic pathway mediated apoptosis in androgen independent prostate cancer cells (PC-3) Mol Cell Biochem. 2010;344:173–184. doi: 10.1007/s11010-010-0540-4. [DOI] [PubMed] [Google Scholar]
- 20.Bhat FA, Sharmila G, Balakrishnan S, Arunkumar R, Elumalai P, Suganya S, Raja Singh P, Srinivasan N, Arunakaran J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014 doi: 10.1016/j.jnutbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Vadgama JV, Said JW, Magyar CE, Doan N, Heber D, Henning SM. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. J Nutr Biochem. 2014;25:73–80. doi: 10.1016/j.jnutbio.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoskes DA, Zeitlin SI, Shahed A, Rajfer J. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology. 1999;54:960–963. doi: 10.1016/s0090-4295(99)00358-1. [DOI] [PubMed] [Google Scholar]
- 23.Beyene D, Daremipouran M, Apprey V, Williams R, Ricks-Santi L, Kassim OO, Naab TJ, Kanaan YM, Copeland RL., Jr Use of Tanning Potential as a Predictor for Prostate Cancer Risk in African-American Men. In Vivo. 2014;28:1181–1187. [PubMed] [Google Scholar]
- 24.Colli JL, Colli A. International comparisons of prostate cancer mortality rates with dietary practices and sunlight levels. Urol Oncol. 2006;24:184–194. doi: 10.1016/j.urolonc.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 3. American Society for Bone and Mineral Research; Lippincott-Raven, Philadelphia: 1996. Vitamin D. Photobiology, Metabolism, etc; pp. 74–81. [Google Scholar]
- 26.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 28.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 29.Coates RJ, Eley JW, Block G, Gunter EW, Sowell AL, Grossman C, Greenberg RS. An evaluation of a food frequency questionnaire for assessing dietary intake of specific carotenoids and vitamin E among low-income black women. Am J Epidemiol. 1991;134:658–671. doi: 10.1093/oxfordjournals.aje.a116138. [DOI] [PubMed] [Google Scholar]
- 30.McCann SE, Ambrosone CB, Moysich KB, Brasure J, Marshall JR, Freudenheim JL, Wilkinson GS, Graham S. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr Cancer. 2005;53:33–41. doi: 10.1207/s15327914nc5301_4. [DOI] [PubMed] [Google Scholar]
- 31.Strom SS, Yamamura Y, Duphorne CM, Spitz MR, Babaian RJ, Pillow PC, Hursting SD. Phytoestrogen intake and prostate cancer: a case-control study using a new database. Nutr Cancer. 1999;33:20–25. doi: 10.1080/01635589909514743. [DOI] [PubMed] [Google Scholar]
- 32.Singh B, Mense SM, Bhat NK, Putty S, Guthiel WA, Remotti F, Bhat HK. Dietary quercetin exacerbates the development of estrogen-induced breast tumors in female ACI rats. Toxicol Appl Pharmacol. 2010;247:83–90. doi: 10.1016/j.taap.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]