Abstract
Background
Hospital readmission is a common, costly problem. Little is known regarding risk factors for readmission in older adults with cancer. This study aims to identify factors associated with 30-day readmission in a cohort of older medical oncology patients.
Setting/Participants
Adults age 65 and over hospitalized to an Oncology Acute Care for Elders Unit at Barnes-Jewish Hospital.
Measurements
Standard geriatric screening tests were administered in routine clinical care. Clinical data and 30-day readmission status were obtained through medical record review.
Results
677 patients met the inclusion criteria. 77% were white and 53% were male. Thoracic (32%), hematologic (20%), and gastrointestinal (18%) malignancies were most common. The 30-day unplanned readmission rate was 35.2%. Multivariable analyses identified complete dependence in feeding (odds ratio [OR], 3.70; 95% confidence interval [CI], 1.29 – 10.65), and some dependence (1.58, 1.04 – 2.41) and complete dependence (2.64, 1.70 – 4.12) in housekeeping, prior to admission, as associated with higher odds of readmission. Age < 75 (1.49, 1.04 – 2.14), African-American race (1.59, 1.06 – 2.39), potentially inappropriate medications (1.36, 0.94 – 1.99), and higher-risk reasons for index admission (1.93, 1.34 – 2.78) also increased odds of readmission. These factors were organized into a prognostic index.
Conclusion
Hospital readmission was common and higher than previously reported rates in general medical populations. We identified several previously unrecognized factors associated with increased risk for readmission, including some geriatric assessment parameters, and developed a practical tool that can be used by clinicians to assess risk of 30-day readmission.
Keywords: Cancer, Readmission, Geriatric assessment
INTRODUCTION
Hospital readmission is a common and costly problem, especially in older adults. From 2003–2004, the readmission rate among Medicare beneficiaries was 19.5%, with an estimated cost of $17.4 billion (1). Hospitalization places older patients at risk for functional decline and institutionalization (2–4). Furthermore, in 2012, the Centers for Medicare and Medicaid Services began reducing payments to hospitals with excessive readmission rates. A large proportion of readmissions are thought to be preventable (5); therefore, reducing readmissions in older adult patient populations is a potentially high-impact strategy to preserve quality of life, improve quality of care, and reduce health care costs.
Among older adults hospitalized to general medical wards, previously identified risk factors for readmission include malnutrition, advancing age, comorbidities, depression, and African-American race (6–8). However, readmission in medical oncology patients remains under-studied; one case-control study of 78 adults with cancer identified gastrointestinal cancer, nausea within 24 hours of discharge, and caregiver difficulty as associated with readmission (9). Risk factors for readmission in older adults with cancer have never been specifically investigated.
Geriatric assessment (GA) is a process of evaluating older adults for functional, psychosocial, or medical vulnerabilities, and developing a multidisciplinary treatment plan to optimize healthy aging. GA can improve outcomes related to survival, avoiding institutionalization, and preservation of functional status (10,11). In older adults with cancer, geriatric assessment can predict chemotherapy toxicity (12–14) or early death (15); however, whether GA predicts hospital readmission in older patients with cancer is unknown.
To investigate risk factors associated with readmission in a medical oncology population of adults over age 65, this study aims to determine whether a brief geriatric assessment predicts 30-day readmission in a cohort of older adults with cancer. We hypothesized that dependence in instrumental activities of daily living (IADLs) would predict 30-day unplanned readmission.
METHODS
Study design and participants
A retrospective cohort study was conducted on patients age 65 and over who were admitted to an Oncology Acute Care for Elders (OACE) unit at Barnes-Jewish Hospital (16), a nonprofit teaching hospital affiliated with Washington University School of Medicine (St. Louis, MO), from 2000–2008. Acute Care for Elders units utilize interdisciplinary teams to address and improve outcomes for hospitalized older adults. Eligible patients had cancer or were within one year of receiving treatment for cancer. The index admission was defined as the earliest hospitalization during which the patient completed the geriatric assessment. Patients who died during the index hospital stay or within 30 days of discharge from the index hospital stay without a preceding readmission were excluded from the cohort.
Measures
As part of routine clinical care on the OACE unit, a brief geriatric assessment battery consisting of the Katz Index of Activities of Daily Living (17), the Lawton Instrumental Activities of Daily Living Scale (18), the Clock Completion Test (19), and the Short Blessed Test of Orientation, Memory, and Concentration (20) was administered to all patients age 65 and older. The Clock Completion Test is scored by assessing accurate placement of clock digits in quadrants of a pre-drawn circle. A score of 4 or greater out of a possible 7 points indicates cognitive impairment. The Short Blessed Test has a score range from 0 to 28, with scores of 9 or higher indicating cognitive impairment. The assessment was administered within 72 hours of admission to the unit. Patients, or their caregivers if patients were unable to participate, were asked to use these scales to self-report their previous level of function (independent, somewhat dependent, or completely dependent), prior to the index admission. Because we hypothesized that some individual (instrumental) activities of daily living would be more predictive of readmission than others, the activities in the Katz and Lawton scales were analyzed individually rather than as a composite score. Comorbidity information was obtained at index admission by trained cancer registrars using the Adult Comorbidity Evaluation 27 (21). We used the 2012 Beers Criteria for Potentially Inappropriate Medication Use in Older Adults (22) to determine if any discharge medications were potentially inappropriate for use in older adults.
Data collection
Demographic and medical data were obtained through medical record review. Functional and cognitive assessment data were collected through review of OACE screening questionnaires. Information about patients’ cancer type, stage, and treatment intent (curative or palliative) was ascertained through review of patients’ oncology notes, pathology reports, radiographic data, and/or medical record review by one of the members of the study team (TW), a board-certified medical oncologist. Other clinical data included primary insurance, clinical diagnoses of dementia or depression, length of stay, patients’ living situation prior to admission, discharge disposition, discharge services such as home health or hospice, and number of medications at admission and discharge. We also recorded hemoglobin and albumin levels within 48 hours of discharge when those laboratory values were available.
The most urgent medical reason requiring hospital admission, or reason for admission (RFA), was ascertained through review of the index admission note, taking into account the patient’s chief complaint, physical exam findings, laboratory and radiographic information, and admitting physician’s assessment and plan. Reasons for admission were consolidated into broader system-based categories. For example, vomiting, dehydration, dysphagia, and diarrhea were classified as gastrointestinal reasons for admission. A taxonomy was iteratively developed to consistently assign RFAs to the correct category. Clinical judgment and consensus by two members of the study team were used to assign a reason for admission for cases that were ambiguous or multifactorial. Reasons for readmission were classified using the same taxonomy.
The primary endpoint, 30-day unplanned readmission, was defined as an unplanned admission to any hospital for any reason within 30 days of discharge from the index hospital stay. Elective or scheduled readmissions, such as for a planned procedure, were excluded from this definition. Evidence of readmission was ascertained from review of the electronic medical record, including outpatient treatment notes for evidence of admission to hospitals outside of the Barnes-Jewish Hospital system.
Data Analysis
Descriptive statistics (e.g., frequencies, mean, standard deviation) were completed on all variables. A univariate logistic regression model was used to examine the association of age, sex, race, place of residence prior to admission, discharge disposition, increase in level of care required after discharge, reason for admission, number of medications on admission and on discharge, increase in number of medications, Katz and Lawton items, Short Blessed Test and Clock Construction test scores, physical therapy consult, occupational therapy consult, skin breakdown, use of restraints or constant observer, dementia diagnosis, BMI, comorbidities, cancer type, cancer stage, treatment intent, presence of a current second cancer, insurance type, discharge services, inappropriate medications, depression, hemoglobin, albumin, and post-discharge emergency department visits against 30-day readmission. Multivariable analysis through stepwise selection was performed to examine the relationship between these covariates and 30-day readmission. A significance level of 0.1 was required to allow a predictor into the model, and a significance level of 0.15 was required for a predictor to stay in the model. The significance of the predictors in the final model was examined by the likelihood ratio test, and the performance of the model was tested by C-statistics. The Hosmer–Lemeshow test was used to test goodness of fit.
Nominal variables with multiple levels, such as cancer type and reason for admission, were dichotomized for analytic purposes into higher- and lower-risk categories based on the unadjusted odds ratio. Age, a continuous variable, was dichotomized into younger than 75 years and 75 years and older, based on previous studies (8) and the cohort’s median age. All analyses were performed using the statistical package SAS 9.3 (SAS Institute Inc., Cary, NC). A prognostic index was developed by assigning points to each variable in the adjusted analysis by dividing the odds ratio of each variable by the smallest odds ratio and rounding to the nearest whole number (23,24).
IRB approval
This study was reviewed and approved by the Washington University Human Research Protection Office.
RESULTS
Patient characteristics
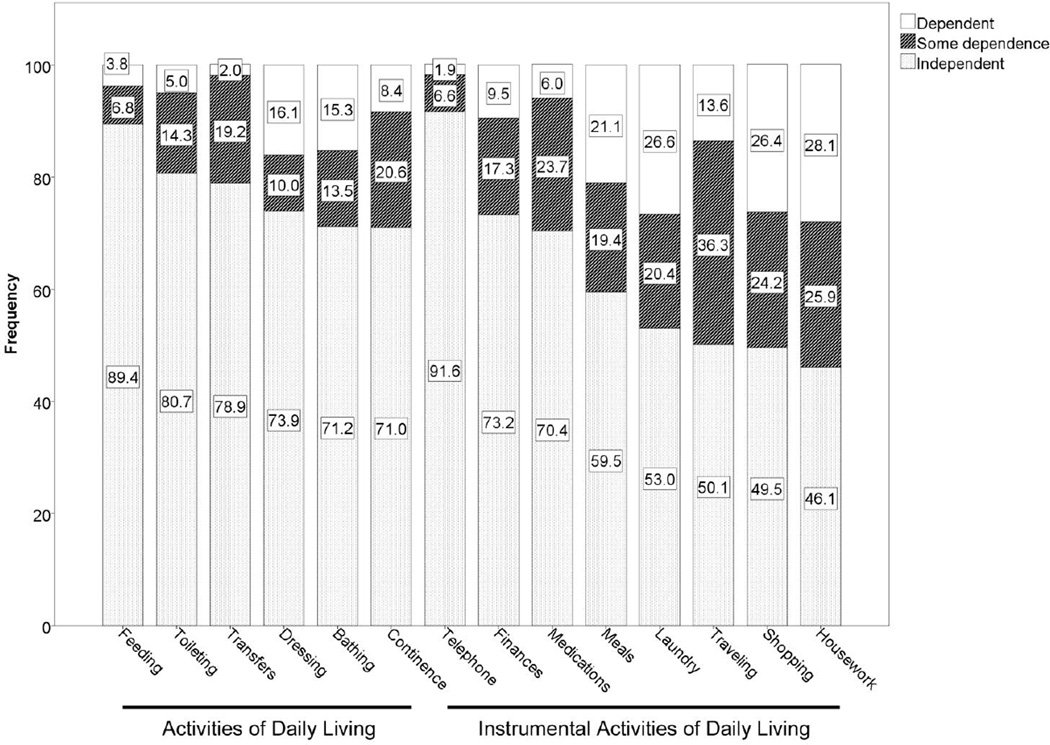
696 index hospitalizations met the inclusion criteria. Demographic, clinical, and geriatric assessment data are shown in Table 1. The median age of the cohort was 72.7 years, and the most common cancer type was solid thoracic tumors, followed by hematologic malignancies. Most cases of cancer were metastatic at the time of the index admission, and the treatment intent was palliative in 74% of cases. The frequency of an abnormal score on the Short Blessed Test or Clock Construction test were 22.3% and 41.8%, respectively, indicating that some degree of cognitive impairment was common. Most patients had mild or moderate comorbidities. Katz and Lawton data are displayed in Figure 1. While most patients were independent in all of their activities of daily living (54%, data not shown), dependence in one or more instrumental activities of daily living was common (72%, data not shown), and the frequency in dependence in the individual Katz and Lawton items was variable.
Table 1.
Patient Characteristics at Index Admission. Measures are reported as median (standard deviation) or n (percent). Denominators reflect missing data.
| Characteristic | Entire cohort (n = 677) | Readmitted patients (n = 238) |
Non-readmitted patients (n = 439) |
|---|---|---|---|
| Age, years | 72.7 (6.4) | 71.5 (5.9) | 73.3 (6.6) |
| Male gender | 356/677 (52.6%) | 120/238 (50.4%) | 236/439 (53.8%) |
| Race | |||
| White | 518/675 (76.7%) | 174/238 (73.1%) | 344/437 (78.7%) |
| African-American | 145 (21.5%) | 61 (25.6%) | 84 (19.2%) |
| Other | 12 (1.8%) | 3 (1.3%) | 9 (2.1%) |
| BMI | 24.0 (5.3) | 23.7 (5.0) | 24.2 (5.5) |
| Length of stay (days) | 6 (8.3) | 6 (7.8) | 5 (8.5) |
| Admitted from home | 618/677 (91.3%) | 223/238 (93.7%) | 400/439 (91.1%) |
| Discharged to home | 573/677 (84.6%) | 196/238 (82.4%) | 377/439 (85.9%) |
| Reason for index admission | |||
| Cardiovascular/Pulmonary | 165/676 (24.4%) | 59/238 (24.8%) | 106 (24.2%) |
| Gastrointestinal/Genitourinary | 161 (23.8%) | 69 (29.0%) | 92 (21.0%) |
| Lab abnormality/Scheduled Procedure | 87 (12.9%) | 19 (8.0%) | 68 (15.5%) |
| Pain control | 67 (9.9%) | 23 (9.7%) | 44 (10.0%) |
| Infection | 69 (10.2%) | 19 (8.0%) | 50 (11.4%) |
| Failure to thrive | 51 (7.5%) | 22 (9.2%) | 29 (6.6%) |
| Neurological | 46 (6.8%) | 21 (8.8%) | 25 (5.7%) |
| Bleeding | 30 (4.4%) | 6 (2.5%) | 24 (5.5%) |
| Reason for Readmission | |||
| Gastrointestinal/Genitourinary | 75/238 (31.5%) | ||
| Cardiovascular/Pulmonary | 53 (22.3%) | ||
| Failure to thrive | 26 (10.9%) | ||
| Infection | 23 (9.7%) | ||
| Neurological | 20 (8.4%) | ||
| Pain control | 18 (7.6%) | ||
| Laboratory abnormality | 15 (6.3%) | ||
| Bleeding | 8 (3.4%) | ||
| Cancer type | |||
| Thoracic | 216/677 (31.9%) | 85/238 (35.7%) | 131/439 (29.8%) |
| Hematologic | 134 (19.8%) | 38 (16.0%) | 96 (21.9%) |
| Gastrointestinal | 121 (17.9%) | 39 (16.4%) | 82 (18.7%) |
| Hepatobiliary | 70 (10.3%) | 32 (13.5%) | 38 (8.7%) |
| Genitourinary | 50 (7.4%) | 15 (6.3%) | 35 (80%) |
| Breast | 46 (6.8%) | 12 (5.0%) | 34 (7.7%) |
| Other | 40 (5.9%) | 17 (7.1%) | 23 (5.2%) |
| Cancer stage | |||
| Unknown | 13/677 (1.9%) | 1/238 (0.4%) | 12/439 (2.73%) |
| Stage I | 18 (2.6%) | 5 (2.1%) | 13 (3.0%) |
| Stage II | 30 (4.4%) | 9 (3.8%) | 21 4.8%) |
| Stage III | 106 (15.7%) | 33 (13.9%) | 73 (16.6%) |
| Stage IV | 362(53.5%) | 147 (61.8%) | 215 (49.0%) |
| Not applicable | 148 (21.9%) | 43 (18.1%) | 105 (23.9%) |
| Treatment intent | |||
| Curative | 175/677 (25.9%) | 52/238 (21.9%) | 123/439 (28.0%) |
| Palliative | 498 (73.6%) | 185 (77.7%) | 313 (71.3%) |
| Not applicable or unknown | 4 (0.6%) | 1 (0.4%) | 3 (0.7%) |
| Current second cancer | 36/677 (5.3%) | 16/238 (6.7%) | 20/439 (4.6%) |
| Insurance | |||
| Medicare with supplement | 412/622 (66.2%) | 144/226 (63.7%) | 268/396 (67.7%) |
| Private insurance | 73 (11.7%) | 28 (12.4%) | 45 (11.4%) |
| Medicare only | 65 (10.5%) | 24 (10.6%) | 41 (10.4%) |
| Medicaid | 48 (7.7%) | 22 (9.7%) | 26 (6.6%) |
| Other | 10 (3.0%) | 6 (2.7%) | 13 (3.3%) |
| Patient-pay | 5 (0.8%) | 2 (0.9%) | 3 (0.8%) |
| Hemoglobin (g/dL) closest to discharge | 10.4 (1.6) | 10.4 (1.5) | 10.4 (1.6) |
| Albumin (g/dL) closest to discharge | 3.1 (0.7) | 2.8 (0.7) | 3.3 (0.6) |
| Total number of medications on admission | 6 (3.7) | 6 (3.8) | 6 (3.6) |
| Total number of medications on discharge | 7 (3.8) | 8 (4.0) | 7 (3.7) |
| Change in number of medications | 1 (3.1) | 1 (3.1) | 1 (3.0) |
| ACE-27 ComorbidityIndex | |||
| None | 78/435 (17.9%) | 38/165 (23.0%) | 40/270 (14.8%) |
| Mild | 203 (46.7%) | 73 (44.2%) | 130 (48.2%) |
| Moderate | 108 (24.8%) | 39 (23.6%) | 69 (25.6%) |
| Severe | 46 (10.6%) | 15 (9.1%) | 31 (11.5%) |
| Dementia diagnosis noted in medical record | 25/669 (3.7%) | 6/237 (2.5%) | 19/432 (4.4%) |
| Any falls during hospitalization | 21/326 (6.4%) | 11/102 (10.8%) | 10/224 (4.5%) |
| Physical therapy consult | 385/568 (67.8%) | 150/202 (74.3%) | 235/366 (64.2%) |
| Occupational therapy consult | 313/528 (59.3%) | 119/188 (63.3%) | 194/340 (57.1%) |
| Presence or development of skin breakdown | 55/393 (14.0%) | 24/133 (18.1%) | 31/260 (11.9%) |
| Constant observer or restraints used | 15/281 (3.9%) | 7/128 (5.5%) | 8/253 (3.2%) |
| Inappropriate medications on discharge | 191/675 (28.3%) | 77/237 (32.5%) | 114/438 (26.0%) |
| Diagnosis of depression or antidepressant medications |
149/675 (22.1%) | 56/237 (23.6%) | 93/438 (21.2%) |
| Abnormal score on Clock Construction Test | 216/517 (41.8%) | 78/175 (44.6%) | 138/342 (40.4%) |
| Abnormal score on Short Blessed Test | 130/584 (22.3%) | 52/199 (26.1%) | 78/385 (20.3%) |
| Discharge services | |||
| None | 374/590 (63.4%) | 112/201 (55.7%) | 262/389 (67.4%) |
| Home health | 129 (21.9%) | 50 (24.9%) | 79 (20.3%) |
| Supportive care | 68 (11.5%) | 32 (15.9%) | 36 (9.3%) |
| Hospice | 19 (3.2%) | 7 (3.5%) | 12 (3.1%) |
Figure 1.
Frequency of Self-Reported Level of Dependence in Six Activities of Daily Living and Eight Instrumental Activities of Daily Living.
30-day readmission rate
The 30-day unplanned readmission rate was 238/677 (35.2%). 32.8% of readmitted patients had the same reason for admission on index and repeat admission. The most common reasons for readmission were from the Gastrointestinal/Genitourinary category (Table 1). In addition to the medical readmission rate, we also identified 3 inpatient psychiatric admissions and 24 emergency room visits that did not result in hospital admission.
Readmission rates in selected subgroups
Table 2 shows the readmission rate for selected subgroups. Notably, higher readmission rates were observed in patients who had any amount of ADL or IADL dependence. Several clinically important variables, such as albumin levels within 48 hours of discharge or the occurrence of falls during hospitalization, were strongly associated with readmission on univariate analysis in the logistic regression model, but could not be included in the final multivariable logistic model due to incomplete data.
Table 2.
Observed Readmission Rate in Selected Subgroups. Higher-risk reasons for index admission included failure to thrive, cardiovascular, pulmonary, gastrointestinal, genitourinary, and neurologic causes. Lower-risk reasons included laboratory abnormalities, scheduled procedure, bleeding, pain control, and infection.
| Variable | Readmission rate |
|---|---|
| Age | |
| 65–69 | 42.6% |
| 70–74 | 31.1% |
| 75–79 | 36.2% |
| Over 80 | 25.7% |
| Race | |
| African-American | 42.1% |
| Non-African-American | 33.4% |
| Reason for index admission | |
| Higher-risk | 40.4% |
| Lower-risk | 26.5% |
| Inappropriate medications | |
| Yes | 40.3% |
| No | 33.1% |
| Katz – Feeding | |
| Independent | 34.4% |
| Some Dependence | 33.3% |
| Dependent | 64.0% |
| Lawton – Housework | |
| Independent | 27.6% |
| Some Dependence | 36.4% |
| Dependent | 48.6% |
| Any ADL dependence | |
| Any dependence | 38.9% |
| No dependence | 32.6% |
| Any IADL dependence | |
| Any dependence | 39.0% |
| No dependence | 27.2% |
| Short Blessed Test score | |
| Normal | 32.4% |
| Abnormal | 40.0% |
| Clock Construction Test score | |
| Normal | 32.2% |
| Abnormal | 36.1% |
| Discharge disposition | |
| Home or domiciliary | 34.2% |
| Medical facility | 40.4% |
| Discharge services | |
| Yes | 41.2% |
| No | 29.9% |
| Falls during index admission | |
| Yes | 52.4% |
| No | 29.8% |
| BMI | |
| < 18.5 | 50.0% |
| 18.5–24.9 | 34.3% |
| 25–29.9 | 38.5% |
| ≥ 30 | 28.1% |
| Albumin (mg/dL) | |
| < 3.5 mg/dL | 42.5% |
| ≥ 3.5 mg/dL | 24.3% |
| Cancer stage | |
| Metastatic | 40.6% |
| Non-metastatic | 28.9% |
Factors associated with readmission
Multivariable analyses identified several factors associated with readmission (Table 3). African-American race was associated with higher odds of readmission (Odds ratio [OR] 1.59; 95% confidence interval [CI] 1.06 to 2.39). Patients younger than 75 years had higher odds of readmission relative to patients age 75 or older (1.49, 1.04 to 2.14). The highest percentage of admissions were for cardiovascular, pulmonary, failure to thrive, or neurologic reasons, which in turn were associated with higher odds for readmission (1.93, 1.34 to 2.78) relative to admissions for laboratory abnormalities, scheduled procedures, bleeding, pain control, or infection. Patients who were discharged with medications considered potentially inappropriate for older adults were at higher risk for readmission than patients who did not receive inappropriate medications (1.36, 0.94 to 1.99) at discharge. Having some dependence (1.58, 1.04 to 2.41) or total dependence (2.64, 1.70 to 4.12) in the housekeeping IADL increased the odds of readmission in a dose-dependent pattern. Compared to having some dependence in the feeding ADL, complete dependence was associated with higher odds of readmission (3.70, 1.29 to 10.65), but independence was not (1.80, 0.88 to 3.66). The c-statistic of the model was 0.666 and the model was a good fit to the data according to the Hosmer-Lemeshow Goodness-of-Fit Test (χ2(8) = 4.24, p = 0.83).
Table 3.
Risk Factors Associated With 30-day Readmission in Multivariable Analyses. Each variable is assigned a point value as part of the OACE Prognostic Index for Hospital Readmission.
| Variable | Adjusted OR (95% CI) | P-value | Points |
|---|---|---|---|
| Age < 75 | 1.49 (1.04 to 2.14) | 0.03 | 1 |
| African-American race | 1.59 (1.06 to 2.39) | 0.02 | 1 |
| Higher-risk reason for index admission |
1.93 (1.34 to 2.78) | < 0.01 | 1 |
| Inappropriate medications | 1.36 (0.94 to 1.99) | 0.11 | 1 |
| Katz – Feeding | 0.04 | ||
| Independent | 1.80 (0.88 to 3.66) | 1 | |
| Some Dependence | Reference | 0 | |
| Dependent | 3.70 (1.29 to 10.65) | 3 | |
| Lawton – Housework | < 0.01 | ||
| Independent | Reference | 0 | |
| Some Dependence | 1.58 (1.04 to 2.41) | 1 | |
| Dependent | 2.64 (1.70 to 4.12) | 2 | |
Prognostic index derivation
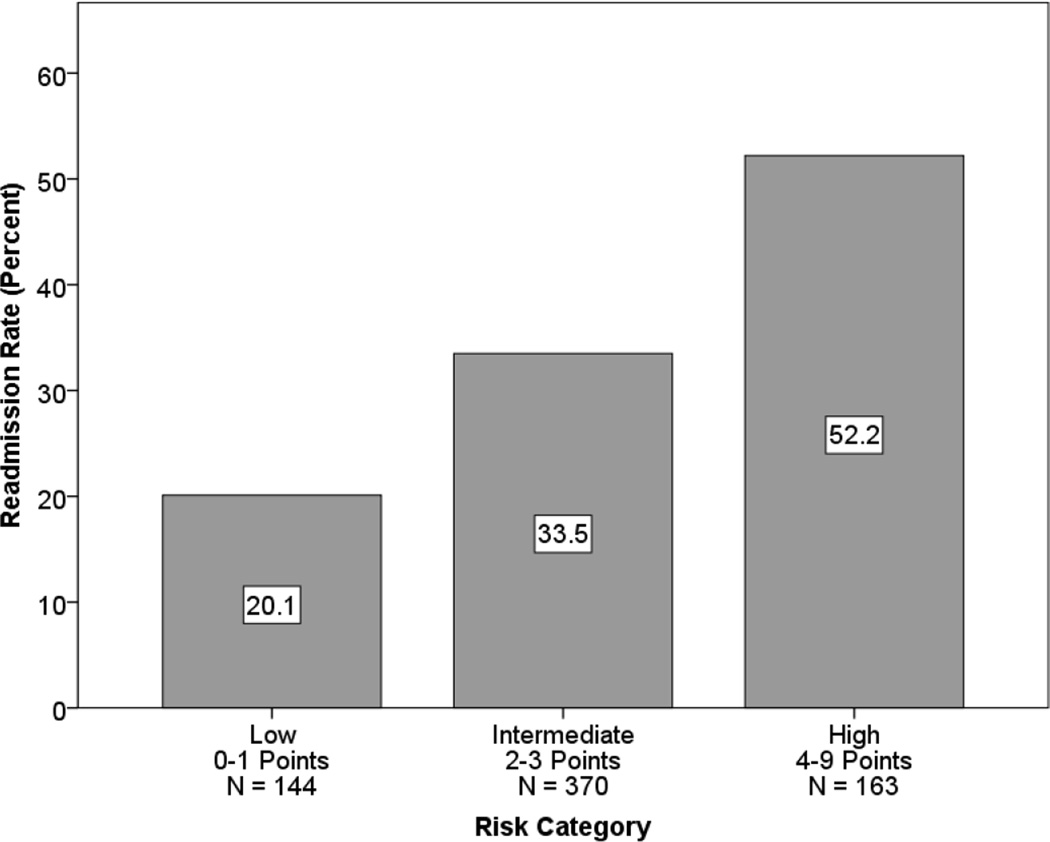
Using the variables in the adjusted analysis, we developed the OACE Prognostic Index for Hospital Readmission to use as a bedside tool to assess an individual patient’s readmission risk. The points assigned to each variable are shown in Table 3. Readmission risk scores were calculated for each patient by adding together the points for each risk factor that was present. The number of possible points ranged from 0 to 9, and patients were divided into three categories of 0–1, 2–3, and 4–9 points, corresponding with low, intermediate, and high risk categories. The readmission rate for each category was 20.1% (low), 33.5% (intermediate), and 52.2% (high), shown in Figure 2. A chi-square test of independence demonstrated that the relationship between readmission rate and risk category was significant (χ2(2) = 35.33, p < 0.0001).
Figure 2.
Readmission Rates by Risk Category.
DISCUSSION
In this retrospective cohort study of older patients with cancer, we identified several previously unrecognized risk factors for hospital readmission, including several geriatric assessment parameters. We discovered a 30-day unplanned readmission rate of 35.2%, which is much higher than previously reported readmission rates in general medical and geriatric patient populations.
Older medical oncology patients likely represent a uniquely vulnerable patient population; active or metastatic cancer has been associated with readmission in adult general medicine patients (25). Frailty and functional impairment may also make our patients more vulnerable to readmission. In our study, the ability to perform a complex functional task independently, housekeeping, was protective against unplanned readmissions, and markers of frailty (such as dependence in feeding or being admitted for failure to thrive) predicted readmission. These findings signal the role of function and frailty as potential targets for future study and interventions to reduce readmissions. Prospective studies are needed to determine if screening patients with brief functional status questions, such as ability to perform housekeeping, can help identify patients at risk of unplanned readmission.
In our study, we found that the use of inappropriate medications, as defined by the 2012 Beers Criteria, was associated with increased odds of readmission. A recent study of general medicine patients over age 60 demonstrated that high-risk medications not included in the Beers Criteria, such as opioid analgesics, also increased risk for readmission (26). As the use of high-risk or inappropriate medications is a potentially modifiable risk factor for readmission, these findings suggest that alternative medications should be used whenever possible to decrease the risk of readmission or other adverse events. However, in cases where no suitable alternatives exist, clinicians could consider observed potentially inappropriate medications at age-adjusted doses, and patients should be watched closely for signs of delirium or physical impairment that could lead to falls.
In our study, we found that older age protected patients against readmission, in contrast to previous reports (7,8). We observed that the incidence of ADL and IADL dependence increased with age and that older patients were more likely to be discharged to another healthcare facility, or discharged to home with home health services, including palliative and hospice care. In a survey of patient and caregiver attitudes about inpatient versus in-home care, Kirk et al found that older adults preferred to be cared for at home (27). We postulate that the recognition of older patients’ frailty led to them receiving more medical care, or changes in their goals of care, which de-emphasized hospitalization and in turn resulted in fewer readmissions. Early data also suggest that care provided by an inpatient palliative care consult team can reduce the probability of a hospital readmission (28). Thus, our findings signal a potential role for more palliative care and home health care involvement as a means to reduce preventable readmission.
This study has several limitations. First, as a tertiary care center, Barnes-Jewish Hospital may serve a patient population with more serious or complex medical issues than what is seen by community hospitals. In our cohort, the OACE team made recommendations that were incorporated into clinical care, limiting the generalizability of our findings to regular medical oncology patients. Randomized controlled trials comparing ACE unit care to usual inpatient care have also demonstrated that the interventions provided on ACE units are effective in reducing readmission (29), so the patients receiving OACE unit care may have had a lower risk of readmission than patients receiving care without any geriatric-specific recommendations. Second, although patient records were reviewed for evidence of readmission to outside hospitals, readmission to hospitals outside the Barnes-Jewish Hospital system may have gone undetected. Considering these limitations, the readmission rate among older medical oncology patients may be even higher than we reported. Additionally, previous studies note that patients overestimate their level of independence in ADLs and IADLs relative to objective clinical assessment (30). Because ADL and IADL information in our study was obtained from patients or caregivers, our patients may have over-reported independence in ADLs/IADLs on the geriatric assessment. Other factors that likely contribute to readmission, but were not evaluated by the brief geriatric assessment administered on the OACE, include the occurrence of delirium and lack of social support. Finally, the retrospective nature of this study limits the generalizability of our findings; we plan to address this limitation by validating our model in a prospective cohort study, which is ongoing.
CONCLUSION
In this retrospective cohort study of hospitalized older adults with cancer, we investigated risk factors for readmission in a population not adequately evaluated in the literature. We found that greater than 1 in 3 patients experienced hospital readmission within 30 days of discharge from the index hospitalization. This readmission rate is much higher than previously reported rates in general medical patient populations and has important implications for both cost and quality of care. Geriatric syndromes such as cognitive impairment and functional dependence were common. We identified several modifiable and previously unrecognized risk factors, obtained during geriatric assessment, that were associated with 30-day readmission. We are also in the process of validating our prognostic index as a practical tool be used by clinicians to identify patients who may be at higher risk of readmission. We conclude that geriatric assessment provides valuable clinical information, and that interventions (31,32) targeted toward some of the risk factors we identified may reduce readmission in this patient population. As oncologists have close follow-up with their patients in the outpatient setting, they may be best positioned to identify and address geriatric care needs in order to prevent hospital readmission.
ACKNOWLEDGMENTS
This publication was supported by the National Cancer Institute of the National Institutes of Health (NIH), Grant Number 1K12CA167540 and the Clinical Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences at the National Institutes of Health, Grant Number UL1RR024992. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
-
-Association for Clinical and Translational Science, 2014 National Meeting
-
-American Geriatrics Society, 2014 Annual Meeting
REFERENCES
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among Patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 2.Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss of Independence in Activities of Daily Living in Older Adults Hospitalized with Medical Illnesses: Increased Vulnerability with Age. J Am Geriatr Soc. 2003;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 3.Gill TM, Allore HG, Holford TR, Guo Z. HOspitalization, restricted activity, and the development of disability among older persons. JAMA. 2004 Nov 3;292(17):2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 4.Palmer RM. Acute hospital care of the elderly: minimizing the risk of functional decline. Cleve Clin J Med. 1995 Apr;62(2):117–128. doi: 10.3949/ccjm.62.2.117. [DOI] [PubMed] [Google Scholar]
- 5.Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ Can Med Assoc J J Assoc Medicale Can. 2011 Apr 19;183(7):E391–E402. doi: 10.1503/cmaj.101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedmann JM, Jensen GL, Smiciklas-Wright H, McCamish MA. Predicting early nonelective hospital readmission in nutritionally compromised older adults. Am J Clin Nutr. 1997 Jun 1;65(6):1714–1720. doi: 10.1093/ajcn/65.6.1714. [DOI] [PubMed] [Google Scholar]
- 7.Marcantonio ER, McKean S, Goldfinger M, Kleefield S, Yurkofsky M, Brennan TA. Factors associated with unplanned hospital readmission among patients 65 years of age and older in a medicare managed care plan. Am J Med. 1999 Jul;107(1):13–17. doi: 10.1016/s0002-9343(99)00159-x. [DOI] [PubMed] [Google Scholar]
- 8.Silverstein MD, Qin H, Mercer SQ, Fong J, Haydar Z. Risk factors for 30-day hospital readmission in patients >=65 years of age. Proc Bayl Univ Med Cent. 2008 Oct;21(4):363–372. doi: 10.1080/08998280.2008.11928429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver C, Schiech L, Held-Warmkessel J, Kedziera P, Haney E, DiLullo G, et al. Risk for Unplanned Hospital Readmission of Patients With Cancer: Results of a Retrospective Medical Record Review. Oncol Nurs Forum. 2006 Jan 1;33(3):E44–E52. doi: 10.1188/06.ONF.E44-E52. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann S, Finger C, Huss A, Egger M, Stuck AE, Clough-Gorr KM. Inpatient rehabilitation specifically designed for geriatric patients: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;340:c1718. doi: 10.1136/bmj.c1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis G, Whitehead MA, Robinson D, O’Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. 2011;343:d6553. doi: 10.1136/bmj.d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012 Jul 1;118(13):3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 13.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol Off J Am Soc Clin Oncol. 2011 Sep 1;29(25):3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wildes TM, Ruwe AP, Fournier C, Gao F, Carson KR, Piccirillo JF, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J Geriatr Oncol. 2013 Jul;4(3):227–234. doi: 10.1016/j.jgo.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soubeyran P, Fonck M, Blanc-Bisson C, Blanc J-F, Ceccaldi J, Mertens C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2012 May 20;30(15):1829–1834. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 16.Flood KL, Carroll MB, Le CV, Ball L, Esker DA, Carr DB. Geriatric Syndromes in Elderly Patients Admitted to an Oncology–Acute Care for Elders Unit. J Clin Oncol. 2006 May 20;24(15):2298–2303. doi: 10.1200/JCO.2005.02.8514. [DOI] [PubMed] [Google Scholar]
- 17.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: The index of adl: a standardized measure of biological and psychosocial function. JAMA. 1963 Sep 21;185(12):914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 19.Watson YI, Arfken CL, Birge SJ. Clock completion: an objective screening test for dementia. J Am Geriatr Soc. 1993 Nov;41(11):1235–1240. doi: 10.1111/j.1532-5415.1993.tb07308.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawas C, Karagiozis H, Resau L, Corrada M, Brookmeyer R. Reliability of the Blessed Telephone Information-Memory-Concentration Test. J Geriatr Psychiatry Neurol. 1995 Oct;8(4):238–242. doi: 10.1177/089198879500800408. [DOI] [PubMed] [Google Scholar]
- 21.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr PRognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004 May 26;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 22.Panel TAGS 2012 BCUE. American Geriatrics Society Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2012;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Concato J, Feinstein AR, Holford TR. The Risk of Determining Risk with Multivariable Models. Ann Intern Med. 1993 Feb 1;118(3):201–210. doi: 10.7326/0003-4819-118-3-199302010-00009. [DOI] [PubMed] [Google Scholar]
- 24.Levine SK, Sachs GA, Jin L, Meltzer D. A Prognostic Model for 1-Year Mortality in Older Adults after Hospital Discharge. Am J Med. 2007 May;120(5):455–460. doi: 10.1016/j.amjmed.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Allaudeen N, Vidyarthi A, Maselli J, Auerbach A. Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54–60. doi: 10.1002/jhm.805. [DOI] [PubMed] [Google Scholar]
- 26.Pavon JM, Zhao Y, McConnell E, Hastings SN. Identifying risk of readmission in hospitalized elderly adults through inpatient medication exposure. J Am Geriatr Soc. 2014 Jun;62(6):1116–1121. doi: 10.1111/jgs.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirk E, Prasad MK, Abdelhafiz AH. Hospital readmissions: patient, carer and clinician views. Acute Med. 2006;5(3):104–107. [PubMed] [Google Scholar]
- 28.Nelson C, Chand P, Sortais J, Oloimooja J, Rembert G. Inpatient Palliative Care Consults and the Probability of Hospital Readmission. Perm J. 2011;15(2):48–51. doi: 10.7812/tpp/10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flood KL, MacLennan PA, McGrew D, Green D, Dodd C, Brown CJ. EFfects of an acute care for elders unit on costs and 30-day readmissions. JAMA Intern Med. 2013 Jun 10;173(11):981–987. doi: 10.1001/jamainternmed.2013.524. [DOI] [PubMed] [Google Scholar]
- 30.Sager MA, Dunham NC, Schwantes A, Mecum L, Halverson K, Harlowe D. Measurement of activities of daily living in hospitalized elderly: a comparison of self-report and performance-based methods. J Am Geriatr Soc. 1992 May;40(5):457–462. doi: 10.1111/j.1532-5415.1992.tb02011.x. [DOI] [PubMed] [Google Scholar]
- 31.Koehler BE, Richter KM, Youngblood L, Cohen BA, Prengler ID, Cheng D, et al. Reduction of 30- day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med Off Publ Soc Hosp Med. 2009 Apr;4(4):211–218. doi: 10.1002/jhm.427. [DOI] [PubMed] [Google Scholar]
- 32.Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA J Am Med Assoc. 1999 Feb 17;281(7):613–620. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]