Abstract
Adenosine-5′-triphosphate is released by neuroendocrine, endocrine, and other cell types and acts as an extracellular agonist for ligand-gated P2X cationic channels and G protein-coupled P2Y receptors in numerous organs and tissues, including the endocrine system. The breakdown of ATP by ectonucleotidases not only terminates its extracellular messenger functions, but also provides a pathway for the generation of two additional agonists: adenosine 5′-diphosphate, acting via some P2Y receptors, and adenosine, a native agonist for G protein-coupled adenosine receptors, also expressed in the endocrine system. This article provides a review of purinergic signaling pathways in the hypothalamic magnocellular neurosecretory cells and neurohypophysis, hypothalamic parvocellular neuroendocrine system, adenohypophysis, and effector glands organized in five axes: hypothalamic-pituitary-gonadal, hypothalamic-pituitary-thyroid, hypothalamic-pituitary-adrenal, hypothalamic-pituitary-growth hormone, and hypothalamic-pituitary-prolactin. We attempted to summarize current knowledge of purinergic receptor subtypes expressed in the endocrine system, including their roles in intracellular signaling, hormone secretion, and other cell functions. We also briefly review the release mechanism for adenosine-5′-triphosphate by neuroendocrine, endocrine and surrounding cells, the enzymes involved in adenosine-5′-triphosphate hydrolysis to adenosine-5′-diphosphate and adenosine, and the relevance of this pathway for sequential activation of receptors and termination of signaling.
Keywords: hypothalamus, pituitary, thyroid gland, adrenal gland, ovary, testis
1. Introduction
The endocrine system controls reproduction, growth and development, homeostasis, and metabolism. Hormones, the endocrine system products, are released from cells directly into the circulation, therefore affecting tissues and cells distant from the site of secretion. Instead of communicating directly with other neurons through synapses, some neurons secrete chemicals that act as hormones. Such a system is called neuroendocrine, and their products are termed neurohormones. Evolutionary, the most primitive endocrine/neuroendocrine system is composed of single endocrine and neuroendocrine cells. Their locally released agents act in a paracrine or autocrine manner, but may also enter the circulation and affect the function of distant cells. In vertebrates, single endocrine and neuroendocrine cells are also present and distributed withinthe pithelium of the airways, cardiovascular system, the gastro-entero-pancreatic region, and the genito-urinary tract; this is defined as the diffuse endocrine system. True endocrine glands, the ductless structures committed to production, storage and release of hormones, evolved much later and are best developed in vertebrates.
The majority of hormone production by endocrine glands is regulated by the hypothalamus and includes the pituitary with two anatomically and physiologically distinct systems. The first is termed the hypothalamo-posterior pituitary system and is composed of hypothalamic magnocellular neurosecretory cells and neurohypophysis. The second, the hypothalamo-anterior pituitary system, is composed of hypothalamic parvocellular neurosecretory cells, median eminence, and adenohypophysis. It also includes the peripheral endocrine glands and numerous tissues regulated by the anterior pituitary hormones and is allocated to five axes: hypothalamic-pituitary-gonadal, hypothalamic-pituitary-thyroid, hypothalamic-pituitary-adrenal, hypothalamic-pituitary-growth hormone, and hypothalamic-pituitary-prolactin (Low, 2011). Likewise, the autonomous nervous system – the endocrine system interplay is conveyed mainly via neuroendocrine control of the pituitary or via hypothalamic control of the preganglionic neurons of the autonomous nervous system located in the brainstem and spinal cord. Direct parasympathetic and/or sympathetic innervation is also present in some endocrine organs, although they are generally poorly innervated. Such innervation plays important roles in the control of endocrine pancreas, adrenal medulla, and adrenal cortex functions (Edwards, 1997; Miller, 1981; Parker et al., 1993).
Thanks to the pioneer efforts of Geoffrey Burnstock (Burnstock, 1972), adenosine-5′-triphosphate (ATP) is now recognized as the signaling molecule acting in the extracellular space and that ATP release pathways, ectonucleotidases, and three classes of purinergic receptors compose the purinergic signaling system.
ATP can be released from virtually every cell, in physiological and pathological conditions, and its extracellular concentrations can rise significantly. Some of the magnocellular, parvocellular, and autonomous nervous system neurons co-secrete ATP; endocrine and/or surrounding cells may release it too (Stojilkovic et al., 2010a). The released ATP acts as an extracellular ligand for two families of purinergic receptors, two-transmembrane domain P2X receptor channels (P2XRs) and seven-transmembrane domain P2Y receptors (P2YRs) (Coddou et al., 2011) (Fig. 1a, top), both being expressed in a variety of endocrine cells (Burnstock, 2014; Stojilkovic et al., 2013).
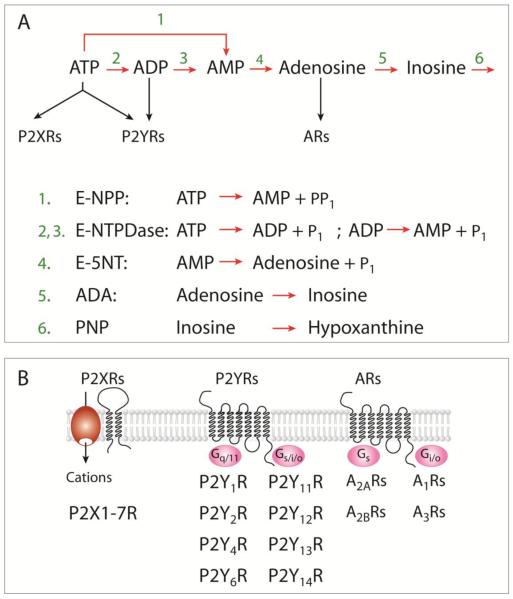
Fig. 1.
Purinergic signaling pathways. A) The nucleotide-hydrolyzing pathways. The extracellularly released ATP is hydrolyzed to AMP by two enzyme families, E-NPP and E-NTDPase, whereas AMP was efficiently hydrolyzed by E-5NT. Adenosine is further deaminated via inosine into hypoxanthine by ADA and purine nucleoside phosphorylase (PNP), respectively. B, ATP is an agonist for two transmembrane domain P2XRs and several seven transmembrane domain P2YRs, whereas ADP activates a few P2YRs but not P2XRs. Adenosine is also an agonist at four G-protein-coupled ARs. Three subunits in homomeric or heteromeric organization are required for formation of functional P2XRs, whereas dimerization is possible for P2YRs and ARs. Derived from (Stojilkovic, 2009).
Seven mammalian purinergic receptor subunits, denoted P2X1 through P2X7, and several spliced forms of these subunits have been cloned. Each subunit is proposed to contain cytoplasmically located N-and C-termini with consensus binding motifs for protein kinases, two transmembrane helices connected by a large extracellular loop, with 10 conserved cysteine residues forming a series of disulfide bridges. Functional channels are organized as trimeric homomers and heteromers (Nicke et al., 2005). P2XR subtypes differ with respect to their ligand selectivity profile, antagonist sensitivity, and cation selectivity. P2XR activation leads to inward currents associated with increased intracellular calcium and C-termini with consensus binding motifs for protein kinases, two transmembrane helices connected by a large extracellular loop, with 10 conserved cysteine residues forming a series of disulfide bridges (Fig. 1b).
Eight mammalian P2YRs have been identified and denoted P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, and P2Y14R. Phylogenetically, these receptors form two subgroups. Members of the first group (1, 2, 4, and 6) signal through Gq/11 pathways (Fig. 1B), activating phospholipase C to generate inositol 1,4,5-trisphosphate and diacylglycerol. In excitable cells, inositol trisphosphate-induced calcium mobilization is frequently accompanied by calcium influx through Cav channels. Activation of MAP kinase and phospholipase D signaling pathways, both secondary to the activation of protein kinase C, has also been reported for P2YRs. The second group (11, 12, 13, and 14) shows variations in coupling to G proteins, including Gi/o, Gs, and G16 (Fischer et al., 2007).
The duration and extent of ATP actions are limited by several ectonucleotidases, which hydrolyze purine nucleotides and nucleosides. Ectonucleotidase enzymes are present in endocrine cells (Burnstock, 2014; Stojilkovic, 2009). These enzymes include members of the ectonucleotide triphosphate diphosphohydrolase family (E-NTPDase), ectonucleotide pyrophosphatase/phosphodiesterase family (E-NPPase) and ecto-5′-nucleotidase (E-5NT), among others (Fig. 1a, bottom). E-NTPDases not only hydrolyze extracellular ATP and/or adenosine-5′-diphosphate (ADP) to adenosine 5′-monophosphate (AMP), but also metabolize other nucleotide tri- and diphosphates, including uridine triphosphate and uridine diphopshate, whereas E-NPPases hydrolyze ATP directly to AMP. AMP is hydrolyzed by E-5NT to adenosine (Zimmermann, 2000). ADP and adenosine act as extracellular ligands too, ADP being a potent agonist for some P2YRs and adenosine an agonist for adenosine receptors (ARs) (Ralevic et al., 1998).
Four different adenosine-activated receptors have been cloned, termed A1R, A2AR, A2BR, and A3R. These receptors signal mainly through adenylyl cyclase. A1R and A3R are negatively coupled to adenylyl cyclase through pertussis toxin-sensitive Gi/o, whereas A2AR and A2BR are positively coupled through cholera toxin-sensitive Gs (Fig. 1b). The human A2BR has also been reported to signal through Gq/11-dependent phospholipase C. The intracellular pathways triggered by these receptors include Cav channels and inwardly rectifying K+ (Kir) channels, and activation of proteins involved in MAP kinase signaling (Burnstock, 2007).
This review focuses on purinergic signaling in the hypothalamo-posterior pituitary system and hypothalamic parvocellular neuroendocrine system and adenohypophysis (the hypothalamic-pituitary axes). For the role of purines in the sympathetic-adrenal medulla system see (Burnstock, 2014).
2. Purinergic regulation of the hypothalamic-pituitary unit
The hypothalamus contains a number of nuclei that are involved in a variety of functions, including control of food and water intake, sexual behavior, reproduction, and daily cycles in physiological state and behavior, temperature regulation, and mediation of emotional responses. Anatomically and based on rostrocaudal landmarks, three hypothalamic regions are discernible: anterior, medial (also known as tuberal), and posterior (Fig. 2A). The anterior hypothalamus contains the paraventricular nucleus (PVN), supraoptic nucleus (SON), and suprachiasmatic nucleus (SCN). The medial hypothalamus contains two major nuclei, the arcuate and the ventromedial, whereas the lateral hypothalamus is more loosely organized. The posterior hypothalamus contains the mammillary and posterior nuclei. The neuroendocrine hypothalamus comprises the PVN, SON, SCN, arcuate nucleus, and preoptic area. By the size of the cell body, two types of neuroendocrine cells in the hypothalamus are discernible: magnocellular (big) and parvocellular (small).
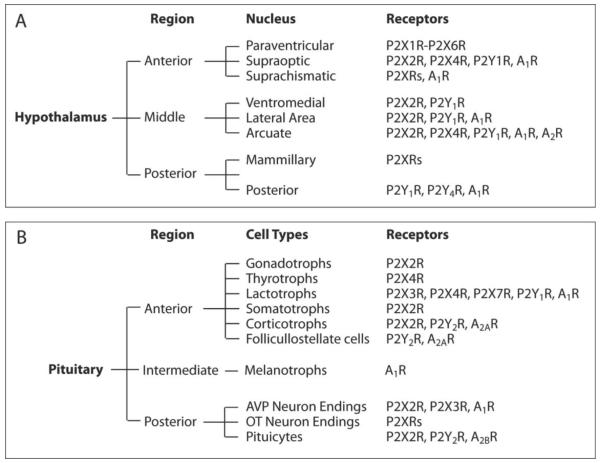
Fig. 2.
Expression of purinergic receptors in hypothalamo-pituitary unit. Receptors and receptor channels expressed in hypothalamic nuclei (A) and pituitary gland (B). P2XRs and A2Rs, unidentified subtype(s) of purinergic receptors. For references see the corresponding sections.
2.1. The hypothalamic magnocellular neurosecretory cells and neurohypophysis
Magnocellular neurons are located within PVN and SON and project non-myelinated axons directly to the posterior pituitary (PP) or neurohypophysis, where the content of their secretory vesicles is released near fenestrated capillaries. The cell bodies of magnocellular PVN neurons, adjacent to the third ventricle, synthesize either vasopressin (VP) or oxytocin (OT) (Clifton et al., 2009), hormones which play important roles in water balance, blood pressure, parturition and lactation (Goodman, 2009). Dynamics of VP and OT release from PP depends on the rate and pattern of neuronal electrical activity, which is neuron type-specific (Armstrong, 2007). Due to convergence of extensive afferent pathways on the magnocellular neurons in the pre- and post-synaptic modes, as well as actions at neuron terminals in the PP, numerous neurotransmitters and humoral factors modulate electrical activity and affect hormone release from the PP (Li et al., 2007; Sladek et al., 2001).
Several lines of evidence indicate that the purinergic signaling pathway is operative in the magnocellular neurosecretory system and PP and plays important role(s) in controlling neuronal activity. Microinjection of ATP in PVN and SON causes VP release (Mori et al., 1992; Mori et al., 1994), indicating the presence of functional purinergic receptors on neuronal cell bodies. Moreover, caudal medulla neurons that project to VP neurons in SON release ATP as a cotransmitter with norepinephrine (Day et al., 1993). Each of these transmitters causes a small and transient amplitude response, whereas their coapplication causes a larger and sustained response in hormone secretion. This synergism appears to involve receptors on the perikaryon of VP and OT neurons, including several subtypes of P2XRs and α1 adrenergic receptors (Gomes et al., 2009; Sladek et al., 2008; Song et al., 2010). Others also indicated the expression of multiple P2XRs in SON neuronal bodies and described their activation induced rise in intracellular calcium concentration ([Ca2+]i) (Shibuya et al., 1999). ATP and uridine triphosphate also depolarize SON neurosecretory cells in vitro, suggesting a role of P2XRs and P2Y2R in this process (Hiruma et al., 1995).
Purinergic signaling in SON and PVN is not limited to the neurons and their nerve endings in the PP, but also includes astrocytes in these nuclei and pituicytes in PP. It was shown that glial cells contribute to ATP release in the PVN (Gordon et al., 2005). Astrocytes in SON express calcium-mobilizing P2Y1R (Espallergues et al., 2007) as well as calcium-controlled small K+ channels (Armstrong et al., 2005). P2Y1R activation may account for stimulation of these channels and synchronization of electrical activity with calcium mobilization. The majority of pituicytes in primary cultures respond to ATP with a rapid phospholipase C-dependent and extracellular calcium-independent rise in [Ca2+]i. indicating the presence of functional P2YRs in these cells (Troadec et al., 1999).
The VP- and OT-containing secretory vesicles in SON and PVN magnocellular neurons contain and release ATP (Troadec et al., 2002; Troadec et al., 1998), but no conclusive evidence was obtained to show the exocytotic nature of this release. Pannexin 2 channels have been implicated in nonvesicular ATP release (Thompson et al., 2008), and they are abundantly expressed on VP positive fibers in PP (Li et al., 2011a). Endogenously released ATP in PP during stimulation is sufficient to depolarize the nerve terminals and potentiate VP release (Knott et al., 2008). Several purinergic receptor subtypes are expressed in VP and OT neurons and nerve terminals (Custer et al., 2012; Gomes et al., 2009; Guo et al., 2009; Knott et al., 2012; Song et al., 2006; Song et al., 2007). ATP may act on nerve terminals in the PP by activating P2XRs, as indicated by [Ca2+]i measurements in isolated PP terminals (Troadec et al., 1998). More recent studies suggested that both P2XR and P2YR subtypes account for ATP-induced increase in [Ca2+]i in SON neurons and hormone release from the PP (Song et al., 2006; Song et al., 2007). Electrophysiological evidence was obtained for the existence of P2XR currents in VP-PP terminals but not in terminals labeled for OT (Knott et al., 2005). However, others suggested that P2XRs are also expressed in OT-containing neurons (Guo et al., 2009).
The endogenously released ATP on SON neurons can be degraded to adenosine, which in turn activates A1R causing modulation of electrical and secretory activity of magnocelullar neurons (Oliet et al., 1999). Extracellularly added ATP is rapidly hydrolyzed in isolated PP, indicating that endogenous ectonucleotidase activity terminates signaling actions of ATP and purinergic stimulation of hormone secretion from nerve endings (Thirion et al., 1996). Adenosine accumulation is also detected upon AMP addition, further suggesting the existence of a complete catalytic cascade, which leads to activation of ARs in PP (Sperlagh et al., 1999). The application of adenosine alone can inhibit voltage-gated calcium (Cav) channels in dissociated SON via A1R signaling through Gz heterotrimeric proteins (Noguchi et al., 2000), leading to inhibition of VP secretion (Song et al., 2005; Wang et al., 2002). A model that can explain how purinergic (through A1R) and/or opioid (through μ receptors) feedback modulation during bursts could mediate differences in the control of neurohypophysial VP vs. OT release was also proposed (Lemos et al., 2012). The presence of A1R immunoreactivity in the SON, the accumulation of endogenous adenosine, and its strong inhibitory influence on magnocellular activity, presumably reflecting activation of inwardly rectifying potassium channels, has also been reported (Ponzio et al., 2005). Endogenous levels of adenosine should be sufficient to activate A1Rs in nerve terminals, leading to inhibition of Cav channels and inhibition of hormone secretion (Knott et al., 2007). Finally, ATP hydrolysis by ectonucleotidases and activation of A1Rs changes pituicytes from a flat to stellate morphology (Rosso et al., 2002) and thus has a profound effect on the three-dimensional relationships within PP.
Taken together, these results indicate that purinergic signaling is present in both SON and PVN neurons with nerve endings in the PP, and that astrocytes and pituicytes contribute to the functional operation of this system. P2X2Rs and probably other P2XRs provide a depolarizing pathway, leading to facilitation of electrical activity, calcium signaling, and hormone release. On the other hand, the rapid hydrolysis of ATP is likely to account for activation of P2Y1R (through ADP) and A1R (through adenosine). Adenosine can end ATP-induced signaling either by attenuating bursts of action potentials in the magnocellular neurons or by terminating peptide release. This sequential pattern of purinergic signaling triggered by degradation of ATP to ADP and adenosine, a stimulatory action on electrical activity mediated by P2XRs and P2YRs and an inhibitory action mediated by ARs, is not unique for the magnocellular neurosecretory system and neurohypophysis (see below).
2. 2. The hypothalamic parvocellular neuroendocrine system
The parvocellular neurosecretory system comprises neurons from several hypothalamic nuclei that project to the median eminence and release neurohormones in the hypophyseal portal blood. The majority of these neurons lie in the arcuate nucleus and medial parts of PVN. Medial parts of PVN contain parvocellular VP and OT neurons. Additional parvocellular neurosecretory neurons are found in preoptic periventricular area, medial septal nucleus and diagonal bands of Broca. These neurons stimulate or inhibit the secretion of anterior pituitary hormones.
Several P2XRs appear to be expressed in the PVN parvocellular neurons (Cham et al., 2006) and the presence of homomeric and heteromeric P2X2Rs was confirmed by single channel analysis (Whitlock et al., 2001). SON neurons express functional presynaptic and extrasynaptic P2X2R and P2X4R that modulate glutamate and GABA release and control the electrical excitability (Vavra et al., 2011). A group of PVN neurons, not belonging to magnocellular or parvicellular group, projects to preganglionic and/or related nuclei and is involved in central control of the autonomic nervous system (Clifton et al., 2009). It has been shown that purinergic neurotransmission within the PVN is also involved in the control of sympathetic nerve activity via P2R activation. The same group also showed an interaction between P2R and non-NMDA glutamate receptors in the PVN, suggesting that it might be important in the regulation of sympathetic outflow (Ferreira-Neto et al., 2013).
In the SCN, the primary circadian pacemaker in mammals, there is a circadian rhythm in ATP intracellular levels (Yamazaki et al., 1994) and release (Womac et al., 2009), the latter suggesting that these oscillations represent a physiological output of the mammalian cellular clock. In addition, ATP release in SCN astrocytes is coupled with mitochondrial calcium signaling (Burkeen et al., 2011). Once in the extracellular space, ATP might activate P2X2R, P2X4R, P2X6R, and/or P2XR7, since these mRNA transcripts were found in rat SCN (Bhattacharya et al., 2013; Collo et al., 1996). The P2X5R protein expression was also confirmed in SCN (Xiang et al., 2006) and its ability to form heterotrimers with P2X2 subunits may be of some functional importance in the nervous tissue (Compan et al., 2012). Activation of presynaptic P2X2R potentiates inhibitory synaptic transmission within the SCN (Bhattacharya et al., 2013). Adenosine signaling in SCN could inhibit glutamatergic retinohypothalamic neurotransmission via A1R, as it has been shown for hamsters (Hallworth et al., 2002) and mice (Sigworth et al., 2003).
The medial preoptic area and arcuate nucleus contain gonadotropin-releasing hormone (GnRH) neurons projecting to the median eminence, where they release this decapeptid, which regulates hypothalamic-pituitary-gonadal axes in a sex-specific manner (see section 3). The arcuate nucleus also contains neurons that control other endocrine functions of the anterior pituitary by secreting both releasing and inhibitory neurohormones at the median eminence, including growth hormone-releasing hormone (GHRH) and dopamine. GHRH regulates the hypothalamic-pituitary-growth hormone axis (see section 6) and dopamine regulates the hypothalamic-pituitary-prolactin axis (see section 7).
The expression of P2X2 mRNA was found in the arcuate nucleus (Kanjhan et al., 1999) and electrophysiological experiments indicated that dissociated rat arcuate neurons express functional homomeric and/or heteromeric P2X2Rs (Wakamori et al., 2004). Imunohistochemical analysis also indicated the expression of these channels in the arcuate nucleus (Collden et al., 2010; Vulchanova et al., 1996; Xiang et al., 1998). Some P2X2R positive neurons coexpress the orexigenic peptides neuropeptide Y and/or agouti-related protein, suggesting that purinergic signaling might be involved in the regulation of food intake (Collden et al., 2010). Immunopositive P2Y1R cells were also detected in the arcuate nucleus and their expression is modified by reduced food availability (Seidel et al., 2006). The arcuate nucleus neurons also express A1R and A2R and adenosine inhibits calcium currents and presynaptically reduces inhibitory GABA neurotransmission (Chen et al., 1997).
2.3. Purinergic receptors in the anterior pituitary
The adenohypophysis comprises the predominant anterior lobe and the minor intermediate lobe. Five distinct hormone-producing cell types are present in the adult anterior pituitary lobe: 1. Corticotrophs express POMC peptides, including adrenocorticotropic hormone (ACTH); 2. Somatotrophs express growth hormone (GH); 3. Lactotrophs express prolactin (PRL); 4. Thyrotrophs express the common glycoprotein α-subunit (αGSU) and the specific β-subunits of thyroid-stimulating hormone (TSH); 5. Gonadotrophs express αGSU and the specific β-subunits of follicle-stimulating hormone (FSH) and/or luteinizing hormone (LH). The intermediate lobe contains melanotrophs, which secrete POMC-derived peptides, including α-melanocyte-stimulating hormone (Fig. 1B).
Single-cell calcium measurements were very helpful in the initial characterization of P2XRs in anterior pituitary cells, along with studies on hormone secretion (Chung et al., 2000; Koshimizu et al., 2000a; Nunez et al., 1997; Tomic et al., 1996; Villalobos et al., 1997). These experiments revealed that functional P2XRs are operative in all secretory cell types and raised the prospect of cell type specific expression. However, this method was of limited use for the identification of receptor subtypes expressed and their functional characterization, especially for the rapidly desensitizing homomeric and heteromeric P2XRs (He et al., 2003b).
RT-PCR analysis revealed the expression of P2X2, P2X3, P2X4, P2X5, and P2X7 mRNA subunit transcripts in a mixed population of anterior pituitary cells, whereas immortalized GH3 pituitary somatolactotroph cells express transcripts for P2X3, P2X4, and P2X7 subunits (Koshimizu et al., 2000a; Zemkova et al., 2010). The mRNA levels of P2X subunits in anterior pituitary cells were examined by in situ hybridization; in parallel to qRT-PCR analysis, mRNA hybrids of the P2X2, P2X3, P2X4, and P2X7 subunits were identified in the rat anterior pituitary (Stojilkovic et al., 2010a). Protein expression of P2X2R, P2X4R, and P2X7R in cultured anterior pituitary cells was confirmed by Western blot (Fig. 2A).
Anterior pituitary cells also express functional G protein-coupled P2YRs and ARs (Rees et al., 2003a; Rees et al., 2003b; Stojilkovic et al., 2010a). Molecular cloning and functional characterization revealed the expression of P2Y2R with a pharmacological profile resembling that of native receptor (Chen et al., 1996b). The RT-PCR analysis also revealed the presence of transcripts for Gq-coupled calcium-mobilizing P2Y1R, P2Y4R, and P2Y6R, as well as Gi-coupled P2Y12R, in mixed anterior pituitary cells while the presence of functional P2Y1R was shown in a fraction of anterior pituitary cells (He et al., 2003a). Normal and immortalized anterior pituitary cells also express A1Rs (Dorflinger et al., 1985; Scorziello et al., 1993; Yu et al., 1998). It has also been suggested that anterior pituitary cells express A2AR, A2BR, and A3R (Dixon et al., 1996; Ohana et al., 2001; Weaver, 1993), but their cell type-specific expression and roles in pituitary functions have not been clarified.
2.4. Storage, release and extracellular metabolism of ATP in the anterior pituitary
In general, ATP is stored in secretory vesicles and released by regulated exocytosis, whereas the non-vesicular ATP is released by ABC-binding cassette transporters, pannexin/connexin channels, and/or dilated P2X7R (Abbracchio et al., 2009). Normal and immortalized anterior pituitary cells release ATP at resting conditions (He et al., 2005). GnRH-induced stimulation of calcium signaling and gonadotropin release is also accompanied by elevation in ATP release (Tomic et al., 1996). This is consistent with an earlier study showing calcium-dependence of ATP release (Chen et al., 1995) and modulation of ATP release by prolactin secretagogues (Nunez et al., 1997). Together, these data suggest that ATP is stored in the secretory vesicles of at least a fraction of these cells and co-secreted with pituitary hormones.
Other pathways may also contribute to ATP release by pituitary cells. These cells express functional multidrug resistance proteins (Andric et al., 2006; Kucka et al., 2010) and P2X7R (Koshimizu et al., 2000a), although their role in ATP release has not been studied. However, there is more information about expression and role of pannexins in ATP release in the pituitary gland. These cells express mRNA and protein transcripts of pannexins 1 and 2. Pannexin 1 is more abundantly expressed in the anterior lobe, and was identified in corticotrophs and a fraction of somatotrophs, as well as in AtT-20 and GH3 immortalized anterior pituitary cells. Pannexin 2 was detected in folliculo-stellate cells of the anterior pituitary and melanotrophs of the intermediate lobe. Overexpression of pannexin 1 and 2 in AtT-20 pituitary cells was shown to enhance the release of ATP, whereas basal ATP release by these cells was suppressed by down-regulating the expression of endogenous pannexin 1. Thus, pannexins may provide a pathway for delivery of ATP to numerous P2XRs and P2YRs endogenously expressed in the pituitary gland (Li et al., 2011a; Li et al., 2011b).
The pituitary gland expresses functional ectonucleotidases, which terminate the extracellular messenger functions of ATP and provide a pathway for the generation of ADP and adenosine (see below). Several lines of evidence indicate the expression and operation of these enzymes in pituitary cells. First, basal ATP release is enhanced in cells treated with ARL67156, an ectonucleotidase inhibitor. Second, perifused pituitary cells are able to degrade between 30% and 70% of extracellularly added ATP. Third, the mRNA transcripts for plasma membrane-located E-NTPDases 1, 2 and 3 are found in pituitary tissues, cultured pituitary cells and immortalized lacto-somatotroph, corticotroph and gonadotroph cell lines (He et al., 2005). Forth, E-5NT, which generates adenosine from AMP, is found by immunohistochemistry to be present in about 20% of anterior pituitary cells (Lewis et al., 2006).
3. Purinergic regulation of the hypothalamic-pituitary-gonadal axis
The hypothalamic-pituitary-gonadal axis consists of three levels: the parvocellular hypothalamic GnRH neurons, the adenohypophysial gonadotrophs, and gonads (testes in the male and ovaries in the females). GnRH, LH, FSH, together with sex steroids, including androgens and estrogens, are the hormonal products of this axis. GnRH is a 10-amino-acid hypothalamic neuropeptide that controls the function of reproductive axis. It is released by hypothalamic GnRH neurons in a pulsatile manner and reaches gonadotrophs through the portal blood, leading to stimulation of synthesis and release of LH and FSH, which in turn control the endocrine and gametogenesis functions of ovaries and testes. The feedback of gonadal steroid hormones at the level of hypothalamus and pituitary plays a major role in synchronized activity of hypothalamic-pituitary-gonadal axis (Jin et al., 2014).
3. 1. Gonadotropin-releasing hormone neurons
GnRH neurons are small bipolar cells not concentrated in discrete nuclei but diffusely located in endocrine hypothalamus with axons predominantly projected to the median eminence and infundibular stalk (Stojilkovic et al., 1994). The initial information about the potential role of purinergic signaling in GnRH neuron functions came from experiments with median eminence explants, showing that application of ATP, ADP, and αβ-methylene ATP, but not AMP and adenosine, stimulate GnRH release (Barnea et al., 1991). In olfactory placode cultures from rhesus monkeys, ATP application leads to synchronization of [Ca2+]i oscillations. This could indicate a role of ATP in pulsatile hormone release (Terasawa et al., 2005). Furthermore, inhibition on E-NTPDases in the neuroendocrine hypothalamus facilitates the midcycle LH surge, suggesting that elevated endogenous ATP concentrations facilitate GnRH neuron secretory activity (Zsarnovszky et al., 2009). Immortalized mouse hypothalamic GnRH-secreting neurons release ATP (He et al., 2005), suggesting that ATP is co-secreted with GnRH and may facilitate secretory activity in median eminence. P2X2 and P2X4 subunits are expressed in monkey GnRH neurons (Terasawa et al., 2005) and this was confirmed in mouse and rat by immunohistochemical analysis done in Brunstock’s lab (Loesch et al., 2001; Loesch et al., 1999; Xiang et al., 1998). They also found the presence of P2X5R and P2X6R immunoreactivity in mouse GnRH neurons (Fu et al., 2009). Transgenic mice and rats expressing GFP-labeled GnRH neurons provide a promising system to investigate the expression and role of purinergic receptors in GnRH neurons more directly.
3.2. Gonadotrophs
Immunohistochemical analysis suggested that P2Y1R and P2Y4R are expressed in gonadotrophs (Yu et al., 2011) and single cell calcium analysis proposed the expression of P2Y2R in these cells (Chen et al., 1995; Chen et al., 1996a; Chen et al., 1996b; Chen et al., 1994). However, electrophysiological analysis in gonadotrophs from embryonic, neonatal, and adult rats revealed that ATP application generates a depolarizing and non-oscillatory current, in contrast to calcium-mobilizing GnRH that triggered an oscillatory hyperpolarizing current driven by small calcium-controlled potassium channels (Stojilkovic et al., 2010b). This indicates that ATP-induced rapid depolarization reflects activation of endogenous P2XRs and questions the presence of functional calcium-mobilizing P2YRs in this particular cell type. ATP-induced depolarization leads to the initiation of firing in quiescent cells, an increase in the frequency of action potentials in spontaneously active cells in a concentration-dependent manner, and a transient stimulation of LH release. ATP also influences GnRH-induced currents and membrane potential oscillations. These inositol trisphosphate-dependent oscillations are facilitated, slowed, or stopped, depending on the ATP concentration, the time of its application, and the level of calcium content in intracellular stores (Zemkova et al., 2006).
The biophysical and pharmacological properties of ATP-induced depolarizing current, i.e., kinetics of activation, deactivation, desensitization, and resensitization, were comparable with those observed in cells expressing recombinant P2X2R cloned from pituitary cell (Zemkova et al., 2006; Zemkova et al., 2004). Pharmacological profile of these receptors (sensitivity of ATP-evoked current to inhibition by pyridoxal 5-phosphate 6-azophenyl-2′,4′-disulfonic acid, reactive blue 2, and suramin, as well as the lack of effect of ivermectin) further confirmed this conclusion. Together, these results indicate that gonadotrophs express the P2X2R subtype of receptors, activation of which facilitates excitability of these cells, calcium signaling and gonadotropin secretion.
3. 3. Ovary
The gamete producing and hormone producing functions of the ovary are under control of FSH and LH and take place in its cortical portion, containing functional units called ovarian follicles. Granulosa and thecal cells of the follicle mediate the endocrine function of the ovary. Granulosa cells surround the oocyte in the follicle and convert androgens (coming from the thecal cells) to estrogens, the dominant hormones in the preovulatory phase of the reproductive cycle, by aromatase. After ovulation, the granulosa cells turn into luteal cells that predominantly produce progesterone and thecal lutein cells continue to produce androgens due to lack the aromatase enzyme that is necessary to produce estrogen (White et al., 2013).
In addition to FSH and LH receptors, ovarian cells also express functional purinergic receptors. In isolated human granulosa-luteal cells, ATP evokes oscillatory calcium release from inositol trisphosphate-sensitive intracellular stores (Lee et al., 1996; Squires et al., 1997). Chicken granulosa cells also respond to ATP application with a rise in [Ca2+]i by activation of P2YRs (Morley et al., 1994). In mouse luteinized-granulosa cells, it appears that in addition to the inositol trisphosphate-sensitive pool, the ryanodine-sensitive pool also contributes to ATP-induced [Ca2+]i oscillations (Morales-Tlalpan et al., 2005). Two calcium-mobilizing receptors, P2Y2R and P2Y4R, were suggested to account for ATP-induced periodic calcium and current oscillations in granulosa cells (Bintig et al., 2009; Tai et al., 2000).
Activation of these receptors does not affect basal progesterone production, but significantly inhibits hCG-induced progesterone production (Tai et al., 2001b). This antigonadotropic action of P2YRs appears not to reflect their calcium signaling functions, but is mediated by mitogen-activated protein kinase signaling pathway (Tai et al., 2001c), presumably through receptor-activated protein kinase C (Tai et al., 2001a). ATP-induced translocation of mitogen-activated protein kinase was also reported (Tai et al., 2004). At the present time, the role of ATP-induced calcium oscillations in these cells has not been clarified.
Granulosa cells express connexin-43 hemichannels, which could provide a pathway for ATP release (Tong et al., 2007). Furthermore, these cells express E-NTPDase1 (Martin-Satue et al., 2009), which probably terminate the agonistic action of ATP and provide a pathway for activation of ARs. Consistent with this hypothesis, it has been reported that adenosine amplifies FSH action in granulosa cells and LH action in luteal cells of rat and human ovaries (Polan et al., 1983). The amplifying role of adenosine on cAMP accumulation in granulosa cells was more robust in response to LH (Ohkawa et al., 1985) and the stimulatory action of adenosine is operative in both preovulatory and luteal granulosa cells and is mediated by A2Rs (Billig et al., 1989; Billig et al., 1988).
Purinergic signaling is operative in other ovarian cell types. Porcine ovarian thecal cells express P2X7R. Their activation causes an increase in [Ca2+]i and calcium-dependent cell apoptosis (Vazquez-Cuevas et al., 2006). This receptor is also expressed in human ovarian surface epithelium of normal and ovarian cancer patients and in the human ovarian carcinoma SKOV3 immortalized cells (Vazquez-Cuevas et al., 2013). The mRNA transcripts for P2X1R and P2X2R are found in ovarian tissues and immunohistochemical analysis revealed the presence of P2X2R in perifollicular and vascular smooth muscle. Furthermore, there is a transition in expression from P2X2R to P2X1R in ovarian smooth muscle cells during pregnancy (Katugampola et al., 2004). Activation of P2Y2R and P2Y6R triggers mitogenic signaling pathways and cell proliferation in porcine ovarian theca cells (Vazquez-Cuevas et al., 2010). Follicular oocytes of Xenopus laevis express P2Y2R, which activation leads to generation of inward currents in a majority of cells (King et al., 1996; Montiel-Herrera et al., 2011), whereas adenosine stimulates hyperpolarizing potassium currents (Fujita et al., 2001).
3.4. Testis
The testis consists of seminiferous tubules, which comprise the bulk of testicular mass and contains three types of cells, spermatogonia, spermatocytes and Sertoli cells, and interstitial space between these tubules containing the androgen-producing Leydig cells and other cell types (White et al., 2013).
In addition to LH and FSH, ATP and adenosine act as modulators of testicular cells, including Sertoli cells (Filippini et al., 1994), spermatogonia (Loir, 1999), and Leydig cells (Ko et al., 2003). The Sertoli cells release ATP endogenously through a still not clarified mechanism (Gelain et al., 2003). However, the basal compartment of the seminiferous epithelium and Leydig cells express pannexin channels, which could account for ATP release (Turmel et al., 2011). In Sertoli cells from immature rats, ATP and ADP are hydrolyzed by E-NTPDase1, whereas E-5NT and ecto-adenosine deaminase (ADA) account for termination of purinergic signaling, hydrolyzing AMP and adenosine, respectively (Casali et al., 2001). Moreover, FSH stimulates ATP and ADP hydrolysis in Sertoli cells (Casali et al., 2003) and therefore increases extracellular adenosine levels (Gelain et al., 2005). A significant increase in ectonucleotidase activity in these cells was observed during sexual maturation, implying that purinergic signaling may be important for male reproduction (Casali et al., 2003). Interstitial macrophages in testis also express E-ENTPDase1 (Martin-Satue et al., 2009), which could contribute to termination of the agonistic action of ATP.
In Sertoli cells, ATP elevates cytosolic calcium and steroid secretion, probably by activating both sodium/calcium influx-dependent P2X4R and P2X7R and calcium mobilizing P2Y1R and P2Y2R (Foresta et al., 1995; Ko et al., 2003; Lalevee et al., 1999; Rossato et al., 2001). Extracellular ATP also increases the sperm fertilizing potential in vitro (Rossato et al., 1999), presumably by activating sodium-conducting ATP-gated channels (Foresta et al., 1996a). The P2X2R, P2X3R, and P2X5R subtypes have been identified in various germ cell types, whereas Sertoli cells express P2X2R, P2X3R, and P2X7R (Glass et al., 2001a) as well as the calcium-mobilizing P2Y2R (Rudge et al., 1995). Calcium signals generated by activated P2XRs and P2YRs in mouse Sertoli cells are coupled to mobilization of mitochondrial calcium (Veitinger et al., 2011). In addition to P2XRs and P2YRs, A1Rs have been found in the crude particulate preparation from rat testis and Sertoli cell enriched cultures (Conti et al., 1988; Monaco et al., 1986; Stiles et al., 1986). Their activation leads to inhibition of the FSH-induced cAMP response (Monaco et al., 1984) in a pertussis toxin-sensitive manner (Monaco et al., 1988). Spermatogenic cells also express cAMP-inhibiting A1Rs (Kangasniemi, 1993; Murphy et al., 1983; Murphy et al., 1981).
In rat and mouse Leydig cells, ATP also increases cytosolic calcium and testosterone secretion, the latter being dependent on sustained calcium influx by activated P2YRs (Foresta et al., 1996b; Perez-Armendariz et al., 1996), indicating its modulatory role in androgen production through activation of P2XRs. Functional P2X2Rs have also been identified in mouse Leydig cells using whole-cell current measurements and specific agonist and antagonists (Poletto Chaves et al., 2006). Western blot experiments revealed that in addition to P2X2R, mouse Leydig cells express P2X4R, P2X6R, and P2X7R and their functionality is confirmed by electrophysiological measurements of the whole-cell current (Antonio et al., 2009).
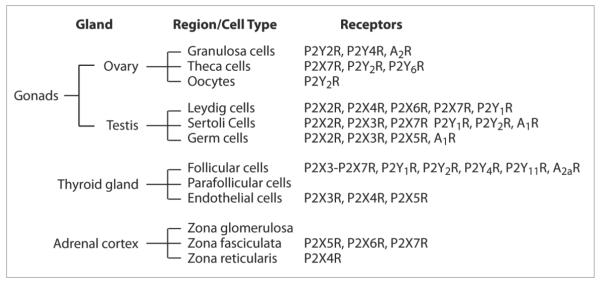
Thus, it appears that ovarian functions are predominantly regulated by P2YRs, in contrast to dominance of P2XRs in control of testicular functions. Also, adenosine amplifies cAMP production in ovarian cells by activating A2Rs and inhibiting it in testicular cells by activating A1Rs (Fig. 3a).
Fig. 3.
Expression of purinergic receptors in peripheral endocrine glands. For references see the corresponding sections.
4. Purinergic regulation of the hypothalamic-pituitary-thyroid axis
Parvocellular TRH-secreting neurons, located predominantly in the PVN of the hypothalamus, project their axon terminals to median eminence and release the tripeptide TRH into the hypophyseal portal system. The released TRH stimulates TSH synthesis and secretion in pituitary thyrotrophs. In the thyroid gland, TSH then stimulates the biosynthesis of the thyroid hormones, thyroxin (T4) and its modified product triiodothyronine (T3). TSH acts primarily via its receptors coupled to the adenylyl cyclase signaling pathway and cross-coupled to the phospholipase C signaling pathway. Thyroid hormones themselves have negative feedback control over the hypothalamus and anterior pituitary, thus controlling the release of both TRH and TSH. The proper function of this axis and its feedback mechanisms is responsible for normal development, differentiation, thermogenesis, and reproduction (Costa-e-Sousa et al., 2012; Nillni, 2010).
4.1. Thyrotropin-releasing hormone neurons
TRH neurons are subject to tight regulation by neuropeptide Y, α-melanocyte-stimulating hormone, and leptin. It was also proposed that TRH secretion in the hypothalamus could be influenced by ATP, because TRH neurons express P2X2R (Collden et al., 2010). However, more studies are needed to elucidate the role of ATP and/or other purines in the control of th hypothalamic part of this axis. Furthermore, having in mind that thyroid hormones profoundly affect ectonucleotidase expression and activity in many tissues, including brain regions such as the cortex and hippocampus (Bruno et al., 2005), and that these enzymes are widely expressed in the hypothalamus, their potential role in the negative feedback of thyroid hormones on TRH secretion should also be investigated.
4.2. Thyrotrophs
The pituitary thyrotrophs represent a relatively small percentage of cells in the anterior pituitary (about 8%) that release TSH, a 28-30 kDa glycoprotein with α and β chains. Once secreted, TSH acts on the thyroid follicular cell through a specific receptor. Rat thyrotrophs express functional P2XRs capable of facilitating calcium influx (Koshimizu et al., 1998), but the specific subtype(s) expressed are still unknown. In human thyrotrophs, ATP stimulates the calcium-phosphatidylinositol cascade, indicating the presence of P2YRs (Raspe et al., 1991). Consistent with these findings, an immunohistochemical study has shown that the majority of rat pituitary thyrotrophs express P2Y1 and P2Y4R (Yu et al., 2011). In addition, pharmacological evidence points to the presence of A1R and A2aR in thyrotrophs. While A1R activation is inhibitory, A2aR activation stimulates TSH release (Kumari et al., 1999). Given that thyrotrophs are a small fraction of pituitary cells and that thyrotroph cell lines are not very widely used, data are still scarce and further studies are needed to elucidate the role of purinergic signaling in pituitary thyrotroph function.
4.3. Thyroid gland
The thyroid gland is a follicular structure composed of two primary cell types: follicular cells or thyrocytes, responsible for thyroglobulin and thyroid hormone synthesis and parafollicular or C-cells which secrete calcitonin involved in control of calcium homeostasis. The thyroid gland is innervated by sympathetic, parasympathetic, and sensory afferents (Grunditz et al., 1988). Therefore, ATP could be co-released from nerve endings, from capillary endothelial cells, or thyrocytes themselves (Kochukov et al., 2004). Very little is known about E-NTPDases in the thyroid gland. A member of the E-NPP family, autotaxin, has been implicated in thyroid carcinoma (Kehlen et al., 2004; Seifert et al., 2008). E-5NT activity was detected in the bovine thyroid gland (Peeters et al., 1988),whereas papillary thyroid carcinoma cells express strong E-5NT immunoreactivity and enzyme activity (Kondo et al., 2006).
Immunohistochemical analysis of adult rat thyroid gland suggested the expression of P2X3R, P2X4R, and P2X5R in follicular and endothelial cells but not in parafollicular cells (Glass et al., 2001b). Human thyrocytes express P2X3, P2X4, P2X5, P2X6, and P2X7 mRNAs (Caraccio et al., 2005). The P2X7R is present in thyroid papillary carcinoma (Solini et al., 2008), and its protein expression is correlated with poor prognosis factors (Kwon et al., 2014), suggesting that enhanced function of these receptors might be a feature of thyroid cancer. Moreover, a specific P2X7R gene polymorphism has been associated with a follicular variant of papillary thyroid cancer (Dardano et al., 2009). Human thyrocytes also express P2Y1, P2Y2, P2Y4, and P2Y11 mRNAs and application of ATP induces interleukin-6 production and release from these cells, presumably through activation of one of these receptors (Caraccio et al., 2005).
Thyrotroph cancer cell lines also express several subtypes of P2XRs and P2YRs. The mRNA transcripts for P2X3, P2X4, and P2X5 subunits are found in rat thyroid FRTL-5 cells, as well as P2Y2, P2Y4, and P2Y6 mRNAs (Ekokoski et al., 2001). The P2YRs are expressed in transformed rat thyroid PCCI3 cell lines (Elia et al., 2003) and their function was documented by single cell calcium measurements and it was shown that ATP induces phospholipase C activation accompanied with calcium entry through capacitative calcium entry channels and L-type Cav channels (Marsigliante et al., 2002). The human thyroid CG3 cancer cell line expresses mRNA transcripts for P2Y1, P2Y4 and P2Y11 receptors (Yang et al., 2009).
The consequences of P2 receptor activation were described in these cell lines. In FRTL-5 cells, ATP induces generation of an inward current and stimulation of membrane internalization presumably by activating endogenous P2X7R (Kochukov et al., 2004; Kochukov et al., 2005). In these cells, ATP-induced calcium signaling is likely to be mediated by P2YRs and P2X5R. It is accompanied with c-Fos and c-Jun expression, through a signaling pathway that includes calcium, protein kinase C, and extracellular regulated kinases ERK1/ERK2 (Ekokoski et al., 2001). The same group also observed the coupling of these receptors to phospholipase A2, leading to release of arachidonic acid through P2 receptors coupled to the Gi/o signaling pathway (Ekokoski et al., 2000). ATP-dependent activation of arachidonate cascade is coupled to calcium-dependent hydrogen peroxide production (Kimura et al., 1995). ATP-induced phosphatidylinositol-calcium signaling in FRTL-5 cells was also observed (Okajima et al., 1989; Raspe et al., 1991).
Functional A2aR are present in primary human thyrocytes, where their activation induces cAMP production (Zhang et al., 2013). Transgenic mice expressing A2aR under the control of the thyroid specific thyroglobulin gene promoter show thyroid hyperplasia and hyperthyroidism (Ledent et al., 1992). In contrast, A1R and A3R were detected in carcinomas, but not in normal human thyroid tissue (Huang et al., 2001; Morello et al., 2008). Data obtained on transformed thyroid cell lines also imply that A1R activation may have multiple effects: stimulation of the sodium iodide symporter gene expression (Harii et al., 1999), inhibition of cAMP and calcium-mediated calcitonin secretion (Zink et al., 1995), and inhibition of TSH-induced cAMP production (Sho et al., 1999).
5. Purinergic regulation of the hypothalamic-pituitary adrenal axis
Maintenance of homeostasis requires continuous adaptation to stressors. Adaptive responses include: i) activation of the autonomic nervous system, leading to increase in cardiovascular and respiratory activity; ii) activation of the hypothalamic–pituitary–adrenal (HPA) axis, leading to increased energy availability; iii) behavioral changes, leading to arousal, defense and escape reactions. The 41-amino acid hypothalamic peptide CRH is the main regulator of the HPA axis activity during stress by stimulating the secretion and synthesis of ACTH in pituitary corticotrophs. The released ACTH stimulates secretion of glucocorticoids and androgens, steroid hormones of the adrenal cortex. Glucocorticoid receptors are expressed in corticotrophs and CRH neurons and contribute to negative feedback actions of glucocorticoids on ACTH secretion. In addition, there are neuronal pathways linked to release of catecholamines from the adrenal medulla, in response to stress (Aguilera et al., 2012).
5.1. Corticotropin-releasing hormone neurons
The highest concentration of CRH-secreting neurons is found in the anterior and medial–dorsal PVN of hypothalamus. Their axons project to hypophyseal portal capillaries in the external zone of the median eminence. These neurons also produce VP and release it into the pituitary portal circulation in response to stress (Swanson et al., 1980). CRH neurons are influenced by multiple inputs, including GABAeric, glutamatergic, and catecholaminergic; NPY and POMC neurons from arcuate nucleus also influence CRH neurons. Whether any of these neurons co-secrete ATP is unknown at the present time, although it is reasonable to suggest it. The potential influence of surrounding glial cells on CRH neuron function through purinergic signaling has also not been investigated. However, P2X2R were shown in some CRH-containing neurons located in the PVN (Collden et al., 2010).
5.2. Corticotrophs
Corticotrophs and melanotrophs are the first secretory pituitary cells to differentiate during embryogenic development. In postnatal pituitaries, corticotrophs represent about 15% of anterior pituitary cells. The main control of ACTH release by corticotrophs is mediated by CRH, which binds to Gs-coupled CRH receptors and facilitates spontaneous electrical activity and ACTH release. In addition to CRH and the CRH family of peptides urocortin 1-3, VP directly acts in synergy with CRH to potentiate ACTH release (Stojilkovic et al., 2010b). Corticotrophs also express P2Y2R and presumably P2X2R. Their activation leads to a rise in [Ca2+]i (Villalobos et al., 1997). Adenosine in low micromolar concentrations stimulates ACTH release in cultures of rat pituitary cells, suggesting the expression of A2R (Anand-Srivastava et al., 1989). Thus, purinergic signaling is operative in corticotrophs, but further studies are needed to clarify its role in cell functions more precisely.
A corticotroph mouse cell line, AtT-20 also synthesizes POMC and packages ACTH into secretory vesicles (Ooi et al., 2004), and thus may provide an alternative cell model to study the role of purinergic signaling in corticotrophs. These cells express several mRNAs, encoding A1R, A2R, P2X1R, P2X3-7R, P2Y1R, P2Y2R, and P2Y4R. In these cells, both adenosine and ATP stimulate POMC gene and transcriptional factors controlling POMC gene expression (Zhao et al., 2006). AtT-20 cells release ATP spontaneously, which can be suppressed by down-regulating the expression of endogenous pannexin 1 but not pannexin 2 with their siRNAs. Furthermore, overexpression of pannexin 1 and 2 in these cells enhances the release of ATP in the extracellular medium, which was blocked by the gap junction inhibitor carbenoxolone (Li et al., 2011a). These results suggest that pannexins may provide a pathway for delivery of ATP to corticotrophs, i.e. that ATP is an autocrine factor for these cells, activating endogenous P2XRs and P2YRs and serving as a source for extracellular adenosine production.
5.3. Adrenal cortex
The adrenal glands are composed of two distinct structures: cortex, the outer part of the gland, and medulla, the inner part of the gland. Anatomically, the adrenal cortex comprises three zones producing and secreting distinct hormones: zona glomerulosa is the outermost layer and is the main site for production of mineralocorticoids, zona fasciculata is situated between the glomerulosa and reticularis and is responsible for producing glucocorticoids, and zona reticularis is the inner most cortical layer and produces adrenal androgens and a small amount of glucocorticoids (Stewart et al., 2011).
The hypothesis that adrenal cortical function is influenced by autonomic innervation was supported by numerous morphological and functional observations (Edwards et al., 1993). The nerve endings lie in close proximity to zona glomerulosa cells without making synaptic contact and release noradrenaline together with the co-transmitter ATP. Ectonucleotidase activity was detected around the nerve profiles, indicating that ADP and adenosine may also influence the adrenal cortex function (Szalay et al., 1998). It appears that release of ATP occurs together, but independently from noradrenaline release in response to field stimulation (Juranyi et al., 1997). It has also been suggested that SCN of the hypothalamus influence adrenal cortex function through the autonomic nervous system (Buijs et al., 1999). Cortex cells also express connexin43, which is a hemichannel known to contribute to ATP release (Davis et al., 2000), but whether ATP is also released by adrenal cortex cells has not been investigated.
ATP-induced rise in [Ca2+]i was observed in bovine zona fasciculata cells (Matsui, 1991) and is accompanied by an increase in steroidogenesis (Kawamura et al., 1991) in a calcium-dependent manner (Niitsu, 1992). ATP also potentiates the steroidogenic effect of ACTH in bovine fasciculate cells (Kawamura et al., 2001). In addition to ATP, ADP and uridine triphosphate stimulate calcium signaling and cortisol secretion (Hoey et al., 1994), ADP may also act through Gs-coupled P2YRs (Nishi et al., 2002), and ATPγS stimulates both inositol phosphate production and cAMP accumulation (Nishi et al., 2004), suggesting a role of more than one P2R subtype in purinergic regulation of steroidogenesis in adrenocortical cells (Fig. 3).
Consistent with this conclusion, immunohistochemical studies revealed the expression of P2X4-7R subtypes in cortical cells. Immunoreactivity for P2X4 was confined to the cells of the zona reticularis, while for P2X5-7 it occurred in cells of the zona fasciculata and the expression of these receptors changes during development (Afework et al., 1999; Afework et al., 2000). RNA blot analysis also indicated significant levels of P2X4R mRNA in the cortex of the adrenal gland (Tanaka et al., 1996). A human adrenal cortex-derived cell line expresses numerous mRNAs for purinergic receptors: P2X5, P2X7, P2Y1, P2Y2, P2Y6, P2Y12, P2Y13, and P2Y14. The same study also showed that P2Y1R are functional and that their activation leads to calcium release from intracellular pools coupled with calcium influx through Orai channels (Nishi et al., 2013).
A human adrenal cortex-derived cell line also expresses mRNAs for A2aR and A2bR (Nishi et al., 2013). Rat adrenocortical cells express mRNA for these two receptors as well as for A1R and A3R. The same study also suggested that the dominant role of adenosine on corticosteroid production is stimulatory, mediated by A2aR and A2bR, activation of which triggers the Janus kinase 2-MAPK-ERK signaling cascade (Chen et al., 2008). The subsequent study by the same group identified the protein kinase-μ in adenosine-dependent activation of this signaling cascade (Chen et al., 2010). On the other hand, in near-term fetal sheep, the A1R type of adenosine receptors may play a role in suppression of adrenal activity (Jensen et al., 2010).
6. Purinergic regulation of the hypothalamic-pituitary-growth hormone axis
GH secretion by pituitary somatotrophs is stimulated by hypothalamic GHRH and inhibited by hypothalamic somatostatin. The released GH exerts a short-loop negative feedback through activation of somatostatin neurons that directly synapse with GHRH neurons. Negative feedback control of GH secretion also occurs at the pituitary level and is mediated by insulin-like growth factor type 1 and by free fatty acids. Ghrelin secreted from the stomach also contributes to the control of GH release at the hypothalamic and pituitary levels (Stojilkovic et al., 2010b).
GHRH neurons are located in arcuate nucleus while somatostatin neurons are located in periventricular nucleus. As discussed earlier, the arcuate nucleus expresses several types of purinergic receptors (section 2), but evidence was not presented that GHRH neurons express these receptors. Purinergic receptors are also expressed in ventromedial nucleus (Seidel et al., 2006) and further studies are needed to investigate their expression and potential role in somatostatin neurons.
About 40% of identified rat somatotrophs respond to ATP application with a rise in [Ca2+]i. The pharmacological profile of these responses is consistent with the expression of P2XR in these cells (Nunez et al., 1997). Additional studies have suggested that these cells express both splice forms of P2X2R, a and b, and that their activation by ATP causes high amplitude calcium signals exclusively dependent on calcium influx through P2X2Rs and L-type Cav channels (Koshimizu et al., 1998; Koshimizu et al., 2000a). These cells also express pannexin 1, suggesting that they may contribute to ATP release (Li et al., 2011a).
7. Purinergic regulation of the hypothalamic-pituitary-prolactin axis
Like somatotrophs, lactotrophs are also under control by the hypothalamus, both stimulatory and inhibitory. The predominant effect is inhibitory and is mediated by tuberohypophyseal dopamine-secreting neurons and dopamine D2 receptors in lactotrophs. Several PRL-releasing factors contribute to the elevation in PRL secretion, including TRH, vasoactive intenstinal polypeptide, and OT. Suckling stimulus, through spinal afferent neurons, inhibits dopaminergic neurons and stimulates PRL-releasing factor neurons. Estrogens sensitize lactotrophs to release PRL, which regulates its own secretion by ultrashort-loop and short-loop feedback (Freeman et al., 2000).
PRL-releasing factor neurons are located in PVN, which expresses purinergic receptors (see section 2). TRH-secreting neurons express P2X2R (Collden et al., 2010), whereas we found no information about the expression of purinergic receptors in vasoactive intenstinal polypeptide-secreting hypothalamic neurons and dopaminergic neurons in the tuberohypophyseal area.
In contrast, there are numerous reports about the expression of purinergic receptors in lactotrophs. Earlier studies have revealed that lactotrophs express Gi-coupled A1Rs (Rees et al., 2003a; Rees et al., 2003b), non-identified calcium-mobilizing P2YR (Carew et al., 1994), and probably several P2XR subtypes (Koshimizu et al., 2000b; Villalobos et al., 1996). Single cell studies revealed that adenosine inhibits basal, vasoactive intenstinal polypeptide-, and TRH-stimulated PRL release via a pertussis toxin-sensitive G protein (Scorziello et al., 1993). The inhibitory effect of an A1R-specific agonist on PRL release was observed in rat pituitary tissue ex vivo (Picanco-Diniz et al., 2006). Consistent with the expression of A1R signaling through heterotrimeric Gi/o proteins, adenosine inhibited cAMP production in rat pituitary cells (Schettini et al., 1990). Numerous experiments with immortalized lacto-somatotrophs (GH3 and GH4C1 cells) indicated the functional expression of A1Rs (Chung et al., 2000; Delahunty et al., 1988; Mollard et al., 1991; Navarro et al., 1997; Navarro et al., 1999; Zapata et al., 1997). It also appears that the A2Rs are expressed in rat pituitary cells and that their activation leads to enhanced PRL and TRH release (Kumari et al., 1999).
The potential role of P2Rs on PRL secretion was shown in dispersed pituitary cells; the authors identified lactotrophs by the reverse hemolytic plaque assay and showed that ATP enhances basal and TRH-stimulated PRL release (Nunez et al., 1997). ATP also stimulates PRL release in perifused pituitary cells (He et al., 2003a). The ability of ATPγS, a nonhydrolysable agonist for P2Rs (Ralevic et al., 1998), to dose-dependently stimulate PRL release in calcium-containing and -deficient medium indicates the role of a P2YR subtype in ATP release. The ligand-selectivity profile of extracellular calcium-independent rise in [Ca2+]i and secretion and the PPADS sensitivity of these responses are consistent with the role of P2Y1R in these processes. ADP and ATP are equipotent in activating this receptor, further confirming that both molecules act as extracellular messengers (He et al., 2003a).
Several lines of evidence support the view that the P2X4R is expressed in lactotrophs and plays a major role in calcium-influx-dependent signaling and secretion. In the presence of 10 μM MRS2500, a highly specific antagonist of P2Y1R (Hechler et al., 2006), ATP still induces a biphasic [Ca2+]i response composed of an early spike response and a sustained plateau response. In the presence of the L-type Cav channel blocker nifedipine, there was a decrease in basal [Ca2+]i levels and the amplitude of ATP-induced [Ca2+]i responses decreased. This indicates that P2XR-activation in these cells leads to facilitation of calcium influx through Cav channels in addition to promoting calcium influx through the pore of the channel. Exogenously added ATP initiates firing of action potentials in quiescent lactotrophs and increases the firing frequency of action potentials in spontaneously firing cells. The pharmacological and biophysical properties of currents in lactotrophs are consistent with the expression of P2X4R in these cells. In agreement with this, ivermectin, a specific allosteric modulator of P2X4Rs (Khakh et al., 1999), induced the progressive increase in the peak amplitude of current in response to repetitive application of 100 μM ATP and delayed the receptor deactivation after washout of ATP. Finally, in perifused pituitary cells, ivermectin increased the amplitude of ATP-induced PRL release.
Anterior pituitary cells also express mRNAs for P2X3, P2X6, and P2X7, but the cell-type-specific expression of these receptor channels has not been firmly established (Zemkova et al., 2010). In immortalized GH3 lacto-somatotrophs, however, the functional expression of P2X7R has been demonstrated (Chung et al., 2000). The GH4C1 pituitary cell line also expresses these receptors (Kimm-Brinson et al., 2001).
8. Summary
ATP, ADP, and adenosine are native agonists for numerous endocrine cells and have dual actions on calcium signaling: stimulatory and inhibitory, depending on the receptor subtype expressed. ATP activates P2XRs, which are expressed in the majority of endocrine cells and conduct calcium through the pore of their channels. In excitable cells, these receptors also facilitate the firing of action potentials and calcium influx through Cav channels. Molecular and physiological evidence for the expression of several calcium-mobilizing and Gs-coupled P2YRs in various endocrine tissues has also been obtained. ADP is a potent agonist for the activation of P2Y1R, which are expressed in several endocrine tissues. Adenosine acts as an agonist for A1Rs, expressed in hypothalamic, pituitary and testicular cells and their activation causes decrease in cAMP production and inhibition of electrical activity and calcium influx through Cav channels. A2Rs are well characterized in ovarian granulosa cells and their activation causes stimulation of cAMP production. The sequential mode of purinergic receptor activation by ATP, ADP, and adenosine is enabled by ectonucleotidases, as illustrated by an example in Fig. 4. While the capacity of ectonucleotidases to hydrolyze ATP and generate ADP and adenosine is well established in several endocrine tissues, further work is required to clarify the mechanism(s) of ATP release.
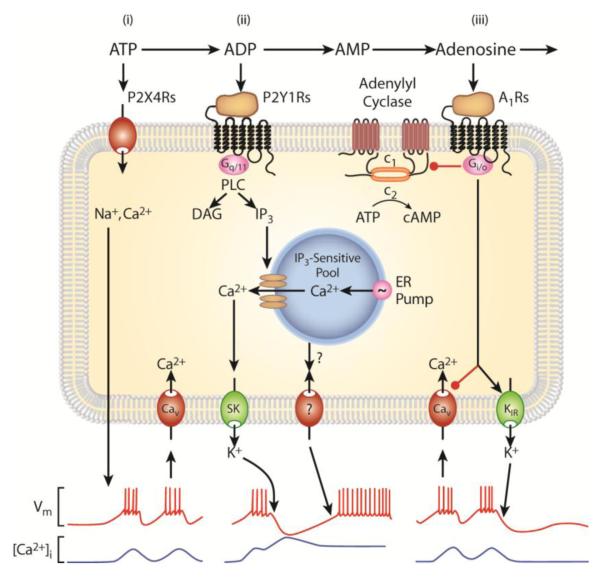
Fig. 4.
Sequential activation of purinergic receptors in lactotrophs: roles of ectonucleotidases. i) ATP binds to P2X4R, causing an inward sodium/calcium-conducting current, which depolarizes lactotrophs and facilitates firing of action potentials and calcium influx through Cav channels. ii) E-NTPDase-mediated generation of ADP from ATP provides a potent agonist for calcium-mobilizing P2Y1R endogenously expressed in lactotrophs. The released calcium triggers transient cell membrane hyperpolarization by activating calcium-controlled small potassium (SK) channels, accompanied with sustained facilitation of electrical activity through still not well-characterized channel(s). iii) Ecto-5′-nucleotidase-mediated production of adenosine provides an agonist for Gi/o-coupled A1R, which is also endogenously expressed in these cells. This in turns leads to inhibition of Cav channels and facilitation of inwardly rectifying potassium (Kir) channels and termination of spontaneous and ADP-induced electrical activity. Derived from (Stojilkovic, 2009).
Acknowledgments
Grant Information: This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development.
Abbreviations
- ACTH
adrenocorticotropic hormone
- ADA
ecto-adenosine deaminase
- ADP
adenosine-5′-diphosphate
- AMP
adenosine-5′-monophosphate
- ARs
adenosine receptors
- ATP
adenosine-5′-triphosphate
- Cav
voltage-gated calcium
- [Ca2+]i
intracellular calcium concentration
- CRH
corticotropin-releasing hormone
- FSH
follicle-stimulating hormone
- GH
growth hormone
- GHRH
growth hormone-releasing hormone
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- E-NPPase
ectonucleotide pyrophosphatase/phosphodiesterase
- E-NTPDases
ecto-nucleoside triphosphate diphosphohydrolases
- E-5NT
ecto-5′-nucleotidase
- OT
oxytocin
- P2XRs
purinergic P2 receptor channels
- P2YRs
purinergic G protein-coupled P2 receptors
- PP
posterior pituitary
- PRL
prolactin
- POMC
pro-opiomelanocortin
- PVN
paraventricular nucleus
- SCN
suprachiasmatic nucleus
- SON
supraoptic nucleus
- TRH
thyrotropin-releasing hormone
- TSH
thyroid-stimulating hormone
- VP
vasopressin
Footnotes
Competing Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Afework M, Burnstock G. Distribution of P2X receptors in the rat adrenal gland. Cell Tissue Res. 1999;298:449–456. doi: 10.1007/s004419900103. [DOI] [PubMed] [Google Scholar]
- Afework M, Burnstock G. Age-related changes in the localization of P2X (nucleotide) receptors in the rat adrenal gland. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2000;18:515–520. doi: 10.1016/s0736-5748(00)00023-x. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012;33:67–84. doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand-Srivastava MB, Cantin M, Gutkowska J. Adenosine regulates the release of adrenocorticotropic hormone (ACTH) from cultured anterior pituitary cells. Molecular and cellular biochemistry. 1989;89:21–28. doi: 10.1007/BF00228276. [DOI] [PubMed] [Google Scholar]
- Andric SA, Kostic TS, Stojilkovic SS. Contribution of multidrug resistance protein MRP5 in control of cyclic guanosine 5′-monophosphate intracellular signaling in anterior pituitary cells. Endocrinology. 2006;147:3435–3445. doi: 10.1210/en.2006-0091. [DOI] [PubMed] [Google Scholar]
- Antonio LS, Costa RR, Gomes MD, Varanda WA. Mouse Leydig cells express multiple P2X receptor subunits. Purinergic Signal. 2009;5:277–287. doi: 10.1007/s11302-008-9128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong WE. The neurophysiology of neurosecretory cells. J Physiol. 2007;585:645–647. doi: 10.1113/jphysiol.2007.145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong WE, Rubrum A, Teruyama R, Bond CT, Adelman JP. Immunocytochemical localization of small-conductance, calcium-dependent potassium channels in astrocytes of the rat supraoptic nucleus. The Journal of comparative neurology. 2005;491:175–185. doi: 10.1002/cne.20679. [DOI] [PubMed] [Google Scholar]
- Barnea A, Cho G, Katz BM. A putative role for extracellular ATP: facilitation of 67copper uptake and of copper stimulation of the release of luteinizing hormone-releasing hormone from median eminence explants. Brain Res. 1991;541:93–97. doi: 10.1016/0006-8993(91)91079-g. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Vavra V, Svobodova I, Bendova Z, Vereb G, Zemkova H. Potentiation of inhibitory synaptic transmission by extracellular ATP in rat suprachiasmatic nuclei. J Neurosci. 2013;33:8035–8044. doi: 10.1523/JNEUROSCI.4682-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig H, Kumai A, Roseberg S. Adenosine receptor-mediated effects on adenylate cyclase activity in rat luteal tissue: a putative local regulatory role of adenosine in the corpus luteum. Biol Reprod. 1989;40:102–110. doi: 10.1095/biolreprod40.1.102. [DOI] [PubMed] [Google Scholar]
- Billig H, Rosberg S. Evidence for A2 adenosine receptor-mediated effects on adenylate cyclase activity in rat ovarian membranes. Mol Cell Endocrinol. 1988;56:205–210. doi: 10.1016/0303-7207(88)90062-7. [DOI] [PubMed] [Google Scholar]
- Bintig W, Baumgart J, Walter WJ, Heisterkamp A, Lubatschowski H, Ngezahayo A. Purinergic signalling in rat GFSHR-17 granulosa cells: an in vitro model of granulosa cells in maturing follicles. J Bioenerg Biomembr. 2009;41:85–94. doi: 10.1007/s10863-009-9199-5. [DOI] [PubMed] [Google Scholar]
- Bruno AN, Diniz GP, Ricachenevsky FK, Pochmann D, Bonan CD, Barreto-Chaves ML, Sarkis JJ. Hypo-and hyperthyroidism affect the ATP, ADP and AMP hydrolysis in rat hippocampal and cortical slices. Neuroscience research. 2005;52:61–68. doi: 10.1016/j.neures.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. The European journal of neuroscience. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Burkeen JF, Womac AD, Earnest DJ, Zoran MJ. Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J Neurosci. 2011;31:8432–8440. doi: 10.1523/JNEUROSCI.6576-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling in endocrine organs. Purinergic Signal. 2014;10:189–231. doi: 10.1007/s11302-013-9396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraccio N, Monzani F, Santini E, Cuccato S, Ferrari D, Callegari MG, Gulinelli S, Pizzirani C, Di Virgilio F, Ferrannini E, Solini A. Extracellular adenosine 5′-triphosphate modulates interleukin-6 production by human thyrocytes through functional purinergic P2 receptors. Endocrinology. 2005;146:3172–3178. doi: 10.1210/en.2004-1527. [DOI] [PubMed] [Google Scholar]
- Carew MA, Wu ML, Law GJ, Tseng YZ, Mason WT. Extracellular ATP activates calcium entry and mobilization via P2U-purinoceptors in rat lactotrophs. Cell Calcium. 1994;16:227–235. doi: 10.1016/0143-4160(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Casali EA, da Silva TR, Gelain DP, Kaiser GR, Battastini AM, Sarkis JJ, Bernard EA. Ectonucleotidase activities in Sertoli cells from immature rats. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica … [et al.] 2001;34:1247–1256. doi: 10.1590/s0100-879x2001001000003. [DOI] [PubMed] [Google Scholar]
- Casali EA, de Souza LF, Gelain DP, Kaiser GR, Battastini AM, Sarkis JJ. Changes in ectonucleotidase activities in rat Sertoli cells during sexual maturation. Molecular and cellular biochemistry. 2003;247:111–119. doi: 10.1023/a:1024150619490. [DOI] [PubMed] [Google Scholar]
- Cham JL, Owens NC, Barden JA, Lawrence AJ, Badoer E. P2X purinoceptor subtypes on paraventricular nucleus neurones projecting to the rostral ventrolateral medulla in the rat. Exp Physiol. 2006;91:403–411. doi: 10.1113/expphysiol.2005.032409. [DOI] [PubMed] [Google Scholar]
- Chen G, van den Pol AN. Adenosine modulation of calcium currents and presynaptic inhibition of GABA release in suprachiasmatic and arcuate nucleus neurons. Journal of neurophysiology. 1997;77:3035–3047. doi: 10.1152/jn.1997.77.6.3035. [DOI] [PubMed] [Google Scholar]
- Chen YC, Chen Y, Huang SH, Wang SM. Protein kinase Cmu mediates adenosine-stimulated steroidogenesis in primary rat adrenal cells. FEBS Lett. 2010;584:4442–4448. doi: 10.1016/j.febslet.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Chen YC, Huang SH, Wang SM. Adenosine-stimulated adrenal steroidogenesis involves the adenosine A2A and A2B receptors and the Janus kinase 2-mitogen-activated protein kinase kinase extracellular signal-regulated kinase signaling pathway. The international journal of biochemistry & cell biology. 2008;40:2815–2825. doi: 10.1016/j.biocel.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Kratzmeier M, Levy A, McArdle CA, Poch A, Day A, Mukhopadhyay AK, Lightman SL. Evidence for a role of pituitary ATP receptors in the regulation of pituitary function. Proc Natl Acad Sci U S A. 1995;92:5219–5223. doi: 10.1073/pnas.92.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZP, Kratzmeier M, Poch A, Xu S, McArdle CA, Levy A, Mukhopadhyay AK, Lightman SL. Effects of extracellular nucleotides in the pituitary: adenosine triphosphate receptor-mediated intracellular responses in gonadotrope-derived alpha T3-1 cells. Endocrinology. 1996a;137:248–256. doi: 10.1210/endo.137.1.8536620. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Krull N, Xu S, Levy A, Lightman SL. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology. 1996b;137:1833–1840. doi: 10.1210/endo.137.5.8612522. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Levy A, McArdle CA, Lightman SL. Pituitary ATP receptors: characterization and functional localization to gonadotropes. Endocrinology. 1994;135:1280–1283. doi: 10.1210/endo.135.3.8070374. [DOI] [PubMed] [Google Scholar]
- Chung HS, Park KS, Cha SK, Kong ID, Lee JW. ATP-induced [Ca(2+)](i) changes and depolarization in GH3 cells. Br J Pharmacol. 2000;130:1843–1852. doi: 10.1038/sj.bjp.0703253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton DK, Steiner RA. Saunders and Elsevier. 6th Edition Philadelphia: 2009. Neuroendocrinology of Reproduction. In: Yen and Jaffe’s Reproductive Endocrinology; pp. 3–31. [Google Scholar]
- Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collden G, Mangano C, Meister B. P2X2 purinoreceptor protein in hypothalamic neurons associated with the regulation of food intake. Neuroscience. 2010;171:62–78. doi: 10.1016/j.neuroscience.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compan V, Ulmann L, Stelmashenko O, Chemin J, Chaumont S, Rassendren F. P2X2 and P2X5 subunits define a new heteromeric receptor with P2X7-like properties. J Neurosci. 2012;32:4284–4296. doi: 10.1523/JNEUROSCI.6332-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Culler MD, Negro-Vilar A. Adenosine receptor-dependent modulation of inhibin secretion in cultured immature rat Sertoli cells. Mol Cell Endocrinol. 1988;59:255–259. doi: 10.1016/0303-7207(88)90111-6. [DOI] [PubMed] [Google Scholar]
- Costa-e-Sousa RH, Hollenberg AN. Minireview: The neural regulation of the hypothalamic-pituitary-thyroid axis. Endocrinology. 2012;153:4128–4135. doi: 10.1210/en.2012-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer EE, Knott TK, Cuadra AE, Ortiz-Miranda S, Lemos JR. P2X purinergic receptor knockout mice reveal endogenous ATP modulation of both vasopressin and oxytocin release from the intact neurohypophysis. J Neuroendocrinol. 2012;24:674–680. doi: 10.1111/j.1365-2826.2012.02299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardano A, Falzoni S, Caraccio N, Polini A, Tognini S, Solini A, Berti P, Di Virgilio F, Monzani F. 1513A>C polymorphism in the P2X7 receptor gene in patients with papillary thyroid cancer: correlation with histological variants and clinical parameters. J Clin Endocrinol Metab. 2009;94:695–698. doi: 10.1210/jc.2008-1322. [DOI] [PubMed] [Google Scholar]
- Davis KT, McDuffie I, Mawhinney LA, Murray SA. Hypophysectomy results in a loss of connexin gap junction protein from the adrenal cortex. Endocrine research. 2000;26:561–570. doi: 10.3109/07435800009048571. [DOI] [PubMed] [Google Scholar]
- Day TA, Sibbald JR, Khanna S. ATP mediates an excitatory noradrenergic neuron input to supraoptic vasopressin cells. Brain Res. 1993;607:341–344. doi: 10.1016/0006-8993(93)91528-z. [DOI] [PubMed] [Google Scholar]
- Delahunty TM, Cronin MJ, Linden J. Regulation of GH3-cell function via adenosine A1 receptors. Inhibition of prolactin release, cyclic AMP production and inositol phosphate generation. Biochem J. 1988;255:69–77. doi: 10.1042/bj2550069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorflinger LJ, Schonbrunn A. Adenosine inhibits prolactin and growth hormone secretion in a clonal pituitary cell line. Endocrinology. 1985;117:2330–2338. doi: 10.1210/endo-117-6-2330. [DOI] [PubMed] [Google Scholar]
- Edwards AV. Aspects of autonomic and neuroendocrine function. Equine veterinary journal. 1997;(Supplement):109–117. doi: 10.1111/j.2042-3306.1997.tb05088.x. [DOI] [PubMed] [Google Scholar]
- Edwards AV, Jones CT. Autonomic control of adrenal function. Journal of anatomy. 1993;183(Pt 2):291–307. [PMC free article] [PubMed] [Google Scholar]
- Ekokoski E, Dugue B, Vainio M, Vainio PJ, Tornquist K. Extracellular ATP-mediated phospholipase A(2) activation in rat thyroid FRTL-5 cells: regulation by a G(i)/G(o) protein, Ca(2+), and mitogen-activated protein kinase. Journal of cellular physiology. 2000;183:155–162. doi: 10.1002/(SICI)1097-4652(200005)183:2<155::AID-JCP2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Ekokoski E, Webb TE, Simon J, Tornquist K. Mechanisms of P2 receptor-evoked DNA synthesis in thyroid FRTL-5 cells. Journal of cellular physiology. 2001;187:166–175. doi: 10.1002/jcp.1070. [DOI] [PubMed] [Google Scholar]
- Espallergues J, Solovieva O, Techer V, Bauer K, Alonso G, Vincent A, Hussy N. Synergistic activation of astrocytes by ATP and norepinephrine in the rat supraoptic nucleus. Neuroscience. 2007;148:712–723. doi: 10.1016/j.neuroscience.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Ferreira-Neto HC, Yao ST, Antunes VR. Purinergic and glutamatergic interactions in the hypothalamic paraventricular nucleus modulate sympathetic outflow. Purinergic Signal. 2013;9:337–349. doi: 10.1007/s11302-013-9352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini A, Riccioli A, De Cesaris P, Paniccia R, Teti A, Stefanini M, Conti M, Ziparo E. Activation of inositol phospholipid turnover and calcium signaling in rat Sertoli cells by P2-purinergic receptors: modulation of follicle-stimulating hormone responses. Endocrinology. 1994;134:1537–1545. doi: 10.1210/endo.134.3.8119196. [DOI] [PubMed] [Google Scholar]
- Fischer W, Krugel U. P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem. 2007;14:2429–2455. doi: 10.2174/092986707782023695. [DOI] [PubMed] [Google Scholar]
- Foresta C, Rossato M, Bordon P, Di Virgilio F. Extracellular ATP activates different signalling pathways in rat Sertoli cells. Biochem J. 1995;311(Pt 1):269–274. doi: 10.1042/bj3110269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresta C, Rossato M, Chiozzi P, Di Virgilio F. Mechanism of human sperm activation by extracellular ATP. Am J Physiol. 1996a;270:C1709–1714. doi: 10.1152/ajpcell.1996.270.6.C1709. [DOI] [PubMed] [Google Scholar]
- Foresta C, Rossato M, Nogara A, Gottardello F, Bordon P, Di Virgilio F. Role of P2-purinergic receptors in rat Leydig cell steroidogenesis. Biochem J. 1996b;320(Pt 2):499–504. doi: 10.1042/bj3200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Fu J, Yu Q, Guo W, He C, Burnstock G, Xiang Z. P2X receptors are expressed on neurons containing luteinizing hormone-releasing hormone in the mouse hypothalamus. Neurosci Lett. 2009;458:32–36. doi: 10.1016/j.neulet.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Fujita R, Kimura S, Kawasaki S, Takashima K, Matsumoto M, Hirano H, Sasaki K. ATP suppresses the K(+) current responses to FSH and adenosine in the follicular cells of Xenopus oocyte. The Japanese journal of physiology. 2001;51:491–500. doi: 10.2170/jjphysiol.51.491. [DOI] [PubMed] [Google Scholar]
- Gelain DP, de Souza LF, Bernard EA. Extracellular purines from cells of seminiferous tubules. Molecular and cellular biochemistry. 2003;245:1–9. doi: 10.1023/a:1022857608849. [DOI] [PubMed] [Google Scholar]
- Glass R, Bardini M, Robson T, Burnstock G. Expression of nucleotide P2X receptor subtypes during spermatogenesis in the adult rat testis. Cells, tissues, organs. 2001a;169:377–387. doi: 10.1159/000047905. [DOI] [PubMed] [Google Scholar]
- Glass R, Burnstock G. Immunohistochemical identification of cells expressing ATP-gated cation channels (P2X receptors) in the adult rat thyroid. Journal of anatomy. 2001b;198:569–579. doi: 10.1046/j.1469-7580.2001.19850569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes DA, Song Z, Stevens W, Sladek CD. Sustained stimulation of vasopressin and oxytocin release by ATP and phenylephrine requires recruitment of desensitization-resistant P2X purinergic receptors. American journal of physiology. Regulatory, integrative and comparative physiology. 2009;297:R940–949. doi: 10.1152/ajpregu.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman HM. Basic Medical Endocrinology. Academic Press -Elsevier; Boston ISBN: 2009. pp. 978-0-12–373975-9. [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nature neuroscience. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Grunditz T, Hakanson R, Sundler F, Uddman R. Neuronal pathways to the rat thyroid revealed by retrograde tracing and immunocytochemistry. Neuroscience. 1988;24:321–335. doi: 10.1016/0306-4522(88)90334-x. [DOI] [PubMed] [Google Scholar]
- Guo W, Sun J, Xu X, Bunstock G, He C, Xiang Z. P2X receptors are differentially expressed on vasopressin- and oxytocin-containing neurons in the supraoptic and paraventricular nuclei of rat hypothalamus. Histochemistry and cell biology. 2009;131:29–41. doi: 10.1007/s00418-008-0493-9. [DOI] [PubMed] [Google Scholar]
- Harii N, Endo T, Ohmori M, Onaya T. Extracellular adenosine increases Na+/I− symporter gene expression in rat thyroid FRTL-5 cells. Mol Cell Endocrinol. 1999;157:31–39. doi: 10.1016/s0303-7207(99)00166-5. [DOI] [PubMed] [Google Scholar]
- He ML, Gonzalez-Iglesias AE, Stojilkovic SS. Role of nucleotide P2 receptors in calcium signaling and prolactin release in pituitary lactotrophs. J Biol Chem. 2003a;278:46270–46277. doi: 10.1074/jbc.M309005200. [DOI] [PubMed] [Google Scholar]
- He ML, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1-3 in hypothalamic and pituitary cells. Purinergic Signal. 2005;1:135–144. doi: 10.1007/s11302-005-6208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ML, Zemkova H, Koshimizu TA, Tomic M, Stojilkovic SS. Intracellular calcium measurements as a method in studies on activity of purinergic P2X receptor channels. Am J Physiol Cell Physiol. 2003b;285:C467–479. doi: 10.1152/ajpcell.00042.2003. [DOI] [PubMed] [Google Scholar]
- Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther. 2006;316:556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma H, Bourque CW. P2 purinoceptor-mediated depolarization of rat supraoptic neurosecretory cells in vitro. J Physiol. 1995;489(Pt 3):805–811. doi: 10.1113/jphysiol.1995.sp021093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey DE, Nicol M, Williams BC, Walker SW. Primary cultures of bovine inner zone adrenocortical cells secrete cortisol in response to adenosine 5′-triphosphate, adenosine 5′-diphosphate, and uridine 5′-triphosphate via a nucleotide receptor that may be coupled to two signal generation systems. Endocrinology. 1994;135:1553–1560. doi: 10.1210/endo.135.4.7925117. [DOI] [PubMed] [Google Scholar]
- Huang Y, Prasad M, Lemon WJ, Hampel H, Wright FA, Kornacker K, LiVolsi V, Frankel W, Kloos RT, Eng C, Pellegata NS, de la Chapelle A. Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci U S A. 2001;98:15044–15049. doi: 10.1073/pnas.251547398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EC, Bennet L, Fraser M, Power GG, Hunter CJ, Gunn AJ. Adenosine A(1) receptor mediated suppression of adrenal activity in near-term fetal sheep. American journal of physiology. Regulatory, integrative and comparative physiology. 2010;298:R700–706. doi: 10.1152/ajpregu.00474.2009. [DOI] [PubMed] [Google Scholar]
- Jin JM, Yang WX. Molecular regulation of hypothalamus-pituitary-gonads axis in males. Gene. 2014;551:15–25. doi: 10.1016/j.gene.2014.08.048. [DOI] [PubMed] [Google Scholar]
- Juranyi Z, Orso E, Janossy A, Szalay KS, Sperlagh B, Windisch K, Vinson GP, Vizi ES. ATP and [3H]noradrenaline release and the presence of ecto-Ca(2+)-ATPases in the capsule-glomerulosa fraction of the rat adrenal gland. J Endocrinol. 1997;153:105–114. doi: 10.1677/joe.0.1530105. [DOI] [PubMed] [Google Scholar]
- Kangasniemi M. Effects of adenosine analog PIA (n-phenylisopropyladenosine) on FSH-stimulated cyclic AMP (cAMP) production in the rat seminiferous epithelium. Mol Cell Endocrinol. 1993;96:141–146. doi: 10.1016/0303-7207(93)90104-r. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. The Journal of comparative neurology. 1999;407:11–32. [PubMed] [Google Scholar]
- Katugampola H, Burnstock G. Purinergic signalling to rat ovarian smooth muscle: changes in P2X receptor expression during pregnancy. Cells, tissues, organs. 2004;178:33–47. doi: 10.1159/000081091. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Matsui T, Niitsu A, Kondo T, Ohno Y, Nakamichi N. Extracellular ATP stimulates steroidogenesis in bovine adrenocortical fasciculata cells via P2 purinoceptors. Japanese journal of pharmacology. 1991;56:543–545. doi: 10.1254/jjp.56.543. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Niitsu A, Nishi H, Masaki E. Extracellular ATP potentiates steroidogenic effect of adrenocorticotropic hormone in bovine adrenocortical fasciculata cells. Japanese journal of pharmacology. 2001;85:376–381. doi: 10.1254/jjp.85.376. [DOI] [PubMed] [Google Scholar]
- Kehlen A, Englert N, Seifert A, Klonisch T, Dralle H, Langner J, Hoang-Vu C. Expression, regulation and function of autotaxin in thyroid carcinomas. International journal of cancer. Journal international du cancer. 2004;109:833–838. doi: 10.1002/ijc.20022. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X(4) receptor channels. J Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimm-Brinson KL, Moeller PD, Barbier M, Glasgow H, Jr., Burkholder JM, Ramsdell JS. Identification of a P2X7 receptor in GH(4)C(1) rat pituitary cells: a potential target for a bioactive substance produced by Pfiesteria piscicida. Environmental health perspectives. 2001;109:457–462. doi: 10.1289/ehp.01109457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Okajima F, Sho K, Kobayashi I, Kondo Y. Thyrotropin-induced hydrogen peroxide production in FRTL-5 thyroid cells is mediated not by adenosine 3′,5′-monophosphate, but by Ca2+ signaling followed by phospholipase-A2 activation and potentiated by an adenosine derivative. Endocrinology. 1995;136:116–123. doi: 10.1210/endo.136.1.7828520. [DOI] [PubMed] [Google Scholar]
- King BF, Wang S, Burnstock G. P2 purinoceptor-activated inward currents in follicular oocytes of Xenopus laevis. J Physiol. 1996;494(Pt 1):17–28. doi: 10.1113/jphysiol.1996.sp021472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott TK, Hussy N, Cuadra AE, Lee RH, Ortiz-Miranda S, Custer EE, Lemos JR. Adenosine trisphosphate appears to act via different receptors in terminals versus somata of the hypothalamic neurohypophysial system. J Neuroendocrinol. 2012;24:681–689. doi: 10.1111/j.1365-2826.2012.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott TK, Marrero HG, Custer EE, Lemos JR. Endogenous ATP potentiates only vasopressin secretion from neurohypophysial terminals. Journal of cellular physiology. 2008;217:155–161. doi: 10.1002/jcp.21485. [DOI] [PubMed] [Google Scholar]
- Knott TK, Marrero HG, Fenton RA, Custer EE, Dobson JG, Jr., Lemos JR. Endogenous adenosine inhibits CNS terminal Ca(2+) currents and exocytosis. Journal of cellular physiology. 2007;210:309–314. doi: 10.1002/jcp.20827. [DOI] [PubMed] [Google Scholar]
- Knott TK, Velazquez-Marrero C, Lemos JR. ATP elicits inward currents in isolated vasopressinergic neurohypophysial terminals via P2X2 and P2X3 receptors. Pflugers Arch. 2005;450:381–389. doi: 10.1007/s00424-005-1471-x. [DOI] [PubMed] [Google Scholar]
- Ko WH, Au CL, Yip CY. Multiple purinergic receptors lead to intracellular calcium increases in cultured rat Sertoli cells. Life Sci. 2003;72:1519–1535. doi: 10.1016/s0024-3205(02)02410-4. [DOI] [PubMed] [Google Scholar]
- Kochukov MY, Ritchie AK. A P2X7 receptor stimulates plasma membrane trafficking in the FRTL rat thyrocyte cell line. Am J Physiol Cell Physiol. 2004;287:C992–C1002. doi: 10.1152/ajpcell.00538.2003. [DOI] [PubMed] [Google Scholar]
- Kochukov MY, Ritchie AK. P2X7 receptor stimulation of membrane internalization in a thyrocyte cell line. J Membr Biol. 2005;204:11–21. doi: 10.1007/s00232-005-0742-y. [DOI] [PubMed] [Google Scholar]
- Kondo T, Nakazawa T, Murata SI, Katoh R. Expression of CD73 and its ecto-5′-nucleotidase activity are elevated in papillary thyroid carcinomas. Histopathology. 2006;48:612–614. doi: 10.1111/j.1365-2559.2005.02277.x. [DOI] [PubMed] [Google Scholar]
- Koshimizu T, Tomic M, Van Goor F, Stojilkovic SS. Functional role of alternative splicing in pituitary P2X2 receptor-channel activation and desensitization. Mol Endocrinol. 1998;12:901–913. doi: 10.1210/mend.12.7.0129. [DOI] [PubMed] [Google Scholar]
- Koshimizu TA, Tomic M, Wong AO, Zivadinovic D, Stojilkovic SS. Characterization of purinergic receptors and receptor-channels expressed in anterior pituitary cells. Endocrinology. 2000a;141:4091–4099. doi: 10.1210/endo.141.11.7737. [DOI] [PubMed] [Google Scholar]
- Koshimizu TA, Van Goor F, Tomic M, Wong AO, Tanoue A, Tsujimoto G, Stojilkovic SS. Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Mol Pharmacol. 2000b;58:936–945. doi: 10.1124/mol.58.5.936. [DOI] [PubMed] [Google Scholar]
- Kucka M, Kretschmannova K, Murano T, Wu CP, Zemkova H, Ambudkar SV, Stojilkovic SS. Dependence of multidrug resistance protein-mediated cyclic nucleotide efflux on the background sodium conductance. Mol Pharmacol. 2010;77:270–279. doi: 10.1124/mol.109.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Buckingham JC, Poyser RH, Cover PO. Roles for adenosine A1- and A2-receptors in the control of thyrotrophin and prolactin release from the anterior pituitary gland. Regul Pept. 1999;79:41–46. doi: 10.1016/s0167-0115(98)00143-8. [DOI] [PubMed] [Google Scholar]
- Kwon JH, Nam ES, Shin HS, Cho SJ, Park HR, Kwon MJ. P2X7 Receptor Expression in Coexistence of Papillary Thyroid Carcinoma with Hashimoto’s Thyroiditis. Korean journal of pathology. 2014;48:30–35. doi: 10.4132/KoreanJPathol.2014.48.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalevee N, Rogier C, Becq F, Joffre M. Acute effects of adenosine triphosphates, cyclic 3′,5′-adenosine monophosphates, and follicle-stimulating hormone on cytosolic calcium level in cultured immature rat Ssertoli cells. Biol Reprod. 1999;61:343–352. doi: 10.1095/biolreprod61.2.343. [DOI] [PubMed] [Google Scholar]
- Ledent C, Dumont JE, Vassart G, Parmentier M. Thyroid expression of an A2 adenosine receptor transgene induces thyroid hyperplasia and hyperthyroidism. The EMBO journal. 1992;11:537–542. doi: 10.1002/j.1460-2075.1992.tb05084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PS, Squires PE, Buchan AM, Yuen BH, Leung PC. P2-purinoreceptor evoked changes in intracellular calcium oscillations in single isolated human granulosa-lutein cells. Endocrinology. 1996;137:3756–3761. doi: 10.1210/endo.137.9.8756543. [DOI] [PubMed] [Google Scholar]
- Lemos JR, Ortiz-Miranda SI, Cuadra AE, Velazquez-Marrero C, Custer EE, Dad T, Dayanithi G. Modulation/physiology of calcium channel sub-types in neurosecretory terminals. Cell Calcium. 2012;51:284–292. doi: 10.1016/j.ceca.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BM, Pexa A, Francis K, Verma V, McNicol AM, Scanlon M, Deussen A, Evans WH, Rees DA, Ham J. Adenosine stimulates connexin 43 expression and gap junctional communication in pituitary folliculostellate cells. Faseb J. 2006;20:2585–2587. doi: 10.1096/fj.06-6121fje. [DOI] [PubMed] [Google Scholar]
- Li C, Tripathi PK, Armstrong WE. Differences in spike train variability in rat vasopressin and oxytocin neurons and their relationship to synaptic activity. J Physiol. 2007;581:221–240. doi: 10.1113/jphysiol.2006.123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bjelobaba I, Yan Z, Kucka M, Tomic M, Stojilkovic SS. Expression and roles of pannexins in ATP release in the pituitary gland. Endocrinology. 2011a;152:2342–2352. doi: 10.1210/en.2010-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Tomic M, Stojilkovic SS. Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels. Gen Comp Endocrinol. 2011b;174:202–210. doi: 10.1016/j.ygcen.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch A, Burnstock G. Immunoreactivity to P2X(6) receptors in the rat hypothalamo neurohypophysial system: an ultrastructural study with extravidin and colloidal gold-silver labelling. Neuroscience. 2001;106:621–631. doi: 10.1016/s0306-4522(01)00288-3. [DOI] [PubMed] [Google Scholar]
- Loesch A, Miah S, Burnstock G. Ultrastructural localisation of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamo-neurohypophysial system. Journal of neurocytology. 1999;28:495–504. doi: 10.1023/a:1007009222518. [DOI] [PubMed] [Google Scholar]
- Loir M. Spermatogonia of rainbow trout: III. In vitro study of the proliferative response to extracellular ATP and adenosine. Molecular reproduction and development. 1999;53:443–450. doi: 10.1002/(SICI)1098-2795(199908)53:4<443::AID-MRD10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Low MJ. Williams Textbook of Endocrinology. Elseviee Saunders; 2011. Neuroendocrinology; pp. 103–174. [Google Scholar]
- Martin-Satue M, Lavoie EG, Pelletier J, Fausther M, Csizmadia E, Guckelberger O, Robson SC, Sevigny J. Localization of plasma membrane bound NTPDases in the murine reproductive tract. Histochemistry and cell biology. 2009;131:615–628. doi: 10.1007/s00418-008-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T. Biphasic rise caused by extracellular ATP in intracellular calcium concentration in bovine adrenocortical fasciculata cells. Biochem Biophys Res Commun. 1991;178:1266–1272. doi: 10.1016/0006-291x(91)91030-g. [DOI] [PubMed] [Google Scholar]
- Miller RE. Pancreatic neuroendocrinology: peripheral neural mechanisms in the regulation of the Islets of Langerhans. Endocr Rev. 1981;2:471–494. doi: 10.1210/edrv-2-4-471. [DOI] [PubMed] [Google Scholar]
- Mollard P, Guerineau N, Chiavaroli C, Schlegel W, Cooper DM. Adenosine A1 receptor-induced inhibition of Ca2+ transients linked to action potentials in clonal pituitary cells. Eur J Pharmacol. 1991;206:271–277. doi: 10.1016/0922-4106(91)90109-u. [DOI] [PubMed] [Google Scholar]
- Monaco L, Conti M. Localization of adenosine receptors in rat testicular cells. Biol Reprod. 1986;35:258–266. doi: 10.1095/biolreprod35.2.258. [DOI] [PubMed] [Google Scholar]
- Monaco L, DeManno DA, Martin MW, Conti M. Adenosine inhibition of the hormonal response in the Sertoli cell is reversed by pertussis toxin. Endocrinology. 1988;122:2692–2698. doi: 10.1210/endo-122-6-2692. [DOI] [PubMed] [Google Scholar]
- Monaco L, Toscano MV, Conti M. Purine modulation of the hormonal response of the rat Sertoli cell in culture. Endocrinology. 1984;115:1616–1624. doi: 10.1210/endo-115-4-1616. [DOI] [PubMed] [Google Scholar]
- Montiel-Herrera M, Zaske AM, Garcia-Colunga J, Martinez-Torres A, Miledi R. Ion currents induced by ATP and angiotensin II in cultured follicular cells of Xenopus laevis. Molecules and cells. 2011;32:397–404. doi: 10.1007/s10059-011-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Tlalpan V, Arellano RO, Diaz-Munoz M. Interplay between ryanodine and IP3 receptors in ATP-stimulated mouse luteinized-granulosa cells. Cell Calcium. 2005;37:203–213. doi: 10.1016/j.ceca.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Morello S, Petrella A, Festa M, Popolo A, Monaco M, Vuttariello E, Chiappetta G, Parente L, Pinto A. Cl-IB-MECA inhibits human thyroid cancer cell proliferation independently of A3 adenosine receptor activation. Cancer biology & therapy. 2008;7:278–284. doi: 10.4161/cbt.7.2.5301. [DOI] [PubMed] [Google Scholar]
- Mori M, Tsushima H, Matsuda T. Antidiuretic effects of purinoceptor agonists injected into the hypothalamic paraventricular nucleus of water-loaded, ethanol-anesthetized rats. Neuropharmacology. 1992;31:585–592. doi: 10.1016/0028-3908(92)90191-q. [DOI] [PubMed] [Google Scholar]
- Mori M, Tsushima H, Matsuda T. Antidiuretic effects of ATP induced by microinjection into the hypothalamic supraoptic nucleus in water-loaded and ethanol-anesthetized rats. Japanese journal of pharmacology. 1994;66:445–450. doi: 10.1254/jjp.66.445. [DOI] [PubMed] [Google Scholar]
- Morley P, Vanderhyden BC, Tremblay R, Mealing GA, Durkin JP, Whitfield JF. Purinergic receptor-mediated intracellular Ca2+ oscillations in chicken granulosa cells. Endocrinology. 1994;134:1269–1276. doi: 10.1210/endo.134.3.8119167. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Goodman RR, Snyder SH. Adenosine receptor localization in rat testes: biochemical and autoradiographic evidence for association with spermatocytes. Endocrinology. 1983;113:1299–1305. doi: 10.1210/endo-113-4-1299. [DOI] [PubMed] [Google Scholar]
- Murphy KM, Snyder SH. Adenosine receptors in rat testes: labeling with 3H-cyclohexyladenosine. Life Sci. 1981;28:917–920. doi: 10.1016/0024-3205(81)90054-0. [DOI] [PubMed] [Google Scholar]
- Navarro A, Zapata R, Canela EI, Mallol J, Lluis C, Franco R. Modulation of GH4 cell cycle via A1 adenosine receptors. J Neurochem. 1997;69:2145–2154. doi: 10.1046/j.1471-4159.1997.69052145.x. [DOI] [PubMed] [Google Scholar]
- Navarro A, Zapata R, Canela EI, Mallol J, Lluis C, Franco R. Epidermal growth factor (EGF)-induced up-regulation and agonist- and antagonist-induced desensitization and internalization of A1 adenosine receptors in a pituitary-derived cell line. Brain Res. 1999;816:47–57. doi: 10.1016/s0006-8993(98)01062-2. [DOI] [PubMed] [Google Scholar]
- Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92:925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- Niitsu A. Calcium is essential for ATP-induced steroidogenesis in bovine adrenocortical fasciculata cells. Japanese journal of pharmacology. 1992;60:269–274. doi: 10.1254/jjp.60.269. [DOI] [PubMed] [Google Scholar]
- Nillni EA. Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front Neuroendocrinol. 2010;31:134–156. doi: 10.1016/j.yfrne.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Arai H, Momiyama T. NCI-H295R, a human adrenal cortex-derived cell line, expresses purinergic receptors linked to Ca(2)(+)-mobilization/influx and cortisol secretion. PLoS One. 2013;8:e71022. doi: 10.1371/journal.pone.0071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Hori S, Niitsu A, Kawamura M. Adenosine 5′-(gamma-thio) triphosphate (ATPgammaS) stimulates both P2Y receptors linked to inositol phosphates production and cAMP accumulation in bovine adrenocortical fasciculata cells. Life Sci. 2004;74:1181–1190. doi: 10.1016/j.lfs.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Nishi H, Kato F, Masaki E, Kawamura M. ADP-sensitive purinoceptors induce steroidogenesis via adenylyl cyclase activation in bovine adrenocortical fasciculata cells. Br J Pharmacol. 2002;137:177–184. doi: 10.1038/sj.bjp.0704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi J, Yamashita H. Adenosine inhibits voltage-dependent Ca2+ currents in rat dissociated supraoptic neurones via A1 receptors. J Physiol. 2000;526(Pt 2):313–326. doi: 10.1111/j.1469-7793.2000.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez L, Villalobos C, Frawley LS. Extracellular ATP as an autocrine/paracrine regulator of prolactin release. Am J Physiol. 1997;272:E1117–1123. doi: 10.1152/ajpendo.1997.272.6.E1117. [DOI] [PubMed] [Google Scholar]
- Ohana G, Bar-Yehuda S, Barer F, Fishman P. Differential effect of adenosine on tumor and normal cell growth: focus on the A3 adenosine receptor. Journal of cellular physiology. 2001;186:19–23. doi: 10.1002/1097-4652(200101)186:1<19::AID-JCP1011>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ohkawa R, Polan ML, Behrman HR. Adenosine differentially amplifies luteinizing hormone-over follicle-stimulating hormone-mediated effects in acute cultures of rat granulosa cells. Endocrinology. 1985;117:248–254. doi: 10.1210/endo-117-1-248. [DOI] [PubMed] [Google Scholar]
- Okajima F, Sato K, Kondo Y. P2-purinergic agonists activate phospholipase C in a guanine nucleotide- and Ca2+-dependent manner in FRTL-5 thyroid cell membranes. FEBS Lett. 1989;253:132–136. doi: 10.1016/0014-5793(89)80945-7. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Poulain DA. Adenosine-induced presynaptic inhibition of IPSCs and EPSCs in rat hypothalamic supraoptic nucleus neurones. J Physiol. 1999;520(Pt 3):815–825. doi: 10.1111/j.1469-7793.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi GT, Tawadros N, Escalona RM. Pituitary cell lines and their endocrine applications. Mol Cell Endocrinol. 2004;228:1–21. doi: 10.1016/j.mce.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Parker TL, Kesse WK, Mohamed AA, Afework M. The innervation of the mammalian adrenal gland. Journal of anatomy. 1993;183(Pt 2):265–276. [PMC free article] [PubMed] [Google Scholar]
- Peeters C, de Wolf M, Van Dessel G, Lagrou A, Hilderson H, Dierick W. Topography, purification and characterization of thyroidal 5′-nucleotidase. The International journal of biochemistry. 1988;20:409–419. doi: 10.1016/0020-711x(88)90209-1. [DOI] [PubMed] [Google Scholar]
- Perez-Armendariz EM, Nadal A, Fuentes E, Spray DC. Adenosine 5′-triphosphate (ATP) receptors induce intracellular calcium changes in mouse leydig cells. Endocrine. 1996;4:239–247. doi: 10.1007/BF02738690. [DOI] [PubMed] [Google Scholar]
- Picanco-Diniz DL, Valenca MM, Antunes-Rodrigues J. Adenosine A1 receptor-mediated inhibition of in vitro prolactin secretion from the rat anterior pituitary. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica … [et al.] 2006;39:1493–1499. doi: 10.1590/s0100-879x2006001100013. [DOI] [PubMed] [Google Scholar]
- Polan ML, DeCherney AH, Haseltine FP, Mezer HC, Behrman HR. Adenosine amplifies follicle-stimulating hormone action in granulosa cells and luteinizing hormone action in luteal cells of rat and human ovaries. J Clin Endocrinol Metab. 1983;56:288–294. doi: 10.1210/jcem-56-2-288. [DOI] [PubMed] [Google Scholar]
- Poletto Chaves LA, Pontelli EP, Varanda WA. P2X receptors in mouse Leydig cells. Am J Physiol Cell Physiol. 2006;290:C1009–1017. doi: 10.1152/ajpcell.00506.2005. [DOI] [PubMed] [Google Scholar]
- Ponzio TA, Hatton GI. Adenosine postsynaptically modulates supraoptic neuronal excitability. Journal of neurophysiology. 2005;93:535–547. doi: 10.1152/jn.01185.2003. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Raspe E, Laurent E, Andry G, Dumont JE. ATP, bradykinin, TRH and TSH activate the Ca(2+)-phosphatidylinositol cascade of human thyrocytes in primary culture. Mol Cell Endocrinol. 1991;81:175–183. doi: 10.1016/0303-7207(91)90216-f. [DOI] [PubMed] [Google Scholar]
- Rees DA, Scanlon MF, Ham J. Adenosine signalling pathways in the pituitary gland: one ligand, multiple receptors. J Endocrinol. 2003a;177:357–364. doi: 10.1677/joe.0.1770357. [DOI] [PubMed] [Google Scholar]
- Rees DA, Scanlon MF, Ham J. Novel insights into how purines regulate pituitary cell function. Clin Sci (Lond) 2003b;104:467–481. doi: 10.1042/CS20030053. [DOI] [PubMed] [Google Scholar]
- Rossato M, La Sala GB, Balasini M, Taricco F, Galeazzi C, Ferlin A, Foresta C. Sperm treatment with extracellular ATP increases fertilization rates in in-vitro fertilization for male factor infertility. Hum Reprod. 1999;14:694–697. doi: 10.1093/humrep/14.3.694. [DOI] [PubMed] [Google Scholar]
- Rossato M, Merico M, Bettella A, Bordon P, Foresta C. Extracellular ATP stimulates estradiol secretion in rat Sertoli cells in vitro: modulation by external sodium. Mol Cell Endocrinol. 2001;178:181–187. doi: 10.1016/s0303-7207(01)00426-9. [DOI] [PubMed] [Google Scholar]
- Rosso L, Peteri-Brunback B, Vouret-Craviari V, Deroanne C, Troadec JD, Thirion S, Van Obberghen-Schilling E, Mienville JM. RhoA inhibition is a key step in pituicyte stellation induced by A(1)-type adenosine receptor activation. Glia. 2002;38:351–362. doi: 10.1002/glia.10072. [DOI] [PubMed] [Google Scholar]
- Rudge SA, Hughes PJ, Brown GR, Michell RH, Kirk CJ. Inositol lipid-mediated signalling in response to endothelin and ATP in the mammalian testis. Molecular and cellular biochemistry. 1995:149–150. 161–174. doi: 10.1007/BF01076574. [DOI] [PubMed] [Google Scholar]
- Schettini G, Landolfi E, Meucci O, Florio T, Grimaldi M, Ventra C, Marino A. Adenosine and its analogue (-)-N6-R-phenyl-isopropyladenosine modulate anterior pituitary adenylate cyclase activity and prolactin secretion in the rat. J Mol Endocrinol. 1990;5:69–76. doi: 10.1677/jme.0.0050069. [DOI] [PubMed] [Google Scholar]
- Scorziello A, Landolfi E, Grimaldi M, Meucci O, Ventra C, Avallone A, Postiglione A, Schettini G. Direct effect of adenosine on prolactin secretion at the level of the single rat lactotroph: involvement of pertussis toxin-sensitive and -insensitive transducing mechanisms. J Mol Endocrinol. 1993;11:325–334. doi: 10.1677/jme.0.0110325. [DOI] [PubMed] [Google Scholar]
- Seidel B, Bigl M, Franke H, Kittner H, Kiess W, Illes P, Krugel U. Expression of purinergic receptors in the hypothalamus of the rat is modified by reduced food availability. Brain Res. 2006;1089:143–152. doi: 10.1016/j.brainres.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Seifert A, Klonisch T, Wulfaenger J, Haag F, Dralle H, Langner J, Hoang-Vu C, Kehlen A. The cellular localization of autotaxin impacts on its biological functions in human thyroid carcinoma cells. Oncology reports. 2008;19:1485–1491. [PubMed] [Google Scholar]
- Shibuya I, Tanaka K, Hattori Y, Uezono Y, Harayama N, Noguchi J, Ueta Y, Izumi F, Yamashita H. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J Physiol. 1999;514(Pt 2):351–367. doi: 10.1111/j.1469-7793.1999.351ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sho K, Narita T, Okajima F, Kondo Y. An adenosine receptor agonist-induced modulation of TSH-dependent cell growth in FRTL-5 thyroid cells mediated by inhibitory G protein. Gi. Biochimie. 1999;81:341–346. doi: 10.1016/s0300-9084(99)80079-0. [DOI] [PubMed] [Google Scholar]
- Sladek CD, Kapoor JR. Neurotransmitter/neuropeptide interactions in the regulation of neurohypophyseal hormone release. Exp Neurol. 2001;171:200–209. doi: 10.1006/exnr.2001.7779. [DOI] [PubMed] [Google Scholar]
- Sladek CD, Song Z. Regulation of vasopressin release by co-released neurotransmitters: mechanisms of purinergic and adrenergic synergism. Progress in brain research. 2008;170:93–107. doi: 10.1016/S0079-6123(08)00409-3. [DOI] [PubMed] [Google Scholar]
- Solini A, Cuccato S, Ferrari D, Santini E, Gulinelli S, Callegari MG, Dardano A, Faviana P, Madec S, Di Virgilio F, Monzani F. Increased P2X7 receptor expression and function in thyroid papillary cancer: a new potential marker of the disease? Endocrinology. 2008;149:389–396. doi: 10.1210/en.2007-1223. [DOI] [PubMed] [Google Scholar]
- Song Z, Gomes DA, Stevens W, Sladek CD. Multiple alpha1-adrenergic receptor subtypes support synergistic stimulation of vasopressin and oxytocin release by ATP and phenylephrine. American journal of physiology. Regulatory, integrative and comparative physiology. 2010;299:R1529–1537. doi: 10.1152/ajpregu.00532.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Sladek CD. Does conversion of ATP to adenosine terminate ATP-stimulated vasopressin release from hypothalamo-neurohypophyseal explants? Brain Res. 2005;1047:105–111. doi: 10.1016/j.brainres.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Song Z, Sladek CD. Site of ATP and phenylephrine synergistic stimulation of vasopressin release from the hypothalamo-neurohypophyseal system. J Neuroendocrinol. 2006;18:266–272. doi: 10.1111/j.1365-2826.2006.01411.x. [DOI] [PubMed] [Google Scholar]
- Song Z, Vijayaraghavan S, Sladek CD. ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;292:R423–431. doi: 10.1152/ajpregu.00495.2006. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Mergl Z, Juranyi Z, Vizi ES, Makara GB. Local regulation of vasopressin and oxytocin secretion by extracellular ATP in the isolated posterior lobe of the rat hypophysis. J Endocrinol. 1999;160:343–350. doi: 10.1677/joe.0.1600343. [DOI] [PubMed] [Google Scholar]
- Squires PE, Lee PS, Yuen BH, Leung PC, Buchan AM. Mechanisms involved in ATP-evoked Ca2+ oscillations in isolated human granulosa-luteal cells. Cell Calcium. 1997;21:365–374. doi: 10.1016/s0143-4160(97)90030-0. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Krone NP. Williams Textbook of Endocrtinology. 12th Edition Elsevieer Saunders; Philadeliphia, PA: 2011. The adrenal cortex. [Google Scholar]
- Stiles GL, Pierson G, Sunay S, Parsons WJ. The rat testicular A1 adenosine receptor-adenylate cyclase system. Endocrinology. 1986;119:1845–1851. doi: 10.1210/endo-119-4-1845. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS. Purinergic regulation of hypothalamopituitary functions. Trends Endocrinol Metab. 2009;20:460–468. doi: 10.1016/j.tem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic SS, He ML, Koshimizu TA, Balik A, Zemkova H. Signaling by purinergic receptors and channels in the pituitary gland. Mol Cell Endocrinol. 2010a;314:184–191. doi: 10.1016/j.mce.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic SS, Koshimizu T. Signaling by extracellular nucleotides in anterior pituitary cells. Trends Endocrinol Metab. 2001;12:218–225. doi: 10.1016/s1043-2760(01)00387-3. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Krsmanovic LZ, Spergel DJ, Catt KJ. Gonadotropin-releasing hormone neurons: intrinsic pulsatility and receptor-mediated regulation. Trends Endocrinol Metab. 1994;5:201–209. doi: 10.1016/1043-2760(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev. 2010b;31:845–915. doi: 10.1210/er.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic SS, Zemkova H. P2X receptor channels in endocrine glands. Wiley interdisciplinary reviews. Membrane transport and signaling. 2013;2:173–180. doi: 10.1002/wmts.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. The Journal of comparative neurology. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Szalay KS, Orso E, Juranyi Z, Vinson GP, Vizi ES. Local non-synaptic modulation of aldosterone production by catecholamines and ATP in rat: implications for a direct neuronal fine tuning. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1998;30:323–328. doi: 10.1055/s-2007-978892. [DOI] [PubMed] [Google Scholar]
- Tai CJ, Chang SJ, Leung PC, Tzeng CR. Adenosine 5′-triphosphate activates nuclear translocation of mitogen-activated protein kinases leading to the induction of early growth response 1 and raf expression in human granulosa-luteal cells. J Clin Endocrinol Metab. 2004;89:5189–5195. doi: 10.1210/jc.2003-032111. [DOI] [PubMed] [Google Scholar]
- Tai CJ, Kang SK, Cheng KW, Choi KC, Nathwani PS, Leung PC. Expression and regulation of P2U-purinergic receptor in human granulosa-luteal cells. J Clin Endocrinol Metab. 2000;85:1591–1597. doi: 10.1210/jcem.85.4.6558. [DOI] [PubMed] [Google Scholar]
- Tai CJ, Kang SK, Choi KC, Tzeng CR, Leung PC. Antigonadotropic action of adenosine triphosphate in human granulosa-luteal cells: involvement of protein kinase Calpha. J Clin Endocrinol Metab. 2001a;86:3237–3242. doi: 10.1210/jcem.86.7.7691. [DOI] [PubMed] [Google Scholar]
- Tai CJ, Kang SK, Leung PC. Adenosine triphosphate-evoked cytosolic calcium oscillations in human granulosa-luteal cells: role of protein kinase C. J Clin Endocrinol Metab. 2001b;86:773–777. doi: 10.1210/jcem.86.2.7231. [DOI] [PubMed] [Google Scholar]
- Tai CJ, Kang SK, Tzeng CR, Leung PC. Adenosine triphosphate activates mitogen-activated protein kinase in human granulosa-luteal cells. Endocrinology. 2001c;142:1554–1560. doi: 10.1210/endo.142.4.8081. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Murate M, Wang CZ, Seino S, Iwanaga T. Cellular distribution of the P2X4 ATP receptor mRNA in the brain and non-neuronal organs of rats. Archives of histology and cytology. 1996;59:485–490. doi: 10.1679/aohc.59.485. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Keen KL, Grendell RL, Golos TG. Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol. 2005;19:2736–2747. doi: 10.1210/me.2005-0034. [DOI] [PubMed] [Google Scholar]
- Thirion S, Troadec JD, Nicaise G. Cytochemical localization of ecto-ATPases in rat neurohypophysis. J Histochem Cytochem. 1996;44:103–111. doi: 10.1177/44.2.8609366. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–1559. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- Tomic M, Jobin RM, Vergara LA, Stojilkovic SS. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J Biol Chem. 1996;271:21200–21208. doi: 10.1074/jbc.271.35.21200. [DOI] [PubMed] [Google Scholar]
- Tong D, Li TY, Naus KE, Bai D, Kidder GM. In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J Cell Sci. 2007;120:4016–4024. doi: 10.1242/jcs.011775. [DOI] [PubMed] [Google Scholar]
- Troadec JD, Thirion S. Multifaceted purinergic regulation of stimulus-secretion coupling in the neurohypophysis. Neuro Endocrinol Lett. 2002;23:273–280. [PubMed] [Google Scholar]
- Troadec JD, Thirion S, Nicaise G, Lemos JR, Dayanithi G. ATP-evoked increases in [Ca2+]i and peptide release from rat isolated neurohypophysial terminals via a P2X2 purinoceptor. J Physiol. 1998;511(Pt 1):89–103. doi: 10.1111/j.1469-7793.1998.089bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troadec JD, Thirion S, Petturiti D, Bohn MT, Poujeol P. ATP acting on P2Y receptors triggers calcium mobilization in primary cultures of rat neurohypophysial astrocytes (pituicytes) Pflugers Arch. 1999;437:745–753. doi: 10.1007/s004240050841. [DOI] [PubMed] [Google Scholar]
- Turmel P, Dufresne J, Hermo L, Smith CE, Penuela S, Laird DW, Cyr DG. Characterization of pannexin1 and pannexin3 and their regulation by androgens in the male reproductive tract of the adult rat. Molecular reproduction and development. 2011;78:124–138. doi: 10.1002/mrd.21280. [DOI] [PubMed] [Google Scholar]
- Vavra V, Bhattacharya A, Zemkova H. Facilitation of glutamate and GABA release by P2X receptor activation in supraoptic neurons from freshly isolated rat brain slices. Neuroscience. 2011;188:1–12. doi: 10.1016/j.neuroscience.2011.04.067. [DOI] [PubMed] [Google Scholar]
- Vazquez-Cuevas FG, Cruz-Rico A, Garay E, Garcia-Carranca A, Perez-Montiel D, Juarez B, Arellano RO. Differential expression of the P2X7 receptor in ovarian surface epithelium during the oestrous cycle in the mouse. Reproduction, fertility, and development. 2013;25:971–984. doi: 10.1071/RD12196. [DOI] [PubMed] [Google Scholar]
- Vazquez-Cuevas FG, Juarez B, Garay E, Arellano RO. ATP-induced apoptotic cell death in porcine ovarian theca cells through P2X7 receptor activation. Molecular reproduction and development. 2006;73:745–755. doi: 10.1002/mrd.20447. [DOI] [PubMed] [Google Scholar]
- Vazquez-Cuevas FG, Zarate-Diaz EP, Garay E, Arellano RO. Functional expression and intracellular signaling of UTP-sensitive P2Y receptors in theca-interstitial cells. Reproductive biology and endocrinology : RB&E. 2010;8:88. doi: 10.1186/1477-7827-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitinger S, Veitinger T, Cainarca S, Fluegge D, Engelhardt CH, Lohmer S, Hatt H, Corazza S, Spehr J, Neuhaus EM, Spehr M. Purinergic signalling mobilizes mitochondrial Ca(2)(+) in mouse Sertoli cells. J Physiol. 2011;589:5033–5055. doi: 10.1113/jphysiol.2011.216309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Alonso-Torre SR, Nunez L, Garcia-Sancho J. Functional ATP receptors in rat anterior pituitary cells. Am J Physiol. 1997;273:C1963–1971. doi: 10.1152/ajpcell.1997.273.6.C1963. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Nunez L, Garcia-Sancho J. Functional glutamate receptors in a subpopulation of anterior pituitary cells. Faseb J. 1996;10:654–660. doi: 10.1096/fasebj.10.5.8621065. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci U S A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamori M, Sorimachi M. Properties of native P2X receptors in large multipolar neurons dissociated from rat hypothalamic arcuate nucleus. Brain Res. 2004;1005:51–59. doi: 10.1016/j.brainres.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Wang G, Dayanithi G, Custer EE, Lemos JR. Adenosine inhibition via A(1) receptor of N-type Ca(2+) current and peptide release from isolated neurohypophysial terminals of the rat. J Physiol. 2002;540:791–802. doi: 10.1113/jphysiol.2002.016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DR. A2a adenosine receptor gene expression in developing rat brain. Brain Res Mol Brain Res. 1993;20:313–327. doi: 10.1016/0169-328x(93)90058-w. [DOI] [PubMed] [Google Scholar]
- White BA, Porterfield SP. Endocrine and Reproductive Physiology. (Elsevier Fourth edition) 2013 [Google Scholar]
- Whitlock A, Burnstock G, Gibb AJ. The single-channel properties of purinergic P2X ATP receptors in outside-out patches from rat hypothalamic paraventricular parvocells. Pflugers Arch. 2001;443:115–122. doi: 10.1007/s004240100624. [DOI] [PubMed] [Google Scholar]
- Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. The European journal of neuroscience. 2009;30:869–876. doi: 10.1111/j.1460-9568.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Bo X, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamus. Brain Res. 1998;813:390–397. doi: 10.1016/s0006-8993(98)01073-7. [DOI] [PubMed] [Google Scholar]
- Xiang Z, He C, Burnstock G. P2X5 receptors are expressed on neurons containing arginine vasopressin and nitric oxide synthase in the rat hypothalamus. Brain Res. 2006;1099:56–63. doi: 10.1016/j.brainres.2006.04.126. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ishida Y, Inouye S. Circadian rhythms of adenosine triphosphate contents in the suprachiasmatic nucleus, anterior hypothalamic area and caudate putamen of the rat--negative correlation with electrical activity. Brain Res. 1994;664:237–240. doi: 10.1016/0006-8993(94)91978-x. [DOI] [PubMed] [Google Scholar]
- Yu Q, Guo W, Song X, Liu X, Xiang Z, He C, Burnstock G. Expression of P2Y receptors in the rat anterior pituitary. Purinergic Signal. 2011;7:207–219. doi: 10.1007/s11302-011-9236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Kimura M, Walczewska A, Porter JC, McCann SM. Adenosine acts by A1 receptors to stimulate release of prolactin from anterior-pituitaries in vitro. Proc Natl Acad Sci U S A. 1998;95:7795–7798. doi: 10.1073/pnas.95.13.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata R, Navarro A, Canela EI, Franco R, Lluis C, Mallol J. Regulation of L-type calcium channels in GH4 cells via A1 adenosine receptors. J Neurochem. 1997;69:2546–2554. doi: 10.1046/j.1471-4159.1997.69062546.x. [DOI] [PubMed] [Google Scholar]
- Zemkova H, Balik A, Jiang Y, Kretschmannova K, Stojilkovic SS. Roles of purinergic P2X receptors as pacemaking channels and modulators of calcium-mobilizing pathway in pituitary gonadotrophs. Mol Endocrinol. 2006;20:1423–1436. doi: 10.1210/me.2005-0508. [DOI] [PubMed] [Google Scholar]
- Zemkova H, He ML, Koshimizu TA, Stojilkovic SS. Identification of ectodomain regions contributing to gating, deactivation, and resensitization of purinergic P2X receptors. J Neurosci. 2004;24:6968–6978. doi: 10.1523/JNEUROSCI.1471-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemkova H, Kucka M, Li S, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Characterization of purinergic P2X4 receptor channels expressed in anterior pituitary cells. Am J Physiol Endocrinol Metab. 2010;298:E644–651. doi: 10.1152/ajpendo.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Xu J, Sun N, Cai H, Ren M, Zhang J, Yu C, Wang Z, Gao L, Zhao J. The presence of adenosine A2a receptor in thyrocytes and its involvement in Graves’ IgG-induced VEGF expression. Endocrinology. 2013;154:4927–4938. doi: 10.1210/en.2012-2258. [DOI] [PubMed] [Google Scholar]
- Zhao LF, Iwasaki Y, Oki Y, Tsugita M, Taguchi T, Nishiyama M, Takao T, Kambayashi M, Hashimoto K. Purinergic receptor ligands stimulate pro-opiomelanocortin gene expression in AtT-20 pituitary corticotroph cells. J Neuroendocrinol. 2006;18:273–278. doi: 10.1111/j.1365-2826.2006.01416.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s archives of pharmacology. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- Zink A, Scherubl H, Hoflich M, Hescheler J, Raue F. Adenosine A1-receptors inhibit cAMP and Ca2+ mediated calcitonin secretion in C-cells. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1995;27:408–414. doi: 10.1055/s-2007-979989. [DOI] [PubMed] [Google Scholar]
- Zsarnovszky A, Bartha T, Frenyo LV, Diano S. NTPDases in the neuroendocrine hypothalamus: possible energy regulators of the positive gonadotrophin feedback. Reproductive biology and endocrinology : RB&E. 2009;7:63. doi: 10.1186/1477-7827-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]