Abstract
Background
Deficits in decision-making and episodic memory are often reported among heavy cannabis users, yet little is known on how they influence negative consequences from cannabis use. Individual differences in decision-making may explain, in part, why some individuals experience significant problems from their cannabis use whereas others do not. We hypothesized that poor decision-making would moderate relationships between amount of cannabis use and problems from cannabis use whereas episodic memory performance would not.
Method
Young adult cannabis users (n = 52) with cannabis as their drug of choice and with minimal comorbidities completed semi-structured interviews, self-report questionnaires, and measures of neurocognitive functioning, with decision-making accessed via the Iowa Gambling Task (IGT), episodic memory via the Hopkins Verbal Learning Test – Revised (HVLT) and problems from cannabis use with the Marijuana Problems Scale.
Results
Strong relationships were observed between amount of cannabis use (lifetime, 12-month, and 30-day) and problems reported from use, but only among participants with Low (impaired) decision-making (R2 = .39 to .51; p < .01). No significant relationships were observed among those with better (low average to high average) decision-making performance (p > .05). In contrast, episodic memory performance was not a significant moderator of the relationship between amount of cannabis use and cannabis problems (p > .05).
Conclusions
Cannabis users with poor decision-making may be at greater risk for experiencing significant negative consequences from their cannabis use. Our results lend further support to emerging evidence of decision-making as a risk factor for addiction and extend these findings to cannabis users.
Keywords: cannabis, decision-making, addiction
1. INTRODUCTION
Deficits in neurocognitive functioning are a well-documented negative consequence of frequent cannabis use (Gonzalez, 2007; Grant et al., 2003; Lisdahl et al., 2014; Pope et al., 2001; Schreiner and Dunn, 2012; Solowij et al., 2002). Meta-analyses on studies of non-intoxicated cannabis users reveal deficits in learning, recall, executive functions, attention, motor abilities, and language (Grant et al., 2003; Schreiner and Dunn, 2012), with deficits in episodic memory (i.e., memory for personally experienced information, as opposed to facts; Tulving, 1984) being one of the most consistently reported (Gonzalez, 2007; Ranganathan and D’Souza, 2006; Solowij and Battisti, 2008). Although several studies document that recovery of most neurocognitive functions may occur after about one month of abstinence (Fried et al., 2005; Hanson et al., 2010; Pope et al., 2001; Schreiner and Dunn, 2012), others have shown that some deficits (e.g., attention and decision-making) persist, especially among very heavy users, those using during early adolescence, and individuals with persistent cannabis use disorders (Bolla et al., 2002, 2005; Hanson et al., 2010; Meier et al., 2012; Pope et al., 2003). However, as with most drugs of abuse, the majority of cannabis users (approximately 91%) do not go on to develop a cannabis use disorder (Anthony et al., 1994; Lopez-Quintero et al., 2011). Although myriad factors may make some cannabis users more vulnerable to cannabis use disorders and to experience more problems from their cannabis use (Kirisci et al., 2013; von Sydow et al., 2002), the role of neurocognitive performance has been relatively understudied, as it is typically examined as a consequence of use in studies of cannabis and neurocognition. A notable exception is a recent study by Day et al. (2013) that reported poorer “working memory” (as assessed by the Trail-Making Test B) was associated with more problems from cannabis use among an adult sample of frequent cannabis users.
Decision-making is a neurocognitive construct that may play an important role in the magnitude of problems experienced from cannabis use. It has been defined in numerous ways (Bechara et al., 2001; Paulus et al., 2003), but essentially refers to making the most advantageous choice under uncertain outcomes that may result in reward or punishment. The Iowa Gambling Task (IGT) is one of the most commonly used measures of decision-making among many available (Monterosso et al., 2001) and requires participants to make choices under conditions of uncertain risk with the goal of obtaining the most advantageous outcome over 100 trials. A seemingly advantageous choice on one trial may be a losing strategy over many trials. Poor overall performance on this task is thought to reflect a preference for immediate rewards even if they are at the expense of longer-term negative outcomes – sometimes referred to as “myopia for the future,” which is observed among patients with lesions affecting the orbitofrontal cortex (OFC; Bechara et al., 1994, 2002). This mirrors to some extent the behavior of individuals with cannabis use disorders, who by definition continue to use cannabis despite experiencing significant negative consequences. Not surprisingly, individuals with various substance use disorders perform poorly on measures of decision-making, including those with alcohol, cocaine, and methamphetamine addiction (e.g., Barry and Petry, 2008; Gonzalez et al., 2007; Grant et al., 2000; van der Plas et al., 2009; Verdejo-Garcia et al., 2007). Similarly, poorer performance on the IGT has also been reported among cannabis users (Bolla et al., 2002, 2005; Fernandez-Serrano et al., 2009; Hermann et al., 2009; Lamers et al., 2006; Verdejo-Garcia et al., 2007; Whitlow et al., 2004). Oftentimes, this is thought to reflect adverse effects of the drug on brain structure, function, or development.
However, emerging evidence lends support to decision-making as an important predictor and moderator in substance use outcomes. For example, poor decision-making performance has been associated with attrition in a weight-management program (Koritzky et al., 2014) and relapse for cocaine (Verdejo-Garcia et al., 2014), methamphetamine (Paulus et al., 2005), alcohol (Bowden-Jones et al., 2005), and opiates (Passetti et al., 2008). With regards to problems from cannabis use, neuroimaging studies have revealed relationships between OFC gray matter volume and more self-reported problems from cannabis (Filbey et al., 2014) and OFC activation with cannabis use relapse (De Bellis et al., 2013), further substantiating the potential role of decision-making deficits in the problems experienced from cannabis use. We recently reported no significant differences between cannabis users and non-users on several measures of inhibitory control (including decision-making), despite finding deficits in episodic memory; Gonzalez et al., 2012). Despite the lack of differences between groups on measures of decision-making and inhibitory control, we found that poorer decision-making performance alone was significantly associated with more DSM-IV symptoms of cannabis use disorder. In a subsequent manuscript with the same sample, decision-making was found to moderate the relationship between amount of cannabis use and engagement in risky sexual behaviors, with greater cannabis use being associated with more risky sexual behaviors, but only among participants with poorer decision-making (Schuster et al., 2012).
In the current study, we set out to examine in more detail how decision-making influences the problems that individuals report experiencing from their cannabis use by using a more fine-grained and well-established measure of cannabis use problems: the Marijuana Problems Scale (Stephens et al., 2000), as well as specifically looking at amount of use across three different time frames (30-day, 12-month, and lifetime). The Marijuana Problems Scale allows for rating the severity of numerous problems that may be experienced by cannabis users as a consequence of their cannabis use. Based on our prior findings, we sought to test the role of decision-making as a potential moderator of the relationship between amount of cannabis use and problems experienced from cannabis use in a sample of young adult cannabis users who identified cannabis as their drug of choice. Contrary to the fairly consistent finding of acute and non-acute cannabis use on episodic-memory that some have reported to resolve with abstinence (noted above), studies on cannabis effects on decision-making have been less consistent, oftentimes showing no acute effects (reviewed in Crean et al., 2011) and a lack of recovery with abstinence (e.g., Bolla et al., 2002, 2005; Crean et al., 2011). Therefore, for comparison and to test for a simple dissociation, in the present study we also examined whether episodic memory performance also served as a moderator of cannabis use and problems from cannabis use. We hypothesized that poorer decision-making and more cannabis use would be related to more cannabis-related problems as measured by the Marijuana Problems Scale (Stephens et al., 2000). More importantly, we anticipated that decision-making performance would moderate the relationship between amount of cannabis use and problems experienced from cannabis use, such that the association between amount of cannabis use and problems from use would be strongest for those with poorer decision-making. We hypothesized that similar relationships would not be observed with measures of episodic memory. Finally, we explored if the moderating effects of decision-making were more relevant for measures of current (30 day) or more distal (12 month and lifetime cumulative use) cannabis use.
2. MATERIAL AND METHODS
2.1 Participants
Participants were a subset (n = 52) of cannabis users from a larger study on inhibitory control and cannabis use among young adults (K23DA023560; PI: Gonzalez) who completed the Marijuana Problems Scale. The Marijuana Problems Scale was introduced after the onset of the larger study and the current sample consists of all eligible participants from the larger study who completed the Marijuana Problems Scale. Participants were recruited from the Chicago area through word-of-mouth and printed flyers placed throughout the community. A small subset was recruited and enrolled from a longitudinal study of trajectories to nicotine dependence (P01 CA098262; PI: Mermelstein). The investigation was approved by the Institutional Review Board of the University of Illinois at Chicago and all participants provided informed consent.
Detailed eligibility criteria are presented in a prior manuscript (Gonzalez et al., 2012). Briefly, participants were screened via telephone, had more than 8 years of education, estimated IQ greater than 75, and no significant neurological, mental health, or developmental problems. All participants used cannabis in the 45 days prior to their evaluation, more than 200 times during their lifetime, at least 4 times per week during peak use, and identified cannabis as their drug of choice. Those evidencing alcohol dependence or recent heavy drinking were excluded, as were those with any other substance use disorder (with the exception of nicotine or caffeine), or history of using other substances more than 10 times in their lifetime or during the 30 days prior to their evaluation (alcohol, nicotine, and hallucinogens notwithstanding). Lifetime frequency of hallucinogen use ranged from 4 to 24 times (Median = 5, IQR = 4, 7.25), with no participant meeting criteria for a hallucinogen use disorder and none reporting use less than 45 days since their study visit. With the exception of cannabis, no participant tested positive on a 10-panel rapid urine toxicology drug test (10-panel Drug Check Cup; Express Diagnostics, Blue Earth, Minnesota) or showed evidence of recent and significant alcohol consumption on alcohol breath test (AlcoMate Prestige Model AL6000; Palisades Park, NJ). Participants were asked to abstain from cannabis on the day of testing and none reported use or appeared intoxicated based on examiner behavioral observations. Participants reported a median of 3 days since last cannabis use (Table 1).
Table 1.
Participant Characteristics
| N =52 | |
|---|---|
| Age | 20.6 (1.9) |
| % Female | 37% |
| Estimated FSIQ | 103.2 (9.7) |
| Years of Education | 13.5 (1.7) |
| Ethnicity/Race | |
| Caucasian | 46% |
| Black | 31% |
| Hispanic | 15% |
| Asian | 6% |
| Other | 2% |
| Annual Household Income in Thousands of Dollars [Md, IQR] | 26.0 [7.8, 76.4] |
| Mother’s Education in years | 14.6 (2.81) |
| Past Major Depressive Disorder (SCID) | 22% |
| BDI-II Total Score [Md, IQR] | 5.5 [2, 9.75] |
| BAI Total Score [Md, IQR] | 5 [1, 9] |
| WURS, % of scores >46 | 6% |
| BIS-11 Total Score | 59.9 (9.5) |
| Age of cannabis initiation | 15.8 (3.3) |
| Years of cannabis use | 4.6 (2.1) |
| Days since last cannabis use | 3 [2.25, 4] |
| % THC+ | 77% |
| Current (30 day) DSM-IV SUD | |
| Alcohol Abuse | 4% |
| Alcohol Dependence | 0% |
| Cannabis Abuse | 31% |
| Cannabis Dependence | 27% |
| Lifetime DSM-IV SUD | |
| Alcohol Abuse | 21% |
| Alcohol Dependence | 0% |
| Cannabis Abuse | 40% |
| Cannabis Dependence | 31% |
| Amount Used in Lifetime [Md, IQR] | |
| Alcoholic drinks | 381 [121.25, 1224] |
| Cigarettes | 586 [19.5, 5382] |
| Cannabis (joints) | 485.1 [182.46, 1452.6] |
| Amount Used in Past 12 months [Md, IQR] | |
| Alcoholic drinks | 132 [33, 261] |
| Cigarettes | 54 [0.25, 936] |
| Cannabis (joints) | 126 [51.25, 471.6] |
| Amount Used in Past 30 days [Md, IQR] | |
| Alcoholic drinks | 10.5 [2, 20] |
| Cigarettes | 7.5 [0, 82.5] |
| Cannabis (joints) | 11.1 [4.25, 35.68] |
| HVLT Immediate Recall (z score) | −.79 (1.4) |
| HVLT Immediate Recall (raw score) | 26.5 (5.0) |
| IGT net score (T-score) | 45.3 (9.3) |
| IGT net score (raw score) | 5.2 (27.6) |
Note. All values are means and standard deviations, unless otherwise noted; Md, Median; IQR, interquartile range; FSIQ, Full Scale IQ; BDI-2, Beck Depression Inventory-2nd Edition; BAI, Beck Anxiety Inventory; WURS, Wender-Utah Rating Scale; BIS, Barratt Impulsiveness Scale-11th version; SCID, Structured Clinical Interview for DSM-IV; SUD Substance Use Disorder diagnosis; THC+, urine positive for cannabis; HVLT, Hopkins Verbal Learning Test—Revised; IGT, Iowa Gambling Task.
2.2 Measures and Procedures
Participants completed a counterbalanced battery of tests administered by trained examiners and requiring 2.5 to 4 hours to complete. Participants completed detailed interviews and questionnaires on medical and mental health history, as well as demographics. Full scale IQ was estimated with the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001). Symptoms of depression and anxiety were assessed with the Beck Depression Inventory – 2nd Edition (BDI-II; Beck et al., 1996) and the Beck Anxiety Inventory (BAI; Beck and Steer, 1990). The Wender-Utah Rating Scale (WURS; Ward et al., 1993) evaluated symptoms of ADHD. The Barratt Impulsiveness Scale-11 (BIS) assessed impulsive personality traits (Patton et al., 1995). The Structured Clinical Interview for DSM-IV disorders (SCID) was used to assess mood disorders (First et al., 2002).
Substance use history was assessed for 13 different substance classes to arrive at estimates of frequency and amount of use during the 12 months and 30 days prior to the evaluation, as well as total cumulative use over the participants’ lifetime (Gonzalez et al., 2012; Rippeth et al., 2004). The Substance Use module of the SCID assessed diagnoses of alcohol and substance use disorders (First et al., 2002).
The Marijuana Problems Scale quantified negative social, personal, occupational, and physical consequences experienced from cannabis use in the past 90 days using 19 self-report items rated on a 3-point scale: (0) No problem; (1) Minor Problem; (2) Serious Problem. Higher total scores indicated more negative consequences. The instrument has been used in various studies and appears to demonstrate adequate validity and reliability (Buckner et al., 2010; Day et al., 2013; Stephens et al., 2000, 2004).
The IGT (Bechara et al., 1994) requires participants to choose from a computerized display of four card decks, with each of the 100 trials resulting in a win of some money and also a loss. Two decks most frequently resulted in small rewards and smaller losses (“good” decks) with other decks more frequently resulting in larger rewards but also larger losses (“bad” decks). The total net score (choices from good decks minus bad decks) was used to quantify overall performance.
The Hopkins Verbal Learning Test-Revised (HVLT; Benedict et al., 1998) assesses episodic auditory memory using a 12-item word list read to participants over three trials. Participants immediately repeat as many words as possible after each trial, and then again after a 25 minute delay which is followed by a forced choice recognition trial. Total Immediate Recall (HVLT-IR) is the total number of correct words spontaneously recalled after the three learning trails, and the index of episodic memory used for this study, as it demonstrated the largest differences in performance between cannabis users and non-users in our prior study (Gonzalez et al., 2012). Standardized scores were calculated using published normative data (Benedict et al., 1998).
2.3 Data Analysis
All analyses were carried out using JMP 9.0 (SAS, Carey, NC). Data were inspected for non-normal distribution and outliers. Square-root transformations or non-parametric procedures were used when indicated. Specifically, measures of alcohol, nicotine, and cannabis use underwent square-root transformation and no analysis resulted in a data point with a Cook’s D value > 1 (Cook and Weisberg, 1982). The primary analyses consisted of multiple linear regressions with centered independent variables of amount of cannabis use, a measure of neurocognitive performance (IGT or HVLT-IR), and their interaction term, regressed on Marijuana Problems Scale scores. Only one measure of cannabis use consumption (lifetime, 12 month, or 30 day) and one measure of neurocognitive function (IGT or HVLT-IR) were included as independent variables in each model, which resulted in six omnibus regression models. Significant two-way interaction effects were followed up with simple slope linear regressions examining the association between amount of cannabis use and Marijuana Problems Scale scores at differing levels neurocognitive performance. All results with p-values < .05 were deemed statistically significant.
3. RESULTS
3.1 Participant Characteristics
The sample consisted of young adult cannabis users with exposure to cannabis, alcohol, and nicotine, but no history of dependence for alcohol or other drugs of abuse (Table 1). The sample was demographically diverse with participants in their early twenties and with some college education. Scores on the BDI-II, BAI, and WURS suggested minimal mental health complaints, with median scores well below clinical cut-points. Table 1 provides detailed information on use of alcohol, nicotine, and cannabis use over three time frames (i.e., cumulative lifetime use, 12-month use, and use during the last 30 days). Median frequency of cannabis use in the sample during the 12 months prior to the evaluation was 4.6 times per week.
3.2 Performance and Responses on Primary Measures of Interest
Based on published normative data (Bechara, 2007), the average IGT performance of the sample was within the average range, whereas performance of the sample on the HVLT-IR was almost one standard deviation below the mean based on age-adjusted norms for healthy individuals (Table 1). Scores on the Marijuana Problems Scale ranged from zero to 15 (mean = 5.27, SD = 3.4, median = 5, IQR = 2.25 to 8).
3.3 Relationships among Cannabis Use, Neurocognitive Performance, and Problems from Cannabis Use
Results of omnibus models are summarized in Table 2. The two-way interactions of IGT by amount of cannabis use on Marijuana Problems Scale scores were statistically significant for lifetime (p = .04), 12-month (p = .03), and last 30 day (p = .009) estimates of amount of cannabis use. Main effects of amount of cannabis use or IGT performance were not significant in these models (all p-values ≥ .18). In contrast, the two-way interactions for regression models with HVLT-IR scores (instead of IGT scores) were not significant regardless of timeframe queried (all p-values > .72). However, lifetime and 12-month amounts of cannabis use, but not 30-day use, evidenced significant main effects in these models, with more cannabis use being associated with more problems from use (see Table 2).
Table 2.
Statistics from Regression Models Examining Interactions between Amount of Cannabis Use and Neuropsychological Performance on Marijuana Problems Scale Scores
| Variable | Lifetime Cannabis Use | 12 month Cannabis Use | 30 day Cannabis Use | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | β | p | R2 | β | p | R2 | β | p | |
| HVLT (Immediate Recall) | .16 | .04 | .11 | .12 | .08 | .27 | |||
| Cannabis Use (sqrt) | .37 | .009 | .28 | .04 | .22 | .14 | |||
| HVLT | .26 | .07 | .21 | .13 | .20 | .16 | |||
| Cannabis Use x HVLT | −.001 | .97 | .05 | .72 | .02 | .90 | |||
| IGT (Net Total) | .18 | .02 | .16 | .04 | .17 | .03 | |||
| Cannabis Use (sqrt) | .15 | .32 | .03 | .87 | −.02 | .92 | |||
| IGT | −.20 | .18 | −.20 | .18 | −.19 | .18 | |||
| Cannabis Use x IGT | −.33 | .04 | −.36 | .03 | −.40 | .009 | |||
Note. Statistically significant findings are indicated by bolded and italicized numbers; R2 and associated p-values are for omnibus models; HVLT, Hopkins Verbal Learning Task; IGT, Iowa Gambling Task
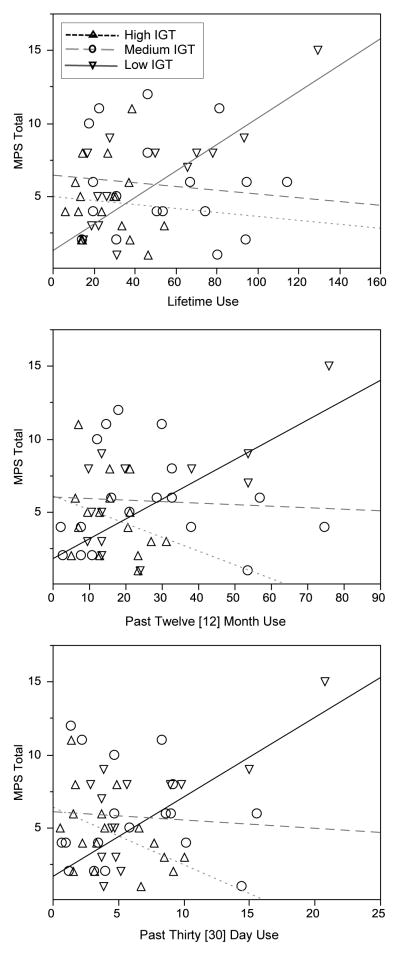
Significant interactions were followed by examining the relationship between amount of cannabis use and Marijuana Problems Scale scores across three levels of IGT performance based on tertiles (Low, n = 17; Medium, n = 18; and High, n = 17) for each of the cannabis use indices (i.e., lifetime, 12-month, 30-day), Table 3. Those in the High (mean IGT T-score = 56.1, SD = 4.9; mean IGT raw-score = 36.8, SD = 15.4) and Medium groups (mean IGT T-score = 43.9, SD = 1.8; mean IGT raw-score = 0.4, SD = 7.9) evidenced performances in the high-average to low-average range, not suggestive of decision-making deficits. However, participants in the Low group demonstrated standardized normative scores in the mildly impaired range (mean IGT T-score = 35.9, SD = 5.6; mean IGT raw-score = −21.3, SD = 17.3). Across all timeframes, greater amounts of cannabis use were associated with higher Marijuana Problems Scale scores among those classified as Low on the IGT (lifetime, p = .001, R2 = .51; 12-month, p = .006, R2 = .41; 30-day, p = .007, R2 = .39), Figure 1. In contrast, amount of cannabis use did not correlate with Marijuana Problems Scale scores among those classified as Medium performers (p-values ≥ .64) or High performers (p-values > .06). Thus, greater amount of cannabis use (regardless of the timeframe assessed) was associated with more problems experienced from cannabis use, but only among the individuals who performed most poorly on the IGT. Despite the relatively low levels of alcohol and nicotine use in the sample (compared to cannabis use), we conducted all analyses again controlling for reported amounts of nicotine and alcohol use (lifetime, 12-month, and 30-day as appropriate) and found the same pattern of significant results.
Table 3.
Statistics from Simple Slope Regressions of Significant IGT x Cannabis Use Interactions on Marijuana Problems Scale Scores
| Variable | Lifetime Cannabis Use | 12 month Cannabis Use | 30 day Cannabis Use | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | β | p | R2 | β | p | R2 | β | p | |
| IGT High | .01 | −.07 | .78 | .09 | −.29 | .25 | .21 | −.46 | .06 |
| IGT Medium | .01 | −.12 | .64 | .001 | −.03 | .90 | .01 | −.08 | .77 |
| IGT Low | .51 | .72 | .001 | .41 | .64 | .006 | .39 | .63 | .007 |
Note. Statistically significant findings are indicated by bolded and italicized numbers; HVLT, Hopkins Verbal Learning Task; IGT, Iowa Gambling Task
Figure 1.
Relationship between lifetime, 12-month, and 30-day cannabis consumption and problems experienced from use stratified by decision-making performance. Amounts of cannabis use are presented in “joints” and are square-root transformed; IGT, Iowa Gambling Task
Finally, we compared the three groups that were stratified on IGT performance on various potential demographic, mental health and substance use confounds. The groups did not differ significantly on age, gender, race/ethnicity, education, WTAR FSIQ, BIS, BDI-II, BAI, WURS, Marijuana Problems Scale, current or past diagnosis of alcohol abuse, current or past diagnosis of cannabis abuse or dependence, amount of alcohol use (lifetime or 12 month), or nicotine use (lifetime, 12 month, or 30 day), all p-values > .05. Significant between-group differences were observed for 30 day alcohol use (p = .05), with post-hoc comparisons with Tukey-Kramer HSD revealing that the Low IGT group reported less 30 day alcohol use than the High IGT group, thus presenting a conservative bias. Finally, we compared age of first cannabis use and age of regular cannabis use (defined as at least once a week for 3 months) stratified by level of IGT performance and found no significant differences (p-values > .17).
4. DISCUSSION
In the current study, decision-making performance (as assessed by the IGT) was a significant moderator of the relationship between amount of cannabis use and problems reported from its use among young adult cannabis users. Amount of cannabis consumed was associated with problems experienced from use only among those participants demonstrating the poorest (impaired) decision-making performance. Among those with better decision-making performance, amount of cannabis use was uncorrelated with problems from use. As hypothesized, the moderating role of neurocognitive functioning appeared specific to decision-making, as episodic memory performance was not found to influence relationships between amount of use and problems from use. Importantly, these relationships could not be accounted for by systematic differences in demographics, education, estimated IQ, trait impulsivity, mental health, nicotine use, alcohol use, or overall number of problems reported from cannabis use across levels of decision-making performance. It is also important to consider that when episodic memory performance was included in the model, instead of decision-making performance, significant associations between amount of cannabis use and problems from use did emerge, such that more cannabis use was associated with more problems from use. In models that included decision-making performance, relationships between amount of cannabis use and problems from use seemed to be specific to a particular subset of our sample: only those individuals with poor decision-making. Our findings provide further evidence that individual differences in decision-making play a role in whether cannabis users experience problems from use, with those cannabis users who have impaired decision-making experiencing problems in proportion to their amount of cannabis use.
Our results illustrate that individual differences in decision-making may have an impact on the consequences experienced from cannabis use. Among the results of a prior study from which the current subset of participants was derived, we reported that poorer decision-making performance was associated with a greater number of DSM-IV symptoms of cannabis use disorder. The current study elaborates on these findings by using a more fine-grained, well-established measure of problems from marijuana use (i.e., the Marijuana Problems Scale), which we began to administer after the parent study was already underway. Despite having a smaller sample in the current study, we nonetheless found strong moderating effects of decision-making on Marijuana Problem Scale scores. It appears that the Marijuana Problems Scale and DSM-IV cannabis use disorder symptoms, although related, are only modestly correlated (i.e., in the current sample, the correlation was .37, p < .01). This could be due in part to different time spans queried (30-day for current DSM-IV symptoms and 90-day for the Marijuana Problems Scale), the specific focus on problems experienced from use in the Marijuana Problems Scale, and the ability to rate symptom severity on the Marijuana Problems Scale rather than simply obtaining a symptom count.
As is the case with observational, cross-sectional studies of neurocognition and substance use, our findings beg the question of whether problems with decision-making among some individuals in our sample emerged as a result of their cannabis use or whether they may have existed prior to initiation of use. Although this important question cannot be answered with the current study design, we offer some speculation. One possible mechanism by which cannabis use may directly impact decision-making is through the expected cannabinoid receptor downregulation that would occur with prolonged cannabis use (Hirvonen et al., 2012). However, contrary to what might be expected, decision-making deficits in the context of cannabis use have been reported to persist beyond the time needed for cannabinoid receptor density to return to normal after abstinence (Bolla et al., 2002, 2005; Crean et al., 2011). Furthermore, the evidence for dose-response effects of cannabis or THC on decision-making performance is lacking for acute effects and is inconsistent for residual and longer-term effects (Crean et al., 2011). A second mechanism for decision-making deficits from cannabis arises from the impact cannabis use may have on the developing brain. Development of prefrontal cortex is ongoing during adolescence (Giedd et al., 1999; Gogtay et al., 2004), a common age of cannabis use initiation. The evidence for more profound neurocognitive deficits among individuals who begin cannabis use earlier (or absence of deficits among those who initiate use in adulthood) support this mechanism (Crane et al., 2012; Ehrenreich et al., 1999; Meier et al., 2012; Pope et al., 2003). However, in the current study, we found that age of first marijuana use and age of regular marijuana use did not differ across levels of decision-making performance. Finally, it is possible that deficits in decision-making predate heavy cannabis use and may contribute to initiation, escalation, and compulsive use. It is important to note that these mechanisms are not exclusive and that decision-making may be both a pre-existing risk factor for development of cannabis addiction, as well as a neurocognitive ability that worsens with prolonged cannabis use, especially when use begins in adolescence.
Other researchers have postulated that at least some neurocognitive deficits (typically measures of executive functions, impulsivity, and behavioral control) may precede and contribute to the development of drug addiction (de Wit, 2009; Goldstein et al., 2006; Iacono et al., 2008). Performances on some laboratory tests of executive functions (e.g. working memory) alone or when taken together with other questionnaires and interview measures of emotion regulation and behavioral control have been associated with the development of substance use and addiction (Day et al., 2013; Giancola and Tarter, 2002; Kirisci et al., 2006; Tarter et al., 2003), as well as escalation in cannabis use (Cousijn et al., 2014). However, evidence for decision-making deficits as a precursor for substance use disorders is far from conclusive. For example, a recent 4-year longitudinal study of adolescents found no significant relationships between decision-making performance and initiation of substance use (Ernst et al., 2010). Moreover, using a strong co-twin control design, Malone and colleagues (2014) concluded that decision-making deficits were a causal effect of alcohol consumption among an adolescent sample, rather than a precursor. Perhaps, it may be that decision-making is more relevant to development of a cannabis use disorder rather than initiation of use, as we have suggested here and in a prior study (Gonzalez et al., 2012).
Our results, along with those of aforementioned studies, may be interpreted in light of dual-process models of addiction and risk-taking, which posit two interacting systems: an impulsive system related to drug-seeking that includes the amygdala and ventral striatum, and a reflective system (involving orbitofrontal regions, anterior cingulate, insula, somatosensory cortex, and hippocampus) that shapes future behavior based on affective states arising from consequences of prior behavior (Bechara, 2005; Steinberg, 2010; Strang et al., 2013). Among individuals who have tried cannabis, a dysfunctional reflective system would contribute to escalating drug use despite experiencing negative consequences. Our results of strong associations between amount of cannabis use and problems from cannabis use only among those individuals with impaired decision-making would be consistent with a dysfunctional reflective system exerting inadequate top-down control of the impulsive system. Cannabis users with impaired decision-making may find themselves smoking cannabis or being intoxicated at times when it conflicts with important role obligations (e.g., schoolwork, job duties, social roles), or they may forgo such obligations altogether in order to obtain, consume, or recover from cannabis and therefore experience more problems from their cannabis use.
Our results should be interpreted in the context of several limitations. Although our study implicates decision-making in shaping how amount of cannabis use influences problems experienced from use, the study design precludes us from making temporal inferences regarding decision-making deficits and cannabis use. We are currently conducting a longitudinal investigation of adolescent cannabis users to better address this issue. Secondly, the larger project from which this study’s sample was derived was specifically designed to assess the impact of cannabis use on decision-making. Thus, participants were carefully selected to be primarily cannabis users who identified cannabis as their drug of choice and had minimal or use of other substances, including alcohol. Despite evidence to suggest that deficits in decision-making may be a common neurocognitive deficit among users of various substances (Bechara and Damasio, 2002; Gonzalez et al., 2007), our particular findings may not generalize to individuals who primarily use other substances or use other drugs heavily in combination with cannabis. It is also important to note that measures of decision-making are complex (Busemeyer and Stout, 2002), involve functioning of various brain systems (Krain et al., 2006; Li et al., 2010), and vary in how much they make risk discernable to participants (Brand et al., 2006, 2007; Hsu et al., 2005; Krain et al., 2006). Indeed, as with any neurocognitive test, many factors may contribute to poor performance on the IGT. Future studies would benefit from inclusion of more decision-making measures and a fine-grained analysis of what specific aspect of decision-making contributes to problems experienced from cannabis use and under what conditions. This may be accomplished through computational modeling approaches that allow for a more nuanced understanding of the mechanisms driving poor performance on the IGT (Busemeyer and Stout, 2002; Fridberg et al., 2010; Vassileva et al., 2013). Furthermore, it remains to be seen if our findings are specific to decision-making or may generalize to other aspects of executive function, as suggested by the findings of Day et al. (2013). Finally, we relied on participant self-report for estimates of cannabis use consumption and problems from use. Collateral information from family members, employers, or teachers regarding the participant’s daily functioning would have strengthened the study design. It will be important to replicate the current findings with a larger sample and compare results to groups of individuals who primarily use other substances.
The importance of understanding contributors to cannabis use disorders is elevated by current trends to facilitate access to cannabis in the US, the high prevalence of cannabis use, and the societal burden of cannabis use disorders. The growing trend toward legalization and decriminalization may result in increased use and dependence (Palamar et al., 2014). This is of concern in light of the approximately 204,000 Americans who met criteria for a cannabis use disorder during 2012, making cannabis the third most common substance (behind alcohol and pain relievers) for which people sought substance use treatment in the United States (Substance and Mental Health Services, 2013). Costs for treatment of cannabis use disorders among adolescents is significant (French et al., 2002) and cannabis dependence is estimated to account for the equivalent of about 2 million years lost due to disability (YLD) worldwide (Degenhardt et al., 2013). Given the mounting evidence for decision-making as an important contributor to the development of cannabis use disorders, it follows that interventions aimed at strengthening decision-making, similar to those employed with polysubstance users (Alfonso et al., 2011), may be promising to help stall escalation of cannabis use and mitigate development of cannabis use disorders. Prevention efforts may benefit from including measures of decision-making among instruments used to identify at-risk youth.
Highlights.
Decision-making, memory, and problems from cannabis were assessed in young adults.
More cannabis use was associated with more negative consequences from cannabis.
Decision-making moderated this relationship, but episodic memory did not.
More use was linked to more problems only among those with impaired decision-making.
Decision-making influences how experiences problems from cannabis use.
Footnotes
Contributors
All authors have materially participated in the research and article preparation. Dr. Gonzalez, participated in conceptualizing the study, conducting data analyses, and writing the manuscript. Dr. Schuster participated in study recruitment, assessment, data analysis, and editing of the manuscript. Dr. Mermelstein is the PI of a study from which a subset of participants were recruited. She assisted with conceptualizing the current study, reviewing results, and contributing to the initial manuscript and subsequent revisions. Dr. Diviak assisted with developing recruitment strategies for the study, identifying eligible participants from Dr. Mermelstein’s study, and editing of the manuscript.
Author Disclosures
Research reported in this article was supported by National Institute on Drug Abuse and the National Cancer Institute of the National Institutes of Health under grant number K23 DA023560, R01 DA031176, R01 DA033156 to Dr. Gonzalez, F31 DA032244 to Dr. Schuster, and P01 CA098262 to Dr. Mermelstein. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other conflicts of interest to declare.
Conflicts of interest: Research reported in this article was supported by National Institute on Drug Abuse and the National Cancer Institute of the National Institutes of Health. The authors have no other conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfonso JP, Caracuel A, Delgado-Pastor LC, Verdejo-García A. Combined goal management training and mindfulness meditation improve executive functions and decision-making performance in abstinent polysubstance abusers. Drug Alcohol Depend. 2011;117:78–81. doi: 10.1016/j.drugalcdep.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Barry D, Petry NM. Predictors of decision-making on the Iowa Gambling Task: independent effects of lifetime history of substane use disorders and performance on the Trail Making Test. Brain Cogn. 2008;66:243–252. doi: 10.1016/j.bandc.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A. The Iowa Gambling Task Professional Manual. Psychological Assessment Resources; Boca Raton, FL: 2007. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. The Psychological Corporation; San Antonio: 1990. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test--Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Bowden-Jones H, McPhillips M, Rogers R, Hutton S, Joyce E. Risk-taking on tests sensitive to ventromedial prefrontal cortex dysfunction predicts early relapse in alcohol dependency: a pilot study. J Neuropsychiatry Clin Neurosci. 2005;17:417–420. doi: 10.1176/jnp.17.3.417. [DOI] [PubMed] [Google Scholar]
- Brand M, Labudda K, Markowitsch H. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Netw. 2006;19:1266–1276. doi: 10.1016/j.neunet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Brand M, Recknor E, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. J Clin Exp Neuropsychol. 2007;29:86–99. doi: 10.1080/13803390500507196. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Ecker AH, Cohen AS. Mental health problems and interest in marijuana treatment among marijuana-using college students. Addict Behav. 2010;35:826–833. doi: 10.1016/j.addbeh.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Busemeyer JR, Stout JC. A contribution of cognitive decision models to clinical assessment: decomposing performance on the Bechara gambling task. Psychol Assess. 2002;14:253–262. doi: 10.1037//1040-3590.14.3.253. [DOI] [PubMed] [Google Scholar]
- Cook RD, Weisberg S. Residuals And Influence In Regression. Chapman and Hall; London: 1982. [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: a prospective fMRI study. Hum Brain Mapp. 2014;35:2470–2482. doi: 10.1002/hbm.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev. 2013;23:117–137. doi: 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AM, Metrik J, Spillane NS, Kahler CW. Working memory and impulsivity predict marijuana-related problems among frequent users. Drug Alcohol Depend. 2013;131:171–174. doi: 10.1016/j.drugalcdep.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Wang L, Bergman SR, Yaxley RH, Hooper SR, Huettel SA. Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug Alcohol Depend. 2013;133:134–145. doi: 10.1016/j.drugalcdep.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Freedman G, Burstein R, Johns N, Engell RE, Flaxman A, Murray CJL, Vos T. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacol. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Ernst M, Luckenbaugh DA, Moolchan ET, Temple VA, Jenness J, Korelitz KE, London ED, Kimes AS. Decision-making and facial emotion recognition as predictors of substance-use initiation among adolescents. Addict Behav. 2010;35:286–289. doi: 10.1016/j.addbeh.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol. 2009;24:1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J. Long-term effects of marijuana use on the brain. Proc Natl Acad Sci. 2014;111:16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Version 2.0. Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- French MT, Roebuck MC, Dennis ML, Diamond G, Godley SH, Tims F, Webb C, Herrell JM. The economic cost of outpatient marijuana treatment for adolescents: findings from a multi-site field experiment. Addiction. 2002;97:84–97. doi: 10.1046/j.1360-0443.97.s01.4.x. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Queller S, Ahn WY, Kim W, Bishara AJ, Busemeyer JR, Porrino L, Stout JC. Cognitive mechanisms underlying risky decision-making in chronic cannabis users. J Math Psychol. 2010;54:28–38. doi: 10.1016/j.jmp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana - a comparison with pre-drug performance. Neurotoxicol Teratol. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Giancola P, Tarter R. Executive cognitive functioning and risk for substance abuse. Psychol Sci. 2002;10:203–205. [Google Scholar]
- Giancola PR, Moss HB, Martin CS, Kirisci L, Tarter RE. Executive cognitive functioning predicts reactive aggression in boys at high risk for substance abuse: a prospective study. Alcohol Clin Exp Res. 1996;20:740–744. doi: 10.1111/j.1530-0277.1996.tb01680.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Alia-Klein N, Cottone L, Volkow N. The orbitofrontal cortex in drug addiction. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford University Press; USA: 2006. pp. 481–522. [Google Scholar]
- Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev. 2007;17:347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: Preliminary observations. J Clin Exp Neuropsychol. 2007;29:155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol. 2012;34:962–976. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Lemenager T, Gelbke J, Welzel H, Skopp G, Mann K. Decision making of heavy cannabis users on the Iowa Gambling Task: stronger association with THC of hair analysis than with personality traits of the Tridimensional Personality Questionnaire. Eur Addict Res. 2009;15:94–98. doi: 10.1159/000189788. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Reynolds M, Vanyukov M. Individual differences in childhood neurobehavior disinhibition predict decision to desist substance use during adolescence and substance use disorder in young adulthood: a prospective study. Addict Behav. 2006;31:686–696. doi: 10.1016/j.addbeh.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter RE, Ridenour T, Reynolds M, Vanyukov M. Longitudinal modeling of transmissible risk in boys who subsequently develop cannabis use disorder. Am J Drug Alcohol Abuse. 2013;39:180–185. doi: 10.3109/00952990.2013.774009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koritzky G, Dieterle C, Rice C, Jordan K, Bechara A. Decision-making, sensitivity to reward, and attrition in weight management. Obesity. 2014;22:1904–1909. doi: 10.1002/oby.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: A meta-analysis of decision-making. NeuroImage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Lamers CTJ, Bechara A, Rizzo M, Ramaekers JG. Cognitive function and mood in MDMA/THC users, THC users and non-drug using controls. J Psychopharmacol. 2006;20:302–311. doi: 10.1177/0269881106059495. [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, D’Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Hum Brain Mapp. 2010;31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K, Wright N, Medina-Kirchner C, Maple K, Shollenbarger S. Considering cannabis: the effects of regular cannabis use on neurocognition in adolescents and young adults. Curr Addict Rep. 2014;1:144–156. doi: 10.1007/s40429-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Quintero C, Perez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115:120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone S, Luciana M, Wilson S, Sparks J, Hunt R, Thomas K, Iacono W. Adolescent drinking and motivated decision-making: a Cotwin-control investigation with monozygotic twins. Behav Genet. 2014:1–12. doi: 10.1007/s10519-014-9651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012;109:E2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O’Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96:1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Palamar JJ, Ompad DC, Petkova E. Correlates of intentions to use cannabis among US high school seniors in the case of cannabis legalization. Int J Drug Policy. 2014;25:424–435. doi: 10.1016/j.drugpo.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Clark L, Mehta MA, Joyce E, King M. Neuropsychological predictors of clinical outcome in opiate addiction. Drug Alcohol Depend. 2008;94:82–91. doi: 10.1016/j.drugalcdep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacol. 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Exp Clin Psychopharmacol. 2012;20:420–429. doi: 10.1037/a0029117. [DOI] [PubMed] [Google Scholar]
- Schuster RM, Crane NA, Mermelstein R, Gonzalez R. The influence of inhibitory control and episodic memory on the risky sexual behavior of young adult cannabis users. J Int Neuropsychol Soc. 2012;18:827–833. doi: 10.1017/S1355617712000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev. 2008;1:81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Fearer SA, Williams C, Picciano JF, Burke RS. The Marijuana Check-up: reaching users who are ambivalent about change. Addiction. 2004;99:1323–1332. doi: 10.1111/j.1360-0443.2004.00832.x. [DOI] [PubMed] [Google Scholar]
- Strang NM, Chein JM, Steinberg L. The value of the dual systems model of adolescent risk-taking. Front Hum Neurosci. 2013;7:223. doi: 10.3389/fnhum.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Agency (SAMHSA) National Survey on Drug Use & Health. United States Department of Health and Human Services. SAMHSA; Washington, D.C: 2013. [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Tulving E. Precis of elements of episodic memory. Behav Brain Sci. 1984;7:223–268. [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31:706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva J, Ahn W, Weber KM, Busemeyer JR, Stout JC, Gonzalez R, Cohen MH. Computational modeling reveals distinct effects of HIV and history of drug use on decision-making processes in women. PLoS One. 2013;8:e68962. doi: 10.1371/journal.pone.0068962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Albein-Urios N, Martinez-Gonzalez J, Civit E, Torre R, Lozano O. Decision-making impairment predicts 3-month hair-indexed cocaine relapse. Psychopharmacology (Berl) 2014;231:4179–4187. doi: 10.1007/s00213-014-3563-9. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. 2007;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Sydow K, Lieb R, Pfister H, Höfler M, Wittchen HU. What predicts incident use of cannabis and progression to abuse and dependence? A 4-year prospective examination of risk factors in a community sample of adolescents and young adults. Drug Alcohol Depend. 2002;68:49–64. doi: 10.1016/s0376-8716(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]