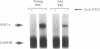Abstract
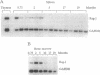
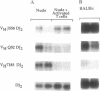
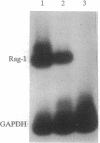
The rearrangement of immunoglobulin genes in B-lymphocyte precursors requires the expression of the recombination activating genes (Rag), which leads to the generation of a highly diverse B-cell repertoire. We can use the level of Rag-1 mRNA in the bone marrow as an index of its capacity to support the maturation of B lymphocytes as all detectable bone marrow Rag-1 mRNA is expressed by B-cell precursors. In mouse bone marrow, Rag-1 mRNA increases during the first 2 months of life to reach its maximal level at 2 months of age. This level is maintained until 5 months of age and thereafter declines to a minimum level by 10 months of age. Thus, bone marrow Rag-1 mRNA is highest at the time when thymic function is maximal in euthymic mice. An association between thymic activity and bone marrow Rag-1 gene expression was supported by showing a low level of bone marrow Rag-1 mRNA in athymic nude mice at an age when this gene is maximally expressed in euthymic mice. Another characteristic of B cells in nude mice is their preferential rearrangement of diversity region (D)-proximal heavy-chain variable region (VH) genes. We demonstrated that injection of syngeneic splenic T cells into nude mice not only stimulates an increase in Rag-1 mRNA in their bone marrow B-cell precursors but also restores their random use of VH genes. Most interestingly, injection of supernatant medium from phytohemagglutinin-activated splenic T-cell cultures from young euthymic mice also induces both Rag-1 mRNA in bone marrow B-cell precursors and random use of VH genes. These findings suggest that thymic function can regulate both Rag-1 gene expression and VH gene use by bone marrow B-cell precursors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chun J. J., Schatz D. G., Oettinger M. A., Jaenisch R., Baltimore D. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell. 1991 Jan 11;64(1):189–200. doi: 10.1016/0092-8674(91)90220-s. [DOI] [PubMed] [Google Scholar]
- Costa T. E., Suh H., Nussenzweig M. C. Chromosomal position of rearranging gene segments influences allelic exclusion in transgenic mice. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2205–2208. doi: 10.1073/pnas.89.6.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas A. A., Lembezat M. P., Rocha B. Selection of antibody repertories: transfer of mature T lymphocytes modifies VH gene family usage in the actual and available B cell repertories of athymic mice. Int Immunol. 1989;1(4):398–408. doi: 10.1093/intimm/1.4.398. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Muegge K., Vila M. P., Durum S. K. Interleukin-7: a cofactor for V(D)J rearrangement of the T cell receptor beta gene. Science. 1993 Jul 2;261(5117):93–95. doi: 10.1126/science.7686307. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Riley R. L., Kruger M. G., Elia J. B cell precursors are decreased in senescent BALB/c mice, but retain normal mitotic activity in vivo and in vitro. Clin Immunol Immunopathol. 1991 May;59(2):301–313. doi: 10.1016/0090-1229(91)90026-7. [DOI] [PubMed] [Google Scholar]
- Rolink A., Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991 Sep 20;66(6):1081–1094. doi: 10.1016/0092-8674(91)90032-t. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Viale A. C., Chies J. A., Huetz F., Malenchere E., Weksler M., Freitas A. A., Coutinho A. VH-gene family dominance in ageing mice. Scand J Immunol. 1994 Feb;39(2):184–188. doi: 10.1111/j.1365-3083.1994.tb03358.x. [DOI] [PubMed] [Google Scholar]