Abstract
Although impulsivity has been associated with cocaine dependence and other addictive behaviors, the biological factors underlying impulsivity have yet to be precisely determined. This study aimed to examine relationships between impulsivity and volumes of the amygdala and hippocampus in cocaine-dependent and healthy comparison individuals. The Barratt Impulsiveness Scale (BIS-11) was used to assess impulsivity. FreeSurfer was used to assess amygdalar and hippocampal volumes from high-resolution structural magnetic resonance images. Relative to healthy comparison subjects, cocaine-dependent individuals scored higher on all three subscales of BIS-11 but did not differ from healthy comparison subjects in amygdalar or hippocampal volumes. Cocaine-dependent individuals showed significant negative correlations between amygdalar volumes and scores on the BIS-11 Attentional subscale, and this relationship differed significantly from the non-significant relationship in healthy comparison subjects. As individual differences in amygdalar structure may contribute to the high impulsivity observed in cocaine-dependent individuals, the findings suggest that future studies should assess the extent to which therapies that target impulsivity in cocaine dependence may operate through the amygdala or alter its structure or function.
Keywords: addiction, neuroimaging, brain volume, amygdala, hippocampus, substance use disorder, MRI, BIS-11
1. Introduction
Impulsivity, characterized by tendencies to act rapidly and rashly without considerable forethought, represents an important intermediate phenotype relating to cocaine dependence and other addictions (Fineberg et al., 2014). Individuals with addiction to cocaine or other drugs often show elevated impulsivity (Bechara, 2005; Garavan and Hester, 2007). As elevated impulsivity has been associated with greater addiction severity and poorer treatment outcome (Helmus et al., 2001; Moeller et al., 2001; Krishnan-Sarin et al., 2007; Poling et al., 2007), impulsivity may represent an important treatment target for addictions (Helmus et al., 2001; Moeller et al., 2001). Thus, understanding the neural correlates of impulsivity may help guide treatment development strategies for addictions. In this regard, neuroimaging has been used to assess the integrity of brain structures in cocaine-dependent individuals and the relationships between these structures and impulsivity. Reduced gray-matter volumes in extensive brain regions including the frontal cortex, cingulate gyrus, insula, and temporoparietal junction have been observed in cocaine-dependent patients, and a significant negative correlation between trait impulsivity and volumes of gray matter in some of these regions, including the orbitofrontal cortex and insula, has been reported (Ersche et al., 2011; Moreno-López et al., 2012; Crunelle et al., 2014)
Data suggest that the amygdala and hippocampus may contribute to self-reported or “trait” impulsivity. For example, genetic factors have been linked to impulsive/aggressive tendencies, which in turn are linked to reduced volumes of the amygdala and hippocampus and to their increased functional response to emotional/motivational stimuli (Pavlov et al., 2012). Healthy individuals have shown positive correlations between impulsivity and activity in the bilateral amygdala while viewing images of emotional faces or appetizing foods (Beaver et al., 2006; Brown et al., 2006) and negative correlations between aggression and volumes of the amygdala (Matthies et al., 2012; Pardini et al., 2014). Veterans with comorbid posttraumatic stress (PTSD) and mild traumatic brain injury (mTBI) have shown reduced volume of the amygdala relative to veterans without PTSD and/or mTBI, and the extent of this reduction correlated with trait impulsivity (Depue et al., 2014). Neuropsychiatric disorders with increased impulsivity and/or aggression, such as psychopathy and borderline personality, are associated with reduced amygdalar and hippocampal volumes (Zetzsche et al., 2007; Huebner et al., 2008; Soloff et al., 2008; Sala et al., 2011) and exaggerated amygdalar reactivity when viewing emotional stimuli. One study reported that after co-varying with impulsivity measures, the difference in amygdalar and hippocampal volumes between individuals with and without borderline personality disorder was no longer significant, suggesting that the reduced volumes may relate to increased impulsivity (Soloff et al., 2008).
Findings of previous studies investigating amygdalar and hippocampal volumes in cocaine-dependent patients appear inconsistent. One study reported increased amygdalar volume (Ersche et al., 2012), several studies reported reduced volume in at least one of the two structures (Makris et al., 2004; Alia-Klein et al., 2011; Barrós-Loscertales et al., 2011; Moreno-López et al., 2012; Rando et al., 2013), and several studies did not find volumetric differences (Jacobsen et al., 2001; Sim et al., 2007; Narayana et al., 2010; Mackey and Paulus, 2013). We are not aware of studies specifically assessing relationships between impulsivity and amygdalar and hippocampal volumes in cocaine-dependent individuals. To examine further amygdalar and hippocampal volumes in cocaine-dependent individuals and their possible relationships with impulsivity, we assessed impulsivity as measured by the Barratt Impulsiveness Scale (BIS-11) (Patton and Stanford, 1995) and examined relationships with amygdalar and hippocampal volumes in cocaine-dependent and healthy comparison subjects. We hypothesized that: 1) cocaine-dependent as compared with healthy comparison subjects would show reduced amygdalar and hippocampal volumes; and 2) BIS-11 scores would correlate inversely with amygdalar and hippocampal volumes in cocaine-dependent individuals.
2. Methods
2.1. Subjects
This study included 34 cocaine-dependent patients and 41 healthy subjects for comparison. Participants were recruited by media advertisements. Some were treatment-seeking cocaine-dependent individuals participating in outpatient randomized clinical trials (Mitchell et al., 2013). They provided written informed consent for participation in the study approved by the Yale School of Medicine Human Investigations Committee. Cocaine-dependent individuals were assessed using the Structured Clinical Interview (SCID) (First et al., 1996; First et al., 1997). They met DSM-IV criteria for current cocaine dependence. Participants were excluded if they had not used cocaine within the past 28 days, less than a 3rd-grade reading level, metal in their body, or any acute or unstable illness including untreated psychotic disorders or current use of prescribed psychotropic medications or were pregnant, breast feeding, color-blind, or left-handed.
Healthy participants were recruited by media advertisements. Urine samples were assessed for recent use of cocaine, opioids, stimulants, marijuana and benzodiazepines. Healthy individuals were excluded if urine samples were positive for any substance. Other exclusionary criteria included left-handedness, pregnancy, current psychiatric diagnoses, or unstable medical conditions.
2.2. Impulsivity Assessment
The BIS-11 is a reliable, valid and widely used 30-item questionnaire measuring self-reported impulsivity and yields Attentional, Motor, and Non-planning subscales (Patton and Stanford, 1995). The Attentional subscale measures the capacity for concentration, the Motor subscale measures the propensity of acting without thinking, and the Non-planning subscale measures the propensity of focusing on present events without considering future events (Patton and Stanford, 1995).
2.3. Image acquisition and processing
High-resolution T1-weighted anatomical images were acquired using a 3-T scanner (Siemens Trio) with the following parameters: TR=1,500 ms, TE=2.83 ms, flip angle=7°, FOV=256×256 mm2, matrix=256×256, 1 mm3 isotropic voxels, 176 slices. The hippocampus and amygdala were automatically segmented using FreeSurfer version 5.1.0 (http:www.//surfer.nmr.mgh.harvard.edu) (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2001). Automated segmentation with the FreeSurfer package has been found to be highly valid and reliable (Morey et al., 2009; Doring et al., 2011). Segmentation of subcortical structures is based on a probabilistic atlas provided by FreeSurfer. The atlas is created by the Center for Morphometric Analysis from 20 unrelated, randomly selected healthy people. The detailed procedure has been described previously (Fischl et al., 2002). In brief, FreeSurfer scripts autorecon 1, 2 and 3 were run in sequence on all imaging data. Processing consisted of removal of non-brain tissue, Talairach transformation, segmentation of subcortical volumetric structures including the hippocampus and amygdala, intensity normalization, tessellation of the gray-matter/white-matter boundary, and labeling of each voxel based on previous probabilistic information. Intracranial volume (ICV) was generated during this processing.
2.4. Data analyses
SPSS Version 19 was used in analyses of the volumetric and clinical data. General-linear-model (GLM) univariate analysis was used to assess between-group differences in scores on BIS-11 and in amygdalar and hippocampal volumes where diagnostic group (cocaine-dependent versus healthy comparison) was included as a between-subject factor and age and gender were included as covariates. ICV was also included as a covariate for comparing volumes between groups. GLM for repeated measure was used to assess potential two-way interactions between group and hemisphere with respect to amygdalar and hippocampal volumes. Partial correlations were used, separately for cocaine-dependent and comparison subjects, to assess the relationships between scores on BIS-11 subscales and volumes of amygdala and hippocampus, controlling for ICV and age. These correlations were compared between cocaine-dependent and comparison individuals using multiple regression (Preacher, 2002). A false-discovery-rate (FDR) approach was used to correct for multiple comparisons (Genovese et al., 2002). The algorithm described in Benjamini & Hochberg was used to perform FDR (Benjamini & Hochberg 1995), and the Q value was set at 0.05. Since the BIS-11 has three subscales, we corrected for three comparisons when assessing group differences in these subscales. We corrected for 12 comparisons when assessing the correlations between the three subscales and four brain structures (3 × 4 = 12). We also explored potential effects of demographic information and comorbidities on differences in BIS-11 scores and volumes of amygdala and hippocampus between patients and comparisons. These exploratory analyses were performed after controlling for demographic information and comorbidities differently. Their results were presented in supplementary materials.
3. Results
3.1. Demographic measures
Demographic information from cocaine-dependent and healthy comparison subjects is presented in Table 1. Cocaine-dependent individuals were older (t=−4.5, df=73, p<0.001) and had received fewer years of education (t=5.3, df=73, p<0.001) relative to healthy comparison subjects. Table 2 presents the employment and clinical characteristics of the cocaine-dependent subjects.
Table 1.
Demographic information of cocaine-dependent and healthy comparison subjects
| Cocaine Dependent (n=34) | Healthy Comparison (n=41) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Demographics | mean | % | mean | % | t/x2 | df | p |
| Age: years (SD) | 39.3 (7.7) | 29.7 (10.1) | 4.5 | 73 | 0.00 | ||
| Education: years (SD) | 12.2 (1.8) | 15.3 (3.0) | 5.3 | 73 | 0.00 | ||
| Female | 9 | 26.5 | 17 | 41.5 | 1.9 | 1 | 0.17 |
| Race | 5.0 | 3 | 0.17 | ||||
| Caucasian | 12 | 35.3 | 23 | 56.1 | |||
| African-American | 16 | 47.1 | 14 | 34.1 | |||
| Hispanic | 4 | 11.8 | 1 | 2.4 | |||
| Multiracial | 2 | 5.9 | 3 | 7.3 | |||
Table 2.
Demographic and clinical characteristics of cocaine-dependent subjects
| Cocaine Dependent (n=34) | ||
|---|---|---|
|
| ||
| Employment and Clinical Characteristics | mean | % |
| Employment Status | ||
| Full-time (35 or more hours per week) | 3 | 8.8 |
| Part-time (less than 35 hours per week) | 8 | 23.5 |
| Unemployed less than one month | 6 | 17.6 |
| Unemployed greater than one month | 14 | 41.2 |
| Not in labor force (student, housewife) | 3 | 8.8 |
| Clinical Characteristics | ||
| Lifetime cocaine use: years (SD) | 7.9 (5.7) | |
| Substance use: Days out of 28 days before treatment (SD) | ||
| Cocaine | 15.2 (6.9) | |
| Cigarettes | 22.0 (11.3) | |
| Heroin | 0.35 (1.7) | |
| Alcohol | 7.1 (8.9) | |
| Marijuana | 2.1 (4.9) | |
| Comorbid Diagnosis | ||
| Lifetime Depressive Disorder | 7 | 20.6 |
| Lifetime Alcohol Dependence/Abuse | 24 | 70.6 |
| Lifetime Marijuana Dependence/Abuse | 17 | 50 |
| Lifetime Substance-induced Mood Disorder | 6 | 17.6 |
| Lifetime Anti-social Personality Disorder | 2 | 5.9 |
| Current Depressive Disorder | 0 | 0 |
| Current Alcohol Dependence/Abuse | 6 | 17.6 |
| Current Marijuana Dependence/Abuse | 6 | 17.6 |
| Current Substance-induced Mood Disorder | 2 | 5.9 |
3.2. Impulsivity scores and amygdalar and hippocampal volumes and their correlations
Relative to healthy comparison subjects, cocaine-dependent individuals scored higher on each BIS-11 subscale (Table 3). These group differences were statistically significant after corrections for 3 comparisons (i.e., one for each subscale). However, cocaine-dependent and healthy comparison subjects did not show significant group differences in the volumes of amygdala and hippocampus, with the possible exception that the left amygdala showed a tendency of smaller volume in patients relative to controls. No significant group-by-hemisphere interactions were identified with respect to the volumes of the two structures.
Table 3.
BIS-11 scores and volumes (mm3) of the amygdala and hippocampus
| Cocaine-Dependent | Healthy Comparison | f | df | p* | Effect Size (d) | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| M | SD | M | SD | |||||
| BIS-11 Total | 68.19 | 10.67 | 55.44 | 8.93 | 20.1 | 1, 71 | 0.00 | 4.04 |
| Attentional | 15.59 | 3.63 | 13.2 | 3.34 | 4.9 | 1, 71 | 0.004 (0.004) | 1.25 |
| Motor | 23.53 | 4.92 | 20.76 | 3.67 | 8.7 | 1, 71 | 0.002 (0.003) | 1.31 |
| Non-planning | 28.82 | 4.88 | 20.66 | 4.86 | 22.2 | 1, 71 | 0.000 (0.000) | 3.24 |
| Volumes | ||||||||
| Left amygdala | 1621.2 | 209.9 | 1734.2 | 288.9 | 3.3 | 1,71 | 0.07 | |
| Right amygdala | 1765.0 | 230.5 | 1817.3 | 263.6 | 1.4 | 1, 71 | 0.24 | |
| Left hippocampus | 3997.6 | 424.2 | 4065.4 | 565.7 | 0.5 | 1, 71 | 0.49 | |
| Right hippocampus | 4109.4 | 438.1 | 4184.4 | 493.4 | 0.7 | 1, 71 | 0.41 | |
number in parentheses are p values after correction for multiple comparisons (i.e., 3 comparisons) using FDR algorithm.
Table 4 shows the correlations between BIS-11 scores and hippocampal and amygdalar volumes. Cocaine-dependent individuals showed significant negative correlations between scores on the Attentional subscale and left (df=30, p=0.006) and right (df=30, p=0.002) amygdalar volumes (Fig. 1). These p values were 0.02 and 0.04, respectively, after correction for 12 comparisons. Healthy controls did not show significant correlations between BIS-11 scores and amygdalar and hippocampal volumes. Cocaine-depedent and comparison subjects showed significant differences in correlations between scores on the Attentional subscale and volumes of the left (z=2.9, p=0.004) and right (z=2.7, p=0.008) amygdala (Fig. 1). Both p values were 0.045 after correction for 12 comparisons. Among cocaine-dependent patients, years of cocaine use and age of first use of cocaine did not show significant correlations with any scores on the BIS-11 and volumes of the amygdala and hippocampus.
Table 4.
Correlations between BIS-11 scores and volumes of the amygdala and hippocampus
| BIS-11
|
|||||
|---|---|---|---|---|---|
| Total | Atten | Mot | Nonpl | ||
| Cocaine-Dependent (n=34)
| |||||
| Amygdala | L | −0.35 | −0.48* | −0.23 | −0.21 |
| R | −0.42 | −0.52* | −0.29 | −0.26 | |
| Hippocampus | L | 0.09 | 0.03 | 0.16 | 0.02 |
| R | 0.02 | 0.01 | 0.10 | −0.04 | |
|
| |||||
| Healthy Comparison (n=41)
| |||||
| Amygdala | L | 0.10 | 0.17 | −0.06 | 0.13 |
| R | 0.08 | 0.07 | 0.03 | 0.08 | |
| Hippocampus | L | −0.07 | 0.05 | −0.03 | −0.14 |
| R | −0.14 | −0.01 | −0.27 | −0.07 | |
p<.05, after correction for 12 comparisons. The highlighted cells indicate significant differences in corresponding correlations between cocaine-dependent and healthy comparison.
Abbreviations: Atten: Attentional; Fun: fun-seeking; L: left; Mot: motor; Nonpl: non-planning; R: right; RR: reward responsiveness
Fig. 1.
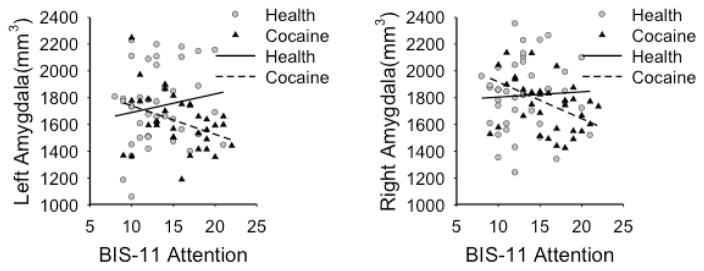
Correlations between impulsivity measures and amygdalar volumes. Scatter plots show correlations between scores on the attentional subscale of the BIS-11 and volumes of the left and right amygdala.
4. Discussion
Consistent with our prediction and previous findings (Gerard Moeller et al., 2002; Patkar et al., 2004; Meda et al., 2009; Ersche et al., 2011), cocaine-dependent individuals scored higher on the BIS-11 relative to healthy comparison subjects. The current cocaine-dependent patients showed a tendency for smaller left amygdala relative to control subjects, but the two groups did not show significant differences in amygdalar and hippocampal volumes. This finding does not support our prediction but is consistent with previous findings of similar volumes of these structures in cocaine-dependent and healthy comparison subjects (Jacobsen et al., 2001; Sim et al., 2007; Narayana et al., 2010; Mackey and Paulus, 2013,). On the other hand, several other studies reported reduced gray-matter volumes in the hippocampus and/or amygdala in cocaine-dependent individuals relative to healthy comparison subjects (Makris et al., 2004; Alia-Klein et al., 2011; Moreno-López et al., 2012; Rando et al., 2013). One possible explanation for these variable findings from different studies may involve small effect sizes of chronic cocaine use on the volumes of the hippocampus and amygdala, although individual differences may also contribute.
Consistent with our prediction, cocaine-dependent patients showed negative correlations between scores on the BIS-11 Attentional subscale and amygdalar volumes. However, they did not show significant correlations between scores on the BIS-11 Motor and Non-planning subscales and volumes of amygdala and hippocampus. The correlations between scores on BIS-11 Attentional subscale and amygdala volumes in the cocaine-dependent group were significantly different from the corresponding correlations exhibited by healthy comparison subjects. Lower amygdalar volumes have been associated with psychiatric conditions with high aggression and impulsiveness such as psychopathy, conduct disorder, and borderline personality disorder (Yang Y, 2009; Fairchild et al., 2011; Jia et al., 2011; Ermer et al., 2012; Bobes et al., 2013). The amygdala can be divided into basolateral (BLA) and central-medial (CMA) divisions. It has been suggested that the BLA is related to calculated behavior while the CMA related to emotional/impulsive behavior (Balleine and Killcross, 2006; Terburg et al., 2012; van Honk et al., 2013). Furthermore, it has been demonstrated that the BLA regulates the activity of the CMA (Tye et al., 2011; Terburg et al., 2012), and selective damage to the BLA can result in impulsive behavioral tendencies (van Honk et al., 2013). The current study cannot determine whether the finding of a tendency of smaller left amygdalar volumes reflects impaired BLA function, and whether impaired BLA function may contribute to increased impulsivity in cocaine-dependent patients. Therefore, future studies should specifically assess whether reduced amygdalar volumes and elevated impulsivity in cocaine-dependent individuals may relate to structural and functional alterations in the BLA and/or CMA and whether these relationships predate or are a consequence or chronic cocaine use.
The current finding of negative correlations between amygdalar volumes and impulsivity does not imply that the amygdala is the only, or even the primary, brain structure related to impulsivity. It has been reported that chronic cocaine use is associated with reduced volumes in extensive brain regions including the medial and lateral prefrontal cortex (Ersche et al., 2011; Hanlon and Canterberry, 2012), orbitofrontal cortex, insula, cingulate, and temporoparietal cortex. Subcortical structures such as the amygdala and hippocampus are regulated by top-down executive control from the frontoparietal cortex (Corbetta et al., 2008; Banich et al., 2009; Hollmann et al., 2012). It has been proposed that these subcortical structures may exert a greater influence on human behavior if the top-down executive control is reduced due to frontoparietal cortical impairment (Everitt and Robbins, 2005; Volkow et al., 2011). As discussed above, chronic cocaine abuse impairs extensive brain regions including the frontoparietal cortex. Therefore, impaired frontoparietal cortex and reduced top-down executive control might contribute to the current finding of negative correlations between impulsivity measures and amygdalar volumes, although this possibility presently remains speculative.
A limitation of this study is that it assessed only amygdalar and hippocampal volumes. This approach was driven by our hypotheses and the relevance of these structures to cocaine dependence and impulsivity (Volkow et al., 2004; Volkow et al., 2011; Pavlov et al., 2012). Future studies should assess relationships between impulsivity and volumetric measures of other brain regions.
In summary, this study observed in cocaine-dependent individuals relative to healthy comparison participants an increased impulsivity as measured by the BIS-11 Attentional subscale and altered relationship between scores on the BIS-11 Attentional subscale and amygdalar volumes. The findings suggest that future studies should further investigate impulsivity as related to the development of and remission from cocaine dependence, and whether interventions targeting amygdalar structure and/or function may reduce impulsivity and cocaine use in cocaine-dependent patients.
Supplementary Material
Highlights.
Cocaine dependent patients reported a greater score on each of BIS-11 subscale than controls.
Patients showed negative correlations between amygdalar volumes and impulsivity measures.
Patients and healthy controls showed significant differences in this correlation.
Acknowledgments
Funding: This study was funded by the following grants: National Institute on Drug Abuse (NIDA) grants K01 DA027750, P50 DA09241, R01 DA020908, P20DA027844, and R01 DA035058.
Footnotes
ClinicalTrials.gov Identifier: NCT00350870
All authors declare no conflict of interest with the content of this manuscript.
Disclosure: Dr. Potenza has consulted for Lundbeck, Ironwood, Shire and INSYS pharmaceuticals and RiverMend, LLC; received research support from Pfizer, Mohegan Sun Casino and the National Center for Responsible Gaming; has participated in surveys, mailings, or telephone consultations related to drug addiction, impulse-control disorders, or other health topics; has consulted for gambling, legal and governmental entities on issues related to addictions or impulse-control disorders; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the NIH and other agencies; has guest edited journal sections; has given academic lectures in grand rounds, Continuing Medical Education events, and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ. Gene× disease interaction on orbitofrontal gray matter in cocaine addiction. Archives of General Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends in Neurosciences. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neuroscience, Biobehavioral Reviews. 2009;33:613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Ávila C. Reduced striatal volume in cocaine-dependent patients. NeuroImage. 2011;56:1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. The Journal of Neuroscience. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yosef H. Controlling the false discovery rate: a ractical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bobes MA, Ostrosky F, Diaz K, Romero C, Borja K, Santos Y, Valdés-Sosa M. Linkage of functional and structural anomalies in the left amygdala of reactive-aggressive men. Social Cognitive and Affective Neuroscience. 2013;8:928–936. doi: 10.1093/scan/nss101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Manuck SB, Flory JD, Hariri AR. Neural basis of individual differences in impulsivity: contributions of corticolimbic circuits for behavioral arousal and control. Emotion. 2006;6:239–245. doi: 10.1037/1528-3542.6.2.239. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelle CL, Kaag AM, van Wingen G, van den Munkhof HE, Homberg JR, Reneman L, van den Brink W. Reduced frontal brain volume in non-treatment-seeking cocaine-dependent individuals: exploring the role of impulsivity, depression, and smoking. Frontiers in Human Neuroscience. 2014:8. doi: 10.3389/fnhum.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Depue BE, Olson-Madden JH, Smolker HR, Rajamani M, Brenner LA, Banich MT. Reduced Amygdala Volume Is Associated with Deficits in Inhibitory Control: A Voxel- and Surface-Based Morphometric Analysis of Comorbid PTSD/Mild TBI. Biomed Research International; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring TM, Kubo TT, Cruz LCH, Juruena MF, Fainberg J, Domingues RC, Gasparetto EL. Evaluation of hippocampal volume based on MR imaging in patients with bipolar affective disorder applying manual and automatic segmentation techniques. Journal of Magnetic Resonance Imaging. 2011;33:565–572. doi: 10.1002/jmri.22473. [DOI] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. Journal of Abnormal Psychology. 2012;121:649–658. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal Brain Structure Implicated in Stimulant Drug Addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Passamonti L, Hurford G, Hagan CC, von dem Hagen EA, van Goozen SH, Goodyer IM, Calder AJ. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. American Journal of Psychiatry. 2011;168:624–633. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJMJ, Gillan CM, Shekar S, Gorwood PAPM, Voon V, Morein-Zamir S, Denys D, Sahakian BJ, Moeller FG, Robbins TW, Potenza MN. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectrums. 2014;19:69–89. doi: 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II): User’s Guide. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-IP, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. Medical Imaging, IEEE Transactions on. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychology Review. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gerard Moeller F, Dougherty DM, Barratt ES, Oderinde V, Mathias CW, Andrew Harper R, Swann AC. Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug and Alcohol Dependence. 2002;68:105–111. doi: 10.1016/s0376-8716(02)00106-0. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Canterberry M. The use of brain imaging to elucidate neural circuit changes in cocaine addiction. Substance Abuse and Rehabilitation. 2012;3:115–128. doi: 10.2147/SAR.S35153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmus TC, Downey KK, Arfken CL, Henderson MJ, Schuster CR. Novelty seeking as a predictor of treatment retention for heroin dependent cocaine users. Drug and Alcohol Dependence. 2001;61:287–295. doi: 10.1016/s0376-8716(00)00153-8. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hellrung L, Pleger B, Schlögl H, Kabisch S, Stumvoll M, Villringer A, Horstmann A. Neural correlates of the volitional regulation of the desire for food. International Journal of Obesity. 2012;36:648–655. doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- Huebner T, Vloet TD, Marx I, Konrad K, Fink GR, Herpertz SC, Herpertz-Dahlmann B. Morphometric brain abnormalities in boys with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:540–547. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. American Journal of Psychiatry. 2001;158:486–489. doi: 10.1176/appi.ajp.158.3.486. [DOI] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biological Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Cavallo DA, Carroll KM, Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Paulus M. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neuroscience and Biobehavioral Reviews. 2013;37:300–316. doi: 10.1016/j.neubiorev.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS, Jr, Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC. Decreased Absolute Amygdala Volume in Cocaine Addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Matthies S, Rüsch N, Weber M, Lieb K, Philipsen A, Tuescher O, Ebert D, Hennig J, van Elst LT. Small amygdala-high aggression? The role of the amygdala in modulating aggression in healthy subjects. World Journal of Biological Psychiatry. 2012;13:75–81. doi: 10.3109/15622975.2010.541282. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, Thomas AD, Muska C, Hylton JL, Pearlson GD. Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behavioural Pharmacology. 2009;20:390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Balodis IM, DeVito EE, Lacadie CM, Yeston J, Scheinost D, Constable RT, Carroll KM, Potenza MN. A preliminary investigation of Stroop-related intrinsic connectivity in cocaine dependence: associations with treatment outcomes. The American Journal of Drug and Alcohol Abuse. 2013;39:392–402. doi: 10.3109/00952990.2013.841711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Sbuse Treatment. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Moreno-López L, Catena A, Fernández-Serrano MJ, Delgado-Rico E, Stamatakis EA, Pérez-García M, Verdejo-García A. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug and Alcohol Dependence. 2012;125:208–214. doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana PA, Datta S, Tao G, Steinberg JL, Moeller FG. Effect of cocaine on structural changes in brain: MRI volumetry using tensor-based morphometry. Drug and Alcohol Dependence. 2010;111:191–199. doi: 10.1016/j.drugalcdep.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini DA, Raine A, Erickson K, Loeber R. Lower amygdala volume in men is associated with childhood aggression, early psychopathic traits, and future violence. Biological Psychiatry. 2014;75:73–80. doi: 10.1016/j.biopsych.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. Pre-Treatment Measures of Impulsivity, Aggression and Sensation Seeking Are Associated with Treatment Outcome for African—American Cocaine—Dependent Patients. Journal of Addictive Diseases. 2004;23:109–122. doi: 10.1300/J069v23n02_08. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pavlov KA, Chistiakov DA, Chekhonin VP. Genetic determinants of aggression and impulsivity in humans. Journal of Applied Genetics. 2012;53:61–82. doi: 10.1007/s13353-011-0069-6. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. The American Journal of Drug and Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Preacher. Calculation for the test of the difference between two independent correlation coefficients. 2002 http://quantpsy.org.
- Rando K, Tuit K, Hannestad J, Guarnaccia J, Sinha R. Sex differences in decreased limbic and cortical grey matter volume in cocaine dependence: a voxel-based morphometric study. Addiction Biology. 2013;18:147–160. doi: 10.1111/adb.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Caverzasi E, Lazzaretti M, Morandotti N, De Vidovich G, Marraffini E, Gambini F, Isola M, De Bona M, Rambaldelli G. Dorsolateral prefrontal cortex and hippocampus sustain impulsivity and aggressiveness in borderline personality disorder. Journal of Affective Disorders. 2011;131:417–421. doi: 10.1016/j.jad.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Soloff P, Nutche J, Goradia D, Diwadkar V. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry Research. 2008;164:223–236. doi: 10.1016/j.pscychresns.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg D, Morgan B, Montoya E, Hooge I, Thornton H, Hariri A, Panksepp J, Stein D, Van Honk J. Hypervigilance for fear after basolateral amygdala damage in humans. Translational Psychiatry. 2012;2:e115. doi: 10.1038/tp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J, Eisenegger C, Terburg D, Stein DJ, Morgan B. Generous economic investments after basolateral amygdala damage. Proceedings of the National Academy of Sciences. 2013;110:2506–2510. doi: 10.1073/pnas.1217316110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Molecular Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, RANKLCPTAW LOcalization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry. 2009;66:986–994. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetzsche T, Preuss UW, Frodl T, Schmitt G, Seifert D, Münchhausen E, Tabrizi S, Leinsinger G, Born C, Reiser M. Hippocampal volume reduction and history of aggressive behaviour in patients with borderline personality disorder. Psychiatry Research: Neuroimaging. 2007;154:157–170. doi: 10.1016/j.pscychresns.2006.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


