Abstract
Purpose
Retropharyngeal adenopathy (RPA) is poor prognostic factor in head and neck (HN) cancer. However, the prognostic significance of RPA in Human Papillomavirus-related (HPV+) oropharyngeal cancer (OPC) is unknown.
Patients and Methods
185 patients with HPV+ OPC were assessed. Pre-therapy images reviewed by a HN radiologist to determine presence of RPA. Doses to the RPAs were determined from treatment plans. Outcomes analyzed using Kaplan-Meier method, log-rank tests, and correlations determined using Spearman’s rank analyses.
Results
29 (16%) of the HPV+ patients had RPA. At median follow-up 49 months, 5-year overall survival (OS), failure-free survival (FFS) and distant failure-free survival (DFFS) were 57% vs. 81% (P=0.02), 63% vs 80% (P=0.015) and 70% vs 91% (p=0.002) for patients with/without RPA, respectively. No differences observed in local/ regional control rates, exceeding 90% in both groups, and No RPA recurrences were observed. In multivariable analysis, stages T4 or N3, and RPA, were independently, statistically significantly associated with both OS and distant failure, while N2c, age, disease site, and smoking status, were not.
Conclusion
RPA in HPV+ OPC is an independent prognostic factor for distant failure, translating into worse OS. Patients with RPA may not be suitable candidates for trials of systemic treatment de-escalation.
Introduction
The location of retropharyngeal lymph nodes is in the space posterior to the nasopharynx and oropharynx and is bound by the constrictor muscles anteriorly and medially, alar (pre-vertebral) fascia posteriorly, carotid sheath laterally, and skull base superiorly, and extends down to the level of C3 inferiorly (1,2). While the RP space is difficult to access surgically, enlarged retropharyngeal lymph nodes, representing pathologic lymphadenopathy, can be identified on imaging such as CT, PET/CT, or MRI.
Retropharyngeal adenopathy (RPA) in non-nasopharyngeal squamous cell carcinoma of the head and neck (HNC) is known to be a poor prognostic factor. Patients with cancer of the larynx, supraglottic larynx, hypopharynx and oropharynx with RPA have worse local and distant control as well as survival (2–4). This is likely because the RP nodes are not usually the primary draining lymph nodes to these sites, as described by Rouviere (1), and metastases to this nodal region likely represent more aggressive and advanced disease. Alternatively, there may be an unfavorable biologic factor that predisposes for both RPA and worse outcomes.
The patients in these previous studies demonstrating poor prognosis related to RPA had mostly smoking and drinking related cancers. In recent years there is a growing incidence of HPV-positive oropharyngeal cancer (OPC), which are not smoking or drinking related, and which were most likely not represented in the prior studies. These patients have a much better prognosis than their HPV-negative counterparts (5–9). As a result, HPV-positive OPC patients have been identified as a population of patients that may benefit from treatment de-escalation by reducing the dose of radiation and reducing or even eliminating chemotherapy (8,9). Several recent studies have identified potentially adverse prognostic factors that may predict for worse outcomes in HPV-positive patients, including high T and N classifications and heavy smoking history (5,6,9–11). However, the significance of the presence of RPA in HPV-positive OPC patients has not been established. We therefore performed a review of consecutive patients with locally advanced (Stage III/IV) HPV+ squamous cell carcinoma of the oropharynx treated with chemo-IMRT at our institution, reviewed RPA involvement in the pre-therapy imaging for each patient, and compared the outcomes of patients with and without RPA.
Patients and Methods
This study was an Institutional Review Board approved review of a prospectively assembled repository of consecutive patients with locally advanced, non-metastatic (stage III/IV) oropharyngeal cancer (OPC), treated from May 2003 to October 2010 at the University of Michigan. The repository includes tissue samples and clinical treatments and outcomes, including surveys of smoking, recorded prospectively for HNC patients seen at our institution and funded by an NIH SPORE (Specialized Programs of Research Excellence). The record of outcome was supplemented by a chart review. All patients had histologically confirmed squamous cell carcinoma of the oropharynx including the tonsils, base of tongue, glossotonsilar sulcus, and pharyngeal wall.
Pretreatment staging was done with clinical exam, direct laryngoscopy, contrast enhanced CT and PET/CT imaging (124/185 patients). MRIs were performed as clinically indicated if there was concern for base of skull or nerve involvement. Patient treatments have previously been described in detail (11). Briefly, patients underwent CT simulation in a 5 point thermoplastic mask. Intensity modulated radiation therapy (IMRT) was used to deliver a total dose of 70 Gy to the gross tumor volume (GTV) expanded by a small (5mm) margin (CTV1), 59–63 Gy to high risk nodal regions (CTV2), and 56–59 Gy to low risk nodal regions (CTV3). The retropharyngeal space was covered as clinically indicated based on the presence of other pathologic cervical lymph nodes on that side of the neck. Non-involved Ipsilateral RP nodes were included as part of CTV2. The contralateral retropharyngeal space was not included in any CTVs unless there was a risk based on evidence of contralateral jugular nodes, in which case it was included in either CTV2 or CTV3. A 3–5 mm margin was added to the CTVs to create a planning target volume (PTV) to account for setup error. Radiation was delivered daily for 35 fractions over the course of 7 weeks in all patients except one who received twice daily RT with 1.25 Gy fractions to a total dose of 75 Gy. All patients were treated with concurrent chemotherapy with Carboplatin (AUC1) and Paclitaxel (30mg/m2) delivered weekly.
Post-treatment, patients were followed by Radiation Oncology, Medical Oncology and Surgical Oncology services with clinical exams every 4–8 weeks. PET-CT to assess response was typically done 3 months after the completion of therapy. Neck dissection practices evolved over the years under study with early patients with bulky nodal disease receiving planned neck dissections adjuvantly, while in later years patients only underwent neck dissection as salvage treatment for clinical, radiographic or scintigraphic evidence of residual disease either at 3 months post-chemoradiation or at the time of clinical suspicion of recurrence.
Human papilloma virus (HPV) expression was determined by polymerase chain reaction (PCR) and in-situ hybridization and p16 expression by immunohistochemistry, as previously described (11). Briefly, HPV was detected using multiplex polymerase chain reaction (PCR) MassArray using QIAamp DNA FFPE tissue kit (Qiagen, Valencia, CA) after DNA extraction from core tissue samples of patients whose tissue was banked prospectively as part of an assembled tissue microarray. In situ hybridization (ISH) for high risk HPV genotypes was performed using the INFORM HPV ISH assay (Ventana Medical Systems Inc., Tucson, AZ). p16INK4a immunohistochemical staining was performed per manufacturer’s protocol (CINtec Histology Kit; MTM Laboratories, Heidelberg, Germany) and visualized using the ultraView polymer detection system. p16 was considered positive if the combined nuclear and cytoplasmic staining was positive in >75% of tumor cells. Patients were considered HPV-positive if either HPV DNA was detected by PCR or ISH or if p16 was positive by immunohistochemistry.
For the purposes of the current study, a radiologist specializing in head and neck imaging (MI) performed an independent review of all imaging studies to identify RPA. All patients had a pre-therapy CT scan of the head and neck, and most patients (124/185 HPV-positive patients) had pre-therapy PET-CT imaging for review. All studies were performed within 4 weeks of beginning treatment. CT imaging is a well-established modality to determine the presence of pathologic RPA with the pathologic lymph nodes presenting as ≥ 7mm in the longest axis (12). While PET imaging has been added to staging protocols and can help identify pathologic lymph nodes, CT imaging remains the most common method of identifying RPA (3,13). For this study, RPA was considered positive if a patient had a lymph node with a maximal axial diameter ≥ 1cm, a necrotic or cystic appearing center, or was FDG avid on PET/CT imaging. These criteria are stricter than others have used and while perhaps lowering the sensitivity, they likely increased the specificity of RPA identifications and reduced the rate of false positives.
All radiation treatment plans for patients with radiographic RPA were reviewed for the purposes of this study to determine the radiation doses to the involved and contralateral retropharyngeal spaces. The retropharyngeal spaces were identified in each patient and radiation doses were determined by isodose lines surrounding the region of interest. The isodose line that covered the entire area at risk was determined to be the minimal dose to the region.
Study endpoints were survival and disease recurrence. Recurrences were classified based on location. Residual or recurrent disease in the GTV or high risk CTV1 was considered local failure, recurrent disease in the regional lymph nodes in the low risk or previously untreated neck above the clavicles was considered regional failure, and disease recurrence outside of the head and neck and below the clavicles was considered distant failure. Time to failure was calculated from the date of radiation treatment start to either the date of histologic confirmation of disease recurrence or, if pathological confirmation was not performed, to the date of conclusive imaging demonstrating recurrence.
The data was analyzed using MedCalc Statistical Software version 14.12.0 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014). We performed Independent T-tests on the baseline characteristics to determine differences in treatment cohorts. The Kaplan-Meier method with log-rank test and cox regression were used to analyze survival outcomes between groups. To evaluate independent correlations between prognostic factors we used Spearman rank correlation (rho) tests. Stepwise (inclusion criteria P<0.05, exclusion criteria P>0.1) cox proportional hazards regression was used to perform a multivariable analysis on potential prognostic factors.
Results
Patient characteristics
Two hundred thirty one patients with OPC treated between 2003–2010 were identified, of whom 198 had HPV testing performed and 184 (93%) were determined to be HPV-positive by PCR and ISH. 169 of the HPV+ patients were also tested for p16 and only 7 (4%) were negative. One patient was HPV negative but p16-positive and was considered positive for the purposes of this study.
Of the 185 HPV+ patients, 29 (16%) were found to have RPA. Nineteen of 29 patients with RPA had PET/CT imaging, and all patients with PET positive RPA had consistent pathologic lymph nodes on CT imaging (≥1cm axial dimension). There were seven instances of pathologic appearing RPA on CT imaging that were not FDG avid. All RPA was laterally located, abutting the medial edge of the carotid artery from the base of skull through the bottom of C2, and none were medial. Baseline patient characteristics are detailed in Table 1A and a comparison of characteristics between patients with or without RPA is provided in Table 1B. There was no statistically significant difference between patients with and without RPA present with regard to age, smoking status, primary tumor or nodal stages, or any other characteristic, besides relatively more women in the RPA group. Patients with RPA were non-significantly more likely to have primary tumors of the tonsil compared with base of tongue (66% vs. 56%, respectively). Among those with RPA, Three patients (10%) had no other involved lymph nodes (stages N1 or N2A, confined to the retropharyngeal nodes), and 26 had additional lymphadenopathy in levels II-IV: 12 had N2b, 9 had N2c and 5 had N3 disease. Only one patient (1/29, 3%) had involved RPA contralateral to the site of the primary tonsil cancer; she had bilateral neck node involvement (N2C disease). Two other patients (2/29, 7%) had bilaterally involved RPA. There was no statistically significant correlation between RPA and other involved lymph nodes, when analyzed by the presence or absence of other involved lymph nodes (ρ=0.07, p=0.36), or by lymph node stage (ρ=0.12, p=0.11).
Table 1.
Characteristics for patients with or without retropharyngeal adenopathy (RPA). Results of independent T-Test assuming equal variance is reported for RPA vs no RPA patients.
| A | |
|---|---|
| Characteristic | Statistic |
| Male (N [%]) | 165 (89%) |
| Age (years): Median (range) | 55 (34 – 78) |
| Primary Tumor site: N (%) | |
| Tonsil | 103 (55%) |
| Base of Tongue | 79 (43%) |
| Pharyngeal wall | 3 (2%) |
| T-stage: N (%) | |
| T1 | 29 (16%) |
| T2 | 73 (39%) |
| T3 | 34 (19%) |
| T4 | 49 (27%) |
| N-stage: N (%) | |
| N0 | 10 (5%) |
| N1 | 13 (7%) |
| N2 | 137 (75%) |
| N2a | 15 (8%) |
| N2B | 77 (42%) |
| N2C | 45 (24%) |
| N3 | 25 (13%) |
| AJCC stage | |
| 3 | 16 (9%) |
| 4 | 169 (91%) |
| Smoking History: N (%) | |
| Never smoker | 73 (39%) |
| Former smoker | 61 (33%) |
| Current smoker | 51 (28%) |
| Pack Years: Median (range) | 5.5 (0 – 140) |
| # pack years | |
| 0 | 75 (40%) |
| 1–10 | 32 (17%) |
| >10 | 78 (42%) |
| Chemotherapy | |
| Carbo-Taxol | 182 (98%) |
| Cisplatin | 3 (2%) |
| RT Fractionation | |
| Once Daily (2 Gy) | 184 (100%*) |
| Twice Daily (1.25 Gy) | 1 (1%*) |
| B | |||
|---|---|---|---|
| Characteristic | No RPA: n (%) | RPA Present: n (%) | T-Test (P value) |
| Patients | 156 (100%) | 29 (100%) | |
| Age (mean) | 56.7 | 55.0 | 0.33 |
| Male Gender | 137 (88%) | 22 (76%) | 0.01 |
| Tonsil cancer | 87 (56%) | 19 (66%) | 0.27 |
| T_Stage | 0.45 | ||
| T1 | 25 (16%) | 4 (14%) | |
| T2 | 63 (40%) | 10 (34%) | |
| T3 | 28 (18%) | 6 (20%) | |
| T4 | 40 (26%) | 9 (31%) | |
| N_Stage | 0.19 | ||
| N1 | 11 (7%) | 2 (7%) | |
| N2 | 115 (74%) | 22 (76%) | 0.25 |
| N2A | 14 (9%) | 1 (3%) | |
| N2B | 65 (42%) | 12 (41%) | |
| N2C | 36 (23%) | 9 (31%) | |
| N3 | 20 (12%) | 5 (17%) | |
| Smoking: | |||
| Pack years (mean) | 15.7 | 22.4 | 0.16 |
| Early neck dissection | 26% | 24% | 0.81 |
Due to rounding some percentage totals are greater than 100%
Chemotherapy and radiotherapy
Twenty eight of 29 patients with RPA received once daily radiation to a total dose of 70 Gy, while one patient (the only patient amongst the entire 185 patients) received twice daily radiation of 1.25 Gy to a total CTV1 dose of 75 Gy. In all patients, the involved RPA PTV received the full prescribed dose (70 Gy, +/− 5%), and all but 6 patients (22/27, 78%) received a minimum of 50 Gy to the uninvolved contralateral retropharyngeal space. All patients received weekly carboplatin (AUC 1) and paclitaxel (30 mg/m2) concurrent with RT, except for 3 patients (2%) who received concurrent cisplatin 40 mg/m2 weekly.
Patterns of failure: Locoregional vs. distant control
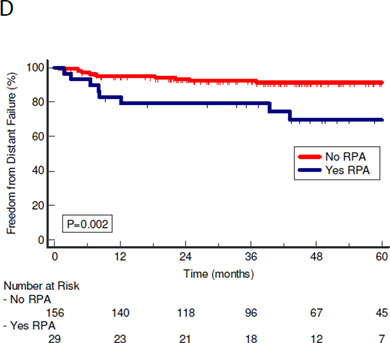
At a median follow-up of 47 months (range 4–108 months), there were no differences in local, regional or combined locoregional control between patients with or without RPA: 5-year rates 96% vs. 95% (P=0.87), 92% vs 90% (P=0.88), and 87% vs 83% (P= 0.33), respectively (Figure 1a–c). There were no recurrences in either the involved retropharyngeal space or contralateral uninvolved retropharyngeal space. There was, however, a significant difference in the incidence of distant failures as first failures in the RPA group (Figure 1d). Freedom from distant failure (FFDF) as first failure at 5 years was 70% in the RPA group compared to 91% in the no- RPA group (P=0.002).
Figure 1.
Kaplan-Meier curves of patterns of failure of patients with and without RPA. P values are calculated using log-rank test. A: Freedom from local failure with local failure as first failure. B: Freedom from regional failure with regional failure as first failure. C: Freedom from locoregional failure D: Freedom from distant failure with distant failure as first failure.
RPA=Retropharyngeal adenopathy; LRF=locoregional failure
Survival Outcomes
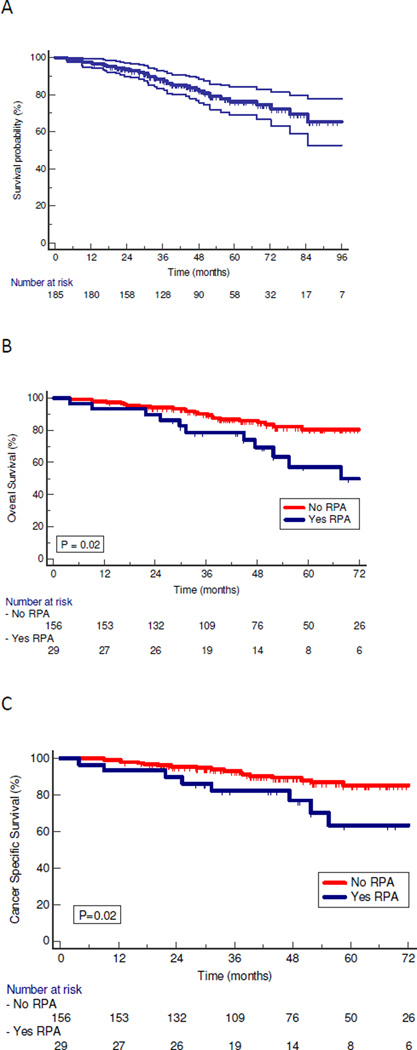
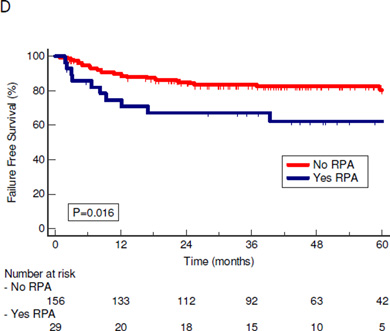
Overall survival for the entire HPV+ cohort is shown in Figure 2a. On univariate analysis, patients with RPA had overall worse outcomes than those without RPA (Figures 2b–d). Median and 5 year overall survival were 87 months and 76 % for the entire cohort, 74 months and 57% for the patients with RPA, and 86 months and 81% for those without RPA, respectively (P=0.02). Cancer specific survival (CSS) was likewise worse for patients with RPA, with a median and 5 year CSS of 81 months and 63% vs 92 months and 85% (P=0.02) for those with and without RPA respectively. Median and 5 year failure free survival (FFS) rates were 72 months and 63% vs. 86 months and 81% (P=0.016) for patients with and without RPA respectively. Thus, the higher rate of distant failure translated into worse CSS and overall survival for RPA patients.
Figure 2.
Kaplan-Meier Curves of survival of patients with HPV-positive oropharyngeal cancer. P values are from log-rank test. A: OS for all HPV-positive patients. B: OS for patients with and without RPA. C: CSS for patients with and without RPA. D: FFS for patients with and without RPA.
CSS=Cancer specific survival; FFS= Failure free survival; OS=Overall survival; RPA= retropharyngeal adenopathy
Risk Factors and Multivariate Analysis
Results of univariate Kaplan-Meier analysis of well-established, published prognostic factors among HPV+ patients: T stage, N stage, and smoking (5–6,9–11), are detailed in Table 2A. In our cohort, smoking was not a significant prognostic factor for OS, CSS, FFS or FFDF whether patients were stratified by pack years (>10 vs ≤10) or any smoking history. T stage was a significant predictor of OS, CSS and FFS, and FFDF. Specifically, T4 patients had poor outcomes with 5 year OS of 61%. Regarding nodal stage, patients staged as N3 had significantly lower OS, CSS, FFS and FFDF compared to N0–N2 patients. There was also a significantly worse CSS and FFDF for patients with nodal stage N2C compared to lower N stages, however there was only a trend for worse OS and FFS (Table 2A).
Table 2.
A: Univariate analysis of risk factors for poor outcomes in HPV+ OPC patients using Cox regression modeling. B: Results of Cox proportional-hazards regression analysis with stepwise selection of established prognostic factors. Factors included in the analysis but found to not be statistically significant, and therefore not included in the model, are age, gender, tumor site (tonsil/glossotonsilar sulcus, base of tongue, pharyngeal wall), and smoking status (number of pack years, pack years and 0,≤10, and >10 pack years, and never, former and current).
| A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | CSS | FFS | FFDF | |||||||||
| P value |
HR | 95% CI | P value |
HR | 95% CI | P value |
HR | 95% CI | P value |
HR | 95% CI | |
| Smoking pk-yrs (>10 vs =10) | 0.27 | 1.43 | 0.76–2.7 | 0.56 | 1.26 | 0.58–2.8 | 0.74 | 1.1 | 0.59–2.1 | 0.44 | 1.4 | 0.59–3.4 |
| Smoking (yes vs no) | 0.11 | 1.8 | 0.86–3.6 | 0.22 | 1.7 | 0.72–4.1 | 0.42 | 1.33 | 0.69–2.7 | 0.17 | 2.0 | 0.74–5.5 |
| T4 vs T1–T3 | 0.0008 | 3.1 | 1.6–5.92 | 0.0064 | 2.98 | 1.35–6.5 | 0.0012 | 3.0 | 1.6–5.8 | 0.0010 | 4.51 | 1.85–11.0 |
| N3 vs N0–N2 | 0.024 | 2.24 | 1.11–4.52 | 0.015 | 2.98 | 1.25–7.12 | 0.0024 | 3.10 | 1.50–6.43 | 0.0019 | 4.3 | 1.72–10.8 |
| N2C vs N0-N2B | 0.059 | 2.1 | 0.98–4.4 | 0.029 | 2.9 | 1.15–7.2 | 0.20 | 1.7 | 0.77–3.7 | 0.011 | 4.3 | 1.4–13.9 |
| B | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | CSS | FFS | FFDF | |||||||||
| P value |
HR | 95% CI | P value |
HR | 95% CI | P value |
HR | 95% CI | P value |
HR | 95% CI | |
| Tstage (T4) | 0.001 | 3.0 | 1.57 to 5.71 | 0.0084 | 2.88 | 1.32 to 6.31 | 0.0004 | 3.27 | 1.70 to 6.28 | 0.0008 | 4.64 | 1.90 to 11.34 |
| Nstage (N3) | 0.020 | 2.45 | 1.16 to 5.20 | 0.0203 | 2.83 | 1.18 to 6.78 | 0.0006 | 3.65 | 1.75 to 7.61 | 0.0016 | 4.53 | 1.78 to 11.56 |
| RPA | 0.034 | 2.11 | 1.04 to 4.27 | 0.048 | 2.34 | 1.01 to 5.44 | 0.030 | 2.25 | 1.08 to 4.67 | 0.0066 | 3.51 | 1.43 to 8.65 |
OS=overall survival; CSS= cancer specific survival; FFS= failure free survival; and FFDF= Freedom from distant failure
These factors, as well as RPA and other covariates (age, primary tumor location and gender) were included in a stepwise cox proportional hazards regression (Table 2B). On multivariable analysis, N3 N-classification (vs. N1–2), T4 T-classification (vs T1–3), and RPA, but not the other factors, were independently associated with worse OS, CSS, FFS and FFDF. These results need to be interpreted with caution because of the low number of events in the RPA group (11 deaths, 10 total failures and 8 distant failures) that may lead to a sparse-data bias. However, these data imply that RPA remains a significant predictor of outcome along with N and T classification.
Discussion
This study demonstrates that patients with HPV-positive OPC with radiographically detected RPA prior to treatment have worse OS, CSS, FFS and freedom from distant failure compared to similar patients without RPA. Locoregional disease control was excellent and equal in both groups, and there were no retropharyngeal recurrences among the patients with RPA.
The incidence of HPV-positive OPC is rising, and in our cohort, of the patients treated during this time period with available HPV status, 93% tested positive for HPV DNA or p16. This percentage is high but is consistent with the range reported recently by others (14–17). As a tertiary referral center for the Midwest, it is possible the referral patterns to our hospital may slightly increase the number of HPV+ patients we see compared to the general population. The reported prevalence of RPA in locoregionally advanced HNC is approximately 12–16%, consistent with our results, and it rises to > 20% in patients with additional jugular lymphadenopathy (2,4,15). These series, which did not examine HPV status, demonstrated correlations between the nodal stage and the risk of RPA. Such a correlation was not found in our HPV+ OPC population. This may be because the large majority of patients with HPV+ disease present with advanced nodal stages (88% stages N2–3 in our series), making a statistically significant correlation between RPA and N stage unlikely.
All patients in our study received full dose radiation (70 Gy) to the involved RPA and 78% received at least 50 Gy to the contralateral, uninvolved RP region. No patients failed within the involved or uninvolved RP regions, and loco-regional control in these patients was excellent, demonstrating the adequacy of the treatment plans. It therefore seems appropriate to continue this strategy and to include the contralateral RP region in these patients within the at- risk clinical target volume. Also, prophylactic ipsilateral RP irradiation is recommended in OPC patients without RPA, especially with involved ipsilateral level II nodes. We have previously described 3 cases of isolated retropharyngeal lymph node recurrences amongst 133 patients, 80 of whom had OPC, before the routine inclusion of the retropharyngeal spaces in the sub-clinical IMRT CTVs (18). Since all disease in the retropharyngeal space, both initial and recurrent, was located in the lateral region, these findings led to recommendations to include the lateral retropharyngeal region as a high risk target for patients with other neck disease (19–21).
The poorer prognosis associated with RPA in HNC in general is well documented (2–4,13), including specifically in OPC (3,13). HPV status was not investigated in these previous studies. A major question is whether the poor prognosis associated with RPA in HNC in general is relevant to patients with HPV+ OPC, who generally do well. In a recent study by Tang et al, 12% of 134 HPV+ OPC patients were found to have RPA, and there was only a non-significant trend in worse event-free and overall survival at 2 years in the RPA vs non-RPA patients (22). However, survival was calculated in that study for all patients who completed therapy by the time of analysis. Patients with HPV+ OPC may have late failures, especially distant metastases, at later times than those with HPV-negative disease, with some events occurring as late as 5 years (9,23). In our study, the greater patient number and especially the longer follow up (median 49 months) contributed to a more robust analysis and facilitated detecting statistically significant differences in outcomes between HPV+ OPC patients with and without RPA.
Others have reported worse prognosis for patients with HPV+ OPC with a substantial smoking history, with as much as a 1% increase risk of reduced survival for every pack year of smoking (5,6). Heavy past smoking has therefore been used as an exclusion criterion for some de-intensification protocols for HPV+ OPC (8,24).. In our cohort, smoking was not found to be a significant prognosticator of outcome when patients were stratified by either smoking status (yes/no) or pack years (≤10 or >10) on univariate or multivariate analysis (Table 2A and 2B). A potential caveat to our findings is that smoking history was obtained through chart review and not prospectively collected as forms filled out by patients at the time of accrual, as was done in the RTOG trials(5). The retrospective nature of our study may have masked the prognostic significance of smoking in our cohort. The Toronto experience reported by O’Sullivan et al reported lesser overall survival in heavy smokers but did not find smoking to be a significant prognostic factor for failure-free survival in their large study of HPV+ OPC patients (9).
In univariate and multivariate analysis, N3 and T4 stage were prognostic for poorer outcomes in our study, as has been previously reported by others (5,9,11). O’Sullivan et al also reported worse outcomes for N2C patients compared to lower N stages in patients with HPV+ OPC treated with radiotherapy alone (9). In our cohort, the univariate analysis of N2C patients (all treated with concurrent chemotherapy) confirmed they have worse CSS and FFDF compared to lower N staged patients. While N2C staging was not significant on multivariate analysis in our study, we recommend proceeding with caution when considering treatment de-escalation for these patients.
The patients in this study were treated consecutively with uniform IMRT dose prescription principles and a uniform chemotherapy regimen. They were unselected, making them fairly representative of the general population of stages III/IV HPV-related OPC treated with chemo-radiotherapy. Still, validation of our results is required. To this end, results from large prospective trials aimed at HPV-related OPC, such as RTOG 1016, will be useful and relevant to our study if they include data regarding RPA.
In conclusion, given the significantly better outcome of patients with locally advanced, HPV-related OPC, studies are underway to assess whether reducing the intensity of therapy may retain these results while reducing the rate and severity of treatment-related sequelae (8,24). These studies typically exclude patients with the adverse prognostic factors reported previously, such as T4, N3, and a history of heavy smoking. Our study demonstrates that RPA is a significant factor associated with increased risk of distant failure and reduced survival in HPV+ OPC, independent of advanced T or N stages. While a validation of our results is required in prospective studies, these data should be taken into account by researchers contemplating de-escalation trials for HPV+ OPC.
Acknowledgments
Funded in part by NIH grant P50CA097248 and the Newman Family Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflict of interest
Contributor Information
Stuart E. Samuels, Department of Radiation Oncology, University of Michigan.
Jeffrey Vainshtein, Department of Radiation Oncology, University of Michigan.
Matthew E. Spector, Department of Otorhinolaryngology-Head Neck Surgery, University of Michigan.
Mohannad Ibrahim, Department of Radiology, University of Michigan.
Jonathan B. McHugh, Department of Pathology, University of Michigan.
Yebin Tao, Department of Biostatistics, University of Michigan.
Matthew Schipper, Department of Radiation Oncology, University of Michigan.
Francis Worden, Department of Medicine – Medical Oncology Division, University of Michigan.
Avraham Eisbruch, Department of Radiation Oncology, University of Michigan.
References
- 1.Rouviere H. Anatomy of the lymphatic system. Ann Arbor MI: Edward Brothers; 1938. [Google Scholar]
- 2.Coskun HH, Ferlito A, Medina JE, et al. Retropharyngeal lymph node metastases in head and neck malignancies. Head Neck. 2011;33:1520–1529. doi: 10.1002/hed.21526. [DOI] [PubMed] [Google Scholar]
- 3.Dirix P, Nuyts S, Bussels B, et al. Prognostic influence of retropharyngeal lymph node metastasis in squamous cell carcinoma of the oropharynx. Int J Radiat Oncol Biol Phys. 2006;65:739–744. doi: 10.1016/j.ijrobp.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin MP, Mendenhall WM, Mancuso AA, et al. Retropharyngeal adenopathy as a predictor of outcome in squamous cell carcinoma of the head and neck. Head Neck. 1995;17:190–198. doi: 10.1002/hed.2880170304. [DOI] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 8.Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: A systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50:2636–2648. doi: 10.1016/j.ejca.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 9.O'Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vainshtein JM, Spector ME, McHugh JB, et al. Refining risk stratification for locoregional failure after chemoradiotherapy in human papillomavirus-associated oropharyngeal cancer. Oral Oncol. 2014;50:513–519. doi: 10.1016/j.oraloncology.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancuso AA, Harnsberger HR, Muraki AS, et al. Computed tomography of cervical and retropharyngeal lymph nodes: Normal anatomy, variants of normal, and applications in staging head and neck cancer. Part ii: Pathology. Radiology. 1983;148:715–723. doi: 10.1148/radiology.148.3.6878692. [DOI] [PubMed] [Google Scholar]
- 13.Gunn GB, Debnam JM, Fuller CD, et al. The impact of radiographic retropharyngeal adenopathy in oropharyngeal cancer. Cancer. 2013;119:3162–3169. doi: 10.1002/cncr.28195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the united states. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the united states. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral hpv infection in the united states, 2009–2010. Jama. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernster JA, Sciotto CG, O'Brien MM, et al. Rising incidence of oropharyngeal cancer and the role of oncogenic human papilloma virus. Laryngoscope. 2007;117:2115–2128. doi: 10.1097/MLG.0b013e31813e5fbb. [DOI] [PubMed] [Google Scholar]
- 18.Eisbruch A, Marsh LH, Dawson LA, et al. Recurrences near base of skull after imrt for head-and-neck cancer: Implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 19.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: Early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 20.Gregoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: A 2013 update. Dahanca, eortc, hknpcsg, ncic ctg, ncri, rtog, trog consensus guidelines. Radiother Oncol. 2014;110:172–181. doi: 10.1016/j.radonc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Bussels B, Hermans R, Reijnders A, et al. Retropharyngeal nodes in squamous cell carcinoma of oropharynx: Incidence, localization, and implications for target volume. Int J Radiat Oncol Biol Phys. 2006;65:733–738. doi: 10.1016/j.ijrobp.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Tang C, Komakula S, Chan C, et al. Radiologic assessment of retropharyngeal node involvement in oropharyngeal carcinomas stratified by hpv status. Radiother Oncol. 2013;109:293–296. doi: 10.1016/j.radonc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Huang SH, Perez-Ordonez B, Weinreb I, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in hpv-related oropharyngeal cancer. Oral Oncol. 2013;49:79–85. doi: 10.1016/j.oraloncology.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Quon H, Forastiere AA. Controversies in treatment deintensification of human papillomavirus-associated oropharyngeal carcinomas: Should we, how should we, and for whom? J clin oncol. United States. 2013:520–522. doi: 10.1200/JCO.2012.46.7746. [DOI] [PubMed] [Google Scholar]