Abstract
Objectives
Stereotactic ablative radiation (SABR) is a promising alternative to lobectomy or sublobar resection for early lung cancer, but the value of SABR in comparison to surgical therapy remains debated. We examined the cost-effectiveness of SABR relative to surgery using SEER-Medicare data.
Materials&Methods
Patients age≥66 years with localized (<5 cm) non-small cell lung cancers diagnosed from 2003-2009 were selected. Propensity score matching generated cohorts comparing SABR with either sublobar resection or lobectomy. Costs were determined via claims. Median survival was calculated using the Kaplan-Meier method. Incremental cost-effectiveness ratios (ICERs) were calculated and cost-effectiveness acceptability curves (CEACs) were constructed from joint distribution of incremental costs and effects estimated by non-parametric bootstrap.
Results
In comparing SABR to sublobar resection, 5-year total costs were $55,120 with SABR vs. $77,964 with sublobar resection (P<0.001) and median survival was 3.6 years with SABR vs. 4.1 years with sublobar resection (P=0.95). The ICER for sublobar resection compared to SABR was $45,683/life-year gained, yielding a 46% probability that sublobar resection is cost-effective. In comparing SABR to lobectomy, 5-year total costs were $54,968 with SABR vs. $82,641 with lobectomy (P<0.001) and median survival was 3.8 years with SABR vs. 4.7 years with lobectomy (P=0.81). The ICER for lobectomy compared to SABR was $28,645/life-year gained, yielding a 78% probability that lobectomy is cost-effective.
Conclusion
SABR is less costly than surgery. While lobectomy may be cost-effective compared to SABR, sublobar resection is less likely to be cost-effective. Assessment of the relative value of SABR versus surgical therapy requires further research.
Introduction
Defining the optimal treatment for early stage lung cancers is an urgent public health priority. As the US population ages, the overall incidence of lung cancers is increasing swiftly, with a projected 50% increase by 2030.1 While the majority of lung cancers present at an advanced stage, dissemination of lung cancer screening into routine practice will likely increase detection of early lung cancers.2 The growing numbers of older adults diagnosed with early lung cancers presents not only a therapeutic challenge, but also an opportunity to treat lung cancers in their most curable stage, thereby maximizing the beneficial impact of therapy on lung cancer mortality.
While lobectomy is well-accepted as a standard of care treatment for fit patients with early, non-small cell lung cancers,3 the high prevalence of smoking-related comorbid illness and advanced age4 in patients diagnosed with non-small lung cancers often necessitates consideration of less radical approaches, such as sublobar resection5 or stereotactic ablative radiotherapy (SABR).6 Recent population-based data suggest comparable survival outcomes with sublobar resection and SABR,7,8 and a recent decision analysis concluded that SABR is cost-effective compared with sublobar resection in patients with marginally operable stage I disease.9
Yet to date, little is known regarding the actual costs of lobectomy, sublobar resection, and SABR relative to their effectiveness in the real world setting of older patients with early, non-small cell lung cancers. Accordingly, in a population-based cohort of older adults with early-stage, non-small cell lung cancer, we sought to measure actual costs and survival outcomes for SABR compared to both sublobar resection and lobectomy. This approach complements randomized trials and decision models by analyzing actual costs and outcomes of these three treatment approaches when employed in everyday practice, thus yielding direct implications for both clinical care and policy decisions.
Materials and Methods
Data source
The Surveillance, Epidemiology, and End Results (SEER)-Medicare population-based database is drawn from 16 tumor registries representing approximately 26% percent of the US population. Records abstracted by tumor registrars are linked to Medicare billing claims, thereby facilitating determination of both specific details regarding the patient's cancer and administration of billed medical procedures. The case ascertainment rate is approximately 98%.10 This analysis included patients diagnosed in 2003 to 2009 with survival information available through December 31, 2012, and cost information available through December 31, 2010.
Cohort creation
We recently published a detailed analysis of survival outcomes for patients diagnosed with non-small cell carcinoma of the lung diagnosed in 2003-2009, treated with either lobectomy, sublobar resection, or SABR, and meeting all of the following criteria: age ≥ 66 years, size ≤ 5 cm, pathologic confirmation, complete fee-for-service Medicare claims from 12 months before to 4 months after diagnosis, no prior cancer, no second cancer diagnosed within 4 months of the index lung cancer, and no distant metastasis or nodal disease at presentation.7 In our prior analysis, propensity score matching was used to create matched cohorts of patients treated with these different treatment modalities, with logistic regression used to calculate the propensity score and 1:1 matching of SABR to surgically treated patients, as outlined in detail in this manuscript. For the current analysis, we began with these matched cohorts contained in the main analysis of the prior publication,7 but subsequently excluded patients whose definitive treatment was coded only in SEER data, as the corresponding claims to determine cost of treatment were missing.
Treatment ascertainment
Treatment was determined using Medicare claims present within 4 months of diagnosis and classified as either lobectomy, sublobar resection, or SABR, in accordance with our prior methods (eTable 1).7 Surgery was further subdivided as video-assisted thoracoscopic surgery (VATS) or non-VATS. The first date any of these claims appears was considered the treatment initiation date.
Covariables
Covariables used for propensity score matching included age at diagnosis, sex, race, use of supplemental oxygen, Charlson comorbidity score in the year prior to diagnosis,11 performance status,12 T stage, use of pathologic staging for the mediastinum, and use of pre-treatment PET scanning (eTable 1).
Measuring cost
Total health care costs are reported from the payer perspective, using reimbursement for each patient's inpatient, outpatient, and carrier claims. We determined cost of care beginning 60 days prior to the initiating lung cancer treatment through the first of the following: death, last follow up, or completion of 5 years after diagnosis. Costs were normalized to 2014 dollars using the Prospective Pricing Index for Part A claims and the Medicare Economic Index for Part B claims.13,14 Costs were adjusted for geographic variation using the geographic adjustment factor for Part A claims and the geographic practice cost index for Part B claims. To account for censoring during the follow up period which leads to incomplete cost information, we calculated mean aggregate costs by using the partitioned version of the simple weighted complete-case estimator.15 Costs are presented using a phase-of-care approach,13 with costs divided a priori as follows: pre-treatment costs (from claims occurring within 60 days prior to initiating treatment), treatment costs (from claims occurring within 30 days of initiating treatment), year 1 costs (from claims occurring from 31 days to 365 days of initiating treatment), year 2 costs (from claims occurring from 366 days to 730 days after initiating treatment), year 3 costs (from claims occurring 731 days to 1095 days after initiating treatment), and so on through year 5. Since very few SABR patients had follow up beyond 5 years, we did not analyze costs after this timepoint.
Survival analysis
Differences in covariate distribution across matched pairs were assessed with a standardized difference threshold of 0.15.16 Median overall survival for each treatment group was calculated using the Kaplan-Meier method, and differences in survival were assessed using proportional hazards regression stratified by matched pair.
Cost-Effectiveness Analysis
We compared overall weighted costs within five years and weighted costs by treatment phase using the Wilcoxon rank-sum test. To explore the influence of costs over time, two different ICERs were calculated, one limited to pre-treatment, treatment, and year 1 costs and the other including pre-treatment, treatment, and year 1-5 costs. This approach yields insight into the cost-effectiveness if only costs associated with treatment and aftercare are considered or if downstream costs due to treatment of recurrence and long-term morbidity are also considered. ICERs were calculated by dividing the difference in mean weighted cost by the difference in median overall survival. Median, as opposed to mean, survival was chosen as this can be readily estimated from right-censored data using the Kaplan-Meier estimator. Sensivity analyses calculated ICERs for patients undergoing VATS in comparison to their matched pairs who underwent SABR.
To assess uncertainty in the estimated ICERs, we performed bootstrap simulation analysis with 1000 bootstrap estimates of the ICER to generate 95% confidence ellipses in the ICER plane. Cost-effectiveness acceptability curves were then generated to illustrate the probabilities that ICERs fall below a spectrum of societal willingness-to-pay thresholds.17,18
All analyses were conducted in SAS v9.3 and used two-tailed statistical tests. This work was approved by our Institutional Review Board.
Results
Composition of matched cohorts
We identified 300 matched pairs of patients treated with either SABR or sublobar resection and 243 matched pairs of patients treated with either SABR or lobectomy, from an original sample of 382 patients treated with SABR, 1,496 patients treated with sublobar resection, and 7,215 patients treated with lobectomy. For the SABR vs. sublobar resection matched pairs, median age was 78 years (interquartile range 73 to 82), 59% were female, and 40% received supplemental oxygen. For the SABR vs. lobectomy matched pairs, median age was 77 years (interquartile range 72 to 82), 61% were female, and 30% received supplemental oxygen. Both sets of matched pairs were well-balanced across all measured covariates, with low standardized differences between covariates (Table 1). Of the patients who underwent surgery, 40% (120/300) of the sublobar resection patients and 27% (66/243) of the lobectomy patients underwent VATS and only 5% (16/300) and 7% (18/243) underwent mediastinal sampling.
Table 1.
Baseline Characteristics of Propensity-Score Matched Cohorts
| SABR and Sublobar Resection (N=600) |
SABR and Lobectomy (N=486) |
|||||
|---|---|---|---|---|---|---|
| SABR | Sublobar Resection | S.D. | SABR | Lobectomy | S.D. | |
| Age | ||||||
| 66-69 | 30 (10%) | 29 (10%) | 0.011 | 28 (12%) | 29 (12%) | −0.013 |
| 70-74 | 65 (22%) | 71 (24%) | −0.048 | 57 (24%) | 60 (25%) | −0.039 |
| 75-79 | 73 (24%) | 76 (25%) | −0.023 | 68 (28%) | 59 (24%) | 0.084 |
| ≥ 80 | 132 (44%) | 124 (41%) | 0.054 | 90 (37%) | 95 (39%) | −0.042 |
| Sex | ||||||
| Male | 126 (42%) | 118 (39%) | 0.054 | 99 (41%) | 92 (38%) | 0.059 |
| Female | 174 (58%) | 182 (61%) | −0.054 | 144 (59%) | 151 (62%) | −0.059 |
| Charlson Comorbidity Index | ||||||
| 0 | 146 (49%) | 142 (47%) | 0.027 | 126 (52%) | 124 (51%) | 0.016 |
| 1 | 84 (28%) | 84 (28%) | 0.000 | 65 (27%) | 63 (26%) | 0.019 |
| ≥2 | 70 (23%) | 74 (25%) | −0.031 | 52 (21%) | 56 (23%) | −0.04 |
| Oxygen Supplementation | ||||||
| No | 181 (60%) | 177 (59%) | 0.027 | 170 (70%) | 171 (70%) | −0.009 |
| Yes | 119 (40%) | 123 (41%) | −0.027 | 73 (30%) | 72 (30%) | 0.009 |
| Performance Score (Medical Assistance) | ||||||
| 0 | 231 (77%) | 224 (75%) | 0.055 | 193 (79%) | 201 (83%) | −0.084 |
| ≥1 | 69 (23%) | 76 (25%) | −0.055 | 50 (21%) | 42 (17%) | 0.084 |
| T-Stage | ||||||
| T1a (0.0 - 2.0 cm) | 139 (46%) | 145 (48%) | −0.040 | 114 (47%) | 113 (47%) | 0.008 |
| T1b (2.1 - 3.0 cm) | 112 (37%) | 101 (34%) | 0.077 | 84 (35%) | 86 (35%) | 0.017 |
| T2a (3.1 - 5.0 cm) | 49 (16%) | 54 (18%) | −0.044 | 45 (19%) | 44 (18%) | 0.011 |
| PET Staging | ||||||
| No | 85 (28%) | 78 (26%) | 0.052 | 80 (33%) | 68 (28%) | 0.107 |
| Yes | 215 (72%) | 222 (74%) | −0.052 | 163 (67%) | 175 (72%) | −0.107 |
| Mediastinal Sampling | ||||||
| No | 282 (94%) | 284 (95%) | −0.029 | 225 (93%) | 225 (93%) | 0.000 |
| Yes | 18 (6%) | 16 (5%) | 0.029 | 18 (7%) | 18 (7%) | 0.000 |
Abbreviations: SABR, stereotactic ablative radiation; S.D., standardized difference.
Survival by treatment
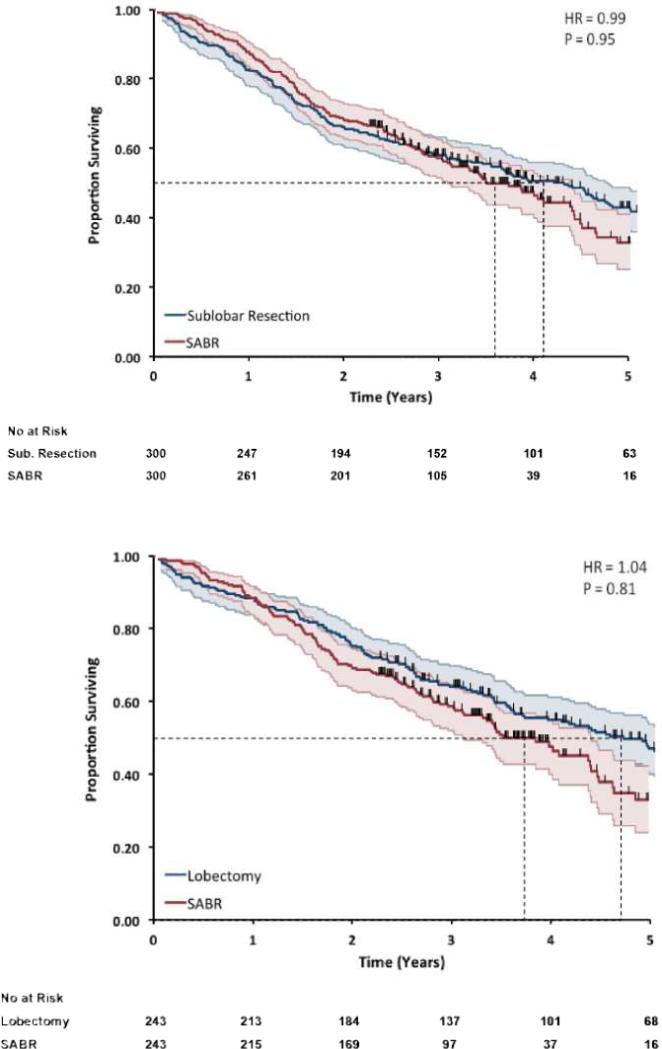
For the comparison of SABR to sublobar resection, median survival was 3.6 years with SABR and 4.1 years with sublobar resection (P=0.95) (Figure 1A). For the comparison of SABR to lobectomy, median survival was 3.8 years with SABR and 4.7 years with lobectomy (P=0.81) (Figure 1B).
Figure 1. Overall survival in matched cohorts.
Matched pairs comparing (A) SABR (red) to sublobar resection (blue) and (B) SABR (red) to lobectomy (blue) for the endpoint of overall survival. There was no significant difference in overall survival for either comparison.
Cost by treatment
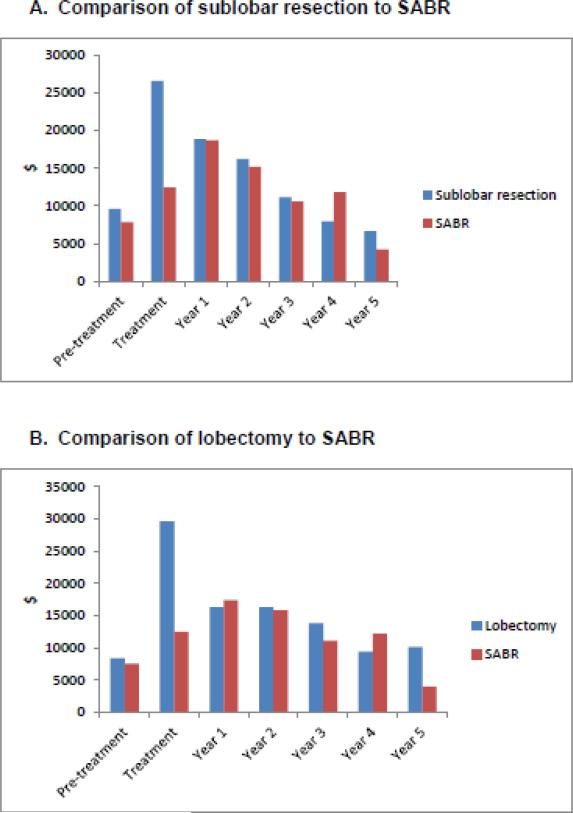
For the comparison of SABR to sublobar resection, mean weighted costs through five years of follow up were $55,120 for SABR and $77,964 for sublobar resection (P<0.001). By treatment phase, costs were lower for SABR as compared to sublobar resection for both the pre-treatment phase ($7,838 vs. $9,615; P=0.02) and the treatment phase ($12,436 vs. $26,522; P<0.001), and were marginally lower for the first year after treatment ($18,698 vs. $18,861; P=0.05), but did not differ significantly during years 2 through 5 of follow up (Figure 2A). The mean cost of VATS-sublobar resection during the treatment phase was $25,001, compared to $27,571 for patients who underwent open sublobar resection (P=0.31)
Figure 2. Cost differences by phase of care.
Difference in cost by phase of care is presented for the comparison of sublobar resection to SABR (A) and lobectomy to SABR (B). The pre-treatment phase includes the 60 days preceding the first date of treatment. Treatment phase includes claims within 30 days of initiating treatment. Year 1 includes the 11 months following the treatment month. Years 2 through 5 include 12 months per year. All costs are reported in 2014 dollars and are adjusted for geographic variation. Abbreviations: SABR (stereotactic ablative radiation).
For the comparison of SABR to lobectomy, mean weighted costs through five years of follow up were $54,968 for SABR and $82,641 for lobectomy (P<0.001). There was no difference in costs of SABR vs. lobectomy in the pre-treatment phase ($7,558 vs. $8,381; P=0.41), but costs were substantially lower with SABR in the treatment phase ($12,468 vs. $29,551; P<0.001). There was no significant difference in cost during years 1-4 of follow up. In year 5 of follow up, SABR was less expensive ($3,967 vs. $10,125; P=0.03) but there were fewer than 11 SABR patients with 5-year data, thus limiting precision of the year 5 estimate (Figure 2B). The mean cost of VATS-lobectomy during the treatment phase was $23,265, compared to $31,895 for patients who underwent open lobectomy resection (P=0.002).
Cost-Effectiveness Analysis
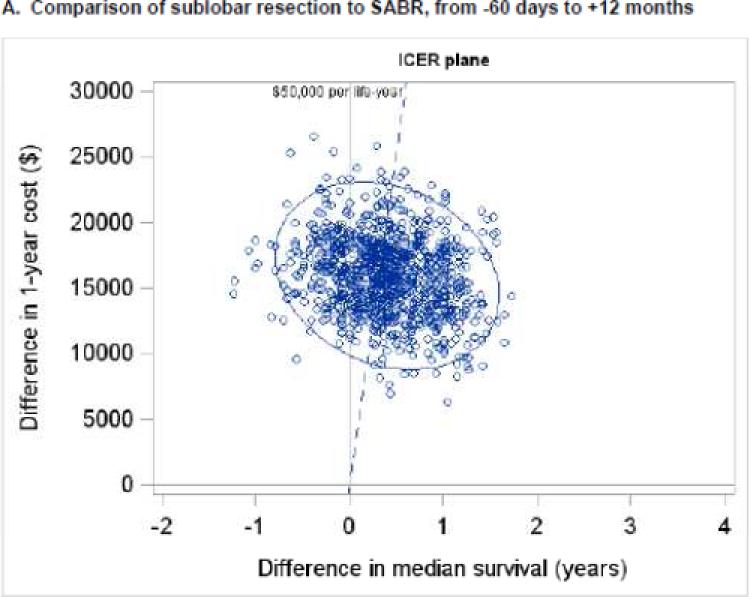
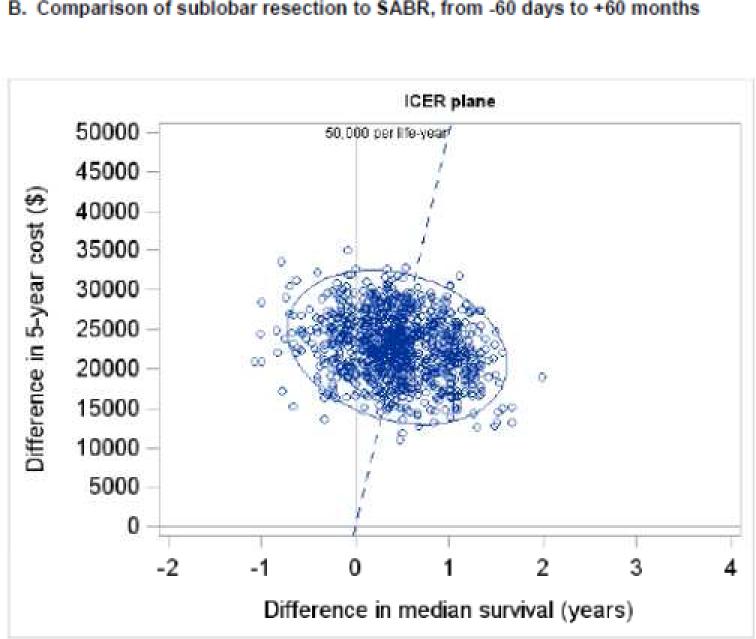
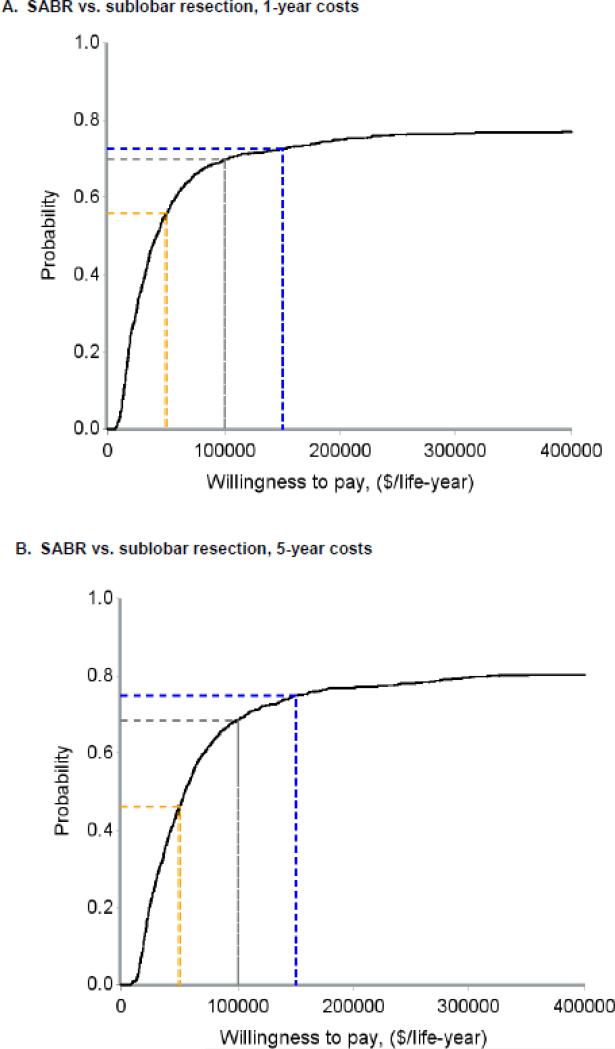
When considering only pre-treatment, treatment, and year 1 costs, the ICER for sublobar resection compared to SABR was $31,572 per life-year gained (95% CI - $224,642 to +$194,160) (Figure 3A). Cost acceptability curves demonstrated that the probabilities that sublobar resection would be cost-effective were 56%, 70%, and 73% at societal willingness-to-pay thresholds of $50,000, $100,000, and $200,000 per life-year gained, respectively (Figure 4A). When considering pre-treatment, treatment, and year 1-5 costs, the ICER for sublobar resection compared to SABR rose to $45,683 per life-year gained (95% CI −$325,572 to +$269,807) (Figure 3B) and the probabilities that sublobar resection would be more cost-effective dropped to 46%, 69%, and 75% at thresholds of $50,000, $100,000, and $200,000 per life-year, respectively (Figure 4B). In a sensitivity analysis, the ICER for VATS-sublobar resection considering pre-treatment, treatment, and year 1 costs was $7,404 per life-year.
Figure 3. Uncertainty analysis for incremental cost effectiveness ratios.
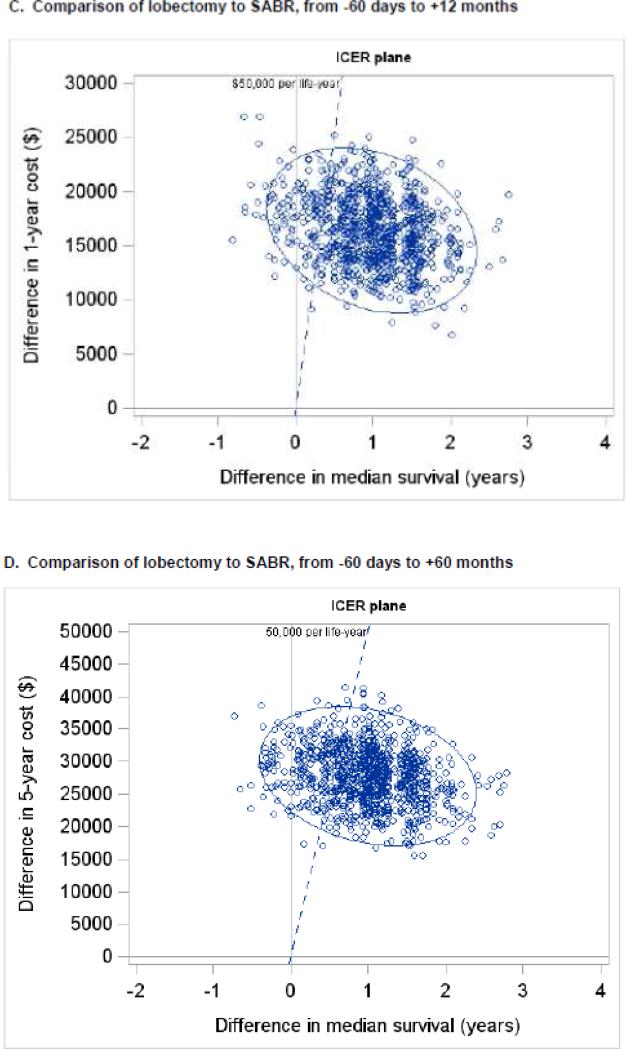
One thousand bootstrap simulations were performed to assess uncertainty in the measured ICER. Each small, open circle represents the ICER calculated from one bootstrapped simulation. The blue ellipse represents the 95% confidence ellipse for the ICER (i.e., the ellipse within which 95% of the bootstrapped simulations measured the ICER). The dashed blue line represents a societal willingness to pay threshold of $50,000 per life-year gained. Panels A and B depict the comparison of sublobar resection to SABR and panels C and D depict the comparison of lobectomy to SABR. Panels A and C depict total costs from −60 days to +12 months, and panels B and D depict total costs from −60 days to + 60 months. Abbreviations: ICER (incremental cost-effectiveness ratio), SABR (stereotactic ablative radiation).
Figure 4. Cost-effectiveness acceptability curves.
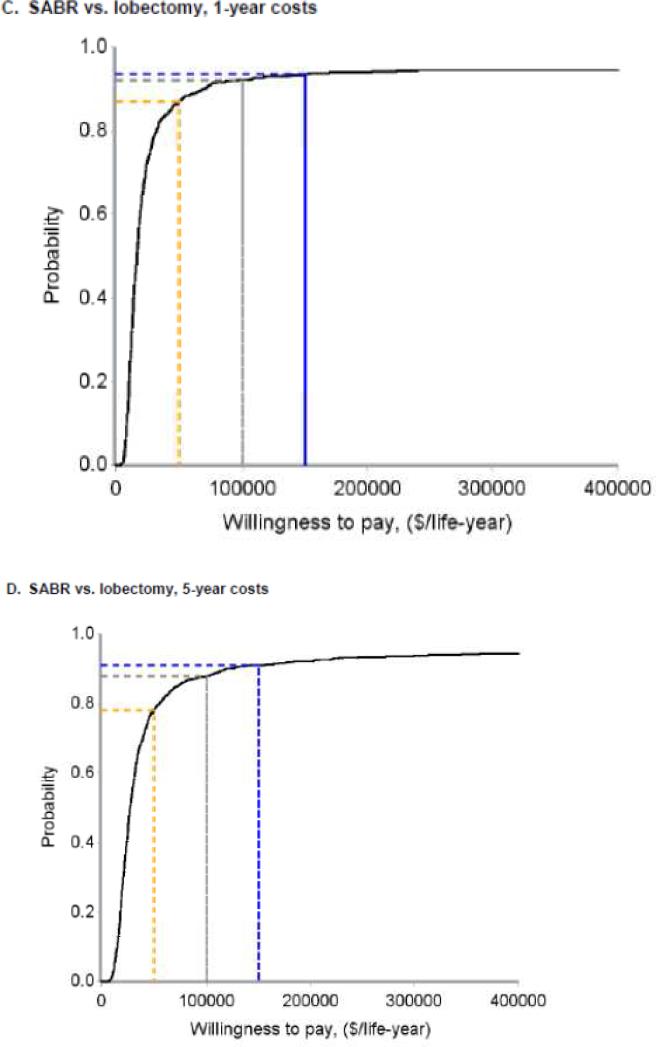
Using the bootstrapping analysis in shown in Figure 3, cost-effectiveness acceptability curves were generated to estimate the probability that surgical therapy would be cost-effective across a spectrum of societal willingness to pay thresholds. The dashed yellow, grey, and blue lines indicate societal willingness to pay thresholds of $50,000, $100,000, and $200,000 per life-year gained, respectively. Panels A and B depict the comparison of sublobar resection to SABR and panels C and D depict the comparison of lobectomy to SABR. Panels A and C depict total costs from −60 days to +12 months, and panels B and D depict total costs from −60 days to + 60 months. Abbreviations: ICER (incremental cost-effectiveness ratio), SABR (stereotactic ablative radiation).
When considering only pre-treatment, treatment, and year 1 costs, the ICER for lobectomy compared to SABR was $17,047 per life-year gained (95% CI −$73,124 to +$108,011) (Figure 3C). Cost acceptability curves demonstrated that the probabilities that lobectomy would be cost-effective were 87%, 92%, and 94% at societal willingness-to-pay thresholds of $50,000, $100,000, and $200,000 per life-year gained, respectively(Figure 4C). When considering pre-treatment, treatment, and year 1-5 costs, the ICER for lobectomy compared to SABR rose to $28,645 per life-year gained (95% CI −$119,828 to +$207,822) (Figure 3D) and the probabilities that lobectomy would be cost-effective dropped to 78%, 88%, and 91% at thresholds of $50,000, $100,000, and $200,000 per life-year, respectively (Figure 4D). In a sensitivity analysis, the ICER for VATS-lobectomy considering pre-treatment, treatment, and year 1 costs was $10,622 per life-year.
Discussion
In this population-based study of older adults with early, non-small cell lung cancers, we calculated that the ICER for sublobar resection compared to SABR was $45,683 per life-year when considering all costs within 5 years, and the probability that sublobar resection would be cost-effective at a societal willingness-to-pay threshold of $50,000 per life-year was 46%. These findings do not provide compelling evidence that sublobar resection is cost-effective compared to SABR in older, frail, marginally operable patients, and are thus consistent with the decision analysis by Shah et al. which concluded that SABR was dominant to sublobar resection in marginally operable patients.9
In contrast, we calculated that the ICER for lobectomy compared to SABR was $28,645 per life-year when considering all costs within 5 years, and the probability that lobectomy would be cost-effective at a societal willingness-to-pay threshold of $50,000 per life-year was 78%. Of note, this ICER is within the same order of magnitude of the findings of Shah et al., who reported an ICER of $13,216 per quality-adjusted-life-year for lobectomy compared to SABR in clearly operable patients. Collectively, these findings suggest that the increased cost of lobectomy has the potential to be worthwhile to patients and society if survival outcomes are indeed improved with lobectomy.
Our findings also illuminate the difference in actual costs of SABR compared to surgery in older patients with early lung cancer. Specifically, within 30 days of initiating therapy, SABR was $14,086 (53%) less expensive than sublobar resection and $17,083 (58%) less expensive than lobectomy. Further, cost differences after the first 30 days of treatment were small, suggesting that the primary difference in cost of these two treatment approaches is the difference in short term costs engendered by the therapies themselves and their associated short-term complications. This finding is noteworthy, in that it suggests that the most benefit to be gained in terms of improving the value of care for early lung cancer is to focus on optimizing the initial treatment interval.
A strength of our analytic approach is the ability to measure real costs of care in an older population that actually received modern lung cancer treatment. Within this context, it is interesting to note that the 5-year mean cost for patients treated with SABR was approximately $55,000, which is $15,000 higher than estimated in the decision analysis by Shah et al. 9 Similarly, 5-year mean cost of sublobar resection was approximately $78,000, which is $27,000 higher than estimated by Shah et al., and 5-year mean cost of lobectomy was approximately $83,000, which is $34,000 higher than estimated by Shah et al. These cost differences could be reflective of variation in reimbursement or practice patterns in SEER regions relative to the reimbursement at University of Pennsylvania, upon which the Shah et al. analysis was based. Alternatively, these cost differences could indicate meaningful differences between the actual patients examined in this study and the idealized 65 year old patient with medically operable stage I lung cancer considered in Shah et al.'s Markov model. A limitation, however, of our analytic approach is the relative imprecision with which differences in median survival between SABR and surgical therapy can be measured. It should be noted that we failed to measure any statistically significant differences in overall survival between SABR and either surgical therapy. If it were the case that survival is truly equivalent between SABR and surgery, then SABR would likely be the cost-effective approach, as it is less costly than either surgical therapy. The difference in cost favoring SABR thus underscores the importance of efforts to collect high quality outcomes data to inform understanding of the relative survival differences between SABR and surgery, as this will determine whether or not the increased cost of surgery could be cost-effective in certain circumstances.
Efforts to date to determine the comparative effectiveness of SABR relative to surgery in operable patients have been problematic, with three randomized clinical trials closed due to poor accrual, and no remaining trials open. In lieu of randomized trials, Radiation Therapy and Oncology Group 0618 sought to evaluate the effectiveness of SABR in operable patients in a non-randomized phase II setting. With 26 evaluable patients and 25 months median follow up, results were promising with a 7.7% 2-year rate of failure in the primary tumor and 19.2% 2-year rate of failure anywhere in the involved lobe.19 Similarly, single-institution, retrospective data comparing sublobar resection to SABR indicated similar cause-specific survival with either treatment, supporting the notion that sublobar resection is unlikely to be cost effective compared to SABR.20
This study is limited in several important ways. First, techniques and doses for SABR continue to evolve, so it is likely that survival outcomes from SABR as implemented in this study, which includes patients diagnosed from 2003 to 2009, may be inferior when compared to contemporary outcomes with SABR. Second, surgical techniques also continue to evolve, and costs of surgical intervention will likely decrease with more widespread adoption of minimally invasive techniques. Third, despite our best efforts to match SABR to surgical patients using advanced statistical techniques and claims-derived covariates including comorbidity, performance status, and oxygen use, there remains potential for residual confounding, which would likely bias in favor of surgical therapy given the physiologic selection criteria imperative in selecting operative candidates. This limitation must be considered when comparing patients treated with SABR or surgical therapy in our analyses, as treatment selection is likely indicative of baseline functional and physiologic differences which are difficult to completely account for within retrospective studies. Fourth, given the relatively small size and limited follow up of our cohort, there is limited precision to estimate differences in median survival across treatment groups, which limits the precision with which the ICER can be measured. Fifth, our ICERs were calculated using actual life-years gained and not quality-adjusted life-years (QALYs) as health utility states for patients treated with SABR are not well understood. Sixth, without a control group of similar patients without cancer, we were unable to determine which costs in our patients were due to cancer itself and which were due to other, non-cancer related problems. Seventh, although the willingness-to-pay threshold is picked from a societal perspective, the study was limited in that only medical costs from a payer's perspective were included. Other costs including patients’ medical costs, indirect costs and overhead costs are difficult or impossible to determine from claims datasets, yet may vary by type of treatment. Eighth, staging of the surgical group was likely based on pathologic assessment of the resected tumor, while staging of the SABR group was based on clinical and radiographic assessment. If upstaging were to commonly occur with pathologic assessment, this could bias survival outcomes in favor of surgical therapy. Further, our surgical cohort included few patients who underwent mediastinal sampling, and thus may not be entirely representative of the general population of patients undergoing lung surgery, for whom mediastinal sampling is commonplace. And finally, we did not include a reference group who received no treatment, although prior work from our group illustrated that elderly patients with early non-small cell lung cancer who did not receive any local therapy experienced much worse overall and lung cancer-specific survival than patients treated with SABR or surgery.8
In summary, SABR is clearly less costly than surgery in older patients with early non-small cell lung cancer. Nevertheless, lobectomy may be cost-effective in patients fit enough to undergo this procedure, assuming that lobectomy truly confers improvements in median overall survival on the order of 1 or more additional years of life. For patients who cannot undergo lobectomy, sublobar resection is less likely to be cost-effective when compared to SABR. Looking to the future, as greater emphasis is placed on value and insurers move toward bundling reimbursement of cancer care, the lower cost of SABR relative to surgery makes it an attractive option for marginally operable elderly patients. This context heightens the need for meticulous cost and outcomes studies to define the value of SABR relative to surgery.
Supplementary Material
Summary.
We used a population-based approach to compare the cost-effectiveness of three local treatment strategies for early lung cancers. We found that stereotactic ablative radiation (SABR) was less expensive than surgery. Survival outcomes tended to favor surgery, but were not statistically significantly better than SABR. We found that lobectomy may be cost-effective at a societal willingness to pay threshold of $50,000/life-year gained, but sublobar resection is less likely to be cost-effective.
Acknowledgements and Conflicts of Interest
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. This work was supported in part by a research grant from Varian Medical Systems. This entity had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation and review of the data; and decision to publish.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith Benjamin D., et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;(27):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research T. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. discussion 622-613. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, Wong ML, Hamilton N, et al. Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans. J Clin Oncol. 2012;30:1447–1455. doi: 10.1200/JCO.2011.39.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–932. doi: 10.1016/j.athoracsur.2007.05.007. discussion 932-923. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, Sublobar Resection, and Stereotactic Radiation for Early-Stage Non-Small Cell Lung Cancers in the Elderly. JAMA Surg. 2014;149:1244–1253. doi: 10.1001/jamasurg.2014.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys. 2012;84:1060–70. doi: 10.1016/j.ijrobp.2012.07.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah A, Hahn SM, Stetson RL, et al. Cost-effectiveness of stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. Cancer. 2013;119:3123–3132. doi: 10.1002/cncr.28131. [DOI] [PubMed] [Google Scholar]
- 10.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV–3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40:IV–26-35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 12.Davidoff AJ, Tang M, Seal B, et al. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 13.Brown ML, Riley GF, Schussler N, et al. Estimating health care costs relatedto cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV–104-117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 14.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika. 2000;87:329–343. [Google Scholar]
- 16.D'Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry. 2005;187:106–108. doi: 10.1192/bjp.187.2.106. [DOI] [PubMed] [Google Scholar]
- 18.Zethraeus N, Johannesson M, Jonsson B, et al. Advantages of using the net-benefit approach for analysing uncertainty in economic evaluation studies. Pharmacoeconomics. 2003;21:39–48. doi: 10.2165/00019053-200321010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Timmerman RD, Paulus R, Pass HI, et al. RTOG 0618: Stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. J Clin Oncol. 2013;31(suppl) abstr 7523. [Google Scholar]
- 20.Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28:928–935. doi: 10.1200/JCO.2009.25.0928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.