Abstract
Locomotion involves complex neural networks responsible for automatic and volitional actions. During locomotion, motor strategies can rapidly compensate for any obstruction or perturbation that could interfere with forward progression. In this pilot study, we examined the contribution of interlimb pathways for evoking muscle activation patterns in the contralateral limb when a unilateral perturbation was applied and in the case where body weight was externally supported. In particular, the latency of neuromuscular responses was measured, while the stimulus to afferent feedback was limited. The pilot experiment was conducted with six healthy young subjects. It employed the MIT-Skywalker (beta-prototype), a novel device intended for gait therapy. Subjects were asked to walk on the split-belt treadmill, while a fast unilateral perturbation was applied mid-stance by unexpectedly lowering one side of the split-treadmill walking surfaces. Subject's weight was externally supported via the body-weight support system consisting of an underneath bicycle seat and the torso was stabilized via a loosely fitted chest harness. Both the weight support and the chest harness limited the afferent feedback. The unilateral perturbations evoked changes in the electromyographic activity of the non-perturbed contralateral leg. The latency of all muscle responses exceeded 100 ms, which precludes the conjecture that spinal cord alone is responsible for the perturbation response. It suggests the role of supraspinal or midbrain level pathways at the inter-leg coordination during gait.
Keywords: locomotion, gait, robotics, training, gait perturbation, interlimb coordination
I. INTRODUCTION
Interlimb coordination, particularly the maintenance of stability under various environmental perturbations, is a problem that has been intriguing researchers for more than one century (Baker 2007). For quadrupeds it has been proven that at slow speeds, the animals coordinate their limbs such that three feet always remain on the ground to provide stability like a tripod while the forth limb advances. Bipedal walking does not have this stability trait and requires greater effort to maintain balance while advancing (Inman et al. 1981). On the other hand, it has been proven that sensory feedback not only assists the transition between the gait phases, but it also affects corrective responses to external perturbations (Nielsen and Sinkjaer 2002). Indeed, interaction of sensory inputs with those circuits’ activity can determine the coordinated pattern of agonist and antagonist muscles (Duysens et al. 2000). This sensory activity contributes to motor control in two ways. It may carry “error signals” following sudden external perturbations, and it may contribute to the pre-programmed motoneuronal activity such as the cutaneous and stretch reflex responses (Zehr and Stein 1999; Nakazawa 2004).
The coordination of the limbs during locomotion can be seen as a rhythmic activity of circuits that control different muscles and are specialized in repeating particular actions over and over again (Bernstein 1967; Bizzi et al. 2000; Dietz 2003). For locomotion, the term used is central pattern generator (CPG), which refers to a functional network of neurons within the spinal cord. This network is responsible for generating the rhythm and shape of the motor pattern (Grillner and Wallen 1985). Although the CPG might receive supraspinal and afferent inputs, it is defined as being able to produce self-sustained patterns of behavior, independent of any sensory input. This understanding of the basic principle of such a CPG is mainly based on data obtained from experimental animals, primarily on experiments with cats (Brown 1911).
Although there is some evidence that a CPG may exist in humans similar to the one in cats (Duysens and de Crommert 1998), it has not yet been proven. Rhythmic activity has only rarely been observed in spinal cord injured (SCI) patients. For patients with a completely transected spinal cord, it is possible to induce, modulate and stop rhythmic contractions of the trunk and lower limb extensor muscles; however, these rhythmic contractions never occurred spontaneously and had only a one-step cycle duration (Bussel et al. 1996). On the other hand, for patients with incomplete lesions, several studies reported subjects with the presence of alternating flexor and extensor activity (Calancie et al. 1994).
In the past decades, several studies on different experimental platforms have been investigating corrective reflex mechanisms during different phases of the gait cycle with experimental protocols focusing on overground walking with a unilateral perturbation during the stance phase (Nakazawa 2004; van der Linden et al. 2007; af Klint et al. 2009). Even though these studies mostly focused on the effect of unilateral perturbations to the ipsilateral leg muscles, the bilateral response has also been studied (Berger et al. 1984; Berger et al. 1987). During posture maintenance, experiments with powerful unilateral displacement of one leg produced bilateral responses both in adults (Berger et al. 1984; Berger et al. 1987) and in healthy human infants (Lam 2003). However, little is known whether this influence is exclusively based on the mechanisms for body stabilization and balance maintenance, or if it is also brought about by interlimb connections from gait pattern generators.
Here we hypothesize that the latency of neuromuscular responses to unilateral mechanical perturbations, when the body weight is supported, will be higher than 100ms suggesting the involvement of the supra-spinal circuitry in the responses.
II. METHODS
A. Apparatus
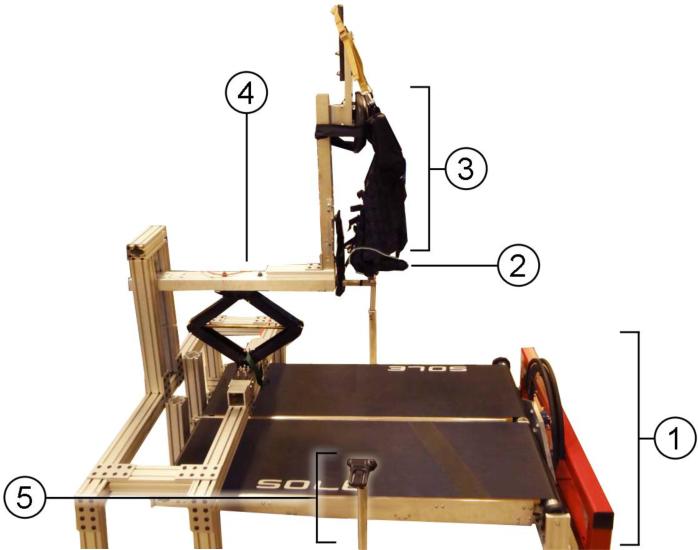
In this study we employed the beta prototype of the MIT Skywalker (Fig. 1). It is a unique, novel device intended for providing robot-assisted gait therapy (Bosecker and Krebs 2009). The overall concept consists of a split-belt treadmill and a body weight support. This system can provide support ranging from 0 to 100% of the patients’ weight and, while keeping the subject safe from falls, still not interfere with the required ranges of leg motion. The body-weight support system (BWSS) includes an underneath bicycle seat and a loosely fitted chest harness providing torso stabilization and preventing falls. Each side of the split-belt treadmill can also be vertically actuated through individual brushless motors. More specifically, each side of the treadmill can be lowered below the ground level and raised back in a controlled fashion (see Fig. 2).
Fig. 1.
The experimental setup mainly consists of (1) a split-belt treadmill (MIT Skywalker) and a body-weight support system (BWSS) composed of (2) a bicycle seat and (3) a chest harness to prevent any upper body movements. The amount of body-weight supported can be adjusted by lowering or lifting the whole BWSS with (4) a car-jack. Additionally an optical motion capture system is used which is based on (5) modified webcams installed on each side of the treadmill.
Fig. 2.
One single split-belt is lowered to apply the vertical perturbation at mid-stance of the right leg. The green arrow illustrates the rotation of the split-belt downwards during the drop of the walking surface and the red arrow illustrates the return of the walking surface upwards, back to horizontal level.
To measure the flexion-extension of the hip and knee joint of both legs in real time and to control the MIT-Skywalker (i.e., determining when to lower the split treadmill panels), we used a custom-made, camera-based motion tracking system. Two low-cost cameras (Logitech Quickcam Pro 9000, Logitech Inc.) were mounted on the sides of the platform at an appropriate distance to be able to capture the whole range of leg movement. Two battery powered systems equipped with two infrared LEDs were placed on each of the subjects’ limbs, one on the shank and the other on the thigh. Fig. 3 depicts the configuration of the sensors. The cameras were modified in order to be able to see the infrared light filtering out the visible spectrum. By modifying the cameras to infrared spectrum, we guaranteed that even in a non-controlled environment such as a therapy clinic, background has limited influence on the readings. Standard image processing techniques were used to monitor the position of the LEDs.
Fig. 3.
Model of human leg walking with LED markers attached at thigh and shank from the view of the tracking camera. The hip and knee angle are determined out of the coordinates of the four markers. With the measured length of thigh and shank, the heel coordinates were calculated to further determine the gait phase in real-time in order to introduce the proper perturbation.
To quantify head movement in real-time and estimate the efficacy of the BWSS in reducing the afferent feedback as the walking surface drops up to 0.8G, a 3-axis analog accelerometer (MMA7361L, Freescale Semiconductor, Austin, TX) was attached to the subjects' head, in the front and centrally located . Each of the three channels has an output range of 3.3V with a sensitivity of 800mV/g. It affords acceleration measurements in the range of 2 g. The accelerometer was calibrated prior to deployment using a 6-point tumble calibration method, i.e. by using gravity as reference acceleration.
B. Experimental Protocol
The experimental protocol was approved by the MIT Committee on the Use of the Humans as Experimental Subjects (COUHES). Six young healthy subjects [5 men, 1 woman, mean age 25±3 years] who were naive to the experimental goals were enrolled in this pilot study. The subjects were “seated” on the bike seat and attached by means of the chest harness to the BWSS. The amount of body-weight support (BWS) was measured with a weight scale so that the vertical position of the seat was adjusted until approximately 80% of the body-weight was supported. The 3-axis analog accelerometer was attached to the front head of the subjects.
Four battery operated LED modules were placed on the subjects’ leg using elastic Velcro straps. The weight of each module did not exceed 0.05 kg and its dimensions of width, length and height were 0.01 m, 0.08 m, and 0.015 m, respectively. The module was small enough and it did not interfere with leg motion. Electromyographic (EMG) signals were acquired from muscles of the left (contralateral) leg using single differential bipolar surface EMG electrodes (DE 2.3, Delsys Inc.) connected to a wireless EMG signal recording device (Myomonitor, Delsys Inc.). The raw signals were digitized at a sampling frequency of 1 kHz. Four muscles were recorded: tibialis anterior (TA), soleus (SOL), rectus femoris (RF), and semitendinosus (ST). The vertical motion of the treadmill was controlled by a programmable servo drive (AKDP003, Kollmorgen Corp.). The subjects were first asked to familiarize themselves with the device and walk for 100 strides on the Skywalker at a speed of 2.6 km/h (0.72m/s). Because we are interested in gait training following a neurological injury, we selected the typical average, self-selected comfortable speed observed in our parallel study involving stroke patients. Gait perturbations were introduced in a random manner with the interval between two successive perturbations randomly varying between 5 and 10 steps. The perturbations were randomly presented at the mid-stance of the perturbed side as defined below (right leg). The detection of the gait phase in real-time was done through the camera-based system, and the appropriate commands were sent to command the motors of the treadmill, which actuated the drop of one split-belt. The split-belt was deflected downwards by 9 degrees in total (11.4cm under the subject’s foot) with an acceleration profile under the foot beginning at 0.24g for the first 33ms, transitioning to 0.57g at 52 ms. The acceleration profile is a function of the nonlinear path of the cam driving the track drop motion. It is important to stress that the camera-based method to detect the gait phase is used only to estimate mid-stance and command the MIT-Skywalker to drop the walking surface; it is not used in the estimation of our primary objective, which is the time delay in in the EMG signal (see below).
C. Data Analysis
All data was analyzed offline except the leg kinematics; this was calculated from the images captured by the camera system and done online in real-time.
1) Leg kinematics
Only sagittal plane motion was considered. Assuming that the pelvis of the subject was constrained by the body-weight support of the device, the hip flexion-extension (qh) and knee flexion-extension (qk) angles were computed by:
| (1) |
| (2) |
where xi, yi (i = 0; 1; 2; 3) are the coordinates of the LEDs position at the image plane (see Fig. 3). The movement of the heel relative to the fixed hip position was computed from:
| (3) |
| (4) |
where Lth, Lsh were the lengths of the thigh and shank respectively measured before the experiment. The heel coordinates were used for determining the gait phase in real-time in order to introduce the proper perturbation. We defined mid-stance as the first time when xh is less than 2 inches after heel strike. The leg kinematics were re-sampled offline to match the EMG acquisition frequency.
2) EMG signal processing
EMG signal processing was conducted off-line. Raw signals were rectified and low pass filtered (fourth-order Butterworth, 50 Hz) to extract an amplitude envelope. Signals from all muscles were synchronized to individual gait cycles according to the leg kinematic measurements. For each subject, EMG signals were then divided into two groups according to the presence of perturbation applied at the corresponding cycle: unperturbed and perturbed trials. For the unperturbed trials, only the last 40 gait cycles before the first perturbation were considered. Thus, data from the gait cycles that succeeded a perturbation were discarded in order to exclude any perturbation-evoked phenomena.
To determine the muscle activity onset latency, we considered the lag between the moment the walking surface dropped and the instant we detected an increase in the EMG signal. A conventional amplitude thresholding method was implemented in MATLAB according to (Di Fabio 1987). Concisely, the latency is measured from the moment when the split-belt deflection is ≥0.05 deg, which corresponds to a total amplitude underneath the subjects’ foot of approx. 0.7 mm and the increased EMG amplitude.
III. RESULTS
The plots presented in Figures 5 to 8 show two different data sets which are marked with the colors blue and red. The blue data set is the average value from the non-perturbed cycles which are conducted before the first perturbation starts. The red line shows the data for the perturbed cycle. The effects of the perturbation are apparent by the difference between the two data sets. The time of the perturbation corresponds to a black vertical line.
Fig. 5.
The 3D acceleration of the head of a representative subject during the treadmill walking is calculated by the root mean square (RMS) of the three acceleration axes. A negative peak occurs directly after the perturbation (red line) with a typical maximum in the range of −0.2 g to −0.3 g.
Fig. 8.
Filtered EMG values of a representative subject of the four muscles measured on the left leg (unpertrubed side) which are rectus femoris (RF), semitendinosus (ST), tibialis anterior (TA) and soleus (SOL).
We are plotting the mean values and one standard deviation (shade of same color). The data shown corresponds to a representative subject.
The time scale is shown as a percentage of gait cycle starting with 0%, which corresponds to heel strike (HS) of the left foot (unperturbed), and ending after two complete gait cycles at 200%, which corresponds again to HS. From around 60% to 100%, the left leg is swinging forward with the perturbation occurring right in the middle, at approximately 80%. This position corresponds to the mid-stance of the right leg where the perturbation is applied.
A. Perturbation movement and head acceleration
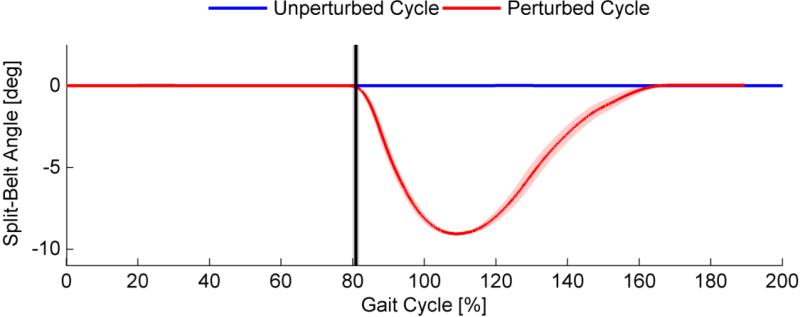
Fig. 4 shows the angle of the right split-belt for the two cases, perturbed and unperturbed. The split-belt is always at neutral position (0 deg) while for the perturbation the split-belt deflects downwards, reaching its maximum deflection at 9 deg. The 3D acceleration of the head is shown in Fig. 5 which is calculated by the root mean square (RMS) of the three acceleration axes. By subtracting the RMS of the offset, 0 g corresponds to no additional acceleration except for gravity.
Fig. 4.
Angle of the right split-belt measured by the incremental encoder. The split-belt is always at neutral position (0 deg) while for the perturbation the split belt deflects downwards about 9 deg with an acceleration of 0.8 g. The starting time of the perturbation, illustrated by the black vertical line, is defined to be the position where the deflection is ≥ 0.05 deg.
B. Leg kinematic responses after unilateral perturbation
While muscle latency is the primary variable of interest, we were able to estimate the knee and hip joint angles from both the perturbed (right) and the unperturbed (left) legs as shown, along with the timing of the perturbation (black vertical line). The results shown here are from a representative subject walking at the specified speed (2.6 km/h). The perturbed hip and knee (Fig. 6) were close to full extension at the moment of the perturbation (perturbation was induced at the mid-stance of the right foot). In general, the consecutive gait cycle was shortened while the kinematics of the perturbed side did not show big differences except that the maximal knee flexion was limited to around 40 deg.
Fig. 6.
Hip and knee joint kinematics of the right leg (perturbed side) of a representative subject. The leg is at stance-phase from 50% to 110% of the gait cycle. The perturbation (vertical black line) starts exactly at mid-stance of the right leg.
In contrast, left leg knee showed a reaction to the perturbation which strongly deviates from the normal unperturbed cycle (Fig. 7). The leg was in the middle of the swing-phase preparing for the HS which was characterized by a knee flexion angle of 0 deg. As a reaction to the perturbation, the knee was typically flexed before HS to an angle of 20 deg. Thus, the left HS for the perturbed trial occurred at the moment where the hip angle showed a small peak (additional extension) at around 95% of the unperturbed gait cycle.
Fig. 7.
Hip and knee joint kinematics of the left leg (non-perturbed side) of a representative subject. The leg is in swing-phase from 60% to 100% of the gait cycle. The perturbation (vertical black line) starts exactly in the middle of the swing-phase.
We must note that the leg kinematics presented here are distinct from normal kinematics found in the literature (Inman et al. 1981). These differences are primarily caused by the constraint imposed on the motion of the pelvis. More specifically, the BWSS restricts vertical motion of the pelvis, which is apparent in overground normal walking. Though the hip kinematics have the same characteristics, they are limited to a range of [−5, +20] deg, while for normal walking have a typical range of [−15, +35] deg (Inman et al. 1981). Furthermore the amount of unloading is quite high and can affect the kinematics. The knee kinematics were similar to the one of normal overground walking with the only difference being that the knee joint was not flexed during the stance-phase.
C. Contralateral muscle responses after unilateral perturbation
Fig. 8 depicts the primary outcome via the EMG activation of the recorded muscles of the left leg compared to the corresponding activation in the unperturbed case. The timing of response was computed from the actual time of the perturbation to the departure point when a significant difference of the muscle activation was observed by using a thresholding algorithm (Di Fabio 1987). At the instant of perturbation, the left leg is approximately at mid-swing; therefore TA and ST are normally active, dorsiflexing the ankle and flexing the knee respectively. The other two muscles, RF and SOL, are normally inactive but, when activated, have time delays similar to the previous two muscles just mentioned (see Tab. 1).
Table 1.
Muscle activity onset time for the four muscles RF, ST, TA and SOL calculated with the amplitude thresholding method by Di Fabio (1987). The values shown are mean ± one standard deviation over all six subjects.
| Muscle Activity Onset Time | |||
|---|---|---|---|
| RF | ST | TA | SOL |
| 163 ± 22 ms | 129 ± 68 ms | 193 ± 80 ms | 207 ± 74 ms |
IV. DISCUSSION
The importance of bilateral sensorimotor signals for the interlimb coordination in locomotion has been demonstrated in animals (Grillner and Rossignol 1978; Duysens and Pearson 1980; Pearson et al. 1992), but it is difficult to ascertain in humans due to the mechanical inter-leg coupling. Evidence from spinalized cats shows that locomotor activity in each hind limb can be generated independently (Grillner and Zangger 1979). In split-belt treadmill conditions, independent rhythm generation in each hind limb is demonstrated by the ability of the hind limbs to walk at different speeds (Forssberg et al. 1980; Morton 2006). On the other hand, following unilateral deafferentation in spinal cats, disruption of both ipsi- and contralateral stepping occurs; this further illustrates the contralateral influence of afferent input (Giuliani and Smith 1987). The investigation of interlimb neural coupling mechanisms in humans is more challenging because central and peripheral influences cannot be explicitly isolated. For example, although perturbations in stance evoke bilateral muscle responses of similar latencies (Dietz and Berger 1982; Berger et al. 1984), the EMG changes in the non-perturbed leg may be attributed to afferent signals generated in that leg due to the joint reaction forces generated in both legs (Yamaguchi and Zajac 1990). In this pilot study, we tried to isolate in the best possible way both the reaction forces and most of the interlimb mechanical coupling by providing body-weight support during walking.
The aim of this study was to investigate the interlimb coordination by applying unilateral perturbations and analyzing the response of the contralateral limb at a slow speed comparable to the preferred speed of our on-going parallel study with stroke patients. We perturb gait by unexpectedly lowering the walking surface under one leg at mid stance. Although the kind of perturbation is similar to ones used in previous studies (Sinkjaer et al. 2000; Nakazawa 2004; Marigold and Patla 2005; van der Linden et al. 2007; af Klint et al. 2009), our experimental paradigm included high body-weight support (80%) and torso stabilization in order to limit the afferent feedback as well as the loading of the legs. Of notice, we repeated the experiments described above providing a different amount of body-weight support (approximately 60% instead of the 80%) obtaining similar time-delay results. To our knowledge, this has never been tested and allows us to explore interlimb coordination not affected by mechanisms for body stabilization. Therefore, the analysis and discussion of results do not focus on the activation of the ipsilateral muscles, which has been widely investigated in the past (van der Linden et al. 2007). In comparison, we focused on analyzing the responses of the contralateral muscles in order to study the inter-limb coordination in body-weight supported walking at slow speeds.
A. Contralateral neuromuscular response to unilateral perturbations
The latency of the effect of the perturbations on the activation of the contralateral ankle flexor and extensor in our experiments (TA: 193±80 ms, SOL: 207±74 ms), which was our primary outcome, was larger than in previous works. More specifically, in an experiment with a movable platform embedded in a walkway that was used to drop the walking surface by 1 cm, the contralateral TA was activated approximately after 100 ms following the perturbation (Nakazawa 2004). The walking surface was dropped when the vertical component of ground reaction force was about 60% of the subject's weight, i.e., close to the mid-stance phase of the gait cycle, very similar to our experimental paradigm. Van der Linden et al. (2007) observed a similarly short latency (approximately 90 ms) in the activation of the contralateral TA. Marigold and Patla (2005) observed a comparable latency (approximately 110 ms) in the activation of the contralateral TA using a ground compliant support that could drop 2 cm with no body-weight support. When compared to existing literature, we observed larger latencies in contralateral muscles. Van der Linden et al. (2007) observed contralateral knee flexors activation approximately 90 ms after the perturbation. In our experiment, increased activity of the contralateral RF was observed at 163±22 ms, and ST was activated with a latency of 129±68 ms. Therefore, the latency of the perturbation effect on the muscles acting on the knee was also larger than observed in similar previous works (Marigold and Patla 2005; van der Linden et al. 2007). The overall mean value and its standard deviation of all contralateral muscle responses are shown in Table 1.
B. Functional role of muscle responses
The kinematics of the right (perturbed) leg is shown in Fig.6. The leg is at neutral position at the moment when the perturbation occurs, and thus the hip and knee kinematics are not directly affected by the perturbation. However, the right knee is only flexed to a maximum angle of 40 deg during the swing phase, which can be explained by the lowered surface not needing such a large knee flexion to achieve sufficient foot clearance.
In contrast, the knee joint of the left leg shows a reaction to the perturbation which strongly deviates from the normal unperturbed cycle (Fig. 7). The leg is in the middle of the swing phase preparing for the HS which is characterized by a knee flexion angle of 0 deg. Thus, as a reaction to the perturbation, the knee is flexed to an angle of 20 deg before HS. This seems intuitive since the body must catch itself from falling. This observation is also in agreement with the findings of van der Linden et al. (2007).
C. Neural mechanisms of interlimb coordination
The significance of afferent input from hip joints, in combination with that from load receptors, for the generation of locomotor activity in the isolated human spinal cord has been reported (Dietz et al. 2002) as well as re-organization of muscle activations during postural and locomotor-like perturbations of standing human subjects (Nashner et al. 1979). Recently it was found that cerebellar damage does not impair the ability to make reactive feedback-driven motor adaptations, but it does significantly disrupt predictive feed-forward motor adaptations during split-belt treadmill locomotion (Morton 2006). On the other hand, it is presumed that in human locomotion, the cerebellum and other supraspinal structures play a more critical role because of the additional demands of bipedal walking (Grillner and Wallen 1985). The cerebellum has been found to also play a significant role in balance, as discovered in experiments involving the phenomenon of the podokinetic adaptation (Earhart et al. 2002). One of the leading hypotheses is that the cerebellum processes sensory inputs and makes immediate alterations of ongoing movement patterns (Bower 1997; Shimansky et al. 2004). Furthermore, it was recently shown that proprioceptive sensory information carried by spinocerebellar tracts provides a major input to the spinocerebellum, which has an important role in coordinating motor output for posture and locomotion (Poppele et al. 2003). Dorsal spinocerebellar tract (DSCT) neurons were shown to be modulated by passive step-like movements of either hind limb, implying they receive a bilateral sensory input.
Distinct from previous work, we supported the body weight during walking. In previous studies, the contralateral limb was maintaining the body center of mass inside the support area or, in general, the body stability (Berger et al. 1984; Berger et al. 1987; Dietz et al. 1989). In those studies the vestibular system could play a significant role in coordinating the lower limb in recovery strategies, minimized in our protocol. Our findings question the hypothesis that a spinal response could fully explain the results and give support to the role of a supraspinal pathway in inter-limb coordination. The large latency in the contralateral leg muscle response makes such an argument plausible. TA activation during unperturbed walking has been characterized by both spinal (CPG) and cortical origin (Duysens et al. 2004). Moreover, it was observed that the disturbance in the sensory information for the perturbed leg evoked muscular activity in the contralateral leg, which is consistent with the role of spinocerebellum (Poppele et al. 2003) and the necessity of supraspinal input for walking (Nielsen 2003; Rossignol 2010). For many motor functions, the cortex is known to be able to control automated functions executed at lower CNS levels. A cerebellar contribution via reticulus spinal neurons has been suggested in humans (Bonnet et al. 1976), while evidence was presented for a cortical control of interlimb coordination in the past (Debaere et al. 2001). Our work adds evidence that the control of stability in walking is a combination of both supraspinal and spinal neural control.
V. CONCLUSION
The aim of this pilot study was to investigate interlimb coordination in healthy subjects at a typical, self-selected comfortable speed of our parallel study involving stroke patients by applying a unilateral perturbation and analyzing the response latencies of the contralateral leg. While similar studies exist, to our knowledge this is the first experimental paradigm including body-weight support that acts to limit afferent feedback. Even though there are some limitations, this study showed that the latency of the effect of the perturbations was larger than in any earlier work. The mean latency of all muscle responses exceeded 100 ms, which precludes the conjecture that spinal cord alone is responsible for the perturbation response, and instead suggests the role of a supraspinal pathway contribution in interlimb coordination. These longer latencies also have to be considered for the specific field of robotic gait rehabilitation where it is common to use a BWS. By unloading the patients’ weight, some of the afferent signals might not be present, a factor that is responsible for the short latencies. In other words to achieve the same conditions in body-weight supported locomotion as in normal walking one needs to pay attention to the loss of sensorimotor signals which mainly concern the vestibular system but may also concern the foot load receptors.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported in part by grants from the Department of Veterans Affairs Rehabilitation Research and Development Service (VA RR&D) “Center of Excellence on Task-Oriented Exercise and Robotics in Neurological Diseases,” (B3688R), NIH R01 HD069776, and the ETH Zurich to S. Seiterle.
H. I. Krebs is a co-inventor in several Massachusetts Institute of Technology-held patents for the robotic technology used in this work. He holds equity positions in Interactive Motion Technologies, Watertown, MA, USA the company that manufactures this type of technology under license to MIT.
R. Riener holds several patents in robotic technology. However, this technology has no relationship to the technology applied in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS’ CONTRIBUTIONS
SS carried out the experiments, collected results, performed data analysis, and wrote the manuscript, TS carried out the experiments, collected results, and performed data analysis, PKA conceived the study and contributed to the writing of the manuscript, RR conceived the study and contributed to the writing of the manuscript, HIK conceived the study, performed data analysis, contributed to the writing of the manuscript and of the revision. All authors read and approved the final manuscript.
REFERENCES
- af Klint R, Nielsen JB, Sinkjaer T, Grey MJ. Sudden Drop in Ground Support Produces Force-Related Unload Response in Human Overground Walking. J Neurophysiol. 2009;101:1705–1712. doi: 10.1152/jn.91175.2008. [DOI] [PubMed] [Google Scholar]
- Baker R. The history of gait analysis before the advent of modern computers. Gait Posture. 2007;26:331–342. doi: 10.1016/j.gaitpost.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Berger W, Dietz V, Horstmann G. Interlimb coordination of posture in man. J Physiol. 1987;390:135. [Google Scholar]
- Berger W, Dietz V, Quintern J. Corrective reactions to stumbling in man: neuronal co ordination of bilateral leg muscle activity during gait. J Physiol. 1984;357:109–125. doi: 10.1113/jphysiol.1984.sp015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein N. The coordination and regulation of movement. 1967 [Google Scholar]
- Bizzi E, Tresch MC, Saltiel P, d'Avella A. New perspectives on spinal motor systems. Nature. 2000;1:101–108. doi: 10.1038/35039000. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Gurfinkel S, Lipchits MJ, popov KE. Central programming of lower limb muscular activity in the standing man. Agressologie. 1976;17:35–42. [PubMed] [Google Scholar]
- Bosecker CJ, Krebs HI. MIT-Skywalker. IEEE Int. Conf. Rehabil. Robot. 2009:542–549. [Google Scholar]
- Bower JM. Control of sensory data acquisition. Int Rev Neurobiol. 1997;41:489–513. doi: 10.1016/s0074-7742(08)60367-0. doi: 10.1016/S0074-7742(08)60367-0. [DOI] [PubMed] [Google Scholar]
- Brown TG. The Intrinsic Factors in the Act of Progression in the Mammal. Proc R Soc London Ser B, Contain Pap A Biol Character. 1911;84:308–319. [Google Scholar]
- Bussel B, Roby-Brami A, Néris OR, Yakovleff A. Evidence for a spinal stepping generator in man. Spinal Cord. 1996;34:91–92. doi: 10.1038/sc.1996.15. [DOI] [PubMed] [Google Scholar]
- Calancie B, Needham-Shropshire B, Jacobs P, et al. Involuntary stepping after chronic spinal cord injury: Evidence for a central rhythm generator for locomotion in man. 1994 doi: 10.1093/brain/117.5.1143. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein R. Amplitude modulation of the soleus {H}{−}reflex in the human during walking and standing. J Neurosci. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Swinnen SP, Béatse E, et al. Brain Areas Involved in Interlimb Coordination: A Distributed Network. Neuroimage. 2001;14:947–958. doi: 10.1006/nimg.2001.0892. [DOI] [PubMed] [Google Scholar]
- Dietz V. Spinal cord pattern generators for locomotion. Clin Neurophysiol. 2003;114:1379–1389. doi: 10.1016/s1388-2457(03)00120-2. [DOI] [PubMed] [Google Scholar]
- Dietz V, Berger W. Spinal coordination of bilateral leg muscle activity during balancing. Exp Brain Res. 1982;47:172–176. doi: 10.1007/BF00239376. [DOI] [PubMed] [Google Scholar]
- Dietz V, Horstmann GA, Berger W. Interlimb coordination of leg-muscle activation during perturbation of stance in humans. J Neurophysiol. 1989;62:680–693. doi: 10.1152/jn.1989.62.3.680. [DOI] [PubMed] [Google Scholar]
- Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-Regulating Mechanisms in Gait and Posture: Comparative Aspects. Physiol Rev. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Duysens J, de Crommert HWAA. Neural control of locomotion; Part 1: The central pattern generator from cats to humans. Gait Posture. 1998;7:131–141. doi: 10.1016/s0966-6362(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Duysens JEJ, Baken BCM, Burgers L, et al. Cutaneous reflexes from the foot during gait in hereditary spastic paraparesis. Clin Neurophysiol. 2004;115:1057–1062. doi: 10.1016/j.clinph.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Earhart GM, Fletcher WA, Horak FB, et al. Does the cerebellum play a role in podokinetic adaptation? Exp Brain Res. 2002;146:538–542. doi: 10.1007/s00221-002-1238-y. [DOI] [PubMed] [Google Scholar]
- Di Fabio RP. Reliability of Computerized Surface Electromyography for Determining the Onset of Muscle Activity. Phys Ther. 1987;67:43–48. doi: 10.1093/ptj/67.1.43. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J, Rossignol S. The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand. 1980;108:283–295. doi: 10.1111/j.1748-1716.1980.tb06534.x. [DOI] [PubMed] [Google Scholar]
- Giuliani CA, Smith JL. Stepping behaviors in chronic spinal cats with one hindlimb deafferented. J Neurosci. 1987;7:2537–2546. [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallen P. Central Pattern Generators for Locomotion, with Special Reference to Vertebrates. Annu Rev Neurosci. 1985;8:233–261. doi: 10.1146/annurev.ne.08.030185.001313. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Inman TI, Ralston HJ, Todd F. Human Walking. Williams and Wilkins; 1981. [Google Scholar]
- Lam T. Stumbling corrective responses during treadmill-elicited stepping in human infants. J Physiol. 2003;553:319–331. doi: 10.1113/jphysiol.2003.043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden MH, Marigold DS, Gabreels FJM, Duysens JEJ. Muscle reflexes and synergies triggered by an unexpected support surface height during walking. J Neurophysiol. 2007;97:3639–3650. doi: 10.1152/jn.01272.2006. [DOI] [PubMed] [Google Scholar]
- Marigold DS, Patla AE. Adapting locomotion to different surface compliances: neuromuscular responses and changes in movement dynamics. J Neurophysiol. 2005;94:1733–1750. doi: 10.1152/jn.00019.2005. doi: 10.1152/jn.00019.2005. [DOI] [PubMed] [Google Scholar]
- Morton SM. Cerebellar Contributions to Locomotor Adaptations during Splitbelt Treadmill Walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K. On the reflex coactivation of ankle flexor and extensor muscles induced by a sudden drop of support surface during walking in humans. J Appl Physiol. 2004;96:604–611. doi: 10.1152/japplphysiol.00670.2003. [DOI] [PubMed] [Google Scholar]
- Nashner LM, Woollacott M, Tuma G. Organization of rapid responses to postural and locomotor-like perturbations of standing man. Exp Brain Res. 1979;36:463–476. doi: 10.1007/BF00238516. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Sinkjaer T. Afferent feedback in the control of human gait. J Electromyogr Kinesiol. 2002;12:213–217. doi: 10.1016/s1050-6411(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Nielsen JB. How we Walk: Central Control of Muscle Activity during Human Walking. Neuroscientist. 2003;9:195–204. doi: 10.1177/1073858403009003012. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Ramirez JM, Jiang W. Entrainment of the locomotor rhythm by group Ib afferents from ankle extensor muscles in spinal cats. Exp Brain Res. 1992;90:557–566. doi: 10.1007/BF00230939. [DOI] [PubMed] [Google Scholar]
- Poppele RE, Rankin A, Eian J. Dorsal spinocerebellar tract neurons respond to contralateral limb stepping. Exp Brain Res. 2003;149:361–370. doi: 10.1007/s00221-003-1378-8. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural Control of Stereotypic Limb Movements. Compr. Physiol. 2010 [Google Scholar]
- Shimansky Y, Wang J-J, Bauer RA, et al. On-line compensation for perturbations of a reaching movement is cerebellar dependent: support for the task dependency hypothesis. Exp Brain Res. 2004;155:156–172. doi: 10.1007/s00221-003-1713-0. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, et al. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi GT, Zajac FE. Restoring unassisted natural gait to paraplegics via functional neuromuscular stimulation: a computer simulation study. Biomed. Eng. (NY) 1990 doi: 10.1109/10.58599. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? . Prog Neurobiol. 1999;58:185–205. doi: 10.1016/s0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]