Abstract
Purpose
To determine the number of drugs exhibiting flip-flop pharmacokinetics following oral dosing from immediate release dosage forms and if they exhibit a common characteristic that may be predicted based on BDDCS classification.
Method
The literature was searched for drugs displaying flip-flop kinetics (i.e. absorption half-life larger than elimination half-life) in mammals in PubMed, via internet search engines and reviewing drug pharmacokinetic data.
Results
Twenty two drugs were identified as displaying flip-flop kinetics in humans (13 drugs), rat (9 drugs), monkey (3 drugs), horse (2 drugs), and/or rabbit (2 drugs). Nineteen of the 22 drugs exhibiting flip-flop kinetics were BDDCS Classes 3 and 4. One of the three exceptions, meclofenamic acid (Class 2), was identified in the horse however it would not exhibit flip-flop kinetics in humans where the oral dosing terminal half-life is 1.4 hr. The second, carvedilol, can be explained based on solubility issues, but the third sapropterin dihydrochloride (nominally Class 1) requires further consideration.
Conclusion
The few drugs displaying oral flip-flop kinetics in humans are predominantly BDDCS Classes 3 and 4. New molecular entities predicted to be BDDCS Classes 3 and 4 could be liable to exhibit flip-flop kinetics when the elimination half life is short and should be suspected to be substrates for intestinal transporters.
Keywords: BDDCS, flip-flop pharmacokinetics, half-life, oral drug absorption, transporters
Introduction
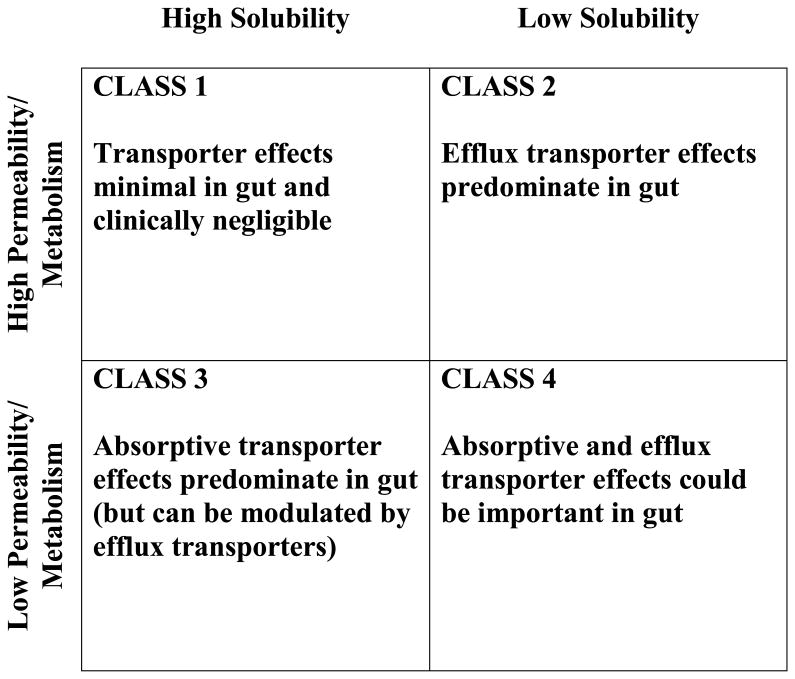
Accurate prediction of in vivo pharmacokinetics from in vitro measurements is an ongoing goal in the field of pharmaceutical sciences and was a primary incentive in the establishment by Amidon and colleagues1 of the Biopharmaceutics Classification System (BCS). Wu and Benet 2 built upon the BCS by modifying it to include information concerning drug elimination, and thus created the Biopharmaceutics Drug Disposition Classification System (BDDCS; Figure 1) to aid in predicting in vivo drug disposition by identifying the role of drug transporters, here presented with respect to effects in the intestine, as reviewed by Shugarts and Benet 3. The BDDCS gives scientists and clinicians a tool for predicting drug disposition and drug-drug interaction characteristics very early in development and with little additional expense. This paper is dedicated to Professor Amidon in recognition of his outstanding and seminal contributions to the pharmaceutical sciences. It would not have been possible to conceive of the BDDCS2, without his prefatory insightful development of BCS1.
Figure 1. The Biophamaceutics Drug Disposition Classification System predicts the effects of transporters on drug absorption in the gut.
Gastrointestinal absorption is generally faster than elimination for most immediate-release, orally dosed drugs. However, there are exceptions characterized as flip-flop pharmacokinetics, in which the rate of absorption of a drug is slower than its rate of elimination. It is termed “flip-flop” because the absorption is the limiting process for elimination, since a drug cannot be cleared from the system any faster than it enters into that system. It follows that observing an increased terminal elimination half-life following oral (p.o.) dosing of a drug, as compared to its intravenous (i.v.) half-life, is indicative of flip-flop pharmacokinetics. That is, while the ratio of a drug's absorption half-life to its elimination half-life (t1/2,abs/t1/2,elim) is usually less than one, in the case of flip-flop kinetics the ratio is greater than one. In 2011, Yáñez et al. 4 published an extensive review of flip-flop pharmacokinetics, identifying 12 drugs exhibiting flip-flop pharmacokinetics following immediate release oral dosing.
It is hypothesized here that drugs exhibiting poor intestinal membrane permeability rate would be those most likely to display flip-flop kinetics, as also noted by Yáñez et al.4 Poorly permeable drugs generally have a low oil-to-water partition coefficient and are classified as BDDCS Classes 3 and 4, which are poorly metabolized. This report describes nineteen drugs that display flip-flop kinetics and are poorly metabolized, although one of these poorly metabolized drugs displays a very weak flip-flop profile. These drugs are all associated with Classes 3 and 4 of the BDDCS. Based on these classifications, the BDDCS predicts that absorptive (uptake) transporters may play an important role in the gastrointestinal absorption of Class 3 and 4 drugs (Figure 1). Here we suggest that in vitro measures of permeability rate and extent of metabolism will predict whether or not a drug would be likely to display flip-flop kinetics in vivo.
Methods
Drugs described in the literature as displaying flip-flop kinetics after oral dosing of immediate-release formulations in mammals (humans, monkeys, horses, rats or rabbits) were identified in a survey of previously reported pharmacokinetic studies. Searches using the term “flip-flop [or flip flop] kinetics [and pharmacokinetics]” were performed in both PubMed, Web of Science, via various internet searches (e.g. Google) and reexaminations of specific drug categories as will be described. The search results were then gleaned to identify reports of flip-flop drugs and their respective i.v. and p.o. half-lives. Studies investigating controlled release formulations, prodrugs or drugs administered via non-oral delivery sites (e.g. intramuscular, inhalation, etc.) were excluded from consideration. Close to 200 studies identifying flip-flop pharmacokinetics were found via the search processes utilized, with the overwhelming majority related to formulations developed to achieve flip-flop pharmacokinetics for drugs with short half-lives. For example, a recent publication reports flip-flop kinetics for the Class 1 drug mycophenolate in transplant patients for an enteric-coated formulation 5. Similarly, drugs with non-enzymatically catalyzed metabolism (e.g. thalidomide 6) or drugs reported to display flip-flop kinetics under conditions of decreased intestinal motility that would affect absorption kinetics were also excluded (e.g. cephradine 7 or dabigatran etexilate 8,9). Two additional drugs were excluded due to a lack of corroborating evidence in the literature: a report of possible flip-flop kinetics of etoposide in children was ambiguous 10; a single report of the i.v. half-life of vildagliptin in humans 11 was within the range of p.o. half-lives reported in other human studies 12,13, and in addition, studies with vildagliptin in rats clearly demonstrated a lack of a flip-flop phenomenon 14.
Where available, the reported terminal half-life after p.o. dosing of a drug was compared to the elimination half-life after i.v. dosing, and a ratio was calculated for each. Drugs were then classified into the BDDCS based on solubility and extent of metabolism following the tabulation of Benet et al. 15. A drug is said to be highly soluble in both BCS and BDDCS when its highest dose strength is soluble in 250 mL or less of aqueous media over the pH range of 1-7.5 at 37°C 2. Drugs were classified as highly metabolized if metabolism accounts for at least 60% of its elimination 15. Of the 22 drugs found to exhibit flip-flop kinetics, 13 had a published BDDCS classification 15. The remaining nine were classified based on the above criteria. Further searches of the literature were done to identify which of these drugs were known substrates of the uptake and efflux transporters expressed on the intestinal lumen and liver.
Results
Acamprosate, amoxicillin, ampicillin, calcium dosbesilate, carbovir, carvedilol, cefuroxime, cephalexin, fexofenadine, florfenicol, furosemide, levovirin, meclofenamic acid, metformin, nitrofurantoin, pravastatin, rebamipide, sapropterin, xamoterol, zanamivir and zidovudine are reported to display flip-flop kinetics, and nedocromil is reported to show a weak trend toward flip-flop kinetics (Table 1). Each of these drugs, except for carvedilol, meclofenamic acid and sapropterin, is eliminated primarily through excretion (i.e. poorly metabolized) and thus is assigned to Classes 3 or 4 of the BDDCS. Interestingly, zidovudine displays flip-flop kinetics in rats 16, where it is poorly metabolized (20-30 % metabolized 17-19, as compared to both monkeys 19,20 and humans 19,21,22 in which zidovudine displays normal kinetics and is extensively metabolized (60-75 % metabolized).
Table 1. Drugs Displaying Flip-Flop Kinetics.
| Drug | BDDCS Class | t1/2, i.v. (h) | t1/2, p.o. (h) | t1/2, p.o./t1/2, i.v. ratio | Reference |
|---|---|---|---|---|---|
| Acamprosate | 3 | 0.32 | 1.87 | 5.8 (rat) | 38,39 |
| 3.2 | 32.7 | 10.2 (human) | |||
| Amoxicillin | 3 | 1.31 | 2.62 | 2.0 (human) | 40 |
| Ampicillin | 3 | 0.78 | 2.35-3.24 | > 3.0 (human) | 41 |
| Calcium dobesilate | 3 | 1.54 | 2.57 | 1.7 (human) | 42 |
| Carbovir | 4 | 0.35 | 1.35 | 3.9 (rat) | 43,44 |
| Carvedilol | 2 | 2.4 | 6.4 (capsule)4.3 (suspension) | 2.7 (human)1.8 (human) | 23 |
| Cefuroxime | 3 | 1.64 | 2.72 | 1.7 (rat) | 45 |
| Cephalexin | 3 | 1.4 | Reported flip-flop | Reported flip-flop (rat) | 46 |
| Fexofenadine | 3 | 2.4 | 5.0 | 2.1 (horse) | 47 |
| 3.7 | 6.6 | 1.8 (monkey) | |||
| Unknown | Varies | Reported flip-flop (human) | |||
| Florfenicol | 3 | 1.7 | 4.8 | 2.8 (rabbit) | 48 |
| Furosemide | 4 | 2.8 | 4.9 | 1.8 (human) | 49 |
| Levovirin | 3 | 3.5 | 12.2 | 3.5 (monkey) | 50 |
| 1.5 | 4.5 | 3.0 (rat) | |||
| 3.7 | 4.1 | 1.1 (dog) | |||
| Meclofenamic acid | 2 | 1.4 | 3.0 | 2.1 (horse) | 24,25 |
| Metformin | 3 | 1.7 | 6.9 | 4.1 (human) | 51,52 |
| Nedocromil | 3 | 13.8 | 15.9 | 1.2 (human) | 53 |
| Nitrofurantoin | 4 | 0.25 | 0.63 | 2.5 (rabbit) | 54 |
| Pravastatin | 3 | 0.78 | 1.77 | 2.3 (human) | 55 |
| Rebamipide | 4 | 0.4 | 5.4 | 13.5 (rat) | 56 |
| Sapropterin | 1 | 0.78 | 2.95 | 3.8 (human) | 26 |
| Xamoterol | 3 | 7.7 | 16 | 2.1 (human) | 57 |
| Zanamivir | 3 | 1.67 | 3.3 | 2.0 (human) | 34 |
| Zidovudine | 3 | 1.6 | 3 to 4 | 2.2 (rat) | 16-22 |
| 1 | 1.14 | 1.65 | 1.4 (monkey) | ||
| 1.1 | 1.0 | < 1 (human) |
In the majority of cases presented in Table 1, the slow absorption process after an oral dose had a very obvious impact on pharmacokinetics and resulted in an observed terminal half-life that was longer by about two-fold or greater as compared to the i.v. dose elimination half-life. One clear exception was nedocromil, for which the flip-flop trend was weak and the absorption half-life to elimination half-life ratio was closer to one (Table 1). Notably, nedocromil has an inherently longer elimination half-life (13.8 h) than any of the other drugs (0.3 – 7.7 h) that displayed convincing flip-flop kinetics. Two BDDCS Class 2 drugs23-25 and one Class 1 drug26 are reported to exhibit flip-flop pharmacokinetics.
Classification into the BDDCS helps to predict whether uptake and/or efflux transporters in the gut will play a role in the absorption of a drug 2,3 (Figure 1). The effects of transporters on the absorption of Class 1 compounds are negligible. For Class 2 compounds, the effects of efflux transporters are expected to dominate in the gut. The BDDCS predicts that absorptive transporter effects will predominate for Class 3 drugs, although efflux transporters in the gut may potentially modulate their disposition. For Class 4 drugs, the BDDCS predicts that the drug's disposition is likely to be to be affected by both absorptive and efflux transporters. Eighteen of the 21 drugs (omitting zidovudine) with flip-flop kinetics identified herein were poorly metabolized and thus classified as either Class 3 or 4. We recently reviewed intestinal drug transporters 27 and only half of the drugs exhibiting flip-flop kinetics have been previously shown to be substrates for at least one uptake and/or efflux transporter (not limited to intestinal transporters) that may play an important role in their pharmacokinetics (Table 2). When the drug is listed as a substrate in the UCSF-FDA Transportal database 28, this reference will provide primary reference sources.
Table 2. Flip-Flop Drugs Are Known Substrates For Transporters.
| Drug | Gut and liver transporters | Reference | |
|---|---|---|---|
| Uptake | Efflux | ||
| Acamprosate | -- | -- | |
| Amoxicillin | PEPT1, PEPT2 | -- | 28 |
| Ampicillin | PEPT1, PEPT2 | MRP4 | 58,59 |
| Calcium dobesilate | -- | -- | |
| Carbovir | Nucleoside, nucleobase | -- | 60 |
| Carvedilol | -- | -- | |
| Cefuroxime | PEPT1, PEPT2 | -- | 58 |
| Cephalexin | PEPT1, OCTs | MATEs | 28 |
| Fexofenadine | OATP1A2, OATP2B1, OATP1B3 | BCRP, MDR1, MRP2, MRP3 | 28 |
| Florfenical | -- | -- | |
| Furosemide | OAT3 | BCRP, MRP2, MRP4 | 28,59,61,62 |
| Levovirin | -- | -- | |
| Meclofenamic acid | -- | -- | |
| Metformin | OCT1, OCT2, OCT3, PMAT | MATE1, MATE2K | 28,63 |
| Nedocromil | -- | -- | |
| Nitrofurantoin | -- | ||
| Pravastatin | MCT1, OATP1B1, OATP2B1, OAT3, OAT4 | MRP2, MRP4, MDR1 | 28,59,64-66 |
| Rebamipide | -- | MRP4 | 59 |
| Sapropterin | -- | -- | |
| Xamoterol | -- | -- | |
| Zanamivir | -- | -- | |
| Zidovudine | CNT1, ENT2, OCTN2, OAT1, OAT2, OAT3, OAT4 | BCRP, MDR1, MRP4, MRP5 | 28,67-72 |
Discussion
There are currently two major drug classification systems in use, the BCS and the BDDCS, which are based on the solubility and nominally the permeability of a drug. The BCS was developed to allow waiver of in vivo bioequivalence studies for highly soluble, highly permeable drugs, where rapid dissolution of immediate release dosage forms could be established 1,29. However, as pointed out by Benet and Larregieu30, the definitive criteria for assignment of Class 1 BCS is ≥ 90% absorption, and, in fact, a number of poor permeability rate drugs relative to metoprolol (e.g., cefadroxil, cephradine, levofloxacin, loracarbef, ofloxacin, pregabalin, and sotalol) showing ≥ 90% absorption are assigned to BCS Class 131. In contrast, BDDCS was developed to predict drug disposition based on solubility and permeability rate, with the recognition that high permeability rate compounds were eliminated primarily by metabolism, while poor permeability rate drugs were eliminated by renal and biliary excretion of unchanged drug 2. A strength of the BDDCS for predicting disposition including drug absorption and flip-flop kinetics lies in the ability to easily obtain values for extent of metabolism that are definitive, reliable and generally consistent from study to study. Alternatively, we have recently shown that in vitro measurements of permeability rate predict BDDCS Class 3 and 4 poor metabolism with 85.6±13.1% accuracy, which is better than utilizing in vitro permeability measurements to predict BDDCS Class 1 and 2 extensive metabolism at 74±7%32 . In the current report, all but three of the drugs found to display flip-flop kinetics were poorly metabolized and thus associated with Classes 3 and 4 of the BDDCS. Zidovudine is a particularly interesting example. It is classified as BDDCS Class 1 for its extensive metabolism in humans, in whom it lacks flip-flop kinetics; however, zidovudine is BDDCS Class 3 in rats in which it is poorly metabolized and displays flip-flop kinetics16,22.
One might expect that flip-flop kinetics would be observed with Class 2 drugs exhibiting poor solubility and/or extensive biliary recycling. However, neither we nor Yáñez et al. 4 were able to identify any BDDCS Class 2 compounds that exhibited flip-flop kinetics in humans except carvedilol 23. Here, in 20 healthy subjects the carvedilol i.v. half-life was 2.4 h, while the terminal half-life was 4.3 h for a 50 mg suspension, and 7.1 h and 6.4 h for a 25mg and 50 mg capsule, respectively 23. It appears here that dissolution of this poorly soluble drug yielded the flip-flop kinetics for the suspension, with disintegration of the capsule (or dissolution of unwetted particles) causing the further terminal half-life increase. As noted in Table 1, an additional BDDCS Class 2 drug, meclofenamic acid, was found to exhibit flip-flop kinetics in horses 24,25. As with the great majority of the drugs in Table 1, meclofenamic acid exhibits a rapid i.v. half-life, 1.4 hr. Meclofenamic acid would not be expected to exhibit flip-flop kinetics in humans since the package insert indicates that following oral dosing in ten subjects the mean elimination half-life was 1.3 hr, ranging from 0.8 to 2.1 hr.
As we had expected more Class 2 poorly soluble drugs to exhibit flip-flop pharmacokinetics, we examined studies for the 60 Class2 drugs listed by Benet et al.15 with the highest dose numbers where oral and iv data are available without finding additional drugs to add to our list. We recognize that this is a very small subset of potential studies to examine. In the BDDCS classification15, 230 Class 2 drugs are dosed orally, with each drug probably studied in 2 to 4 animal species plus humans. Thus, approximately 500 to 900 studies could be investigated outside of those identified as exhibiting flip-flop pharmacokinetics. Similarly 188 Class 3 and 4 orally dosed drugs may be found in the compilation15. Of these 188 drugs, we were able to identify 113 compounds where bioavailability following oral and iv dosing was reported in the Goodman and Gilman Pharmacokinetic Data compilations (7th through 12th editions). One of these drugs, zanamivir, exhibited slower oral absorption than elimination that was not identified as flip-flop pharmacokinetics in the publication34. We had previously identified zanamivir as exhibiting flip-flop pharmacokinetics following inhalation and intranasal administration, but did not identify the oral dosing data, and no oral dosage form of this drug had been approved (or submitted for approval), but we have included zanamivir in our listing in Table 1. Following identification of zanamivir, we carefully reviewed other drugs approved for inhalation or nasal administration. It is possible that albuterol may exhibit flip-flop pharmacokinetics following oral dosing35, but our confidence in the report is not sufficient to list it here. We believe that the other two inhalation Class 3 and 4 drugs listed in Benet et al. 15, ipratropium and terbutaline, do not exhibit flip-flop pharmacokinetics.
During the review process for this paper flip-flop pharmacokinetics was reported for the drug sapropentin dihydrochloride in infants and young children with phenylketonuria26. Sapropentin dihydrochloride is a synthetic preparation of the naturally occurring phenylalanine hydroxylase cofactor tetrahydrobiopterin (BH4). We have listed the drug as BDDCS Class 1 because it is dehydrated by the enzyme PCD/DCoH (pterin-4a-carbinolamine dehydratase/dimerization cofactor of hepatocyte nuclear factor 1α)33. However, the body regenerates BH4 in vivo and the major route of elimination in humans is via the bile. Thus, in fact, because of the regeneration process, sapropentin dihydrochloride might be considered to have BDDCS Class 3 characteristics. However, as noted in Table 2 transporter effects on sapropentin (or BH4) have not been identified.
The current findings describe a further benefit of employing the BDDCS early in drug development. It is proposed that Class 3 and 4 drugs are primarily susceptible to flip-flop kinetics in humans, and the data suggest that such disposition is likely to be most apparent and a more important consideration for drugs with relatively short half-lives. One would like to know whether a drug exhibits flip-flop pharmacokinetics so as to be able to define the rate limiting step in drug elimination, so as to be able to predict potential drug interactions and the potential liability for toxicity-lack of efficacy outcomes.
Given that transit through the small intestine takes only a few hours following gastric emptying36, the presence of a flip-flop phenomenon for immediate-release drugs would intuitively only be possible for drugs with half-lives not exceeding their gastrointestinal transit time. Additionally, classification as BDDCS Class 3 or 4 has implications for both drug-drug interactions as well as pharmacogenetics. In the former case, concomitant administration of another drug that affects the expression or function of a given transporter or enzyme may alter the pharmacokinetics of the drug of interest and potentially result in drug concentrations in either subtherapeutic or toxic ranges. In the latter situation, the natural variation of transporter expression or function also has the potential to affect a drug's disposition. For example, polymorphisms in OCT1 affect the pharmacokinetics of metformin in humans37. Thus, use of the BDDCS during early-stage development of novel drugs will help to identify those drugs that may encounter important transporter effects that require more directed characterization of their disposition.
Although flip-flop pharmacokinetics is a topic found in almost all pharmacokinetics textbooks and a topic of presentation in courses taught both in academia and in short courses taught to industrial scientists, there are very few drugs that inherently exhibit slower oral absorption than elimination (versus many controlled release drug products designed to achieve this phenomenon). This was found by Yáñez et al.4 and in the review here. In fact, of the 698 orally dosed drugs examined by Benet et al.15, only 9 are here documented to exhibit flip-flop pharmacokinetics in humans.
In summary, this report demonstrates that poorly metabolized drugs in BDDCS Classes 3 and 4 are associated with flip-flop kinetics in cases where the drugs have relatively short half-lives. Furthermore, absorptive and efflux transporters may potentially play important roles in the disposition of BDDCS Class 3 and 4 drugs. It might be expected that poorly soluble Class 2 drugs should also exhibit flip-flop kinetics where absorption is limited by dissolution, although only one example was identified. The implications of these findings are that simple in vitro measures of solubility and permeability rate can be used with the BDDCS early in development in order to predict whether or not flip-flop pharmacokinetics might occur (although few drugs would actually be expected to do so) and gut transporters are likely to influence the in vivo pharmacokinetic behavior of a new molecular entity. Thus, BDDCS classification helps to identify compounds early on for which increased characterization of transporter interactions may be necessary for the purpose of predicting potential drug-drug interactions including transporter-enzyme interplay, as well as assessing the potential importance of pharmacogenetic variability in a population.
Acknowledgments
The work presented here was supported in part by NIH grants GM75900 and GM61390.
Abbreviations
- BCRP
breast cancer resistance protein
- BCS
Biopharmaceutics Classification System
- BDDCS
Biopharmaceutics Drug Disposition Classification System
- CNT
tetrahydrobioterin
- BH4
concentrative nucleoside transporter
- ENT
equilibrative nucleoside transporter
- FDA
Food and Drug Administration
- i.v
intravenous
- MATE
multi-antimicrobial extrusion protein
- MCT
monocarboxylate transporter
- MDR
multidrug resistance transporter
- MRP
multidrug resistance protein
- OAT
organic anion transporter
- OATP
organic anion-transporting polypeptide
- OCT
organic cation transporter
- OCTN
organic cation transporters novel
- PEPT
peptide transporter
- PMAT
plasma membrane monamine transporter
- p.o
per os
- UCSF
University of California, San Francisco
References
- 1.Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 2.Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 3.Shugarts S, Benet LZ. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res. 2009;26(9):2039–2054. doi: 10.1007/s11095-009-9924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yáñez JA, Remsberg CM, Sayre CL, Forrest ML, Davies NM. Flip-flop pharmacokinetics--delivering a reversal of disposition: challenges and opportunities during drug development. Ther Deliv. 2011;2(5):643–672. doi: 10.4155/tde.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han N, Yun HY, Kim IW, Oh YJ, Kim YS, Oh JM. Population pharmacogenetic pharmacokinetic modeling for flip-flop phenomenon of enteric-coated mycophenolate sodium in kidney transplant recipients. Eur J Clin Pharmacol. 2014;70(10):1211–1219. doi: 10.1007/s00228-014-1728-4. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher H, Smith RL, Williams RT. The metabolism of thalidomide: the spontaneous hydrolysis of thalidomide in solution. Br J Pharmacol Chemother. 1965;25(2):324–337. doi: 10.1111/j.1476-5381.1965.tb02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philipson A, Stiernstedt G, Ehrnebo M. Comparison of the pharmacokinetics of cephradine and cefazolin in pregnant and non-pregnant women. Clin Pharmacokinet. 1987;12(2):136–144. doi: 10.2165/00003088-198712020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36(2):386–399. doi: 10.1124/dmd.107.019083. [DOI] [PubMed] [Google Scholar]
- 9.Troconiz IF, Tillmann C, Liesenfeld KH, Schafer HG, Stangier J. Population pharmacokinetic analysis of the new oral thrombin inhibitor dabigatran etexilate (BIBR 1048) in patients undergoing primary elective total hip replacement surgery. J Clin Pharmacol. 2007;47(3):371–382. doi: 10.1177/0091270006297228. [DOI] [PubMed] [Google Scholar]
- 10.Edick MJ, Gajjar A, Mahmoud HH, van de Poll ME, Harrison PL, Panetta JC, Rivera GK, Ribeiro RC, Sandlund JT, Boyett JM, Pui CH, Relling MV. Pharmacokinetics and pharmacodynamics of oral etoposide in children with relapsed or refractory acute lymphoblastic leukemia. J Clin Oncol. 2003;21(7):1340–1346. doi: 10.1200/JCO.2003.06.083. [DOI] [PubMed] [Google Scholar]
- 11.He YL, Sadler BM, Sabo R, Balez S, Wang Y, Campestrini J, Laurent A, Ligueros-Saylan M, Howard D. The absolute oral bioavailability and population-based pharmacokinetic modelling of a novel dipeptidylpeptidase-IV inhibitor, vildagliptin, in healthy volunteers. Clin Pharmacokinet. 2007;46(9):787–802. doi: 10.2165/00003088-200746090-00006. [DOI] [PubMed] [Google Scholar]
- 12.He H, Tran P, Yin H, Smith H, Batard Y, Wang L, Einolf H, Gu H, Mangold JB, Fischer V, Howard D. Absorption, metabolism, and excretion of [14C]vildagliptin, a novel dipeptidyl peptidase 4 inhibitor, in humans. Drug Metab Dispos. 2009;37(3):536–544. doi: 10.1124/dmd.108.023010. [DOI] [PubMed] [Google Scholar]
- 13.Hu P, Yin Q, Deckert F, Jiang J, Liu D, Kjems L, Dole WP, He YL. Pharmacokinetics and pharmacodynamics of vildagliptin in healthy Chinese volunteers. J Clin Pharmacol. 2009;49(1):39–49. doi: 10.1177/0091270008325152. [DOI] [PubMed] [Google Scholar]
- 14.He H, Tran P, Yin H, Smith H, Flood D, Kramp R, Filipeck R, Fischer V, Howard D. Disposition of vildagliptin, a novel dipeptidyl peptidase 4 inhibitor, in rats and dogs. Drug Metab Dispos. 2009;37(3):545–554. doi: 10.1124/dmd.108.023002. [DOI] [PubMed] [Google Scholar]
- 15.Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13(4):519–547. doi: 10.1208/s12248-011-9290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melvin GC, Ellison SR, Monk CM, Bates TR. Existence of a flip-flop kinetic model for zidovudine (AZT) after oral administration. Res Commun Chem Pathol Pharmacol. 1990;70(2):193–204. [PubMed] [Google Scholar]
- 17.Mays DC, Dixon KF, Balboa A, Pawluk LJ, Bauer MR, Nawoot S, Gerber N. A nonprimate animal model applicable to zidovudine pharmacokinetics in humans: inhibition of glucuronidation and renal excretion of zidovudine by probenecid in rats. J Pharmacol Exp Ther. 1991;259(3):1261–1270. [PubMed] [Google Scholar]
- 18.Patel BA, Chu CK, Boudinot FD. Pharmacokinetics and saturable renal tubular secretion of zidovudine in rats. J Pharm Sci. 1989;78(7):530–534. doi: 10.1002/jps.2600780704. [DOI] [PubMed] [Google Scholar]
- 19.Good SS, Durack DT, de Miranda P. Biotransformation in various species and in humans of 3′-azido-3′-deoxythymidine, a potential agent for the treatment of AIDS. Fed Proc. 1986;45 [Google Scholar]
- 20.Qian MX, Finco TS, Mehta M, Viswanathan CT, Gallo JM. Pharmacokinetic evaluation of drug interactions with zidovudine. I: Probenecid and zidovudine in monkeys. J Pharm Sci. 1991;80(11):1007–1011. doi: 10.1002/jps.2600801102. [DOI] [PubMed] [Google Scholar]
- 21.Blum MR, Liao SH, Good SS, de Miranda P. Pharmacokinetics and bioavailability of zidovudine in humans. Am J Med. 1988;85(2A):189–194. [PubMed] [Google Scholar]
- 22.Klecker RW, Jr, Collins JM, Yarchoan R, Thomas R, Jenkins JF, Broder S, Myers CE. Plasma and cerebrospinal fluid pharmacokinetics of 3′-azido-3′-deoxythymidine: a novel pyrimidine analog with potential application for the treatment of patients with AIDS and related diseases. Clin Pharmacol Ther. 1987;41(4):407–412. doi: 10.1038/clpt.1987.49. [DOI] [PubMed] [Google Scholar]
- 23.von Möllendorff E, Reiff K, Neugebauer G. Pharmacokinetics and bioavailability of carvedilol, a vasodilating beta-blocker. Eur J Clin Pharmacol. 1987;33(5):511–513. doi: 10.1007/BF00544245. [DOI] [PubMed] [Google Scholar]
- 24.Johansson IM, Kallings P, Hammarlund-Udenaes M. Studies of meclofenamic acid and two metabolites in horses--pharmacokinetics and effects on exercise tolerance. J Vet Pharmacol Ther. 1991;14(3):235–242. doi: 10.1111/j.1365-2885.1991.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 25.Snow DH, Baxter P, Whiting B. The pharmacokinetics of meclofenamic acid in the horse. J Vet Pharmacol Ther. 1981;4(2):147–156. doi: 10.1111/j.1365-2885.1981.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 26.Qi Y, Mould DR, Zhou H, Merilainen M, Musson DG. A prospective population pharmacokinetic analysis of sapropterin dihydrochloride in infants and young children with phenylketonuria. Clin Pharmacokinet. 2015;54(2):195–207. doi: 10.1007/s40262-014-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estudante M, Morais JG, Soveral G, Benet LZ. Intestinal drug transporters: an overview. Adv Drug Deliv Rev. 2013;65(10):1340–1356. doi: 10.1016/j.addr.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 28.Morrissey KM, Wen CC, Johns SJ, Zhang L, Huang SM, Giacomini KM. The UCSF-FDA TransPortal: a public drug transporter database. Clin Pharmacol Ther. 2012;92(5):545–546. doi: 10.1038/clpt.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Food and Drug Administration. Guidance for Industry: Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. www.fda.gov/cder/guidance/index.htm, part of U.S. Food and Drug Administration Center for Drug Evaluation and Research. http://www.fda.gov/Cder/
- 30.Benet LZ, Larregieu CA. The FDA should eliminate the ambiguities in the current BCS biowaiver guidance and make public the drugs for which BCS biowaivers have been granted. Clin Pharmacol Ther. 2010;88(3):405–407. doi: 10.1038/clpt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen M, Yu L. The use of drug metabolism for prediction of intestinal permeability. Mol Pharmaceutics. 2009;6(1):74–81. doi: 10.1021/mp8001864. [DOI] [PubMed] [Google Scholar]
- 32.Hosey CM, Benet LZ. Predicting the extent of metabolism using in vitro permeability rate measurements and in silico permeability rate predictions Mol Pharmaceutics Epub ahead of print. 2015 doi: 10.1021/mp500783g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 34.Cass LM, Efthymiopoulos C, Bye A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin Pharmacokinet. 1999;36(Suppl 1):1–11. doi: 10.2165/00003088-199936001-00001. [DOI] [PubMed] [Google Scholar]
- 35.Boulton DW, Fawcett JP. Enantioselective disposition of salbutamol in man following oral and intravenous administration. Br J Clin Pharmacol. 1996;41(1):35–40. doi: 10.1111/j.1365-2125.1996.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 36.Benmair Y, Fischel B, Frei EH, Gilat T. Evaluation of a magnetic method for the measurement of small intestinal transit time. Am J Gastroenterol. 1977;68(5):470–475. [PubMed] [Google Scholar]
- 37.Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, Sheardown SA, Yue L, Burchard EG, Brett CM, Giacomini KM. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83(2):273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saivin S, Hulot T, Chabac S, Potgieter A, Durbin P, Houin G. Clinical pharmacokinetics of acamprosate. Clin Pharmacokinet. 1998;35(5):331–345. doi: 10.2165/00003088-199835050-00001. [DOI] [PubMed] [Google Scholar]
- 39.Zornoza T, Cano-Cebrián MJ, Hipólito L, Granero L, Polache A. Evidence of a flip-flop phenomenon in acamprosate pharmacokinetics: an in vivo study in rats. Biopharm Drug Dispos. 2006;27(7):305–311. doi: 10.1002/bdd.513. [DOI] [PubMed] [Google Scholar]
- 40.Paintaud G, Alván G, Dahl ML, Grahnén A, Sjövall J, Svensson JO. Nonlinearity of amoxicillin absorption kinetics in human. Eur J Clin Pharmacol. 1992;43(3):283–288. doi: 10.1007/BF02333024. [DOI] [PubMed] [Google Scholar]
- 41.Tanigawara Y, Yamaoka K, Nakagawa T, Uno T. Moment analysis for the separation of mean in vivo disintegration, dissolution, absorption, and disposition time of ampicillin products. J Pharm Sci. 1982;71(10):1129–1133. doi: 10.1002/jps.2600711013. [DOI] [PubMed] [Google Scholar]
- 42.Franke G, Schneider T, Siegmund W, Scherber A. Bioavailability studies of calcium dobesilate--a case of flip-flop kinetics. Pharmazie. 1985;40(8):562–563. [PubMed] [Google Scholar]
- 43.Walsh JS, Patanella JE, Unger SE, Brouwer KR, Miwa GT. The metabolism and excretion of carbovir, a carbocyclic nucleoside, in the rat. Drug Metab Dispos. 1990;18(6):1084–1091. [PubMed] [Google Scholar]
- 44.Yeom YH, Remmel RP, Huang SH, Hua M, Vince R, Zimmerman CL. Pharmacokinetics and bioavailability of carbovir, a carbocyclic nucleoside active against human immunodeficiency virus, in rats. Antimicrob Agents Chemother. 1989;33(2):171–175. doi: 10.1128/aac.33.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz-Carretero P, Nacher A, Merino-Sanjuan M, Casabo VG. Pharmacokinetics and absolute bioavailability of oral cefuroxime axetil in the rat. Int J Pharm. 2000;202(1-2):89–96. doi: 10.1016/s0378-5173(00)00420-8. [DOI] [PubMed] [Google Scholar]
- 46.Padoin C, Tod M, Perret G, Petitjean O. Analysis of the pharmacokinetic interaction between cephalexin and quinapril by a nonlinear mixed-effect model. Antimicrob Agents Chemother. 1998;42(6):1463–1469. doi: 10.1128/aac.42.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C. Some pharmacokinetic aspects of the lipophilic terfenadine and zwitterionic fexofenadine in humans. Drugs R D. 2007;8(5):301–314. doi: 10.2165/00126839-200708050-00004. [DOI] [PubMed] [Google Scholar]
- 48.Abd El-Aty AM, Goudah A, Abo El-Sooud K, El-Zorba HY, Shimoda M, Zhou HH. Pharmacokinetics and bioavailability of florfenicol following intravenous, intramuscular and oral administrations in rabbits. Vet Res Commun. 2004;28(6):515–524. doi: 10.1023/b:verc.0000040241.06642.49. [DOI] [PubMed] [Google Scholar]
- 49.Fredrick MJ, Pound DC, Hall SD, Brater DC. Furosemide absorption in patients with cirrhosis. Clin Pharmacol Ther. 1991;49(3):241–247. doi: 10.1038/clpt.1991.23. [DOI] [PubMed] [Google Scholar]
- 50.Lin CC, Luu T, Lourenco D, Yeh LT, Lau JY. Absorption, pharmacokinetics and excretion of levovirin in rats, dogs and cynomolgus monkeys. J Antimicrob Chemother. 2003;51(1):93–99. doi: 10.1093/jac/dkg046. [DOI] [PubMed] [Google Scholar]
- 51.Pentikäinen PJ, Neuvonen PJ, Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. 1979;16(3):195–202. doi: 10.1007/BF00562061. [DOI] [PubMed] [Google Scholar]
- 52.Sambol NC, Chiang J, Lin ET, Goodman AM, Liu CY, Benet LZ, Cogan MG. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol. 1995;35(11):1094–1102. doi: 10.1002/j.1552-4604.1995.tb04033.x. [DOI] [PubMed] [Google Scholar]
- 53.Neale MG, Brown K, Foulds RA, Lal S, Morris DA, Thomas D. The pharmacokinetics of nedocromil sodium, a new drug for the treatment of reversible obstructive airways disease, in human volunteers and patients with reversible obstructive airways disease. Br J Clin Pharmacol. 1987;24(4):493–501. doi: 10.1111/j.1365-2125.1987.tb03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nobutoshi W, Tomoo F, Kazumasa A, Nobuyoshi K. A flip-flop model for nitrofurantoin disposition in the rabbit following oral administration. International Journal of Pharmaceutics. 1984;21(1):85–98. [Google Scholar]
- 55.Singhvi SM, Pan HY, Morrison RA, Willard DA. Disposition of pravastatin sodium, a tissue-selective HMG-CoA reductase inhibitor, in healthy subjects. Br J Clin Pharmacol. 1990;29(2):239–243. doi: 10.1111/j.1365-2125.1990.tb03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin BS, Kim CH, Jun YS, Yoon CH, Rho JI, Lee KC, Han HS, Yoo SD. Oral absorption and pharmacokinetics of rebamipide and rebamipide lysinate in rats. Drug Dev Ind Pharm. 2004;30(8):869–876. doi: 10.1081/ddc-200034577. [DOI] [PubMed] [Google Scholar]
- 57.Bastain W, Boyce MJ, Stafford LE, Morton PB, Clarke DA, Marlow HF. Pharmacokinetics of xamoterol after intravenous and oral administration to volunteers. Eur J Clin Pharmacol. 1988;34(5):469–473. doi: 10.1007/BF01046704. [DOI] [PubMed] [Google Scholar]
- 58.Luckner P, Brandsch M. Interaction of 31 beta-lactam antibiotics with the H+/peptide symporter PEPT2: analysis of affinity constants and comparison with PEPT1. Eur J Pharm Biopharm. 2005;59(1):17–24. doi: 10.1016/j.ejpb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Uchida Y, Kamiie J, Ohtsuki S, Terasaki T. Multichannel liquid chromatography-tandem mass spectrometry cocktail method for comprehensive substrate characterization of multidrug resistance-associated protein 4 transporter. Pharm Res. 2007;24(12):2281–2296. doi: 10.1007/s11095-007-9453-7. [DOI] [PubMed] [Google Scholar]
- 60.Mahony WB, Domin BA, Daluge SM, Zimmerman TP. Membrane permeation characteristics of abacavir in human erythrocytes and human T-lymphoblastoid CD4+ CEM cells: comparison with (-)-carbovir. Biochem Pharmacol. 2004;68(9):1797–1805. doi: 10.1016/j.bcp.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 61.Bakos E, Evers R, Sinkó E, Váradi A, Borst P, Sarkadi B. Interactions of the human multidrug resistance proteins MRP1 and MRP2 with organic anions. Mol Pharmacol. 2000;57(4):760–768. doi: 10.1124/mol.57.4.760. [DOI] [PubMed] [Google Scholar]
- 62.Hasegawa M, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Multidrug resistance-associated protein 4 is involved in the urinary excretion of hydrochlorothiazide and furosemide. J Am Soc Nephrol. 2007;18(1):37–45. doi: 10.1681/ASN.2005090966. [DOI] [PubMed] [Google Scholar]
- 63.Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35(10):1956–1962. doi: 10.1124/dmd.107.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamai I, Takanaga H, Maeda H, Sai Y, Ogihara T, Higashida H, Tsuji A. Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochem Biophys Res Commun. 1995;214(2):482–489. doi: 10.1006/bbrc.1995.2312. [DOI] [PubMed] [Google Scholar]
- 65.Tokui T, Nakai D, Nakagomi R, Yawo H, Abe T, Sugiyama Y. Pravastatin, an HMG-CoA reductase inhibitor, is transported by rat organic anion transporting polypeptide, oatp2. Pharm Res. 1999;16(6):904–908. doi: 10.1023/a:1018838405987. [DOI] [PubMed] [Google Scholar]
- 66.Yamazaki M, Akiyama S, Ni'inuma K, Nishigaki R, Sugiyama Y. Biliary excretion of pravastatin in rats: contribution of the excretion pathway mediated by canalicular multispecific organic anion transporter. Drug Metab Dispos. 1997;25(10):1123–1129. [PubMed] [Google Scholar]
- 67.Abla N, Chinn LW, Nakamura T, Liu L, Huang CC, Johns SJ, Kawamoto M, Stryke D, Taylor TR, Ferrin TE, Giacomini KM, Kroetz DL. The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. J Pharmacol Exp Ther. 2008;325(3):859–868. doi: 10.1124/jpet.108.136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Georges B, Galland S, Rigault C, Le Borgne F, Demarquoy J. Beneficial effects of L-carnitine in myoblastic C2C12 cells. Interaction with zidovudine. Biochem Pharmacol. 2003;65(9):1483–1488. doi: 10.1016/s0006-2952(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 69.Jorajuria S, Dereuddre-Bosquet N, Becher F, Martin S, Porcheray F, Garrigues A, Mabondzo A, Benech H, Grassi J, Orlowski S, Dormont D, Clayette P. ATP binding cassette multidrug transporters limit the anti-HIV activity of zidovudine and indinavir in infected human macrophages. Antivir Ther. 2004;9(4):519–528. [PubMed] [Google Scholar]
- 70.Pan G, Giri N, Elmquist WF. Abcg2/Bcrp1 mediates the polarized transport of antiretroviral nucleosides abacavir and zidovudine. Drug Metab Dispos. 2007;35(7):1165–1173. doi: 10.1124/dmd.106.014274. [DOI] [PubMed] [Google Scholar]
- 71.Ritzel MW, Yao SY, Huang MY, Elliott JF, Cass CE, Young JD. Molecular cloning and functional expression of cDNAs encoding a human Na+-nucleoside cotransporter (hCNT1) Am J Physiol. 1997;272(2 Pt 1):C707–714. doi: 10.1152/ajpcell.1997.272.2.C707. [DOI] [PubMed] [Google Scholar]
- 72.Yao SY, Ng AM, Sundaram M, Cass CE, Baldwin SA, Young JD. Transport of antiviral 3′-deoxy-nucleoside drugs by recombinant human and rat equilibrative, nitrobenzylthioinosine (NBMPR)-insensitive (ENT2) nucleoside transporter proteins produced in Xenopus oocytes. Mol Membr Biol. 2001;18(2):161–167. doi: 10.1080/09687680110048318. [DOI] [PubMed] [Google Scholar]