Abstract
Background
Effects of cannabis, the most commonly encountered non-alcohol drug in driving under the influence cases, are heavily debated. We aimed to determine how blood Δ9-tetrahydrocannabinol (THC) concentrations relate to driving impairment, with and without alcohol.
Methods
Current occasional (≥1×/last 3months, ≤3days/week) cannabis smokers drank placebo or low-dose alcohol, and inhaled 500mg placebo, low (2.9%)-THC, or high (6.7%)-THC vaporized cannabis over 10min ad libitum in separate sessions (within-subject design, 6 conditions). Participants drove (National Advanced Driving Simulator, University of Iowa) simulated drives (~0.8h duration). Blood, oral fluid (OF) and breath alcohol samples were collected before (0.17h, 0.42h) and after (1.4h, 2.3h) driving that occurred 0.5–1.3h after inhalation. We evaluated standard deviations of lateral position (lane weave, SDLP) and steering angle, lane departures/min, and maximum lateral acceleration.
Results
In N=18 completers (13 men, ages 21–37years), cannabis and alcohol increased SDLP. Blood THC concentrations of 8.2 and 13.1μg/L during driving increased SDLP similar to 0.05 and 0.08g/210L breath alcohol concentrations, the most common legal alcohol limits. Cannabis-alcohol SDLP effects were additive rather than synergistic, with 5μg/L THC+0.05g/210L alcohol showing similar SDLP to 0.08g/210L alcohol alone. Only alcohol increased lateral acceleration and the less-sensitive lane departures/min parameters. OF effectively documented cannabis exposure, although with greater THC concentration variability than paired blood samples.
Conclusions
SDLP was a sensitive cannabis-related lateral control impairment measure. During-drive blood THC ≥8.2μg/L increased SDLP similar to notably-impairing alcohol concentrations. Despite OF’s screening value, OF variability poses challenges in concentration-based effects interpretation. KEYWORDS: Cannabis, Alcohol, Driving, Lateral Control, THC, Oral Fluid
Graphical Abstract
1. INTRODUCTION
Reducing drugged driving is a U.S. and worldwide priority (ONDCP, 2013). Cannabis is the most frequently detected illicit drug in drivers (Berning et al., 2015; Lacey et al., 2009; Legrand et al., 2013; Pilkinton et al., 2013); 12.6% of weekend nighttime drivers were positive for Δ9-tetrahydrocannabinol (THC, primary psychoactive phytocannabinoid), in 2013–2014, a 48% increase since 2007 (Berning et al., 2015). Although blood THC is associated with increased crash risk and driver culpability (Asbridge et al., 2012; Drummer et al., 2004; Gjerde et al., 2011; Laumon et al., 2005; Li et al., 2012), cannabis effects on driving remain heavily debated. Road tracking and ability to remain within the lane are crucial driving skills. Lane weaving, an observable effect of drug-impaired driving, is a common measure for assessing driving performance. Standard deviation of lateral position (SDLP) is a sensitive vehicular control indicator, often employed in drugged driving research (Anderson et al., 2010; Lenné et al., 2010; Ramaekers et al., 2006a; Verster et al., 2006). In previous studies, cannabis increased SDLP and straddling lanes, but results were assessed by dose rather than blood THC concentrations (Ramaekers et al., 2000; Robbe, 1998; Downey et al., 2013).
To date, 23 states and the District of Columbia (DC) approved medical marijuana; 4 states and DC legalized recreational cannabis for adults (ProCon.org, 2014). Cannabis legalization is a crucial road safety issue. Since legalizing medical marijuana (2000), Colorado observed increased driving under the influence of cannabis (DUIC) cases (Urfer et al., 2014), and fatal motor vehicle crashes with cannabis-positive drivers; whereas no significant change was observed in 34 states without legalized medical marijuana (Salomonsen-Sautel et al., 2014). Establishing evidence-based per se laws for DUIC remains challenging, with varying laws across the US (Armentano, 2013; Grotenhermen et al., 2007; Lacey et al., 2010). Many are concerned that implementing concentration-based cannabis-driving legislation will unfairly target individuals not acutely intoxicated, because residual THC can be detected in blood for up to a month of sustained abstinence in chronic frequent smokers (Bergamaschi et al., 2013). Appropriate blood THC concentrations that universally reflect driving impairment remain elusive. Determining blood THC concentrations associated with lateral control impairment in occasional users would benefit forensic interpretation.
There is interest in linking driving impairment with oral fluid (OF) THC concentrations. OF is easy to collect, non-invasive, and associated with recent cannabis intake (Bosker and Huestis, 2009; Drummer, 2006; Wille et al., 2014). OF-based DUIC legislation exists in some jurisdictions (Drummer et al., 2007; Huestis et al., 2011; Van der Linden et al., 2012); however, limited simultaneous driving and OF concentration data preclude direct association with impairment.
Alcohol is the most common drug identified in drivers (Berning et al., 2015; Legrand et al., 2013). Cannabis and alcohol, frequently detected together (Legrand et al., 2013), produced greater impairing effects together than either separately (Robbe, 1998; Ronen et al., 2010), but it is unclear whether effects are additive or synergistic.
This is the first in a series of manuscripts evaluating cannabis’ effects, with and without concurrent alcohol, on driving. We present here effects, relative to THC concentrations, on drivers’ lateral control. We hypothesized cannabis and alcohol would each impair lateral control, with synergistic effects when combined.
2. METHODS
2.1 Participants
Healthy adults provided written informed consent for this Institutional Review Board-approved study. Inclusion criteria were ages 21–55years; self-reported cannabis consumption ≥1×/3months but ≤3days/week over the past 3months (Cannabis Use Disorders Identification Test [CUDIT]; Adamson and Sellman, 2003); self-reported “light” or “moderate” alcohol consumption according to a Quantity-Frequency-Variability (QFV) scale (Sobell and Sobell, 2003); or, if “heavy”, not more than 3–4 servings on a typical drinking occasion; licensed driver for ≥2years with currently valid unrestricted license; and self-reported driving ≥1300miles in the past year. Exclusion criteria included past or current clinically significant medical illness; history of clinically significant adverse event associated with cannabis or alcohol intoxication or motion sickness; ≥450mL blood donation in 2weeks preceding drug administration; pregnant/nursing; interest in drug abuse treatment within past 60days; currently taking drugs contraindicated with cannabis or alcohol or known to impact driving; requirements for nonstandard driving equipment; and prior participation in a similar driving simulator study.
2.2 Study Design/Procedures
Participants entered the clinical research unit 10–16h prior to drug administration to preclude acute intoxication. Participants drank 90% grain alcohol in fruit juice to reach approximately 0.065% peak breath alcohol concentration [BrAC], or placebo (juice with alcohol-swabbed rim and topped with 1mL alcohol to mimic alcohol taste and odor) ad libitum over 10min. After drinking, they inhaled 500mg placebo (0.008±0.002% THC), low (2.9±0.14%)-, or high (6.7±0.05%)-THC vaporized (Volcano® Medic, Storz & Bickel, Tuttlingen, Germany) cannabis (NIDA Chemistry and Physiological Systems Research Branch) ad libitum over 10min. Participants received all six alcohol/cannabis combinations in randomized order, with sessions separated by ≥1week.
Simulated drives occurred 0.5–1.3h after start of cannabis dosing. Blood collection times were 0.17, 0.42, 1.4, and 2.3h post-inhalation. Blood was collected via indwelling peripheral venous catheter into grey-top (potassium oxalate/sodium fluoride) Vacutainer® tubes (Becton, Dickinson and Company, Franklin Lakes, NJ), and stored on ice ≤2h. Specimens were stored in 3.6mL Nunc® cryotubes (Thomas Scientific, Swedesboro, NJ) at −20°C, and analyzed within 3months, based on known cannabinoid stability (Scheidweiler et al., 2013). OF was collected simultaneously with blood (except 0.42h), with the Quantisal™ collection device (Immunalysis, Pomona, CA). BrAC was measured via Alco-Sensor® IV (Intoximeters, St. Louis, MO) at the same times as blood, reporting alcohol in g/210L breath (limit of quantification [LOQ] 0.006g/210L), equivalent to approximate blood alcohol concentration (BAC).
2.3 National Advanced Driving Simulator
Driving simulations were conducted in NADS-1, the high-fidelity, full-motion simulator at the National Advanced Driving Simulator (NADS), Iowa City, IA (Figure 1). A 1996 Malibu sedan is mounted in a 7.3m-diameter dome with a motion system providing 400m2 acceleration space, ±330° rotation, and high-frequency motion (Lee et al., 2010). Drivers experience acceleration, braking, steering cues, road conditions (e.g., gravel), and realistic sounds (e.g., wind, motor). NADS-1 produces a complete record of vehicle state (e.g., lane position) and driver inputs (e.g., steering wheel position).
Figure 1.
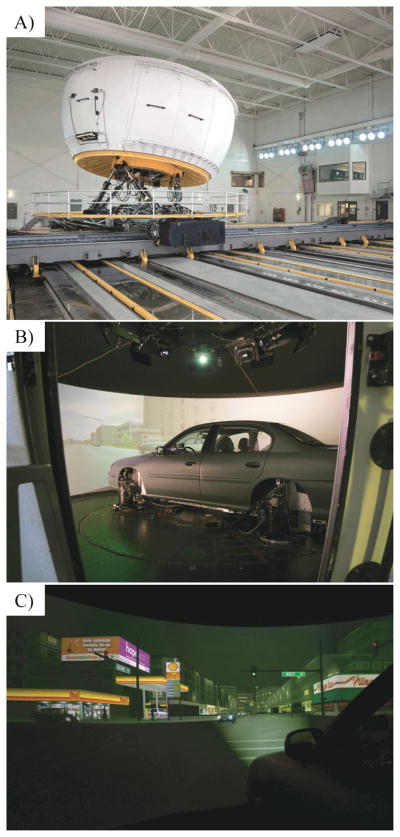
The National Advanced Driving Simulator: A) exterior, dome mounted in room; B) dome interior with car mounted inside; C) view of night-drive simulation.
2.4 Drives
The 45min drive challenged multiple driving skills affected by cannabis, including SDLP. Each drive had urban, interstate and rural nighttime segments. The urban segment involved a two-lane city roadway with posted speed limits 25–45miles/h (40–72km/h) and signal-controlled and uncontrolled intersections; interstate, a four-lane divided expressway with posted 70miles/h (113km/h) speed limit; rural, two-lane undivided road with curves, a gravel portion, and a 10min timed straightaway. Because each participant drove six times, three scenarios with varied event orders were utilized to minimize practice effects. Each scenario contained the same number of curves and turns, in varied order and position. Other traffic, pedestrians, and potential hazards were present throughout the drive. Hundreds of performance variables were monitored; the lateral control (necessary for road tracking, lane keeping) subset is presented here.
2.5 Specimen Analysis
Blood THC concentration was quantified by a previously-published method (Schwope et al., 2011). Briefly, 0.5mL blood was protein-precipitated with ice-cold acetonitrile, and supernatants diluted and solid-phase extracted. THC’s linear range was 1–100μg/L. Inter-assay (n=30) analytical bias and imprecision were ≤3.7% and ≤8.7%, respectively. OF THC quantification is described in detail elsewhere (Hartman et al., 2015a). We utilized a published validated method (Milman et al., 2010), modified by adding 0.4mL hexane to solid-phase extraction columns before the initial elution solvent. THC’s linear range was 0.5–50μg/L. Inter- and intra-assay imprecision were ≤6.6%; analytical bias, ≤14.4% (n=21). If concentrations exceeded the upper LOQ, OF specimens were diluted with drug-free QuantisalTM buffer to achieve concentrations within the method’s linear range.
2.6 Data Analysis
Blood THC concentrations during drives were modeled via individual power-curve regression from pre-drive (0.17 and 0.42h) and post-drive (1.4 and 2.3h) specimens. BrAC concentrations during drives were modeled by linear interpolation, as alcohol was in the post-absorptive phase, during which its pharmacokinetics are linear (Jones and Andersson, 2003). Driving data were analyzed by participants’ modeled concentrations during drives.
Data were reviewed to determine which events were suitable for analysis. Events for which dependent measures were not meaningful (e.g., SDLP during turn), were excluded. For each dependent measure, events with similar means were grouped for analytic purposes. Data were analyzed using SAS v9.4 General Linear Model (GLM) Select function to identify appropriate regression models. This procedure was selected due to its ability to accommodate continuous dependent measures and combinations of continuous and categorical independent measures (Neerchal et al., 2014). The stepwise selection method was chosen; the Schwarz Bayesian Information Criterion determined model entry/removal (Schwarz, 1978). Effect hierarchy was not enforced on model parameters. Available model parameters were blood THC, BrAC, interaction term THC*BrAC, speed limit, inverse curvature, and subject. Dependent measures of drivers’ lateral control included SDLP, standard deviation of steering wheel angle, lane departures/min (“lane departure” defined as edge of vehicle crossing a lane boundary; per minute allowed for normalization across drive events), and maximum lateral acceleration in events without sharp turns. For final regression models, the analysis of variance for the model fit is presented, along with estimates, t-values, and p-values for model parameters.
3. RESULTS
3.1 Participants
Nineteen healthy adults (13 men, ages 21–37 years, 74% white) participated (Table 1). Most consumed cannabis ≥2×/month (but ≤3days/week), and reported last intake within a week prior to admission. Participants self-reported driving 6–23 years, and all reported driving ≥1×/week. Data review revealed one participant (#12) was consistently an extreme outlier in almost all measures and dosing conditions, including placebo cannabis/placebo alcohol. Driving videos indicated markedly erratic and abnormal driving behavior, inattention, and distractibility in all conditions, suggesting invalid data. These data were excluded from all driving analyses, yielding N=18 completing drivers.
Table 1.
Self-reported demographic characteristics, recent cannabis and alcohol consumption and driving history of 19 healthy adult occasional cannabis smokers
| Participant | Sex | Age (years) |
Race and ethnicity |
BMI (kg/m2) |
Alcohol intake frequency |
Typical drinks per occasion |
Cannabis intake frequency |
Hours “stoned” on typical cannabis occasiona |
Time since last cannabis consumed (days) |
Amount last consumedb (joint or joint equivalent) |
Years of driving experience |
Driving frequency |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 23.7 | W | 24.3 | 2–3×/wk | 2–4 | 2–4×/m | 1–2 | 1 | 1 | 7 | ≥1×/d |
| 2 | F | 28.4 | AA | 23.8 | ≥4×/wk | 2–4 | 2–4×/m | 3–4 | 14 | 1 | --c | --c |
| 3 | M | 21.9 | W | 24.7 | 2–3×/wk | 5–6 | 2–4×/m | 1–2 | 6 | 1 | 7 | ≥1×/d |
| 4 | M | 37.8 | W | 26.1 | 2–3×/wk | 2–4 | 2–3×/wk | 1–2 | 3 | 2.5 | 23 | ≥1×/d |
| 5 | M | 26.6 | W | 21.6 | ≤1×/m | 2–4 | ≤1×/m | 1–2 | 11 | 3.5 | 12 | ≥1×/d |
| 6 | F | 26.3 | W | 20.0 | 2–3×/wk | 2–4 | 2–3×/wk | 3–4 | 1 | 0.25 | 12 | ≥1×/d |
| 7 | M | 25.8 | W | 40.6 | 2–4×/m | 2–4 | 2–3×/wk | 1–2 | 0.3 | 0.5 | 11 | ≥1×/d |
| 8 | M | 26.1 | H | 31.5 | 2–4×/m | 1–2 | 2–3×/wk | 1–2 | 3 | 1 | 10 | ≥1×/d |
| 9 | M | 23.2 | W | 19.5 | 2–3×/wk | 2–4 | 2–3×/wk | 3–4 | 2 | 1 | 7 | ≥1×/wk |
| 10 | M | 23.1 | W | 23.9 | 2–4×/m | 2–4 | ≤1×/m | 1–2 | 2 | 0.25 | 9 | ≥1×/d |
| 11 | M | 32.3 | O, H | 28.9 | 2–3×/wk | 2–4 | 2–3×/wk | 1–2 | 4 | 1 | 16 | ≥1×/d |
| 12d | F | 23.4 | W | 23.3 | 2–3×/wk | 2–4 | 2–4×/m | 3–4 | 4 | 1 | 8 | ≥1×/wk |
| 13 | F | 30.3 | AA | 24.1 | 2–3×/wk | 2–4 | ≤1×/m | <1 | 120 | 1 | 14 | ≥1×/d |
| 14 | M | 24.6 | W | 23.3 | 2–3×/wk | 2–4 | 2–4×/m | 1–2 | 7 | 0.8 | 8 | ≥1×/wk |
| 15 | M | 21.8 | W | 32.7 | ≤1×/m | 1–2 | 2–4×/m | 1–2 | 7 | 0.13 | 6 | ≥1×/d |
| 16 | F | 21.7 | AA, W | 23.0 | 2–4×/m | 1–2 | 2–3×/wk | 1–2 | 1.1 | 1.5 | 7 | ≥1×/d |
| 17 | M | 28.7 | W | 18.3 | 2–3×/wk | 2–4 | ≤1×/m | 3–4 | 45 | 0.5 | 12 | ≥1×/wk |
| 18 | M | 28.1 | W | 48.3 | 2–4×/m | 2–4 | 2–4×/m | 3–4 | 5 | 1 | 12 | ≥1×/d |
| 19 | F | 22.9 | W | 21.6 | 2–4×/m | 5–6 | 2–3×/wk | 3–4 | 1 | 1 | 6 | ≥1×/d |
| Median (all) | 25.8 | 23.9 | 4.0 | 1.0 | 10 | |||||||
| Mean (all) | 26.1 | 26.3 | 12.5 | 1.0 | 10 | |||||||
| StDev (all) | 4.1 | 7.5 | 27.9 | 0.8 | 4 | |||||||
| Median (N=18) | 25.9 | 24.0 | 3.5 | 1.0 | 10 | |||||||
| Mean (N=18) | 26.3 | 26.5 | 13.0 | 1.1 | 11 | |||||||
| StDev (N=18) | 4.2 | 7.7 | 28.6 | 0.8 | 4 |
Hours “stoned” ‘ wording originates from Cannabis Use Disorders Identification Test, source of self-reported cannabis frequency data
Cannabis amount last consumed is based on empirically-normalized joint consumption, to account for various administration routes and self-reported “sharing” between multiple individuals
Participant did not provide response
Participant excluded from driving analyses due to consistently outlying behavior
Abbreviations: W, White; AA, African American; H, Hispanic or Latino; As, Asian; O, Other; AI, American Indian/Native American; StDev, standard deviation
3.2 Driving
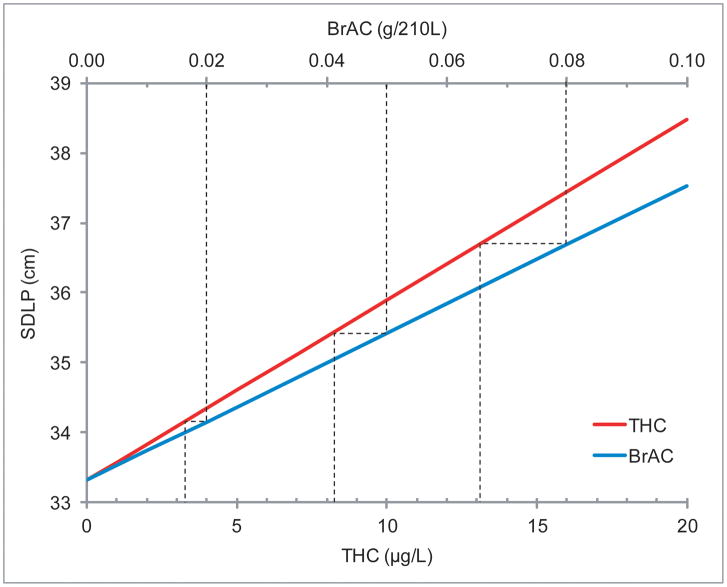
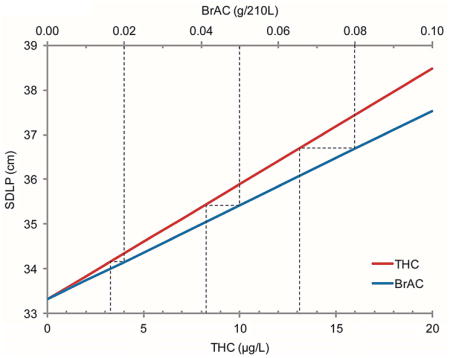
GLM Select model results are depicted in Table 2. THC concentration and BrAC significantly associated with SDLP, but the interaction (THC*BrAC) was not selected into the model. This indicates additive, rather than synergistic, cannabis and alcohol effects. To account for a possible ceiling effect of increasing concentrations, quadratic terms THC2 and BrAC2 were added to the list of potential predictors; neither was included in the resultant model. The model predicts that blood THC and BrAC increased SDLP 0.26 cm per μg/L THC and 0.42 cm per 0.01g/210L BrAC (Table 3), representing 0.8% and 1.3% increases relative to median baseline (drug-free) SDLP per μg/L THC or 0.01g/210L BrAC, respectively. Participants displayed high inter-individual variability in baseline (drug-free) SDLP (Supplemental Figure 11). BrAC concentrations of 0.05% and 0.08%, the most common per se alcohol limits worldwide, were associated with similar SDLP to 8.2 and 13.1μg/L THC concentrations, respectively (Figure 2). Low (1 and 2μg/L) blood THC concentrations were associated with SDLP increases similar to 0.01g/210L BrAC. At 5μg/L THC, a 4.1% increase in SDLP was observed; at 10μg/L, SDLP increased 8.2%. This change was comparable to 0.05g/210L BrAC (6.7% increase) and 0.08g/210L BrAC (11% increase).
Table 2.
General Linear Model (GLM) Select results of effects on lateral control measures in 18 volunteer drivers after controlled vaporized cannabis with or without oral alcohol.
| Parameter | DF | Estimate (b) | t | Standard Error | p-value |
|---|---|---|---|---|---|
| Standard Deviation of Lateral Position (SDLP) | |||||
|
| |||||
| THC | 1 | 0.26 | 3.6 | 0.07 | 0.0004 |
|
| |||||
| BrAC | 1 | 0.42 | 2.9 | 0.15 | 0.0037 |
|
| |||||
| THC*BrAC | |||||
|
| |||||
| Speed Limit | 1 | 0.50 | 19 | 0.03 | <0.0001 |
|
| |||||
| Inverse Curvature | 1 | 464 | 9.5 | 49 | <0.0001 |
|
| |||||
| Intercept | 1 | 17.3 | 8.3 | 2.1 | <0.0001 |
|
| |||||
| Subject | 17 | ||||
|
| |||||
| Model df: | 21 | ||||
| Model F-value | 28.24 | ||||
| Error df: | 1916 | ||||
|
| |||||
| Standard Deviation of Steering Angle (Curvy) | |||||
|
| |||||
| THC | |||||
|
| |||||
| BrAC | |||||
|
| |||||
| THC*BrAC | |||||
|
| |||||
| Speed Limit | 1 | 0.07 | 5.4 | 0.01 | <0.0001 |
|
| |||||
| Inverse Curvature | 1 | −122 | −7.7 | 16 | <0.0001 |
|
| |||||
| Intercept | 1 | 5.2 | 9.0 | 0.6 | <0.0001 |
|
| |||||
| Subject | |||||
|
| |||||
| Model df: | 2 | ||||
| Model F-value | 29.59 | ||||
| Error df: | 427 | ||||
|
| |||||
| Standard Deviation of Steering Angle (Straight) | |||||
|
| |||||
| THC | |||||
|
| |||||
| BrAC | |||||
|
| |||||
| THC*BrAC | |||||
|
| |||||
| Speed Limit | 1 | −0.40 | −17 | 0.02 | <0.0001 |
|
| |||||
| Inverse Curvature | 1 | 1389 | 27 | 51 | <0.0001 |
|
| |||||
| Intercept | 1 | 25 | 21 | 1.2 | <0.0001 |
|
| |||||
| Subject | |||||
|
| |||||
| Model df: | 2 | ||||
| Model F-value | 657.9 | ||||
| Error df: | 1936 | ||||
|
| |||||
| Lane Departures/min | |||||
|
| |||||
| THC | |||||
|
| |||||
| BrAC | 1 | 0.030 | 2.8 | 0.009 | 0.0055 |
|
| |||||
| THC*BrAC | |||||
|
| |||||
| Speed Limit | 1 | 0.010 | 6.8 | 0.001 | <0.0001 |
|
| |||||
| Inverse Curvature | 1 | 10.9 | 5.2 | 2.1 | <0.0001 |
|
| |||||
| Intercept | 1 | 1.4 | 10.3 | 0.14 | <0.0001 |
|
| |||||
| Subject | 17 | ||||
|
| |||||
| Model df: | 20 | ||||
| Model F-value | 19.59 | ||||
| Error df: | 840 | ||||
|
| |||||
| Maximum Lateral Acceleration (Non-Sharp Events) | |||||
|
| |||||
| THC | |||||
|
| |||||
| BrAC | 1 | 0.0023 | 3.5 | 0.0007 | 0.0005 |
|
| |||||
| THC*BrAC | |||||
|
| |||||
| Speed Limit | 1 | 0.0012 | 11.4 | 0.0001 | <0.0001 |
|
| |||||
| Inverse Curvature | |||||
|
| |||||
| Intercept | 1 | 0.091 | 10.0 | 0.0091 | <0.0001 |
|
| |||||
| Subject | 17 | ||||
|
| |||||
| Model df: | 19 | ||||
| Model F-value | 17.37 | ||||
| Error df: | 2026 | ||||
|
| |||||
| Maximum Lateral Acceleration (Sharp Events) | |||||
|
| |||||
| THC | |||||
|
| |||||
| BrAC | |||||
|
| |||||
| THC*BrAC | |||||
|
| |||||
| Speed Limit | |||||
|
| |||||
| Inverse Curvature | 1 | −1.8 | −4.3 | 0.43 | <0.0001 |
|
| |||||
| Intercept | 1 | 0.45 | 17 | 0.027 | <0.0001 |
|
| |||||
| Subject | 17 | ||||
|
| |||||
| Model df: | 18 | ||||
| Model F-value | 8.61 | ||||
| Error df: | 304 | ||||
Driving occurred 0.5h after drinking placebo or active alcohol (calculated to produce approximate peak 0.065% BrAC) and inhaling placebo, 2.9% THC, or 6.7% THC vaporized bulk cannabis (500 mg, Volcano® Medic vaporizer). Estimate represents parameter (coefficient) estimate [effect size scaled to the unit] for each factor (negative b indicates the parameter decreases the effect; positive b indicates the parameter increases the overall effect).
Boldface indicates parameter included in the final GLM Select model. All p-values for final overall analysis of variance of model fits were <0.0001.
Abbreviations: DF, degrees of freedom; THC, blood Δ9-tetrahydrocannabinol concentration; BrAC, breath alcohol concentration
Table 3.
GLM Select model estimates for predicted standard deviation of lateral position (SDLP), lane departures/min, and maximum lateral acceleration associated with specific blood Δ9-tetrahydrocannabinol (THC) concentrations and breath alcohol concentrations (BrAC) during driving
| During-Drive Concentration |
Standard Deviation of Lateral Position (SDLP) |
Lane Departures/min | Maximum Lateral Acceleration (Non-Sharp Events) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| THC (μg/L) |
BrAC (g/210L) |
Median [range] predicted SDLP (cm) |
Difference (cm) |
Percent Increasea (%) |
Median [range] predicted lane departures/min (N) |
Difference (N) |
Percent Increasea (%) |
Median [range] predicted maximum lateral acceleration (m/s2) |
Difference (m/s2) |
Percent Increasea (%) |
| 0 | 0 | 31.4 [24.7–44.8] | -- | -- | 0.38 [0.05–1.95] | -- | -- | 1.17 [0.87–1.54] | -- | -- |
| 1 | 0 | 31.7 [25.0–45.1] | 0.26 | 0.8 | 0.38 [0.05–1.95] | 0 | 0 | 1.17 [0.87–1.54] | 0 | 0 |
| 2 | 0 | 32.0 [25.3–45.4] | 0.52 | 1.6 | 0.38 [0.05–1.95] | 0 | 0 | 1.17 [0.87–1.54] | 0 | 0 |
| 5 | 0 | 32.7 [26.0–46.1] | 1.3 | 4.1 | 0.38 [0.05–1.95] | 0 | 0 | 1.17 [0.87–1.54] | 0 | 0 |
| 7 | 0 | 33.3 [26.5–46.7] | 1.8 | 5.8 | 0.38 [0.05–1.95] | 0 | 0 | 1.17 [0.87–1.54] | 0 | 0 |
| 10 | 0 | 34.0 [27.3–47.4] | 2.6 | 8.2 | 0.38 [0.05–1.95] | 0 | 0 | 1.17 [0.87–1.54] | 0 | 0 |
| 20 | 0 | 36.6 [29.9–50.0] | 5.2 | 16 | 0.38 [0.05–1.95] | 0 | 0 | 1.17 [0.87–1.54] | 0 | 0 |
| 0 | 0.01 | 31.9 [25.2–45.3] | 0.42 | 1.3 | 0.41 [0.08–1.97] | 0.026 | 6.9 | 1.19 [0.90–1.56] | 0.022 | 1.9 |
| 0 | 0.02 | 32.3 [25.6–45.7] | 0.84 | 2.7 | 0.43 [0.11–2.00] | 0.053 | 14 | 1.21 [0.92–1.58] | 0.045 | 3.8 |
| 0 | 0.05 | 33.6 [26.8–47.0] | 2.1 | 6.7 | 0.51 [0.19–2.08] | 0.13 | 35 | 1.28 [0.98–1.65] | 0.11 | 9.5 |
| 0 | 0.08 | 34.8 [28.1–48.2] | 3.4 | 11 | 0.59 [0.26–2.16] | 0.21 | 55 | 1.35 [1.05–1.72] | 0.18 | 15 |
| 0 | 0.10 | 35.7 [29.0–49.1] | 4.2 | 13 | 0.64 [0.32–2.21] | 0.26 | 69 | 1.39 [1.10–1.76] | 0.22 | 19 |
| 2 | 0.05 | 34.1 [27.4–47.5] | 2.6 | 8.4 | 0.51 [0.19–2.08] | 0.13 | 35 | 1.28 [0.98–1.65] | 0.11 | 9.5 |
| 5 | 0.05 | 34.9 [28.1–48.3] | 3.4 | 11 | 0.51 [0.19–2.08] | 0.13 | 35 | 1.28 [0.98–1.65] | 0.11 | 9.5 |
Data generated from 18 healthy occasional cannabis smokers 0.5–1.3h after ingesting placebo or active oral alcohol and inhaling placebo or active vaporized bulk cannabis. Values obtained by assessing general linear model (GLM) Select results of each measure at specific THC concentrations and BrAC. All estimates are for speed 55 miles/h (89 km/h), straight road.
Relative to median baseline (blood THC 0 μg/L, BrAC 0 g/210L) value
Figure 2.
GLM Select modeled standard deviation of lateral position (SDLP) versus blood Δ9-tetrahydrocannabinol (THC) concentration (lower x-axis) and versus breath alcohol concentration (BrAC, upper x-axis). Note x-axis scales are different so slopes cannot be directly compared; dotted lines indicate THC concentrations producing equivalent SDLP to 0.02, 0.05, and 0.08g/210L BrAC.
Natural-log SDLP transformation is common analytical practice due to non-normal distribution. Results obtained from ln(SDLP; Supplemental Tables 1 and 22) were similar to untransformed SDLP; therefore, we report the more straightforward and conservative SDLP results.
BrAC significantly increased lane departures/min and maximum lateral acceleration; these measures were not sensitive to cannabis. Neither THC nor BrAC affected standard deviation of steering wheel angle.
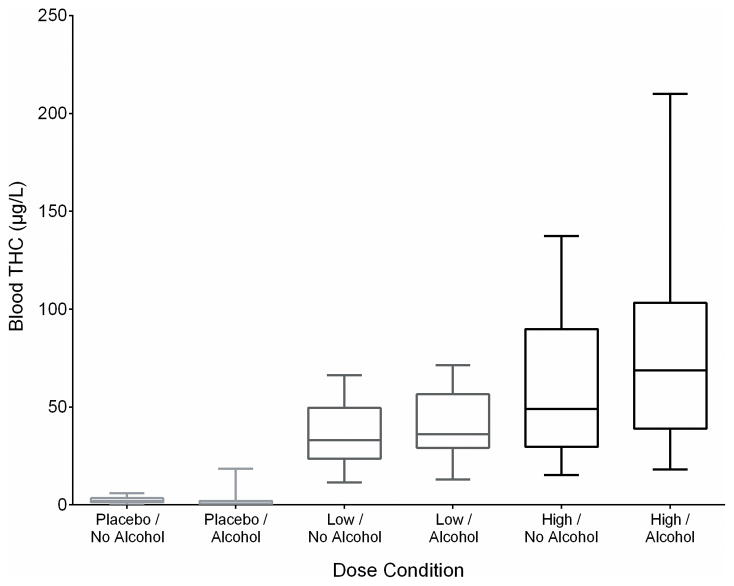
THC concentration-based statistical analysis was utilized because of substantial overlap in achieved THC blood Cmax across the active-THC dose groups (Figure 3): 6 participants achieved higher Cmax after the low than high-THC dose and 4 had low and high Cmax within 20% of one another despite a 2-fold dose difference. This overlap makes statistical analysis by dose group (Table 4) not scientifically meaningful, illustrating the importance of analyzing effects by actual blood THC. THC concentration peaks prior to finishing inhalation (Huestis et al., 1992), and inhalation variability causes THC concentration variability (Azorlosa et al., 1995, Hartman et al., 2015b). Table 5 presents mean (SD) results by THC and alcohol condition.
Figure 3.
Box plot of maximum blood Δ9-tetrahydrocannabinol (THC) concentration by administered cannabis (placebo, 0.008% THC; low, 2.9% THC; high, 6.7% THC) and alcohol (placebo, active) doses for 18 participants.
Table 4.
Participant distribution into 3 (placebo, low, high cannabis) × 2 (placebo, alcohol) repeated measures design and results of repeated measures linear mixed model, accounting for achieved Δ9-tetrahydrocannabinol (THC) blood maximum concentration. Due to inhaled dose self-titration and interindividual variability, some participants are represented multiple times in certain cells (e.g., THC <8.6 μg/L/placebo alcohol) and not at all in others.
| Structural problem with analysis by condition | Placebo Cannabis | THC Cmax <8.6 μg/L (median) “Low” | THC Cmax >8.6 μg/L (median) “High” | ||
|---|---|---|---|---|---|
| Placebo Alcohol | 18 data points | 17 data points | 19 data points | ||
| 0 repeating points | 6 repeating points (same participant falls into this category for low and high administered doses) | 7 repeating points (same participant falls under this category for low and high administered doses) | |||
| 18 unique cases | |||||
| 11 unique cases | 12 unique cases | ||||
|
| |||||
| Active Alcohol | 18 data points | 19 data points | 17 data points | ||
| 0 repeating points | 1 repeating point (same participant falls into this category for low and high administered doses) | 1 repeating point (same participant falls into this category for low and high administered doses) | |||
| 18 unique cases | |||||
| 18 unique cases | 16 unique cases | ||||
| Results of analysis by conditiona | Standard Deviation of Lateral Position (SDLP) | Lane Departures/min | Maximum Lateral Acceleration (Non-Sharp Events) |
|---|---|---|---|
| pTHC group (P,L,H) | 0.2801 | 0.4537 | 0.2543 |
| palcohol (P,A) | 0.0673 | 0.1286 | 0.0918 |
| pTHC-alcohol | 0.2398 | 0.1245 | 0.4949 |
| pdrive event | <0.0001 | <0.0001 | <0.0001 |
Due to unequal cells and resultant invalid statistical assumptions for within-subjects (repeated measures) design and “missing” or duplicate data, linear mixed model analysis (for which resultant p-values are displayed) has low power and uncertain interpretation.
Table 5.
Mean (standard deviation) results for standard deviation of lateral control (SDLP), lane departures/min, and maximum lateral acceleration during driving, grouped by achieved THC/alcohol concentration conditions and by administered THC and alcohol dose conditions.
| Achieved THC, Alcohol Conditions (THC Grouped by Median Blood Concentration) |
Standard Deviation of Lateral Position (SDLP) |
Lane Departures/min | Maximum Lateral Acceleration (Non-Sharp Events) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THC Group | Alcohol Dose |
N | Mean (cm) |
St Dev (cm) |
Difference (cm) |
Percent Increasea (%) |
Mean (N) |
St Dev (N) |
Difference (N) |
Percent Increasea (%) |
Mean (m/s2) |
St Dev (m/s2) |
Difference (m/s2) |
Percent Increasea (%) |
| Placebo | Placebo | 18 | 28.8 | 17.8 | -- | -- | 0.52 | 0.71 | - | - | 0.115 | 0.080 | - | - |
| <Median (<8.6 μg/L) | Placebo | 11 | 32.3 | 21.7 | 3.5 | 12% | 0.69 | 0.93 | 0.17 | 33% | 0.112 | 0.083 | −0.003 | −3% |
| >Median (>8.6 μg/L) | Placebo | 12 | 29.8 | 16.4 | 1.0 | 3% | 0.54 | 0.70 | 0.02 | 4% | 0.110 | 0.079 | −0.005 | −4% |
| Placebo | Active | 18 | 32.3 | 21.7 | 3.5 | 12% | 0.74 | 0.98 | 0.22 | 42% | 0.130 | 0.091 | 0.015 | 13% |
| <Median (<8.6 μg/L) | Active | 18 | 34.6 | 22.0 | 5.8 | 20% | 0.76 | 0.90 | 0.24 | 46% | 0.126 | 0.086 | 0.011 | 10% |
| >Median (>8.6 μg/L) | Active | 16 | 32.2 | 17.8 | 3.4 | 12% | 0.77 | 0.98 | 0.25 | 48% | 0.121 | 0.088 | 0.006 | 5% |
| Administered Dose Conditions | SDLP | Lane Departures/min | Maximum Lateral Acceleration (Non-Sharp Events) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THC | Alcohol | N | Mean (cm) | St Dev (cm) | Difference (cm) | Percent Increasea (%) | Mean (N) | St Dev (N) | Difference (N) | Percent Increasea (%) | Mean (m/s2) | St Dev (m/s2) | Difference (m/s2) | Percent Increasea (%) |
| Placebo | Placebo | 18 | 28.8 | 17.8 | - | - | 0.52 | 0.71 | - | - | 0.115 | 0.080 | - | - |
| Low | Placebo | 18 | 31.3 | 20.3 | 2.5 | 9% | 0.64 | 0.85 | 0.12 | 23% | 0.116 | 0.084 | 0.001 | 1% |
| High | Placebo | 18 | 31.2 | 19.1 | 2.4 | 8% | 0.61 | 0.84 | 0.09 | 17% | 0.106 | 0.078 | −0.009 | −8% |
| Placebo | Active | 18 | 32.3 | 19.3 | 3.5 | 12% | 0.74 | 0.98 | 0.22 | 42% | 0.130 | 0.091 | 0.015 | 13% |
| Low | Active | 18 | 34.2 | 21.6 | 5.4 | 19% | 0.73 | 0.94 | 0.21 | 40% | 0.123 | 0.083 | 0.008 | 7% |
| High | Active | 18 | 32.2 | 17.4 | 3.4 | 12% | 0.80 | 0.96 | 0.28 | 54% | 0.123 | 0.092 | 0.008 | 7% |
Data are from 18 healthy occasional cannabis smokers 0.5–1.3h after ingesting placebo or active oral alcohol and inhaling placebo or active (low/2.9%, high/6.7% Δ9-tetrahydrocannabinol [THC]) vaporized bulk cannabis. Due to the resultant unbalanced design in low- and high-THC conditions imposed by participants’ self-titration, statistical analysis of variance could not be conducted by dose condition.
Relative to placebo-placebo condition
3.3 Pre- and Post-drive Blood and OF THC Concentrations
Table 6 presents pre- and post-drive blood and OF concentrations. Full blood and OF pharmacokinetic data are presented in Hartman et al. (2015b and 2015a, respectively). Between-subject blood concentration variability (coefficient of variation) was substantially lower than matched OF concentration variability at all time points: 45–65% vs. 125–207%, respectively, immediately post-dose; 39–69% vs. 129–184% at 1.4h; and 61–82% vs. 139–174% at 2.3h (Table 6).
Table 6.
Blood and oral fluid THC and variability prior to and after driving (N=19) after controlled vaporized active (2.9% THC and 6.7% THC) cannabis with or without alcohol.
| Time post-dose (h) | Blood THC (μg/L) | OF THC (μg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No Alcohol | Alcohol | No Alcohol | Alcohol | ||||||
| 2.9% | 6.7% | 2.9% | 6.7% | 2.9% | 6.7% | 2.9% | 6.7% | ||
| −0.8 (baseline) | Median range |
0 0–6.2 |
0 0–5.4 |
0 0–4.9 |
0 0–6.3 |
0.5 0–30.7 |
0 0–11.7 |
0 0–72.9 |
0.6 0–34.2 |
|
| |||||||||
| Mean (SD) | 0.5 (1.5) | 0.4 (1.3) | 0.5 (1.2) | 0.6 (1.5) | 4.6 (8.7) | 2.6 (4.0) | 6.3 (17.0) | 4.7 (8.9) | |
|
| |||||||||
| %CV | 284% | 332% | 245% | 282% | 191% | 157% | 272% | 189% | |
|
| |||||||||
| 0.17 (pre-drive 1) | Median range |
32.7 11.4–66.2 |
42.2 15.2–137 |
35.3 13.0–71.4 |
67.5 18.1–210 |
848 32.1–18,230 |
764 25.1–23,680 |
735 72.9–7,494 |
952 22.7–66,200 |
|
| |||||||||
| Mean (SD) | 35.9 (16.7) | 56.2 (36.4) | 40.5 (18.2) | 75.0 (48.1) | 2,101 (4,142) | 3,220 (5,645) | 1,599 (2,005) | 7,652 (15,842) | |
|
| |||||||||
| %CV | 46% | 65% | 45% | 64% | 197% | 175% | 125% | 207% | |
|
| |||||||||
| 0.42 (pre-drive 2) | Median range |
10.0 1.6–17.9 |
13.2 2.4–40.8 |
10.6 5.5–17.4 |
16.2 5.3–43.9 |
-- | -- | -- | -- |
|
| |||||||||
| Mean (SD) | 10.0 (4.5) | 16.8 (10.9) | 10.4 (3.4) | 19.0 (11.9) | -- | -- | -- | -- | |
|
| |||||||||
| %CV | 45% | 65% | 33% | 63% | -- | -- | -- | -- | |
|
| |||||||||
| 1.4 (post-drive 1) | Median range |
3.7 0–10.7 |
4.6 0–14.7 |
3.6 1.4–6.3 |
6.2 1.3–18.4 |
52.5 3.0–662 |
91.0 9.3–1,028 |
69.5 7.0–1,822 |
138 5.2–3,940 |
|
| |||||||||
| Mean (SD) | 3.9 (2.3) | 5.7 (3.9) | 3.6 (1.4) | 6.8 (4.6) | 91.3 (145) | 213 (275) | 228 (418) | 637 (1,097) | |
|
| |||||||||
| %CV | 59% | 69% | 39% | 68% | 159% | 129% | 184% | 172% | |
|
| |||||||||
| 2.3 (post-drive 2) | Median range |
1.9 0–8.5 |
2.6 0–9.6 |
1.8 0–4.9 |
3.2 0–9.5 |
33.1 1.8–374 |
46.9 1.9–542 |
35.4 8.7–473 |
91.0 1.6–1,541 |
|
| |||||||||
| Mean (SD) | 2.2 (1.8) | 3.2 (2.6) | 1.8 (1.1) | 3.2 (2.5) | 47.7 (81.1) | 92.1 (128) | 86.4 (124) | 263 (458) | |
|
| |||||||||
| %CV | 82% | 82% | 61% | 77% | 170% | 139% | 144% | 174% | |
Abbreviations: THC, Δ9-tetrahydrocannabinol; OF, oral fluid; SD, standard deviation; CV, coefficient of variation
4. DISCUSSION
Using a sophisticated driving simulator and rigorous placebo-controlled, within-subject design, we found a positive association between blood THC concentration and one (SDLP) of 3 alcohol-sensitive lateral control impairment measures (SDLP, normalized lane departures, maximum acceleration). Cannabis-alcohol combination effects were additive, not synergistic.
Decreased lateral control was associated with blood THC concentrations and BrAC, based on descriptive models. SDLP is among the most sensitive and consistently utilized driving impairment measures (Charlton and Starkey, 2013; Ramaekers et al., 2006a; Verster and Roth, 2011, 2012). Given that most countries have 0.05 or 0.08% BAC per se laws, the observed SDLP increase may be substantial enough to be considered impairment. Although SDLP (experimental measure) is not directly validated to predict crash risk (epidemiological measure), it is an objective measure of continuous behavior while driving (Lococo and Staplin, 2006). The lowest criterion of drug-induced driving impairment is considered to be SDLP consistent with 0.05 BAC, approximately 2.4cm (Lococo and Staplin, 2006). In this study, ≥8.2μg/L THC met that criterion. The increase associated with 10μg/L THC also was similar to 2μg/L THC+0.05g/210L BrAC (8.4% increase). At higher 20μg/L THC, SDLP increased 16%, comparable to 0.10g/210L BrAC (13% increase). In an on-road study (Ramaekers et al., 2000; Robbe, 1998), 100, 200 and 300μg/kg THC doses (~7mg, ~14mg, ~21mg) significantly increased SDLP 1.7–3.5cm relative to placebo. These increases are consistent with our 7–10μg/L during-drive THC (5.8–8.2% increase) or 0.05–0.08g/210L BrAC (6.7–10.7% increase, Table 3). Our final lane departures/min and maximum lateral acceleration GLM Select models did not include THC, indicating increasing THC concentrations did not increase these measures. Alcohol concentration-dependently increased lane departures/min and maximum lateral acceleration, with 0.05g/210L corresponding to 35% and 9.5% increases, respectively.
Combining cannabis with alcohol produced an additive — rather than synergistic—effect on SDLP, with no interaction term. Past simulator studies were inconsistent regarding SDLP cannabis-alcohol interactions. Ronen et al (2010) observed significant increases in lane position variability when 13mg THC and 0.05% (BAC) alcohol were combined, despite neither producing an independent significant effect. Conversely, Lenné et al (2010) observed significant main effects of cannabis and alcohol independently, but no interaction (combined effects not synergistic), similar to our findings. Combining 100 or 200μg/kg THC with 0.04% target BAC in the on-road study described above significantly increased SDLP by 5.3 and 8.5cm, classified as “severe” performance decrements (Ramaekers et al., 2000; Robbe, 1998). In our model, this increase is similar to ≥20μg/L blood THC alone. Although epidemiological studies do not quantify crash risk by SDLP, increases in lane weave may lead to more lane departures (detected by Downey et al., 2013) and, in turn, more crashes. Cannabis approximately doubled crash risk in two recent epidemiological meta-analyses (Li et al., 2012; Asbridge et al., 2012).
Unlike cannabis, alcohol affected additional lateral control parameters besides SDLP. Lane departures/min and maximum lateral acceleration also increased with BrAC, consistent with prior NADS alcohol findings (Lee et al., 2010). This suggests more extreme reaction to lateral position when DUI alcohol, compared to DUIC. Cannabis-influenced drivers may attempt to drive more cautiously to compensate for impairing effects, whereas alcohol-influenced drivers often underestimate their impairment and take more risks (Sewell et al., 2009). Alcohol’s strong effects on driving are well-established (Charlton and Starkey, 2013, 2015; Moskowitz and Fiorentino, 2000; Van Dyke and Fillmore, 2014). Alcohol increased center and edge lane crossings, and time over the edge line in a simulated drive (Charlton and Starkey, 2013). Lack of observed cannabis effects on lane departures contrasts with prior findings. Downey et al. (2013) observed dose-dependent cannabis effects on straddling lane barrier or solid lines, with or without alcohol, in simulated nighttime driving. That study had more participants (80), possibly providing higher power to detect weak effects. In one on-road study, only cannabis-alcohol combinations significantly increased time out of lane (Ramaekers et al., 2000; Robbe, 1998); neither cannabis nor alcohol (0.04% BAC) alone produced a significant effect. Because increasing lane departures and “time out of lane” require more substantial lane weaving than SDLP, this discrepancy may result from the low alcohol dose administered in that study. SDLP is more sensitive, with observable impairment at BACs as low as 0.04% (Moskowitz and Fiorentino, 2000).
Neither cannabis nor alcohol affected standard deviation of steering angle. To our knowledge, only one prior simulator study found a significant alcohol effect on this parameter: 0.6g/kg alcohol (peak BACs ~0.05%) produced a significant but small increase in standard deviation of steering angle (Lenné et al., 2010). Lower 0.4g/kg (peak BACs ≤ 0.025%) had no effect. Although cannabis alone (19, 38mg) did not significantly increase steering angle variability (main effect), there was significant interaction with driver experience. Experienced drivers (≥7 years driving) showed unchanged or decreased steering angle variability with increasing cannabis dose relative to placebo; inexperienced drivers (<2 years) had increased variability (Lenné et al., 2010). All of our participants had ≥6 years of driving experience, perhaps accounting for this discrepancy. Lenné et al. (2010) also analyzed effects by dose rather than concentration, possibly resulting in greater apparent effect size because dose-wise (categorical) variable analyses generally have higher power than continuous variables. Multiple other studies found no cannabis-only effect on steering wheel position variability (Anderson et al., 2010; Ronen et al., 2010), although one observed increased steering variability in occasional smokers after alcohol alone and alcohol-cannabis combination (Ronen et al., 2010). Standard deviation of steering angle appears insensitive, due to the amplifying effect of steering mechanisms. Minor steering adjustments can substantially alter course and change lane position due to forward motion, despite re-straightening the wheel.
By controlling ad libitum inhalation topography (e.g., inhalation rate, depth, hold time), smokers can self-titrate cannabis dose to achieve desired pharmacological response (Azorlosa et al., 1995). We infer self-titration from the observed disjunction between dose and THC concentration; there is often poor correlation between THC dose and blood concentration, making concentration-based analysis more meaningful and robust than dose-based analysis (see Tables 4–5, Figure 3). In our sample, 52.6% of participants showed evidence of self-titration (Hartman et al 2015b). Substantial concentration variability was observed, consistent with prior cannabis research (Desrosiers et al., 2014). This further underscores the robustness of concentration-based—rather than dose-based—analysis.
There is substantial interest in relating driving performance directly to OF concentrations due to screening advantages. THC enters OF primarily by oromucosal contamination during inhalation, and consequently is less representative of systemic concentrations shortly after intake. OF concentration variability was 2–5-fold higher than for paired blood concentrations, making interpretation of effects more challenging. Similar to blood, low OF THC concentrations are difficult to interpret because intake history and individual variability affect detection time and later concentrations. However, in this sample, OF THC >1600μg/L indicated intake within the last 1.4h, and >600μg/L indicated intake within the last 2.3h. In a roadside study, the percentage of people displaying observable cannabis-related impairment increased with increasing OF concentrations when aggregated into wide ranges (≤3μg/L, 3–25μg/L, 25–100μg/L, >100μg/L) (Fierro et al., 2014).
4.1 Strengths and limitations
Major study strengths include the double-blind, placebo-controlled, within-subject design; drive scenarios controlling for other road conditions (speed limit and curvature), which potentially affect drivers’ lateral control and road tracking performance; administration of multiple doses of cannabis (THC) with/without alcohol; concentration-based analysis; and multiple specimen collections before and after driving (allowing during-drive pharmacokinetic modeling), to better relate driving impairment to THC concentrations.
In authentic DUIC cases, measured THC concentrations do not reflect those present during driving. Blood collection is typically delayed 90min to 4h after the event (Biecheler et al., 2008; Jones et al., 2008). During this delay, there is rapid THC distribution from blood into highly-perfused tissues, resulting in rapid blood THC concentration decrease in the first hour post-inhalation. Later, THC concentration continues to decrease, albeit more slowly. This results in lower measured THC concentrations than were present during driving. In contrast, we examined driving performance relative to THC concentrations and BrAC that were present during driving. Thus, to our knowledge, the current study is among the most robust analyses of cannabis and alcohol effects on lateral control at specific THC concentrations. For context, we report driving performance results at concentrations typically considered or established for per se laws around the world (1, 2, 5, 7μg/L THC; 0.02, 0.05, 0.08% BrAC) (Armentano, 2013; Grotenhermen et al., 2007; Karakus et al., 2014; Lacey et al., 2010; Ramaekers et al., 2006b; Verstraete A, 2011). However, these per se limits are applied to THC concentrations that may substantially underestimate concentrations during driving. Thus, our reported THC 1–5μg/L SDLP changes may be understated compared to forensic DUIC cases. In the present study, median blood and OF THC concentrations immediately post-dose were >30μg/L and >700μg/L, respectively. Blood THC ≥20μg/L indicated intake within the last 0.42h and THC ≥10μg/L indicated intake within the last 1.4h. Thus, if people drive during or soon after cannabis inhalation, during-drive THC concentrations could exceed 20μg/L. Our SDLP increase associated with THC ≥20μg/L (~5.2cm) was considered “severe” by other researchers (Ramaekers et al., 2000; Robbe, 1998), representing a 16% increase in our observed lane position variability. Despite lack of significant THC effect on lane departures/min, our results suggest substantial lateral control performance decrements, consistent with effects produced by known impairing alcohol concentrations. Verster and Roth (2014) determined that lane departures alone were not sufficiently sensitive to experimentally detect impaired driving or effect size differences. SDLP is a sensitive marker, serving as experimental proxy for rarer events such as lane departures. Even minor lateral control decrements may be dangerous in narrow or winding roads, or in heavy traffic where navigational precision or defensive driving may be required.
This study has several limitations. We approached data analyses via a stepwise GLM Select procedure, with the goal of describing data without assumptions of which parameters (THC, BrAC, other) would produce fixed effects. In research settings, participants are aware driving is constantly under observation, and may drive with greater caution or focus. Other participants may have wanted to demonstrate that cannabis does not affect driving; public attitudes toward DUIC are less negative than for DUI alcohol (McCarthy et al., 2007; Terry and Wright, 2005). However, self-perception of driving performance or impairment—even without drugs—may be unreliable (Van Dyke and Fillmore 2014; Verster and Roth, 2012).
This study was limited to occasional smokers. Frequent cannabis smokers demonstrate tolerance to some acute cannabis intoxication effects (Ramaekers et al., 2011), but tolerance did not compensate for all effects (Downey et al., 2013). There is currently substantial interest in comparing occasional to frequent smokers and assessing potential tolerance (Ramaekers et al., 2009; Toennes SW et al., 2008; Wright and Terry, 2002), especially as medical and recreational cannabis becomes more commonplace.
We do not believe that conducting this study in a driving simulator, rather than on the road, represents a significant limitation. Rather, simulators offer advantages for assessing impaired driving. Participants can engage in risky driving behavior without endangering themselves or others. Simulators provide controlled reproducible research environments and ability to make detailed real-time measurements. Modern simulators produce highly realistic driving scenarios (Hartman and Huestis, 2012). The NADS-1 is the world’s most sophisticated simulator, and was successfully utilized to assess distracted and drugged driving (Garrott et al., 2005; Lee et al., 2010).
4.2 Conclusion
In this rigorous, double-blind, placebo-controlled study, cannabis and alcohol were significantly associated with impaired driving lateral control. Cannabis only affected SDLP; whereas alcohol affected SDLP, lane departures/min, and maximum acceleration. During-drive 8.2μg/L blood THC was associated with SDLP increases similar to 0.05g/210L BrAC (~0.05% BAC), and SDLP at 13.1μg/L THC approximated 0.08g/210L BrAC. Combining alcohol and cannabis produced an additive effect on SDLP; 5μg/L THC with 0.05g/210L BrAC was similar to 0.08g/210L SDLP impairment. These THC concentrations during driving are higher than those generally measured hours later during sample collection. OF concentration variability was substantially greater than blood concentration variability, suggesting better performance as a screening tool than impairment gauge.
Supplementary Material
Highlights.
We model cannabis’ effects on driving lateral control via sophisticated simulator.
Models are based on blood THC and breath alcohol concentrations during driving.
THC increased standard deviation of lateral position (SDLP); 0.26 cm per μg/L THC.
Alcohol increased SDLP 0.42 cm per 0.01g/210L; additional lateral control measures.
During-drive 7–10μg/L blood THC produced similar SDLP to 0.05g/210L breath alcohol.
Concurrent alcohol and cannabis produced additive rather than synergistic effects.
Acknowledgments
We thank the nurses and staff of the University of Iowa Clinical Research Unit and National Advanced Driving Simulator staff, especially Cheryl Roe, Jennifer Henderson, Rose Schmitt, and Kayla Smith, for their excellent contributions to successful completion of the study. We also thank Drs. Dereece Smither and Richard Compton, National Highway Traffic Safety Administration (NHTSA) for valuable input and Omar Ahmad for technical assistance. We acknowledge the University of Maryland, Baltimore Toxicology Program and the Graduate Partnership Program, National Institutes of Health (NIH).
Role of Funding Source
This research was funded by the United States Office of National Drug Control Policy; the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health; and the National Highway Traffic Safety Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
Authors Hartman, Brown, Gorelick, Gaffney, and Huestis participated in the research design. Authors Hartman, Brown, Milavetz, Spurgin, and Gaffney participated in research conduct, under oversight from Author Huestis. Authors Hartman, Brown, Milavetz, Spurgin, Pierce, Gaffney, and Huestis participated in data analysis, under the substantial guidance of Author Pierce. Author Hartman wrote the initial draft of the manuscript, Authors Gorelick and Huestis contributed substantially to the draft revision process, and all authors contributed to the finalized version.
Conflicts of Interest
Volcano® and Quantisal™ devices and supplies (Storz & Bickel, Tuttlingen, Germany and Immunalysis, Pomona, CA) were provided by manufacturers through Materials Transfer Agreements. No commercial entity played any role in study design and conduct, data analysis, manuscript drafting, or the decision to publish. The authors declare no personal conflicts of interest.
Clinical Trial Registration: Effects of Inhaled Cannabis on Driving Performance, NCT01620177 https://clinicaltrials.gov/ct2/show/NCT01620177?term=Cannabis+AND+driving&rank=1
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson SJ, Sellman JD. A prototype screening instrument for cannabis use disorder: the Cannabis Use Disorders Identification Test (CUDIT) in an alcohol-dependent clinical sample. Drug Alcohol Rev. 2003;22:309–315. doi: 10.1080/0959523031000154454. [DOI] [PubMed] [Google Scholar]
- Anderson BM, Rizzo M, Block RI, Pearlson GD, O’Leary DS. Sex differences in the effects of marijuana on simulated driving performance. J Psychoactive Drugs. 2010;42:19–30. doi: 10.1080/02791072.2010.10399782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano P. Should per se limits be imposed for cannabis? equating cannabinoid blood concentrations with actual driver impairment: practical limitations and concerns. Humboldt J Social Relations. 2013;35:45–55. [Google Scholar]
- Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: Systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. doi: 10.1136/bmj.e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azorlosa JL, Greenwald MK, Stitzer ML. Marijuana smoking: Effects of varying puff volume and breathhold duration. J Pharmacol Exp Ther. 1995;272:560–569. [PubMed] [Google Scholar]
- Bergamaschi MM, Karschner EL, Goodwin RS, Scheidweiler KB, Hirvonen J, Queiroz RH, Huestis MA. Impact of prolonged cannabinoid excretion in chronic daily cannabis smokers’ blood on per se drugged driving laws. Clin Chem. 2013;59:519–526. doi: 10.1373/clinchem.2012.195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berning A, Compton R, Wochinger K. Traffic Safety Facts: Research Note. National Highway Traffic Safety Administration, US Department of Transportation; Washington, DC: 2015. Results of the 2013–2014 National Roadside Survey of Alcohol and Drug Use by Drivers. Report No. DOT HS 812–118. [Google Scholar]
- Biecheler MB, Peytavin JF, Facy F, Martineau H. SAM survey on “drugs and fatal accidents”: search of substances consumed and comparison between drivers involved under the influence of alcohol or cannabis. Traffic Inj Prev. 2008;9:11–21. doi: 10.1080/15389580701737561. [DOI] [PubMed] [Google Scholar]
- Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem. 2009;55:1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton SG, Starkey NJ. NZ Transport Agency Research Report Traffic and Road Safety Research Group. University of WaikatO; Hamilton, NZ: 2013. Driver Risk From Blood Alcohol Levels Between 50mg/100ml And 80mg/100ml. Doc. No. 541. [Google Scholar]
- Charlton SG, Starkey NJ. Driving while drinking: performance impairments resulting from social drinking. Accid Anal Prev. 2015;74:210–217. doi: 10.1016/j.aap.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Desrosiers NA, Himes SK, Scheidweiler KB, Concheiro-Guisan M, Gorelick DA, Huestis MA. Phase I and II cannabinoid disposition in blood and plasma of occasional and frequent smokers following controlled smoked cannabis. Clin Chem. 2014;60:631–643. doi: 10.1373/clinchem.2013.216507. [DOI] [PubMed] [Google Scholar]
- Downey LA, King R, Papafotiou K, Swann P, Ogden E, Boorman M, Stough C. The effects of cannabis and alcohol on simulated driving: influences of dose and experience. Accid Anal Prev. 2013;50:879–886. doi: 10.1016/j.aap.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Drummer OH. Drug testing in oral fluid. Clin Biochem Rev. 2006;27:147–159. [PMC free article] [PubMed] [Google Scholar]
- Drummer OH, Gerostamoulos D, Chu M, Swann P, Boorman M, Cairns I. Drugs in oral fluid in randomly selected drivers. Forensic Sci Int. 2007;170:105–110. doi: 10.1016/j.forsciint.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Drummer OH, Gerostamoulos J, Batziris H, Chu M, Caplehorn J, Robertson MD, Swann P. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36:239–248. doi: 10.1016/s0001-4575(02)00153-7. [DOI] [PubMed] [Google Scholar]
- Fierro I, Gonzalez-Luque JC, Alvarez FJ. The relationship between observed signs of impairment and THC concentration in oral fluid. Drug Alcohol Depend. 2014;144:231–238. doi: 10.1016/j.drugalcdep.2014.09.770. [DOI] [PubMed] [Google Scholar]
- Garrott WR, Mazzae EN, Goodman MJ. NHTSA’s National Advanced Driving Simulator Research Program. National Highway Traffic Safety Administration; 2005. Paper No. 05–0377. [Google Scholar]
- Gjerde H, Normann PT, Christophersen AS, Samuelsen SO, Mørland J. Alcohol, psychoactive drugs and fatal road traffic accidents in Norway: a case-control study. Accid Anal Prev. 2011;43:1197–1203. doi: 10.1016/j.aap.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F, Leson G, Berghaus G, Drummer OH, Krüger HP, Longo M, Moskowitz H, Perrine B, Ramaekers JG, Smiley A, Tunbridge R. Developing limits for driving under cannabis. Addiction. 2007;102:1910–1917. doi: 10.1111/j.1360-0443.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Hartman RL, Anizan S, Jang M, Brown TL, Yun K, Gorelick DA, Milavetz G, Spurgin A, Gaffney G, Huestis MA. Cannabinoid disposition in oral fluid after controlled vaporizer administration with and without alcohol. Forensic Toxicol. 2015a doi: 10.1007/s11419-015-0269-6. [DOI] [Google Scholar]
- Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney G, Huestis MA. controlled cannabis vaporizer administration: blood and plasma cannabinoids with and without alcohol. Clin Chem. 2015b;61:850–69. doi: 10.1373/clinchem.2015.238287. [DOI] [PubMed] [Google Scholar]
- Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013;59:478–492. doi: 10.1373/clinchem.2012.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–82. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Verstraete A, Kwong TC, Morland J, Vincent MJ, De La Torre R. Oral fluid testing: promises and pitfalls. Clin Chem. 2011;57:805–810. doi: 10.1373/clinchem.2010.152124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW, Andersson L. Comparison of ethanol concentrations in venous blood and end-expired breath during a controlled drinking study. Forensic Sci Int. 2003;132:18–25. doi: 10.1016/s0379-0738(02)00417-6. [DOI] [PubMed] [Google Scholar]
- Jones AW, Holmgren A, Kugelberg FC. Driving under the influence of cannabis: a 10-year study of age and gender differences in the concentrations of tetrahydrocannabinol in blood. Addiction. 2008;103:452–461. doi: 10.1111/j.1360-0443.2007.02091.x. [DOI] [PubMed] [Google Scholar]
- Karakus A, Idiz N, Dalgic M, Ulucay T, Sincar Y. Comparison of the effects of two legal blood alcohol limits: the presence of alcohol in traffic accidents according to category of driver in Izmir, Turkey. Traffic Inj Prev. 2014 doi: 10.1080/15389588.2014.968777. [DOI] [PubMed] [Google Scholar]
- Lacey J, Brainard K, Snitow S. Drug Per Se Laws: A Review of Their Use in States. National Highway Traffic Safety Administration; 2010. Report No. DOT HS 811–317. [Google Scholar]
- Lacey JH, Kelley-Baker T, Furr-Holden D, Voas RB, Romano E, Ramirez A, Brainard K, Moore C, Torres P, Berning A. 2007 National Roadside Survey of Alcohol and Drug Use by Drivers: Drug Results. National Highway Traffic Safety Administration Office of Behavioral Safety Research; 2009. Report No. DOT HS 811–249. [Google Scholar]
- Laumon B, Gadegbeku B, Martin JL, Biecheler MB. Cannabis intoxication and fatal road crashes in France: population based case-control study. BMJ. 2005;331:1371. doi: 10.1136/bmj.38648.617986.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Fiorentino D, Reyes ML, Brown TL, Ahmad O, Fell J, Ward N, Dufour R. Assessing the Feasibility of Vehicle-Based Sensors to Detect Alcohol Impairment. National Highway Traffic Safety Administration; 2010. Report No. DOT HS 811–358. [Google Scholar]
- Legrand SA, Isalberti C, der Linden TV, Bernhoft IM, Hels T, Simonsen KW, Favretto D, Ferrara SD, Caplinskiene M, Minkuviene Z, Pauliukevicius A, Houwing S, Mathijssen R, Lillsunde P, Langel K, Blencowe T, Verstraete AG. Alcohol and drugs in seriously injured drivers in six European countries. Drug Test Anal. 2013;5:156–165. doi: 10.1002/dta.1393. [DOI] [PubMed] [Google Scholar]
- Lenné MG, Dietze PM, Triggs TJ, Walmsley S, Murphy B, Redman JR. The effects of cannabis and alcohol on simulated arterial driving: influences of driving experience and task demand. Accid Anal Prev. 2010;42:859–866. doi: 10.1016/j.aap.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Li MC, Brady JE, DiMaggio CJ, Lusardi AR, Tzong KY, Li G. Marijuana use and motor vehicle crashes. Epidemiol Rev. 2012;34:65–72. doi: 10.1093/epirev/mxr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lococo KH, Staplin L. Literature Review Of Polypharmacy And Older Drivers: Identifying Strategies To Collect Drug Usage And Driving Functioning Among Older Drivers. National Highway Traffic Safety Administration, US Department Of Transportation; Washington, DC: 2006. Report No. DOT HS 810–558. [Google Scholar]
- McCarthy DM, Lynch AM, Pederson SL. Driving after use of alcohol and marijuana in college students. Psychol Addict Behav. 2007;21:425–430. doi: 10.1037/0893-164X.21.3.425. [DOI] [PubMed] [Google Scholar]
- Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J Chrom A. 2010;1217:1513–1521. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz H, Fiorentino D. A Review Of The Literature On The Effects Of Low Doses Of Alcohol On Driving-Related Skills. National Highway Traffic Safety Administration; 2000. Report No. DOT HS 809–028. [Google Scholar]
- Neerchal NK, Morel JG, Huang X, Moluh A. A Stepwise Algorithm for Generalized Linear Mixed Models. SAS Global Forum; Washington, DC: 2014. pp. 1822–2014. [Google Scholar]
- O.N.D.C.P. Office of National Drug Control Policy. White House; Washington, DC: 2013. [Accessed on 7 August 2013]. http://www.whitehouse.gov/ondcp. [Google Scholar]
- Pilkinton MW, Robertson A, McCluskey DL. Drugged driving: increased traffic risks involving licit and illicit substances. J Drug Educ. 2013;43:183–201. doi: 10.2190/DE.43.2.f. [DOI] [PubMed] [Google Scholar]
- ProCon.org. [Accessed on 2 Dec 2014];23 Legal Medical Marijuana States and DC: Laws, Fees, and Possession Limits. 2014 http://medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
- Ramaekers J, Kauert G, Theunissen E, Toennes S, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- Ramaekers J, Theunissen E, de Brouwer M, Toennes S, Moeller M, Kauert G. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology. 2011;214:391–401. doi: 10.1007/s00213-010-2042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Kuypers KP, Samyn N. Stimulant effects of 3,4-methylenedioxymethamphetamine (MDMA) 75 mg and methylphenidate 20 mg on actual driving during intoxication and withdrawal. Addiction. 2006a;101:1614–1621. doi: 10.1111/j.1360-0443.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of Δ9-THC concentration in serum and oral fluid: limits of impairment. Drug Alcohol Depend. 2006b;85:114–122. doi: 10.1016/j.drugalcdep.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Robbe HWJ, O’Hanlon JF. Marijuana, alcohol and actual driving performance. Hum Psychopharmacol. 2000;15:551–558. doi: 10.1002/1099-1077(200010)15:7<551::AID-HUP236>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Robbe H. Marijuana’s impairing effects on driving are moderate when taken alone but severe when combined with alcohol. Hum Psychopharmacol. 1998;13:S70–S78. [Google Scholar]
- Ronen A, Chassidim HS, Gershon P, Parmet Y, Rabinovich A, Bar-Hamburger R, Cassuto Y, Shinar D. The effect of alcohol, THC and their combination on perceived effects, willingness to drive and performance of driving and non-driving tasks. Accid Anal Prev. 2010;42:1855–1865. doi: 10.1016/j.aap.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Salomonsen-Sautel S, Min SJ, Sakai JT, Thurstone C, Hopfer C. Trends in fatal motor vehicle crashes before and after marijuana commercialization in Colorado. Drug Alcohol Depend. 2014;140:137–144. doi: 10.1016/j.drugalcdep.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidweiler KB, Schwope DM, Karschner EL, Desrosiers NA, Gorelick DA, Huestis MA. In vitro stability of free and glucuronidated cannabinoids in blood and plasma following controlled smoked cannabis. Clin Chem. 2013;59:1108–1117. doi: 10.1373/clinchem.2012.201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- Schwope D, Scheidweiler K, Huestis M. Direct quantification of cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem. 2011;401:1273–1283. doi: 10.1007/s00216-011-5197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18:185–193. doi: 10.1080/10550490902786934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Wilson VB, editors. Assessing Alcohol Problems: A Guide for Clinicians and Researchers. National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health; Bethesda, MD: 2003. pp. 75–99. [Google Scholar]
- Terry P, Wright KA. Self-reported driving behaviour and attitudes towards driving under the influence of cannabis among three different user groups in England. Addict Behav. 2005;30:619–626. doi: 10.1016/j.addbeh.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Comparison of cannabinoid pharmacokinetic properties in occasional and heavy users smoking a marijuana or placebo joint. J Anal Toxicol. 2008;32:470–477. doi: 10.1093/jat/32.7.470. [DOI] [PubMed] [Google Scholar]
- Urfer S, Morton J, Beall V, Feldmann J, Gunesch J. Analysis of Delta9-tetrahydrocannabinol driving under the influence of drugs cases in Colorado from January 2011 to February 2014. J Anal Toxicol. 2014;38:575–581. doi: 10.1093/jat/bku089. [DOI] [PubMed] [Google Scholar]
- Van der Linden T, Legrand SA, Silverans P, Verstraete AG. DUID: oral fluid and blood confirmation compared in Belgium. J Anal Toxicol. 2012;36:418–421. doi: 10.1093/jat/bks038. [DOI] [PubMed] [Google Scholar]
- Van Dyke N, Fillmore MT. Alcohol effects on simulated driving performance and self-perceptions of impairment in DUI offenders. Exp Clin Psychopharmacol. 2014;22:484–493. doi: 10.1037/a0038126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster JC, Roth T. Standard operation procedures for conducting the on-the-road driving test, and measurement of the standard deviation of lateral position (SDLP) Int J Gen Med. 2011;4:359–371. doi: 10.2147/IJGM.S19639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster JC, Roth T. Drivers can poorly predict their own driving impairment: a comparison between measurements of subjective and objective driving quality. Psychopharmacology (Berl) 2012;219:775–781. doi: 10.1007/s00213-011-2400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster JC, Roth T. Excursions out-of-lane versus standard deviation of lateral position as outcome measure of on-the-road driving test. Hum Psychopharmacol Clin Exp. 2014;29:322–329. doi: 10.1002/hup.2406. [DOI] [PubMed] [Google Scholar]
- Verster JC, Veldhuijzen DS, Patat A, Olivier B, Volkerts ER. Hypnotics and driving safety: meta-analyses of randomized controlled trials applying the on-the-road driving test. Curr Drug Saf. 2006;1:63–71. doi: 10.2174/157488606775252674. [DOI] [PubMed] [Google Scholar]
- Verstraete AKA, Jantos R, Skopp G, Gjerde H, Vindenes V, et al. Per se limits – Methods of defining cut-off values for zero tolerance. DRUID (Driving under the Influence of Drugs, Alcohol and Medicines) 2011 Doc. No. TREN-05-FP6TR-S07.61320-518404-DRUID. [Google Scholar]
- Wille SM, Di Fazio V, Toennes SW, van Wel JH, Ramaekers JG, Samyn N. Evaluation of Delta9-tetrahydrocannabinol detection using DrugWipe5S screening and oral fluid quantification after Quantisal collection for roadside drug detection via a controlled study with chronic cannabis users. Drug Test Anal. 2014;7:178–86. doi: 10.1002/dta.1660. [DOI] [PubMed] [Google Scholar]
- Wright K, Terry P. Modulation of the effects of alcohol on driving-related psychomotor skills by chronic exposure to cannabis. Psychopharmacology. 2002;160:213–219. doi: 10.1007/s00213-001-0955-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.