Abstract
Background
The older population size has increased substantially, and a considerable proportion of older adults are cigarette smokers. Quitting smoking is associated with reduced health risk. This review is among the first to quantitatively assess the relative efficacy of types of cessation interventions for smokers aged ≥ 50 years.
Methods
We conducted searches of the Cochrane Library, Embase, MEDLINE, and PsycINFO to identify smoking cessation studies on adults aged ≥ 50 years. Twenty-nine randomized clinical trials met the inclusion criteria. Three main types of interventions were identified. We analyzed relative cessation rates or Risk Ratios (RRs) between the type of intervention groups and the control group by fixed- and random-effects meta-analyses at the study level. We conducted a weighted least squares meta-regression of cessation rates on trial and sample characteristics to determine sources of outcome heterogeneity.
Results
Fixed-effects analysis showed significant treatment effects for pharmacological (RR=3.18, 95% CI: 1.89–5.36), non-pharmacological (RR=1.80, 95% CI: 1.67–1.94), and multimodal interventions (RR=1.61, 95% CI: 1.41–1.84) compared with control group. Estimations based on meta-regression suggested that pharmacological intervention (mean point prevalence abstinence rate (PPA) = 26.10%, CI: 15.20–37.00) resembled non-pharmacological (27.97%, CI: 24.00–31.94), and multimodal interventions (36.64%, CI: 31.66–41.62); and non-pharmacological and multimodal interventions had higher PPAs than the control group (18.80%, CI: 14.48–23.12), after adjusting for a number of trial and sample characteristics.
Conclusions
A small number of smoking cessation studies examined smokers aged ≥ 50 years. Additional research is recommended to determine smoking cessation efficacy for diverse older population groups (e.g., ethnic minorities).
Keywords: smoking cessation interventions, older adults, meta-analysis, weighted least squares meta-regression
1. INTRODUCTION
The older population (aged 65 and older) in the United States is projected to double from 40.2 million in 2010 to 88.5 million in 2050, largely driven by the aging of the ‘baby boomers’ born between 1946 and 1964 (Vincent and Velkoff, 2010). In 2014, the youngest ‘baby boomers’ turned 50 years old. By 2030, all the ‘baby boomers’ will have become over 65 years old (Vincent and Velkoff, 2010).
Based on data from the 2012 National Health Interview Survey (NHIS), it was estimated that about 18.1% (42.1 million) of U.S. adults were current cigarette smokers, who reported smoking at least 100 cigarettes in their lifetime, and reported smoking every day or some days at the time of the interview (Agaku et al., 2014). The prevalence of current smoking was lower among older adults aged ≥ 65 years (8.9%) than those aged 18–24 (17.3%), 25–44 years (21.6%) or 45–64 years (19.5%).
Survey data show that older cigarette smokers are less likely than younger adults to be interested in quitting smoking, make quit attempts, and achieve smoking cessation (CDC, 2011). From the 2010 NHIS, 53.8% of current smokers aged ≥ 65 years indicated that they would like to quit smoking completely, as compared with 70.2% among those aged 18–64 years (CDC, 2011). Prevalence of current and former smokers who made a quit attempt in the year before the interview were 62.4%, 56.9%, 45.5%, and 43.5% for adults aged 18–24, 25–44, 45–64, and 65 years and over, respectively. Accordingly, past-year smoking cessation rate was lower among people aged 45–64 years (4.7%) and ≥ 65 years (5.3%) than people aged 18–24 (8.2%) and 25–44 years (7.1%). This has important clinical implications as older adults are more likely than young adults to have aging-related medical illnesses (e.g., heart or lung related conditions) that may be exacerbated by smoking (DHHS, 2014).
As a leading cause of premature morbidity and mortality in the U.S., smoking may impact almost all organs in the human body, and is linked to a multitude of cancers and other diseases (DHHS, 2014). Based on self-reported and spirometry data from the US National Health and Nutrition Examination Survey, it was estimated that adults aged 35 years or older had about 14 million (95% CI, 12.9–15.1 million) major medical conditions attributable to smoking in 2009 (Rostron et al., 2014).
There are important health benefits to quitting smoking at any age (DHHS, 2014; Doll et al., 2004). Findings from a study of 34,439 male British doctors indicate that smoking cessation at age 60, 50, 40, and 30 would increase life expectancy by 3, 6, 9, 10 years, respectively (Doll et al., 2004).
Whilst population-level data suggest that there is a lower smoking cessation rate among older smokers than younger groups (CDC, 2011), when interventions have included or deliberately targeted older adults, they tend to perform as well as younger smokers (Abdullah and Simon, 2006; Doolan and Froelicher, 2008). Previous reviews have suggested that older smokers respond to smoking cessation interventions at similar rate to younger smokers and that cessation brings health benefits (Abdullah and Simon, 2006; Doolan and Froelicher, 2008). Intervention studies reporting stratified results by age group found that the abstinence rate for senior smokers was comparable to or even higher than younger age groups (Dale et al., 2001; Doolan et al., 2008; McFall et al., 2010; Tashkin et al., 2011). A study employing comprehensive behavioral intervention indicated that the quitting rate for older smokers aged 62 years and over (52.0%) was significantly higher than that for smokers younger than 62 years old (38.1%) at the 12-month follow-up (Doolan et al., 2008). However, such studies only represent a small proportion of cessation interventions for adults in general, as most studies do not report outcomes for different age groups. In addition, the number of interventions targeting specifically at the elderly is small. It highlights a critical knowledge gap in the efficacy of interventions for older smokers (Zbikowski et al., 2012).
Previous reviews tried to fill the gap by summarizing findings on smoking cessation for older smokers. Pharmacological interventions seemed to be effective in smoking cessation for older smokers (Zbikowski et al., 2012). There were various non-pharmacological interventions such as physician-delivered interventions, behavioral interventions, and interventions through printed materials. Physician-delivered interventions achieved moderate quit rates (Pilowsky and Wu, 2014; Zbikowski et al., 2012). Cessation outcomes from counseling/behavioral interventions varied with intensities of interventions and length of follow-ups (Pilowsky and Wu, 2014; Zbikowski et al., 2012). More intensive interventions and multimodal interventions involving both medications and counseling often resulted in best outcomes (Zbikowski et al., 2012). However, none of the past reviews on adults aged ≥ 50 years employed meta-analysis/regression to quantitatively assess the relative effectiveness of different intervention strategies, and how intervention outcomes are associated with trial and sample characteristics (Abdullah and Simon, 2006; Doolan and Froelicher, 2008; Pilowsky and Wu, 2014; Zbikowski et al., 2012).
This review focused on smokers aged 50 and over for several reasons. First, there are few intervention studies exclusively focusing on smokers aged ≥ 65 years, but there are relatively more studies on those aged ≥ 50 years. Second, a tailored smoking cessation guide for smokers aged ≥ 50 years (Clear Horizons) was developed by the National Cancer Institute, and tested in multiple studies on older smokers (Orleans et al., 2000; Ossip-Klein et al., 1997; Rimer et al., 1994). Third, the Clinical Practice Guideline on treating tobacco use and dependence referred to studies on smokers aged ≥ 50 years as evidence for treating older smokers (Fiore et al., 2008). Fourth, past reviews on smoking cessation for older smokers included adults aged ≥ 50 years (Abdullah and Simon, 2006; Doolan and Froelicher, 2008; Pilowsky and Wu, 2014; Zbikowski et al., 2012).
Randomized controlled trials (RCTs) provide the evidence to help evaluate causal inferences, and decrease allocation bias and other types of bias if well designed (Levin, 2007). Following Zbikowski et al. (2012), we searched for RCTs published on and after January 1, 1990. The aims of this study were to 1) synthesize results published between January 1, 1990 and January 28, 2015 from RCTs on smoking cessation interventions for smokers aged ≥ 50 years, 2) determine relative effectiveness of intervention types (pharmacological, non-pharmacological, and multimodal interventions), and 3) examine whether trial and sample features were associated with intervention outcomes (abstinence rates).
2. METHODS
2.1. Eligibility Criteria
We used the PRISMA to guide our literature review and analysis (Moher et al., 2009). We included RCTs of smoking cessation that report abstinence rates for adult smokers aged ≥ 50 years, published in English between January 1, 1990 and January 28, 2015. Three main inclusion criteria were: 1) reporting results from smoking cessation RCTs; 2) presenting smoking abstinence either in the form of proportions or the numbers of abstainers and sample sizes; 3) including adult smokers aged ≥ 50 years, or reporting abstinence rates by age groups that included those ≥ 50 years.
2.2. Information Sources and Search Strategy
We conducted a systematic search of the Cochrane Library, Embase, MEDLINE, and PsycINFO for eligible studies published between January 1, 1990 and January 28, 2015. We evaluated review articles related to this area to identify additional studies for including in the meta-analysis (Abdullah and Simon, 2006; Doolan and Froelicher, 2008; Zbikowski et al., 2012). The following search terms were used for the four databases: “(nicotine OR tobacco OR cigarette OR smoking) AND (program OR intervention)”. The results shown in Embase and MEDLINE were restricted to “randomized controlled trial”, “middle aged, aged, very elderly”, “human” and “English”. The results in PsycINFO were restricted to “treatment outcome/clinical trial”, “middle aged, aged, very old”, “human”, and “English”, “peer reviewed journal”. We restricted the results from the Cochrane Library to “trials” (other restrictions were not available), and screened the results manually for eligible studies.
2.3. Study Identification and Data Extraction
Records identified through the four databases and review articles were initially screened for duplication, and then by titles, abstracts, and full-texts sequentially by the first author (D.C.). After removal of duplicates, we excluded irrelevant titles and abstracts based primarily on the three inclusion criteria. Full-text files of the remaining articles were retrieved and reviewed systematically. Studies meeting all three criteria were included in the outcome-level analysis. Studies without an inactive or minimal control group were excluded from the study-level analysis.
We recorded the trial outcome, selected trial features, and baseline characteristics of study participants in each randomized arm from the included studies in a pre-specified excel worksheet. Each arm was classified into one of the four categories: pharmacotherapy (e.g., nicotine patches/gums, bupropion), non-pharmacological interventions (e.g., physician/nurse counseling, group counseling, counselor-initiated calls, Clear Horizons Guide, computer tailored messages, quitline), and multimodal interventions (pharmacotherapy plus counselling/behavioral interventions), and inactive or minimal control. The sample size of available numbers of studies precludes further grouping of interventions. Categorization was based on treatment actions of each randomized arm instead of case-control divisions in a study. For example, as the comparison group, the standard treatment in Hall et al. (2009) incorporated 12 weeks of bupropion, 10 weeks of nicotine gum plus 5 group counseling sessions, and thus was defined as a multimodal treatment rather than a control group.
2.4. Analysis at the Study Level
We conducted conventional fixed- and random-effects meta-analyses of risk ratios (RR) at the study level, using Mantel-Haenszel and DerSimonian and Laird methods, respectively. To determine the effect sizes of the studies identified in this review, our analyses were based primarily on results from fixed-effects analysis. Results from random-effects analysis were provided to aid understanding of the estimates of a larger population of studies (Hedges and Vevea, 1998). Heterogeneity in effect sizes was examined by I2 statistic, and values of 25%–50%, 50%–75%, and ≥75% are considered low, moderate, and high, respectively (Higgins et al., 2003; Higgins and Thompson, 2004).
Studies with both a treatment group and an inactive control (comparison) group with minimum intervention were included in this level of analysis. Trials that involved an active comparison group were excluded from the analysis, because they did not examine the same effect size as studies with inactive controls. Studies testing more than one type of treatment strategies provided more than one pair of outcomes. A funnel plot was subsequently obtained to examine potential bias of the meta-analyses (Egger et al., 1997). Both Egger’s test and Harbord’s modified test were used to test for small-study effects—a tendency of larger treatment effects in smaller studies (Harbord et el., 2009; Sterne et al., 2000).
The reporting quality of the included studies was assessed by the 25-item Consolidated Standards of Reporting Trials (CONSORT) 2010 checklist (Moher et al., 2010). Twelve of the 25 items included two sub-items, so there was a total number of 37 items in the checklist. The quality of the evidence was rated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (Guyatt et al., 2011).
2.5. Weighted Least Squares (WLS) Meta-regression at the Outcome Level
2.5.1. Study Variables
The dependent variable of the meta-regression was abstinence rate from a trial arm. We used intention-to-treat (ITT) outcomes wherever possible. The majority of the studies reported 7-day or longer point prevalent abstinence from smoking, based on participants’ self-report with/without biochemical verification. In the study-level analysis, we extracted cessation outcomes observed with highest dosage, longest follow-up time, and for the combined older age group. The unit of analysis for the WLS meta-regression was any abstinence rate reported in a trial. For example, a number of studies reported abstinence rates at different follow-up times (Doolan et al., 2008; Ferketich et al., 2012; Hall et al., 2009) or various age subgroups (Dale et al., 2001; McFall et al., 2010; Vetter and Ford, 1990), thus supplying more than one data point to the regression.
The thirteen independent variables contained ten variables of trial characteristics, two variables of sample demographics, and an intercept. The key trial characteristics were three dummy variables representing intervention types, with control as the reference; and two dummy variables indicating the mode of delivery (i.e., printed materials/mailings and phone intervention), with face-to-face intervention as the reference group. Face-to-face discussion especially with doctors is the preferred mode of delivery, although other modes have been shown to improve patients’ knowledge and compliance with interventions (Harris et al., 2002). Past meta-analyses suggested that non-treatment factors such as definitions of abstinence, follow-up length, sample and setting may all contribute to variations in abstinence rates (Hughes et al., 2003). Measurements of reported abstinence rates were categorized into point prevalence abstinence (reference group), prolonged abstinence and continuous abstinence. It is expected that point prevalence is higher than prolonged or continuous abstinence at a given follow-up time (Hughes et al., 2003). Biochemical verification was dichotomized, indicating whether self-reported smoking cessation status was verified by biochemical methods, such as examining the carbon monoxide (CO) level in exhaled air and cotinine level in saliva or urine. It has been reported that self-reported cessation rates were higher than biochemically verified rates in a given study (Noonan et al., 2013; Rigotti et al., 2014). Follow-up times reported in different units were converted to values in months, and they were further classified into three categories: up to 3 months (reference), 3 to 12 months (binary variable), and over 12 months (binary variable). Regarding the sample demographics, we controlled for age and gender composition, since national survey data show varied cessation rates by age group and gender (CDC, 2011). While mean age could be used to characterize each randomized group, due to missing information in many observations, the lower bound of an age group was employed. Specifically, the lower bound of an age group referred to the minimum age of the sample for studies on smokers aged ≥ 50 years, or the minimum age of an age group for studies reporting stratified results by age group. The last covariate in the regression was percentage of female subjects in the trial arm.
We also recorded the upper bound of the age group, mean age, percentage of white participants, average number of cigarettes consumed per day, mean duration of smoking in years, and mean pack-years in each group where available. However, they were not covariates in the regression because substantial missing information on these variables would decrease the sample size, leading to lower analysis power. To evaluate one additional covariate, more than 10 additional observations are needed (Baker et al., 2009). The ratio of independent variables to observations in this meta-regression (13 to 138) fell within the recommended range (lower than 1 to 10).
2.5.2. Model Description
Publication bias was defined as “The result of the tendency of authors to submit, organizations to encourage, reviewers to approve, and editors to publish articles containing ‘positive’ findings (e.g., a gene-disease association), especially ‘new’ results, in contrast to findings or reports that do not report statistically significant or ‘positive’ results” (Porta, 2008). In the presence of publication selection bias, WLS meta-regression has been shown to outperform random-effects meta-regression, especially when large heterogeneity was present (Stanley and Doucouliagos, 2013). Specifically, simulation results indicated that WLS meta-regression yielded smaller bias and mean squared error than random-effects meta-regression, regardless of publication bias (Stanley and Doucouliagos, 2013). Meta-analysts in medical research have long recognized the important role of weighted regression in adjusting for publication bias and heterogeneity (Moreno et al., 2009; Thompson and Sharp, 1999).
Following notations from Cameron and Trivedi (2010), we first specified a linear regression model with heteroskedastic independent errors as follows:
| (1) |
Where Yi represented abstinence rates, xi was a vector of independent variables abovementioned, μi denoted an error term equaling to σ(zi)εi. It was assumed that E(εi|xi, zi) = 0, E(εiεj|xi, zi, xj, zj) = 0 when i ≠ j, and . The errors were heteroskedastic with variance σ2(zi), so Ordinary Least Squares (OLS) estimates were consistent but not efficient. Transforming model (1) by multiplying each term by Wi = 1/σ(zi) would yield the following linear regression model with homoskedastic errors.
| (2) |
Based on a robust estimate of the error variance, an OLS estimator for the transformed model yielded a WLS estimator, which was more efficient than OLS estimates for model (1).
In this study, the skedasticity function was specified as , where vi was a vector of factors including the log of sample size and trial location from each study that influenced the skedasticity function, and α represented the corresponding coefficients. Differences in study methodology might result in heteroskedastic errors. Large trials usually yield lower standard errors (higher precision) than small trials (Egger et al., 1997). A systematic review of controlled trials indicated that unusually high proportions of favorable treatment effects were found in trials conducted in certain countries (China, in particular) than other countries (e.g., England, U.S.; Vickers et al., 1998). The trial location in this study was a binary variable indicating whether the trial was conducted in the U.S.
Based on the estimated regression coefficients (β̂), we calculated the average abstinence rates of different treatment categories by setting the treatment dummies at specified values, and other covariates at sample values. For instance, average point prevalence abstinence rate for multimodal treatment was calculated by setting the dummies for pharmacological, non-pharmacological, multimodal interventions, prolonged abstinence, and continuous abstinence, to 0, 0, 1, 0, 0, respectively, for all observations. All statistical analyses were carried out in STATA Version 13.1 (Cameron and Trivedi, 2010).
3. RESULTS
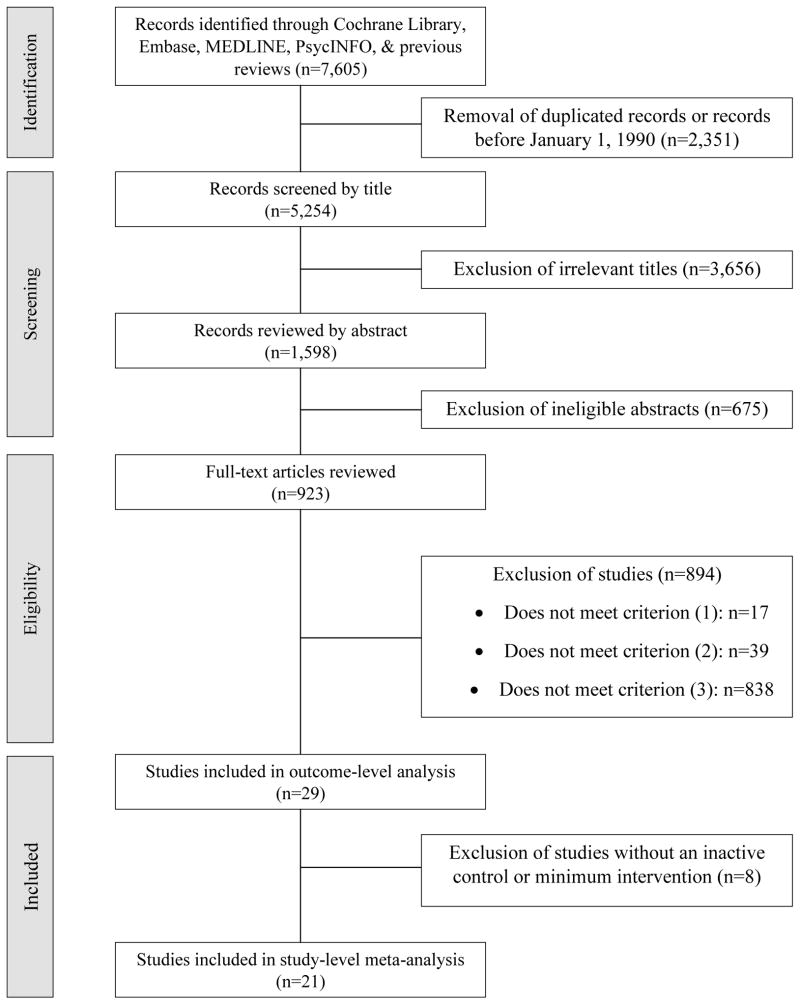
Searches through databases and review articles located 7,605 records (Figure 1). After removing 2,351 duplicated records or records before January 1, 1990, 3,656 irrelevant titles, and 675 ineligible abstracts, 923 articles were retained for full-text review. Of them, 894 were eliminated for not fulfilling one of the criteria. Thus, 29 studies were included for outcome-level analysis. Eight studies without an inactive control or minimum intervention were excluded; 21 studies were retained for study-level meta-analysis.
Figure 1.
Flow chart of article identification
3.1. Study-level Results
3.1.1. Descriptive Results
Of the 29 studies, 21 studies compared efficacies between interventions and inactive or minimal controls, yielding 25 comparisons in the study-level meta-analysis (See Table S11 for additional details). Two studies adopted pharmacological treatments alone; 16 employed non-pharmacological interventions; 8 used multimodal interventions. Two studies adopted both non-pharmacological and multimodal interventions; and one study employed both pharmacological and multimodal interventions. The majority of the trials were conducted in the U.S. (n=17); the rest (n=12) came from other countries with distinct demographic features. Sample sizes of the 29 studies ranged from 18 participants (Ferketich et al., 2012) to 7,354 Medicare beneficiaries (Joyce et al., 2008). Eighteen of the 29 identified trials focused on middle and older smokers aged ≥ 50 years. Others included wider age ranges, but reported abstinence rates for older age subgroups. Seven studies indicated that their trials were blinded either on the participant or investigator side, and four reported no blinding. It was unclear whether blinding was implemented in other studies. Studies also differed in some inclusion criteria such as pre-existing diseases and smoking intensities.
The 29 studies on average reported 20 items (range: 6–28) out of the 37 items in the CONSORT 2010 checklist. The numbers and percentages of reporting for individual items varied (Table S22). Three of the items were reported in all the studies (items 2b, 5, 6a); twelve of the items were reported in over 25 of the studies (1b, 1a, 3a, 4a, 12a, 12b, 13a, 15, 16, 18, 22, 25). Some of the items were poorly reported with fewer than 5 reports out of 29 studies, including allocation concealment mechanism (9), implementation (10), blinding (11a, 11b), presentation of both absolute and relative effect sizes for binary outcomes (17b), harms (19) and protocol (24). Four items largely concerning changes to the trial design and outcome after commencement, and interim analysis and stopping rules were not mentioned in any of the studies (3b, 6b, 7b, 14b).
By the GRADE criteria, the quality of evidence was high for studies on pharmacological and multimodal interventions (Table S33). There were only two studies on pharmacological interventions alone, and they generated a relatively large pooled effect (strong association), with a wide confidence interval (moderate imprecision). The quality of evidence for studies of non-pharmacological interventions was low with serious risks of bias and inconsistency.
3.1.2. Estimates from Meta-analysis
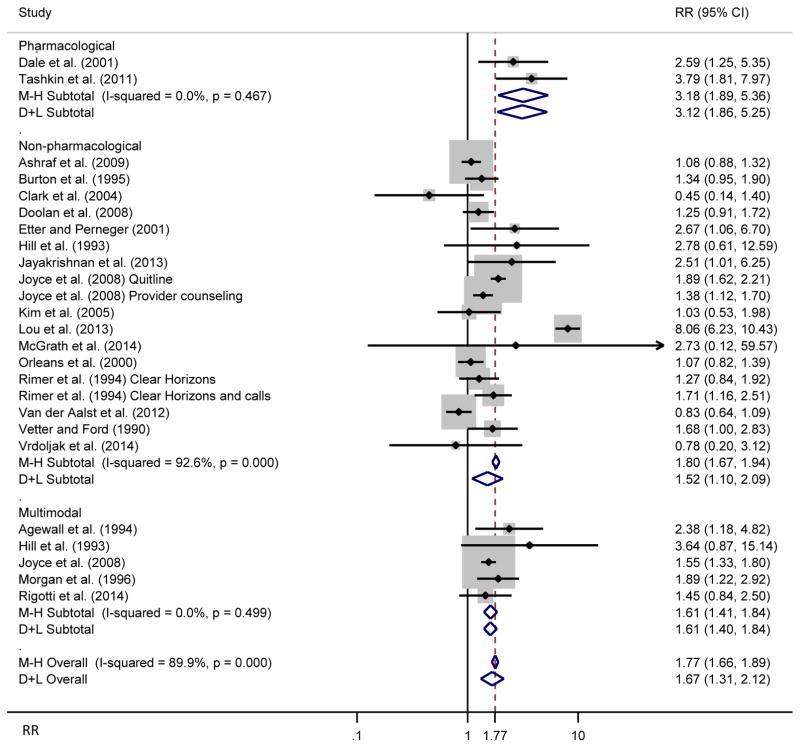
Stratified meta-analysis results by treatment type are presented in Figure 2. Fixed-effects models indicated that pharmacological (Relative Cessation Rate or Risk Ratio [RR]=3.18, 95% confidence intervals [CI]: 1.89–5.36), non-pharmacological (RR=1.80, 95% CI: 1.67–1.94), and multimodal interventions (RR=1.61, 95% CI: 1.41–1.84) were associated with higher cessation rates than control groups. There was high heterogeneity among studies testing the effects of non-pharmacological interventions (I2=92.6%); there was no evidence of heterogeneity among pharmacological (I2=0.0%) and multimodal trials (I2=0.0%).
Figure 2.
Forest plot showing a fixed-effects meta-analysis of smoking cessation interventions on abstinence stratified by treatment category, along with overall random effects
Note: RR stands for risk ratio, which could be interpreted as relative cessation rates. 25 RRs were obtained from 21 studies.
Random-effects analyses tended to give lower pooled treatment effects in all models and conservative estimates of treatment effects (wider confidence intervals) than fixed-effects models when there was substantial heterogeneity. The treatment effects of non-pharmacological interventions were lower with wider confidence intervals (RR=1.52, 95% CI: 1.10–2.09).
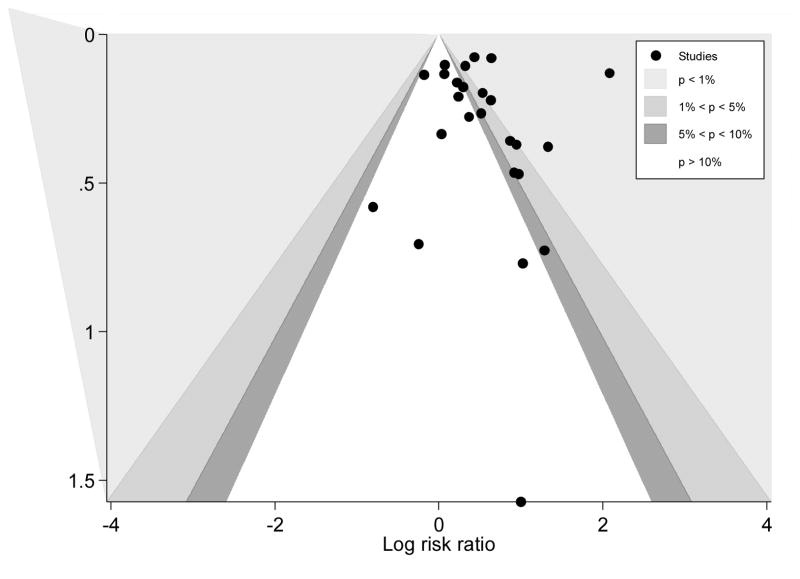
The funnel plot with contours of statistical significance indicated missing observations on the left-hand side of the plot, suggesting a lack of studies reporting non-significant and significantly lower cessation rates in intervention groups (Figure 3). If it was assumed that studies were selected based on two-sided p-values, then publication bias was likely to be one of the factors for funnel asymmetry (Palmer et al., 2008). However, there was no significant evidence of small-study effects by Egger’s test (p=0.781) and Harbord’s modified test (p=0.357).
Figure 3.
Funnel plot with contours of statistical significance
3.2. Outcome-level Analysis
3.2.1. Descriptive Results
Among 138 observations drawn from the 29 studies, the average abstinence rate was 26.31% (Table 1). Mean cessation rates from pharmacological, non-pharmacological, multimodal interventions, and control arms were 29.69% (n=11), 23.72% (n=46), 36.67% (n=45), and 15.64% (n=36), respectively. Most of the outcomes were point prevalence abstinences (78.26%), biomedically verified (63.77%), delivered face-to-face (71.01%), and conducted in the United States (73.91%). One-way ANOVA indicated that the mean cessation rates, lower and upper bound of age range, mean smoking duration, and pack-years differed significantly among the four categories. Chi-square test or Fisher’s exact test (if one or more of the cells has an expected frequency of five or less) for binary variables suggested that there were statistically significant relationships between biomedical verification, delivery of intervention, follow-up length, and location with treatment (control) categories.
Table 1.
Summary statistics by intervention category
| Whole Sample
|
Pharmacological
|
Non-Pharmacological
|
Multimodal
|
Control
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) or % | Min | Max | N | Mean (SD) or % | N | Mean (SD) or % | N | Mean (SD) or % | N | Mean (SD) or % | |
|
|
|
|
|
|
||||||||
| Abstinence Rate (%) | 138 | 26.31(17.46)*** | 0 | 66 | 11 | 29.69(20.94) | 46 | 23.72(14.33) | 45 | 36.67(17.43) | 36 | 15.64(12.41) |
| Point Prevalence Abstinence (1=Yes, 0=No) | 138 | 78.26% | 0 | 1 | 11 | 54.55% | 46 | 82.61% | 45 | 84.44% | 36 | 72.22% |
| Prolonged Abstinence (1=Yes, 0=No) | 138 | 18.84%† | 0 | 1 | 11 | 45.45% | 46 | 13.04% | 45 | 15.56% | 36 | 22.22% |
| Continuous Abstinence (1=Yes, 0=No) | 138 | 2.90% | 0 | 1 | 11 | 0.00% | 46 | 4.35% | 45 | 0.00% | 36 | 5.56% |
| Biomedical Verification (1=Yes, 0=No) | 138 | 63.77%*** | 0 | 1 | 11 | 100.00% | 46 | 41.30% | 45 | 82.22% | 36 | 58.33% |
| Face-to-face Intervention (1=Yes, 0=No) | 138 | 71.01%*** | 0 | 1 | 11 | 100.00% | 46 | 50.00% | 45 | 95.56% | 36 | 58.33% |
| Printed Materials/Mailings (1=Yes, 0=No) | 138 | 21.74%*** | 0 | 1 | 11 | 0.00% | 46 | 30.43% | 45 | 2.22% | 36 | 41.67% |
| Phone Intervention (1=Yes, 0=No) | 138 | 7.25%** | 0 | 1 | 11 | 0.00% | 46 | 19.57% | 45 | 2.22% | 36 | 0.00% |
| Follow-up (Up to 3 months) (1=Yes, 0=No) | 138 | 23.19%* | 0 | 1 | 11 | 63.64% | 46 | 21.74% | 45 | 17.78% | 36 | 19.44% |
| Follow-up (3 to 12 months) (1=Yes, 0=No) | 138 | 50.72%* | 0 | 1 | 11 | 9.09% | 46 | 58.70% | 45 | 51.11% | 36 | 52.78% |
| Follow-up (Over 12 months) (1=Yes, 0=No) | 138 | 26.09% | 0 | 1 | 11 | 27.27% | 46 | 19.57% | 45 | 31.11% | 36 | 27.78% |
| US (1=Yes, 0=No) | 138 | 73.91%** | 0 | 1 | 11 | 81.82% | 46 | 69.57% | 45 | 91.11% | 36 | 55.56% |
| Sample Size | 138 | 269.93(481.43) | 4 | 2605 | 11 | 71.82(57.50) | 46 | 313.52(421.97) | 45 | 206.76(526.09) | 36 | 353.75(549.76) |
| Lower Bound of Age Range | 138 | 54.82(6.74)*** | 50 | 80 | 11 | 56.73(6.05) | 46 | 56.54(7.37) | 45 | 51.53(3.64) | 36 | 56.14(7.76) |
| Upper Bound of Age Range | 37 | 69.41(6.99)*** | 56 | 79 | 5 | 61.20(3.90) | 12 | 73.50(3.58) | 8 | 62.50(6.65) | 12 | 73.33(3.60) |
| Mean Age | 72 | 59.44(7.73) | 9.5 | 72.3 | 0 | 0.00(0.00) | 23 | 61.70(5.24) | 30 | 57.48(2.15) | 19 | 59.78(13.48) |
| Female (%) | 138 | 47.49(25.34) | 0 | 100 | 11 | 37.60(20.87) | 46 | 54.52(23.73) | 45 | 44.96(22.27) | 36 | 44.72(30.48) |
| White (%) | 96 | 76.57(28.61) | 0 | 100 | 11 | 83.11(16.79) | 28 | 77.09(37.08) | 36 | 78.14(9.57) | 21 | 69.77(40.65) |
| Mean No. of Cigarettes Per Day | 93 | 22.89(4.35)† | 14.6 | 32.8 | 9 | 25.11(2.82) | 28 | 24.06(4.19) | 39 | 21.74(4.37) | 17 | 22.41(4.61) |
| Mean Smoking Duration (Year) | 74 | 39.01(3.72)*** | 30.4 | 46 | 5 | 37.22(2.90) | 21 | 42.11(3.09) | 36 | 36.90(2.89) | 12 | 40.67(2.73) |
| Mean Pack-years | 72 | 44.25(8.94)*** | 23.9 | 58.1 | 3 | 37.56(0.00) | 22 | 51.09(7.98) | 36 | 40.38(7.41) | 11 | 45.06(8.36) |
Notes: “N” denoted number of observations. 138 observations were obtained from 29 studies. For binary variables, we presented the percentages of observations equal to 1 in the table. Significance levels were obtained by conducting one-way ANOVA for continuous variables, and Chi-square test or Fisher’s exact test (if one or more of the cells has an expected frequency of five or less) for binary variables across treatment categories.
p<0.05;
p<0.01;
p<0.001.
3.2.2. WLS meta-regression
The study-level analyses cannot examine the sources of heterogeneity, and random-effects meta-regression is not the best solution in the presence of publication bias and large heterogeneity. Therefore, we conducted WLS meta-regressions to explore whether abstinence rates differ by intervention strategies and whether the heterogeneity in effect sizes were related to design and sample characteristics.
Results from the WLS meta-regression are shown in Table 2. Sample size and the location variable, US, were not associated with error variance. At the 5% significance level, non-pharmacological and multimodal interventions yielded higher abstinence rates than control groups, after adjusting for heteroskedastic errors related to the trial location and sample size, and controlling for other covariates. However, the coefficient for pharmacotherapy was not statistically significant. Prolonged abstinence rates were lower than point prevalence abstinence rates. Cessation rates with biochemical confirmation were higher than unverified rates. Intervention delivered over the phone had lower abstinence rates than face-to-face intervention. Having 3–12 months of follow-up was marginally associated with decreased cessation rates compared with having ≤3 months of follow-up. A 1 year increase in the lower bound of the age range was associated with 0.42 percentage point increase in abstinence rate. A 1 percentage point increase in the percentage of female participants was associated with a 0.14 percentage point increase in abstinence rate.
Table 2.
Results from weighted least square meta-regression with abstinence rates as the dependent variable
| Variable Name
|
Coefficient β
|
Standard Error
|
P value
|
|---|---|---|---|
| Control (reference) | |||
| Pharmacological | 7.30 | 5.90 | 0.218 |
| Non-pharmacological | 9.17 | 2.87 | 0.002 |
| Multimodal | 17.84 | 3.46 | <0.001 |
| Face-to-face Intervention (reference) | |||
| Printed Materials/Mailings | −3.69 | 3.75 | 0.327 |
| Phone Intervention | −8.43 | 3.69 | 0.024 |
| Point Prevalence Abstinence (reference) | |||
| Prolonged Abstinence | −10.95 | 4.02 | 0.007 |
| Continuous Abstinence | 0.61 | 5.07 | 0.904 |
| Biomedical Verification | 12.90 | 3.65 | 0.001 |
| Follow-up (Up to 3 months) (reference) | |||
| Follow-up (3 to 12 months) | −6.21 | 3.00 | 0.041 |
| Follow-up (Over 12 months) | −0.47 | 4.03 | 0.907 |
| Lower Bound of Age Range | 0.42 | 0.21 | 0.047 |
| Female Percentage | 0.14 | 0.07 | 0.046 |
| Intercept | −14.60 | 11.96 | 0.224 |
| Error Variance Equation
|
Coefficient α
|
||
| Log of sample size | −0.02 | 0.09 | 0.796 |
| US | −0.17 | 0.25 | 0.494 |
| Intercept | 5.37 | 0.51 | <0.001 |
R2 = 0.45, F(12, 138) = 9.19, P <0.001
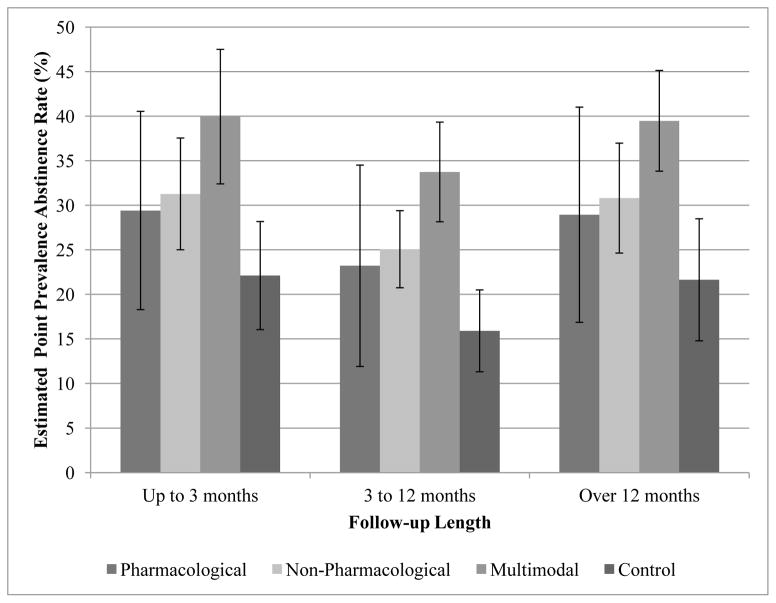
Based on estimated coefficients from the meta-regression, we calculated the average point prevalence abstinence rates by treatment groups (including the control group), adjusting for other covariates in the regression. Pharmacological intervention (mean point prevalence abstinence rate = 26.10%, CI: 15.20–37.00) resembled non-pharmacological (27.97%, CI: 24.00–31.94), and multimodal interventions (36.64%, CI: 31.66–41.62); and non-pharmacological and multimodal interventions had higher abstinence rates than the control group (18.80%, CI: 14.48–23.12). The estimated point prevalence abstinence rates were further stratified by both intervention category and follow-up length (Figure 4).
Figure 4.
Estimated mean abstinence rate by treatment group and follow-up length
4. DISCUSSION
4.1. Main Findings
This study identified 29 RCTs of smoking cessation interventions for smokers aged 50 and older, compared to 13 studies identified in a recent review (Zbikowski et al., 2012). Results based on the CONSORT checklist suggested that the reporting quality of these studies was moderate. Using GRADE criteria, the quality of evidence was high for studies on pharmacological and multimodal interventions, and low for studies of non-pharmacological interventions. This study is among the first to quantitatively review relative effect sizes of different treatment modalities for older smokers. By employing both study-level and outcome-level analyses, it identified various trial and sample characteristics related to heterogeneous abstinence rates.
First, descriptive statistics at the outcome-level showed that mean cessation rates differed significantly among pharmacological, non-pharmacological multimodal interventions, and control groups. Second, fixed-effects meta-analyses at the study level indicated that there were significant treatment effects in pharmacological, non-pharmacological and multimodal interventions. Random-effects analyses generated lower pooled effect sizes in all types of interventions and wider confidence intervals in non-pharmacological interventions than fixed-effects analyses. Third, WLS meta-regression results at the outcome level similarly showed that non-pharmacological and multimodal interventions had significantly higher abstinence rates than the inactive control or minimum intervention group. We also found that use of biochemical verification (vs. non-verification), lower bound of age range, and percentages of female subjects were positively associated with cessation rates, whereas use of phone intervention (vs. face-to-face intervention), prolonged abstinence (vs. point prevalence), follow-up at 3–12 months (vs. follow-up at 3 months or less) was negatively related to abstinence rates.
Our findings are consistent with prior studies on adult smokers in general, showing that multimodal interventions usually produce the highest abstinence rates (APA, 2006; Zbikowski et al., 2012). Pharmacotherapy and behavioral interventions have been considered two complimentary modalities to improve smoking cessation synergistically, so a combined approach is often recommended by clinical practice guidelines (Stead and Lancaster, 2012). Pharmacotherapy alone is an effective treatment for smoking cessation, but it may not be as effective as the combined approach, partly because patients’ compliance with medication could be improved by various behavioral interventions such as motivational interviewing, family-assisted approaches, or cognitive behavioral therapies (Carroll et al., 2004).
Our results suggested that face-to-face interventions generated higher cessation rates than interventions by phone after controlling for a number of other trial and sample characteristics. This is in line with findings that face-to-face discussions with health professionals such as physicians and nurses are the preferred means of information delivery for patients (Harris et al., 2002; James et al., 1999).
We also found that studies using biochemical verification showed higher cessation rates than those without, which was seemingly contradictory to evidence that self-reported cessation rates were overestimated (Noonan et al., 2013; Rigotti et al., 2014). For instance, one study investigating the validity of self-reported cessation by a biochemical test found that 15 out of 72 self-reported quitters did not pass the urine cotinine test, resulting in an over-reporting rate of 21% (Noonan et al., 2013). However, the meta-regression analysis compared results from studies with vs. those without using biochemical verification (instead of comparing unverified and verified results from the same study); thus, the results should be interpreted differently.
This review suggests an association between a trial’s follow-up times and cessation rates. Some studies showed lower cessation rates at longer follow-up times (Ferketich et al., 2012; Tashkin et al., 2011) or fluctuations but a generally downward trend over time (Hall et al., 2009; Hill et al., 1993). Potential reasons for this pattern may be related to relapse in the post-treatment period (Tashkin et al., 2011) or a decline in compliance to intervention over time (Costello et al., 2011). On the other hand, two studies using self-help materials (i.e., Clear Horizons Guide tailored to older smokers) generated comparatively high cessation rates at longer follow-up times (Ossip-Klein et al., 1997; Rimer et al., 1994). Rimer et al. (1994) concluded that “referring to the Clear Horizons Guide repeatedly over time” would increase the time of exposure to intervention. In such a situation, follow-up time may be suggestive of some levels of treatment exposure. In this analysis, follow-up times were based on study time points reported by each study. The regression results showing higher cessation rates in the short-term category (≤ 3 months of follow-up) were specific to the studies included in the analysis and generally agreed with findings from most studies except for the two studies on self-help manuals.
The positive association between lower bound of age range and cessation rates indicates that increased age may be associated with higher cessation rates among middle and older aged smokers. This is consistent with evidence from interventions stratified by age groups (Dale et al., 2001; Doolan et al., 2008; McFall et al., 2010; Tashkin et al., 2011). The gender differences in cessation rates are in line with observational findings from surveys in that a lower proportion of male smokers than female smokers quitted smoking (CDC, 2011). However, previous studies were mixed regarding gender differences in the efficacy of nicotine replacement therapy (NRT). While an earlier review did not find gender difference in quitting rate (Munafò et al., 2004), two prior reviews showed that women were less likely than men to quit when using NRT (Cepeda-Benito et al., 2004; Perkins and Scott, 2008). Biological, psychological, and social factors may be related to gender differences in cessation outcome (Torchalla et al., 2011). Women have special genetic variations that may reduce the benefit of NRT, have higher nicotine and cotinine metabolism levels due to hormone changes in the body or more weight concerns, and they are more likely than men to develop negative mood and depression during a quit attempt (Torchalla et al., 2011). On the other hand, there was evidence that, compared to men, female smokers were more likely to adopt treatment in a quit attempt, especially behavioral and multimodal treatment (Shiffman et al., 2008).
Methodologically, this study not only analyzed the effect sizes of smoking cessation RCTs by fixed- and random-effects models at the study level, but also used WLS meta-regression to explore the sources of heterogeneity at the outcome level. The lack of non-significant and unfavorable results for cessation interventions in the funnel plot suggested possible publication bias, though we did not find any small-study effects in studies included for the meta-analysis. WLS meta-regression performed well in the presence of publication bias and substantial heterogeneity. We adjusted for possible heteroskedatic errors associated with trial locations and sample sizes in the meta-regression, but their effects were not significant.
4.2. Limitations
First, sample size at the study level was small, reflecting a pattern that smoking cessation interventions among older adults have been understudied. Indeed, only a small portion of trials have focused on older adults specifically or reported cessation outcomes stratified by age groups. Older smokers are at high risk of morbidity and mortality, so cessation interventions targeted at the elderly deserve research. The number of observations in the pharmacotherapy only group was quite small, which resulted in a wider confidence interval than other categories. The estimation for this category should be interpreted with caution.
Second, there are a number of sources for funnel plot asymmetry. Only 29 studies published in English were included in the analyses. That is, published studies are more likely to report significant results than unpublished ones, and studies published in languages other than English may have different preferences. Although we used WLS meta-regression to reduce the estimation bias in response to potential publication bias, not every source of funnel plot asymmetry was explored by the analyses. For example, studies reporting “negative” results are less likely to be cited, which has decreased their probability of being located during the literature search process (Egger et al., 1997).
Third, covariates in the meta-regression were not exhaustive. Survey data have shown that whites (compared to ethnic minorities) and heavier smokers were more likely to undertake treatment (Shiffman et al., 2008), so racial composition and pre-treatment smoking intensity were both good candidates for the model. However, univariate regression did not show that percentage of white participants and mean pack-years in the trial arm were significant predictors of abstinence rates (results not shown). In addition, there was missing information for these two variables in a large proportion of the observations, so they were not included in the final model. Mean age was substituted by the lower bound of the age group for the same reason. Overall, this highlights an important issue in the quality of trial reporting, and it calls for a need to improve reporting for older smokers’ racial/ethnic backgrounds and smoking history.
Other unmeasured factors related to outcome heterogeneity are not accounted for. Regardless of treatment types, more intensive treatment tends to show better outcome; but it is difficult to find a variable that measures treatment intensity consistently across studies of various intervention strategies. Not all the studies reported ITT results (almost half of the observations in the outcome level analysis), which might increase reported abstinence rates and bias. It should be emphasized that different categorization of treatment methods might yield different results. Finally, our results are also limited by the number of databases searched and by not searching non-academically published articles. We restricted our review to studies published in English between January 1, 1990 and January 28, 2015. The search results were screened systematically by a single researcher (the first author). A multilingual and larger research team may identify more relevant studies and increase the reliability of search results.
4.3. Conclusions and Recommendations
Overall, this meta-analysis adds quantitative evidence to the field regarding smoking cessation interventions for older smokers. Regardless of the type of method being used, study-level analyses show that there are significant treatment effects in pharmacological, non-pharmacological, and multimodal interventions, in relation to the control group. Similarly, outcome-level regression results indicate that all the intervention types have higher cessation rates than the control group except for pharmacotherapy. The regression results suggest that cessation rates vary with a number of trial and sample characteristics.
The overall results are based on the available information reported by identified studies, and they should be considered as preliminary estimates and interpreted within the context of study limitations, including a small sample size of available studies for analysis. One important reason for focusing on smokers aged 50 and over is the lack of studies on older smokers aged 65 and over. This review reveals a need for more controlled trials on smoking cessation for both middle-aged and older smokers to better understand how and whether combining pharmacotherapy with behavioral interventions improve abstinence rates for different gender and racial/ethnic groups. This review also suggests that multimodal interventions delivered face-to-face, with rigorous methodology (e.g., with biochemical verification of cessation), and strict implementation are preferred designs.
Given a high proportion of white population in extant studies, future studies should reach a more diverse population by recruiting more minorities. There was a low probability of adopting treatment among less-educated, low-income, and minority smokers (Shiffman et al., 2008).
The lack of information on sequence generation, allocation concealment, and blinding increased the risk of bias in studies using non-pharmacological interventions. Missing information on trial and sample characteristics, such as mean age and smoking intensity of each randomized arm, has limited the number of covariates in the meta-regression. As such, we urge researchers to report randomization methods, key demographics, smoking history and patterns more thoroughly, which will facilitate the process of evaluation and improve the quality of estimates in future meta-analyses.
Non-pharmacological interventions yielded highly heterogeneous outcomes across studies. Additional research is needed to identify the sources of serious inconsistency in this type of intervention particularly.
Supplementary Material
Highlights (for review).
We conducted a meta-analysis of smoking cessation trials of adults aged ≥50 years.
Pharmacological, non-pharmacological, and multimodal interventions were examined.
Nearly all the intervention types had higher cessation rates than control groups.
Cessation rates varied depending on a number of trial and sample characteristics.
This review shows a need to increase smoking cessation studies in older minorities.
Acknowledgments
Role of the Funding Source: This work was made possible by research support from the U.S. National Institutes of Health (R01MD007658, R01DA019623, R01DA019901, R33DA027503; PI, Li-Tzy Wu) and by Duke University Department of Psychiatry and Behavioral Sciences. The sponsoring agency had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. The opinions expressed in this paper are solely those of the authors.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors: Danhong Chen contributed to study designs, conducted literature searches and data analyses, and drafted the manuscript. Li-Tzy Wu contributed to study designs and analyses, drafted the manuscript, and supervised the work. Both contributing authors, Danhong Chen and Li-Tzy Wu, have participated in the revision and concurred with the resubmission.
Conflict of Interest: No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah ASM, Simon JL. Health promotion in older adults: evidence-based smoking cessation programs for use in primary care settings. Geriatrics. 2006;61:30–34. [PubMed] [Google Scholar]
- Agaku IT, King BA, Dube SR. Current cigarette smoking among adults-United States, 2005–2012. MMWR. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- APA. Practice guideline for the treatment of patients with substance use disorders. (2) 2006 Retrieved 8 Oct. 2014 from http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243188&PDFSource=6.
- Baker WL, Michael White C, Cappelleri JC, Kluger J, Coleman CI From the Health Outcomes, P., Economics Collaborative, G. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract. 2009;63:1426–1434. doi: 10.1111/j.1742-1241.2009.02168.x. [DOI] [PubMed] [Google Scholar]
- Cameron AC, Trivedi PK. Microeconometrics Using Stata. Stata Press; Texas: 2010. Revised Edition. [Google Scholar]
- Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug Alcohol Depend. 2004;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Quitting smoking among adults--United States, 2001–2010. MMWR. 2011;60:1513–1519. [PubMed] [Google Scholar]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol. 2004;72:712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Costello M, Sproule B, Victor JC, Leatherdale S, Zawertailo L, Selby P. Effectiveness of pharmacist counseling combined with nicotine replacement therapy: a pragmatic randomized trial with 6,987 smokers. Cancer Causes Control. 2011;22:167–180. doi: 10.1007/s10552-010-9672-9. [DOI] [PubMed] [Google Scholar]
- Dale LC, Glover ED, Sachs DPL, Schroeder DR, Offord KP, Croghan IT, Hurt RD. Bupropion for smoking cessation: predictors of successful outcome. Chest. 2001;119:1357–1364. doi: 10.1378/chest.119.5.1357. [DOI] [PubMed] [Google Scholar]
- DHHS. The Health Consequences Of Smoking—50 Years Of Progress: A Report Of The Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan DM, Froelicher ES. Smoking cessation interventions and older adults. Prog Cardiovasc Nurs. 2008;23:119–127. doi: 10.1111/j.1751-7117.2008.00001.x. [DOI] [PubMed] [Google Scholar]
- Doolan DM, Stotts NA, Benowitz NL, Covinsky KE, Froelicher ES. The Women’s Initiative for Nonsmoking (WINS) XI: age-related differences in smoking cessation responses among women with cardiovascular disease. Am J Geriatr Cardiol. 2008;17:37–47. doi: 10.1111/j.1076-7460.2007.07360.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferketich AK, Otterson GA, King M, Hall N, Browning KK, Wewers ME. A pilot test of a combined tobacco dependence treatment and lung cancer screening program. Lung Cancer. 2012;76:211–215. doi: 10.1016/j.lungcan.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Jaen CR, Baker T, Bailey W, Benowitz N, Curry S, Dorfman S, Froelicher E, Goldstein M, Healton C. Treating tobacco use and dependence: 2008 update. A U.S Public Health Service Report. Am J Prev Med. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Muñoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction. 2009;104:1043–1052. doi: 10.1111/j.1360-0443.2009.02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbord RM, Harris RJ, Sterne JA. Updated tests for small-study effects in meta-analyses. Stata J. 2009;9:197. [Google Scholar]
- Harris M, Bayer A, Tadd W. Addressing the information needs of older patients. Rev Clin Gerontol. 2002;12:5–11. [Google Scholar]
- Hedges LV, Vevea JL. Fixed-and random-effects models in meta-analysis. Psychol Methods. 1998;3:486. [Google Scholar]
- Higgins J, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RD, Rigdon M, Johnson S. Behavioral smoking cessation treatment for older chronic smokers. Behav Ther. 1993;24:321–329. [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- James C, James N, Davies D, Harvey P, Tweddle S. Preferences for different sources of information about cancer. Patient Educ Counsel. 1999;37:273–282. doi: 10.1016/s0738-3991(99)00031-2. [DOI] [PubMed] [Google Scholar]
- Jayakrishnan R, Uutela A, Mathew A, Auvinen A, Mathew PS, Sebastian P. Smoking cessation intervention in rural Kerala, India: findings of a randomised controlled trial. Asian Pac J Cancer Prev. 2013;14:6797–6802. doi: 10.7314/apjcp.2013.14.11.6797. [DOI] [PubMed] [Google Scholar]
- Joyce GF, Niaura R, Maglione M, Mongoven J, Larson-Rotter C, Coan J, Lapin P, Morton S. The effectiveness of covering smoking cessation services for medicare beneficiaries. Health Serv Res. 2008;43:2106–2123. doi: 10.1111/j.1475-6773.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin KA. Study design VII. Randomised controlled trials. Evidence-Based Dentistry. 2007;8:22–23. doi: 10.1038/sj.ebd.6400473. [DOI] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Malte CA, et al. Integrating tobacco cessation into mental health care for posttraumatic stress disorder: a randomized controlled trial. JAMA. 2010;304:2485–2493. doi: 10.1001/jama.2010.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux P, Elbourne D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:e1–e37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:332–336. [PMC free article] [PubMed] [Google Scholar]
- Moreno SG, Sutton AJ, Ades AE, Stanley TD, Abrams KR, Peters JL, Cooper NJ. Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Med Res Methodol. 2009;9 doi: 10.1186/1471-2288-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan GD, Noll EL, Orleans CT, Rimer BK, Amfoh K, Bonney G. Reaching midlife and older smokers: tailored interventions for routine medical care. Prev Med. 1996;25:346–354. doi: 10.1006/pmed.1996.0065. [DOI] [PubMed] [Google Scholar]
- Munafò M, Bradburn M, Bowes L, David S. Are there sex differences in transdermal nicotine replacement therapy patch efficacy? A meta-analysis. Nicotine Tob Res. 2004;6:769–776. doi: 10.1080/14622200410001696556. [DOI] [PubMed] [Google Scholar]
- Noonan D, Jiang Y, Duffy SA. Utility of biochemical verification of tobacco cessation in the Department of Veterans Affairs. Addict Behav. 2013;38:1792–1795. doi: 10.1016/j.addbeh.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossip-Klein DJ, Carosella AM, Krusch DA. Self-help interventions for older smokers. Tob Control. 1997;6:188–193. doi: 10.1136/tc.6.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TM, Peters JL, Sutton AJ, Moreno SG. Contour-enhanced funnel plots for meta-analysis. Stata J. 2008;8:242–254. [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–1251. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Pilowsky D, Wu LT. Chapter 15: Tobacco use cessation. In: Crome I, Wu LT, Rao R, Crome P, editors. Substance Use and Older People. John Wiley & Sons, Ltd; UK: 2014. pp. 212–222. [Google Scholar]
- Porta M. Publication bias. In: Porta M, editor. A Dictionary Of Epidemiology. Oxford University Press; 2008. Retrieved 8 Oct. 2014, from http://www.oxfordreference.com.proxy.lib.duke.edu/view/10.1093/acref/9780195314496.001.0001/acref-9780195314496-e-1542. [Google Scholar]
- Rigotti NA, Regan S, Levy DE, Japuntich S, Chang Y, Park ER, Viana JC, Kelley JH, Reyen M, Singer DE. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312:719–728. doi: 10.1001/jama.2014.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimer BK, Orleans CT, Fleisher L, Cristinzio S, Resch N, Telepchak J, Keintz MK. Does tailoring matter? The impact of a tailored guide on ratings and short-term smoking-related outcomes for older smokers. Health Educ Res. 1994;9:69–84. doi: 10.1093/her/9.1.69. [DOI] [PubMed] [Google Scholar]
- Rostron BL, Chang CM, Pechacek TF. Estimation of cigarette smoking–attributable morbidity in the United States. JAMA Intern Med. 2014;174:1922–1928. doi: 10.1001/jamainternmed.2014.5219. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Individual differences in adoption of treatment for smoking cessation: demographic and smoking history characteristics. Drug Alcohol Depend. 2008;93:121–131. doi: 10.1016/j.drugalcdep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Stanley T, Doucouliagos H. Deakin Working Paper No. 2013_2. Deakin University; 2013. Better than random: weighted least squares meta-regression analysis. Retrieved 8 Oct. 2014, from http://www.deakin.edu.au/buslaw/aef/workingpapers/papers/2013_2.pdf. [Google Scholar]
- Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2012;10:CD008286. doi: 10.1002/14651858.CD008286.pub2. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC. Effects of varenicline on smoking cessation in patients with mild to moderate copd: a randomized controlled trial. Chest. 2011;139:591–599. doi: 10.1378/chest.10-0865. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–2708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Torchalla I, Okoli CTC, Hemsing N, Greaves L. Gender differences in smoking behaviour and cessation. J Smok Cessat. 2011;6:9–16. [Google Scholar]
- Vetter NJ, Ford D. Smoking prevention among people aged 60 and over: a randomized controlled trial. Age Ageing. 1990;19:164–168. doi: 10.1093/ageing/19.3.164. [DOI] [PubMed] [Google Scholar]
- Vincent GK, Velkoff VA. The Next Four Decades: The Older Population In The United States: 2010 To 2050. US Department of Commerce, Economics and Statistics Administration, US Census Bureau; 2010. [Google Scholar]
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials. 1998;19:159–166. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]
- Zbikowski SM, Magnusson B, Pockey JR, Tindle HA, Weaver KE. A review of smoking cessation interventions for smokers aged 50 and older. Maturitas. 2012;71:131–141. doi: 10.1016/j.maturitas.2011.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.