Abstract
Activation induced deaminase (AID) initiates somatic hypermutation and class switch recombination of the Ig genes in antigen-activated B cells, underpinning antibody affinity maturation and isotype switching. AID can also be pathogenic by contributing to autoimmune diseases and oncogenic mutations. Moreover, AID can exert non-canonical functions when aberrantly expressed in epithelial cells. The lack of specific inhibitors prevents therapeutic applications to modulate AID functions. Here, we have exploited our previous finding that the HSP90 molecular chaperoning pathway stabilizes AID in B cells, to test whether HSP90 inhibitors could target AID in vivo. We demonstrate that chronic administration of HSP90 inhibitors decreases AID protein levels and isotype switching in immunized mice. HSP90 inhibitors also reduce disease severity in a mouse model of acute B-cell lymphoblastic leukemia in which AID accelerates disease progression. We further show that human AID protein levels are sensitive to HSP90 inhibition in normal and leukemic B cells, and that HSP90 inhibition prevents AID-dependent epithelial to mesenchymal transition in a human breast cancer cell line in vitro. Thus, we provide proof-of-concept that HSP90 inhibitors indirectly target AID in vivo and that endogenous human AID is widely sensitive to them, which could have therapeutic applications.
Keywords: Activation induced deaminase (AID), HSP90 inhibitors, antibody response, class switch recombination, leukemia
Introduction
The humoral immune response generates high affinity antibodies to neutralize invading antigens, as well as different classes of antibodies (IgM, IgG, IgA, IgE) that impart specialized biological functions. Both these characteristics of the antibodies require the enzyme Activation Induced Deaminase (AID) [1]. AID underpins antibody affinity maturation by introducing point mutations over the V region of the Ig genes in antigen-activated B cells, via the mechanism of somatic hypermutation (SHM) [2]. B cells harboring mutations resulting in higher affinity antibodies are selected during the germinal center reaction [3]. AID also initiates the DNA breaks that trigger class switch recombination (CSR), a rearrangement that replaces the Igh exons encoding for IgM for those encoding another isotype [1, 4]. Affinity maturation and isotype switching play important roles in autoimmune diseases and AID can thereby contribute to pathogenesis. AID levels correlate with pathogenic autoantibodies in mouse autoimmune arthritis [5] and MRLlpr/lpr mice, a model of systemic lupus erythematosus (SLE) in which AID function contributes to nephritis [6-8]. Human patients with rheumatoid arthritis and SLE also show higher levels of AID and this is associated to a worst disease [9, 10]. In this context, AID inhibition could be therapeutic but no specific inhibitor is available.
AID has oncogenic side effects that are intrinsically associated with the mechanisms of SHM and CSR. AID overexpression is oncogenic [11, 12] but normal levels of AID can also mutate and induce chromosomal translocations affecting oncogenes and tumor suppressors [13, 14]. AID is most likely etiological in the GC-derived B-cell neoplasms diffuse large B-cell lymphoma and Burkitt’s lymphoma (BL) [14, 15]. AID is also expressed in non GC-derived hematological malignancies such as chronic myelogenous leukemia (CML) [16], B-cell acute lymphoblastic leukemia (B-ALL) [17, 18] and, chronic lymphocytic leukemia (CLL) [19-21]. In these leukemia, AID favors disease progression and correlates with poorer outcome [16, 17, 22-24]. Hence, also in this context AID inhibition could have therapeutic value [25].
Some human epithelial cancers express AID [26], albeit it only seems to produce substantial numbers of mutations in neoplasms of B-cell origin [27]. Nevertheless, AID could still contribute to the progression of certain epithelial cancers through non-canonical functions such as DNA demethylation and transcriptional regulation [28]. Indeed, low levels of AID expression can influence epigenetic reprograming of pluripotent cells and alter the gene expression profile in human fibroblasts [28, 29]. We have shown that AID is necessary for the cytokine-induced epithelial to mesenchymal transition (EMT) in mammary epithelial cell lines: ZR75.1 breast cancer cells depleted of AID fail to upregulate genes required for the EMT and lose metastatic characteristics i.e.: the ability to invade and migrate under EMT-inducing conditions [30]. While the mechanism/s of these non-canonical functions of AID are unknown and their biological relevance is controversial, these evidences indicate that AID has at least the capacity to influence gene expression in certain settings [28]. Thus, inhibiting AID expressed in epithelial malignancies could also have therapeutic value.
Multiple mechanisms regulate AID to permit optimal antibody diversification while minimizing pathological side-effects [31, 32]. Controlling AID protein stability is an important regulatory instance [31]. We have shown that AID interacts with HSP90 and that treating human and mouse B cell lines with HSP90 inhibitors leads to ubiquitin-dependent proteasomal degradation of endogenous and transfected AID in the cytoplasm [33]. Since 90% of AID is cytoplasmic [34], inhibiting the HSP90 molecular chaperoning pathway causes a dose-responsive decrease in the cellular AID levels through protein destabilization, and reduces SHM and CSR in vitro [33, 35]. HSP90 inhibitors show promising clinical activity against various cancers [36, 37] and have gone through security, toxicity and bioavailability tests in animals and humans; providing a practical possibility for targeting AID in vivo. It is also important to determine whether they affect AID and the antibody response to better evaluate the outcome of those clinical trials. Here, we provide evidence that AID protein levels and activity can be reduced in vivo by the HSP90 inhibitor 17-DMAG, currently in clinical trials [38-41]. We additionally show that AID levels in normal and cancerous human B cells, as well as the non-canonical functions of AID in epithelial cells, are sensitive to HSP90 inhibition.
Results
The HSP90 inhibitor 17-DMAG reduces AID levels in vivo
AID is not constitutively expressed in B cells but induced in the GC [42]. Naïve B cells display a wide repertoire of random antibody specificities and only a few respond to any given antigen [3]. Thus, only ~2-4% of the splenic B cells express AID after immunization, which is not sufficient for analyzing AID protein levels by western blot. To circumvent this limitation, we used the reporter mouse model AID-GFPtg, which carries a bacterial artificial chromosome including the whole mouse Aicda locus with GFP knocked-in as an in-frame C-terminal fusion [42]. AID-GFPtg mice have been extensively characterized: AID-GFP displays identical gene expression regulation and tissue specificity than endogenous AID and complements AID-deficiency [42, 43]. This mouse line allows directly monitoring AID-GFP in primary B cells by flow cytometry, with the MFI of GFP being proportional to the protein levels. We have shown that human AID and AID-GFP are similarly sensitive to the HSP90 inhibitors geldanamycine and its derivative 17-AAG, while GFP itself is insensitive [33]. In AID-GFPtg mice, the mouse AID-GFP protein, which is 2.5-fold more abundant than endogenous AID, was sensitive to 17-AAG (Fig. 1A), without affecting Aicda mRNA levels (Supporting Information Fig. 1A). For in vivo work we used the 17-AAG analog 17-DMAG, of identical mechanism of action [36], because it is water soluble, shows low toxicity and has better bioavailability than 17-AAG when administered orally, with widespread tissue distribution [38].
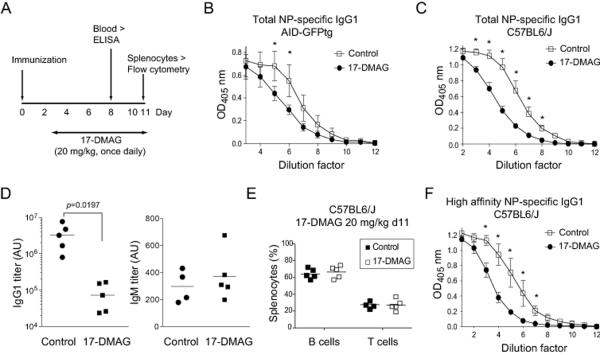
Figure 1. HSP90 inhibition reduces AID levels in germinal center B cells.
A. Left, Western blot probed with an anti-AID antibody recognizing both endogenous AID (E) and AID-GFP (Tg) in C57BL6/J or AID-GFPtg splenic B cells activated with anti-CD180, which induces proliferation, with and without IL-4 and LPS, which induce AID expression. Asterisks indicate non-specific bands that serve as loading controls. One representative blot out of 2 experiments is shown. Right, Purified naïve B cells from AID-GFPtg mice were stimulated with LPS + IL-4 and 48 h later (t0) treated with DMSO (Ctrl) or 2 μM (blue) or 8 μM (red) 17-AAG. The level of AID-GFP was determined by flow cytometry at various time points and plotted normalized to t0=100%. Means + SEM of 2 mice each from a single experiment are plotted.
B. AID-GFPtg mice were treated with 50 mg/kg 17-DMAG or water twice a day for 2 days starting at day 3 post-immunization with 100 μg of NP-CGG and splenocytes analyzed at day 5 post-immunization. Left: One representative flow cytometry dot plot is shown. Middle, overlapping histograms of GFP MFI of B220+ GFP+ cells. Right, MFI values normalized to the average MFI of control mice set as 100% for 4 mice from 2 independent experiments. Each dot represents one mouse, bars show means.
C. The proportion of B and T cells in the spleen of the 4 mice from (B) was determined by flow cytometry. Each dot represents one mouse, bars show means.
D. Splenic cells from AID-GFPtg mice that were immunized with 25 μg NP-CGG and 72 h later treated with 20 mg/kg 17-DMAG or water vehicle, once daily. AID-GFP levels were determined by flow cytometry at day 11 post-immunization as in (B). Left: One representative histogram out of 2 independent experiments is shown. Right: MFI values normalized to the average MFI of control mice set as 100% for 4 mice from 2 independent experiments. Each dot represents one mouse, bars show means.
E. The proportion of B and T cells in the spleen of the 4 mice from (D) was determined by flow cytometry. Each dot represents one mouse, bars show means.
F. Flow cytometry analysis of AID-GFP levels in Peyer’s patches B cells from 3 mice from (D). (B-F) Statistically significant differences are indicated by p-values from unpaired two-tailed Student’s t-test (α=0.05).
We immunized AID-GFPtg mice with 4-Hydroxy-3-nitrophenylacetyl-Chicken Gamma Globulin (NP-CGG) and three days later administered 50 mg/kg of 17-DMAG by gavage twice a day, for two days. Analysis of splenic cells showed that AID-GFP levels were reduced by ~40% in the 17-DMAG-compared to the vehicle-treated mice, as judged by the MFI of GFP+ B cells (Fig. 1B and supporting information Fig. 1B). This 17-DMAG dose and regime slightly reduced the proportion of B cells found in the spleen (Fig. 1C) but indicated that it was possible to consistently decrease AID levels in vivo through HSP90 inhibition. We repeated the experiment using 20 mg/kg 17-DMAG administered only once, daily. While two days treatment did not significantly reduce AID-GFP levels (data not shown), by day 11 this regime reduced the MFI of AID-GFP in immunized mice by 22% (Fig. 1D) without affecting the levels of B220, IgH or IgL in the same cells (Supporting information Fig. 1C), nor the proportion of splenic B or T cells compared to control mice (Fig. 1E). We also saw a consistent decrease in AID-GFP levels in B cells from chronic GC in the Peyer’s patches of the three mice we analyzed for that purpose at day 11 of this regime (Fig. 1F), indicating that 17-DMAG is distributed to other lymphoid tissues as well. This regime showed no signs of toxicity when continued for up to 20 days (data not shown). We conclude that daily oral doses of 17-DMAG at 20 mg/kg can reduce the level of AID protein in mice, without affecting the proportion of splenic B and T cells and is suitable for prolonged treatments.
HSP90 inhibition in vivo reduces AID physiological function
We asked whether the decrease in AID levels caused by HSP90 inhibition in vivo would be sufficient to reduce its biological activity. To determine the efficiency of isotype switching in vivo, we measured the levels of NP-specific IgG1 production by ELISA in the primary response at day 8 post-immunization [44] of the AID-GFPtg mice that had been immunized with NP-CGG and treated daily with 20 mg/kg 17-DMAG or vehicle from day 3 post-immunization (Fig. 2A, same mice as in Fig. 1DF). AID-GFPtg mice treated with 17-DMAG showed significantly lower titers of anti-NP-specific IgG1 (Fig. 2B), without affecting the levels of total serum IgG (data not shown). The reduction in anti-NP IgG1 in these mice coincided with decreased AID-GFP levels while B and T cell populations were unaffected (Figs. 1D, E). The effect of 17-DMAG was not restricted to AID-GFPtg mice; treating immunized C57BL6/J mice led to a similar decrease in total anti-NP-specific IgG1 titers (Fig. 2C, D). In contrast, anti-NP IgM titers were not significantly different, serving as control of similar immunization levels in all mice and showing that 17-DMAG did not cause a general depression in the antibody response; i.e. antigen-specific IgM was unaffected and even showed an upward trend (Fig. 2D), as it would be expected from inhibiting CSR. B and T cell populations in C57BL6/J were unaffected by 20 mg/kg 17-DMAG at day 11 post-immunization (Fig. 2E). The level of high-affinity anti-NP IgG1 was also reduced (Fig. 2F). We did not detect a significant effect on their affinity using the NaSCN ELISA method (data not shown), which is not surprising since the antibody response against NP is clonally restricted and high affinity can be achieved through a single point mutation [44, 45]. We conclude that oral administration of 17-DMAG is sufficient to significantly reduce CSR in vivo, at least in part by reducing AID protein levels.
Figure 2. HSP90 inhibitors reduce isotype switching in vivo.
A. Scheme of the experiments.
B. AID-GFPtg mice were immunized with 25 μg NP-CGG and treated with 17-DMAG or water as in (A), and total anti-NP-specific IgG1 in the serum was measured by ELISA at day 8 using NP25-BSA for capture. The mean ± SD of the OD values for 4 mice per group compiled from 2 experiments are plotted at various dilutions.
C. C57BL/6 mice were immunized, treated and analyzed as in (A,B) and total anti-NP-specific IgG1 was measured by ELISA at day 8. The mean ± SD of the OD values for 4-6 mice per group compiled from 2 experiments are plotted at various dilutions.
D. Titers of total anti-NP IgG1 and anti-NP IgM in arbitrary units (AU) were calculated as the dilution factor for OD50 for the mice from (C) mice from ELISA curves performed as in (C). Each dot represents a mouse from 4-6 mice per group compiled from 2 experiments, bars show means. Statistically significant differences are indicated by p-values from unpaired two-tailed Student’s t-test (α=0.05).
E. The proportion of B and T cells in the spleen of the mice from (C) was determined by flow cytometry. Each dot represents one mouse out of 4-5 mice from 2 independent experiments, bars show means.
F. High affinity anti-NP-specific IgG1 was measured in the serum of the mice from (C) by ELISA using NP4-BSA for capture. The mean ± SD of the OD values for 4-6 mice per group compiled from 2 experiments are plotted at various dilutions.
(B, C, F) Control and 17-DMAG curves were significantly different by Wilcoxon test (p<0.05). Individual dilutions were compared by t-test corrected for multiple comparisons by the Holm-Sidak method. Asterisks denote significant differences (p<0.05).
Targeting of AID in a leukemia model by HSP90 inhibition
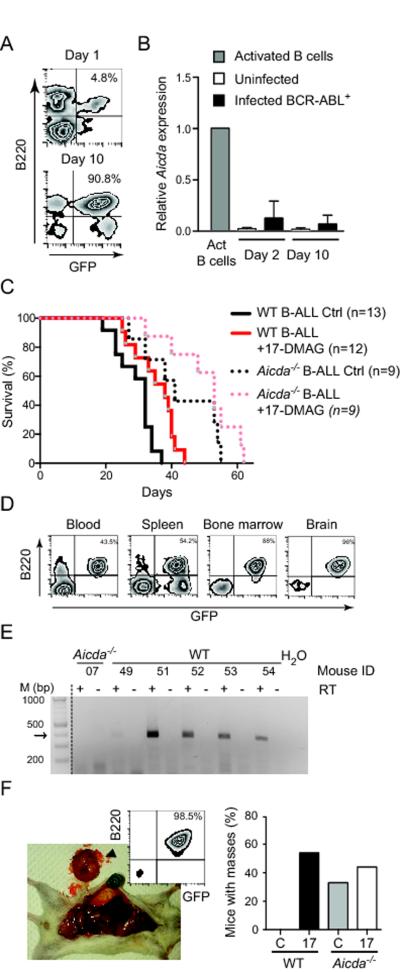
To ask whether HSP90 inhibition would also affect AID in leukemic cells we used a B-ALL model in which BM cells from donor mice are transformed in vitro by the oncogenic kinase BCR-ABL1 and transplanted into recipient mice, which develop leukemia within 4-6 weeks. AID is an important disease progression factor in this model [17]. We cultured BM cells from either WT or Aicda−/− mice with IL-7 and Flt3L to promote differentiation to the B-cell lineage and infected them with a retroviral vector encoding BCR-ABL1p210 and GFP as marker (Fig. 3A). The presence of BCR-ABL1 induced the expression of AID in WT BM cells to ~10% of the mRNA levels present in cytokine-activated splenic B cells (Fig. 3B). We transplanted the transformed cells into lymphocyte-deficient NOD.Rag1KO.IL2RγcKO (NRG) mice and followed up the development of clinical signs. Mice were sacrificed when paralyzed or moribund and analyzed. Leukemia derived from Aicda−/− BM cells was significantly less aggressive than leukemia derived from WT BM cells (Fig. 3C), indicating that the relatively low AID expression detected in WT cells was sufficient to cause a more aggressive disease in this model. Leukemic cells were of B-cell lineage, as shown by B220 expression, and were found in multiple organs including blood, BM, spleen and brain (Figs 3D and Supporting Information Fig. 2). Importantly, leukemic cells derived from WT BM continued to express low levels of AID at the time of euthanasia (Fig. 3E). Treatment with 17-DMAG for 20 days significantly increased the survival of mice with AID+ leukemia (Fig. 3C). We expected that 17-DMAG would also improve survival of mice bearing Aicda−/− leukemia because HSP90 is required for cell groWTh [37] and we indeed observed such a trend (Fig. 3C). However, the difference with the untreated mice did not reach statistical significance suggesting that the effect of 17-DMAG on Aicda−/− leukemia was relatively less robust than on the WT leukemia. Moreover, upon necropsy we noticed that NRG mice transplanted with Aicda−/− leukemia developed visible masses, typically one at the MLN or close to the spine or two symmetrical masses in the legs, which were not found in mice with WT leukemia in these experiments (Fig. 3F). Flow cytometry showed that these masses were just a local accumulation of leukemic GFP+ cells (Fig. 3F). We posit that since Aicda−/− leukemia is less aggressive, it simply allowed time for the formation of the masses we observed. Interestingly, treating the NRG mice bearing WT leukemia with 17-DMAG led to the appearance of similar masses (Fig. 3F). We conclude that AID+ leukemia are at least equally sensitive to HSP90 inhibitor and that the destabilizing effect of 17-DMAG on AID could also produce qualitative changes, in this case visible as masses that seem to correlate with a relatively more benign disease.
Figure 3. HSP90 inhibition delays AID+ B-ALL in mice.
A, B. BM cells from C57BL6/J mice were cultured with IL-7 and Ftl3L and infected with a retrovirus encoding BCR-ABL1-ires-GFP.
A. Cells were stained with anti-B220 and analyzed by flow cytometry for GFP expression at days 1 and 10 post-infection. One representative plot out of 3 independent experiments is shown.
B. Aicda expression levels were determined by qRT-PCR and normalized to Hprt expression within each sample. Results are shown relative to Aicda expression in mouse splenic B cells activated for 24 h with LPS and IL-4. Means + SEM of 2 samples per time point from 2 independent experiments are shown.
C. Overall survival of NRG mice transplanted with C57BL6/J (WT) or Aicda−/− BM cells 24 h after being infected with BCR-ABL1-ires-GFP retroviruses as in (A). Mice from each group were treated with either water (Ctrl) or 17-DMAG. Curves were compared by the Mantel-Cox long rank test: WT Ctrl versus WT 17-DMAG (p=0.0055); WT Ctrl vs Aicda−/− Ctrl (p=0.0035); Aicda−/− Ctrl vs Aicda−/− 17-DMAG (p=0.1875). The graph compiles n mice from 3 independent experiments.
D. Flow cytometry plots showing the proportion of B220+ GFP+ (i.e. BCR-ABL1+) cells in different organs from one representative transplanted NRG mouse. Results for all mice are shown in Supporting Information Fig. 2.
E. One representative agarose gel out of 2 independent experiments to detect Aicda mRNA by RT-PCR, with (+) or without (−) reverse transcriptase, in Aicda−/− or WT leukemia extracted from randomly chosen NRG mice from (C). The arrow indicates the expected size of the PCR product.
F. All NRG mice transplanted with BCR-ABL1-transformed BM were analyzed by necropsy. Left: One representative photograph of a mass in a mouse with Aicda−/− leukemia out of 18 mice analyzed. The inset shows the flow cytometry analysis of the cells in the tumor. Right: The bars graph shows the proportion of mice treated with vehicle (C) or 17-AAG (17) showing masses of GFP+ cells at necropsy compiled from the 2 experiments with 9-13 mice per group.
Human AID is sensitive to HSP90 inhibition in normal and cancerous B cells
To complement our preclinical analysis of AID destabilization by HSP90 inhibition, we tested the sensitivity of AID to HSP90 inhibition in normal and cancerous human B cells. We analyzed B cells freshly isolated from tonsils or PBMCs from six healthy donors, which were activated with CD40L and IL-4 to induce AID expression. AID protein levels were reduced by 30-80% by the treatment with 17-AAG (Fig. 4A). To analyze the sensitivity of AID to HSP90 inhibition in human malignancies we first analyzed three BL B-cell lines that constitutively express AID. AID protein was reduced by ~30-50% by 17-AAG in these BL lines when analyzed by either western blot or intracellular staining and flow cytometry (Fig. 4B). We then tested freshly isolated leukemic cells from three CLL patients, which expressed AID upon activation with IL-4 and CD40L. In each case intracellular staining and flow cytometry showed that 17-AAG decreased AID protein levels, albeit to a lesser extent than in the BL lines (Fig. 4C). We have previously demonstrated that HSP90 inhibitors destabilized endogenous and overexpressed human AID protein [33]. However, in CLL cells, HSP90 inhibition reduces NF-κB-mediated transcription [40], which induces AICDA [46]. We therefore asked whether HSP90 inhibitors might also affect AICDA transcription. We found that 17-AAG decreased AID mRNA levels in two CLL samples but not in CLL#2 and it actually increased them in the BL cells (Supporting Information Fig. 3A). Treatment with 17-AAG did not significantly change Aicda mRNA levels in mouse B cells after 48 h, despite clearly decreasing CSR and AID protein levels (Supporting Information Fig. 3B-C). We conclude that AID in human B cells is broadly sensitive to HSP90 inhibition through protein destabilization; although in some CLL cases there could also be a contribution from gene repression or mRNA destabilization.
Figure 4. Human AID is sensitive to HSP90 inhibition in normal and transformed B cells.
A. Human B cells were purified from blood or tonsils, activated with IL-4 and sCD40L and 3 days later (t0) treated with 2 μM 17-AAG (+) or DMSO (−) for 24 h and analyzed by western blot using anti-AID and β-actin as loading control. The experiment was performed with 6 different donors; one representative blot of each is shown. Right, AID to actin ratio in treated cells is plotted as % of the same ratio in the corresponding untreated sample, as determined by densitometry.
B. BL cell lines EL1BL, Daudi and Ramos that constitutively express endogenous AID were treated with 8 μM 17-AAG for 24 h and AID levels analyzed by Western blot, as in (A) including densitometry of each sample (Left) or by intracellular staining and flow cytometry, with the reduction (%) in AID levels after treatment with 17-AAG indicated for each sample (Right). One representative out of 2 independent experiments is shown for each technique.
C. AID levels were analyzed by intracellular staining and flow cytometry in PBMCs from three CLL patients activated with IL-4 and sCD40L and 3 days later treated with 8 μM 17-AAG or DMSO for 24 h. The reduction (%) in AID levels after treatment with 17-AAG indicated for each sample. One representative plot from each patient is shown.
HSP90 inhibition prevents AID-dependent EMT in a human breast cancer cell line
To assay the effect of HSP90 inhibition on an AID non-canonical function (i.e. transcriptional regulation) we used the human breast cancer cell line ZR75.1, in which we have previously shown that cytokine-induced EMT is dependent on AID expression [30]. ZR75.1 cells express very low but detectable levels of AID mRNA, which increase ~7-fold after treatment with TNFα [30]. We eliminated inducible AID expression through a specific shRNA (AIDkd) (Fig. 5A), which completely abolishes the EMT in these cells [30]. Here, we show that treating AID-proficient ZR75.1 cells with 17-AAG had the same effect than AID ablation. Treatment with 17-AAG decreased the basal transcription levels of mesenchymal factors Vimentin (VIM) and N-cadherin (CDH2), and key EMT transcription factors Slug (SNAI2), ZEB1 and ZEB2 (Fig. 5B) without affecting the expression of E-cadherin (CDH1) and Snail (SNAI1) (Fig. 5B), which are not reduced by AID knockdown [30]. Furthermore, treating AID-deficient ZR75.1 cells with 17-AAG did not change the expression of EMT factors (Fig. 5C), indicating that an important part of the effect of 17-AGG was by targeting AID. Since AID knockdown blocks the EMT [30], we asked whether 17-AAG would do the same. We induced the EMT in ZR75.1 cells with TNFα and TGFβ and at the same time inhibited HSP90. Consistent with the results shown above, 17-AAG treatment abolished the EMT similarly to AID knockdown, as shown by cell morphology as well as by its ability to prevent the upregulation of the mesenchymal markers Vimentin (VIM) and N-cadherin (CDH2) (Figs. 5D-F). Once again, in EMT-inducing conditions, 17-AAG did not affect the expression of genes that are not affected by AID knockdown (Fig. 5F). These results strongly suggest that HSP90 inhibition targets AID in human epithelial cells and can prevent its non-canonical functions.
Figure 5. HSP90 inhibition prevents AID-dependent epithelial to mesenchymal transition.
A. AICDA mRNA levels were measured by qRT-PCR in ZR75.1 cells expressing shRNA control (shScr) or shRNA against AID (AIDkd). TNF-α (50ng/ml) was used to induce AICDA. Bars show mean + SD out of 3-4 samples for each condition from ≥ 3 independent experiments.
B. Relative mRNA levels of various genes measured by qRT-PCR in ZR75.1 cells untreated or treated with 1 μM 17-AAG for 72 h. Expression of mesenchymal markers VIM and CDH2, and transcription factors SNAI2 (Slug), ZEB1 and ZEB2 (*: p<0.0005 VIM, CDH2 and ZEB2; p<0.005 SNAI2 and ZEB1) and, CDH1 (E-cadherin) and SNAI1 (Snail) were measured. Results are expressed as fold change relative to the levels seen in cells treated with DMSO. Bars show mean + SD out of 3-5 samples for each gene from ≥ 3 independent experiments. Expression was normalized to GAPDH and HPRT1.
C. Relative mRNA levels of EMT markers and transcription factors in ZR75.1 AIDkd cells, untreated or treated with 1 μM 17-AAG for 72 h were measured and plotted as in (B). Bars show mean + SD out of 3-5 samples for each gene from ≥3 independent experiments. Expression was normalized to GAPDH and HPRT1.
D. Bright field images (20X) of ZR75.1 cells treated with vehicle (DMSO) or 1 μM 17-AAG and/or TNF-α and TGF-β to induce the EMT (indicated as EMT). Treatment with 17-AAG decreases cell scattering at baseline and in EMT-inducing conditions, similarly to the effect we previously reported for AID knockdown in both conditions [30]. One representative image out of 3 independent experiments performed is shown.
E. Expression of Vimentin assessed by western blot in whole cell extracts of ZR75.1 cells, treated with DMSO, EMT inducers (TNF-α and TGF-β) and/or 17-AAG as indicated, or MDA-MB-231 breast cancer cells (as a positive control). β-actin was used as loading control. One representative blot out of 3 independent experiments performed is shown.
F. VIM, CDH2, CDH1 and SNAI1 mRNA levels measured by qRT-PCR in vehicle-treated ZR75.1 cells (DMSO) or EMT induced in the presence or absence of either shRNA targeting AID (AIDkd) or the HSP90 inhibitor 17-AAG. Bars show mean + SD out of 3-5 samples per gene from ≥ 3 independent experiments. Expression was normalized to GAPDH and HPRT1.
A-C, F. Differences in gene expression were compared by paired two-tailed t-test (assuming unequal variance, α=0.05).
Discussion
We have previously demonstrated that AID stability depends on the HSP90 molecular chaperoning pathway in vitro [33, 35]. Herein we determined the feasibility of using HSP90 inhibitors to destabilize AID in vivo in mice. This strategy could provide a useful alternative to pharmacologically target AID, until clinically apt specific inhibitors can be developed. Since HSP90 inhibitors are being actively pursued [36, 37], our results also establish AID as a relevant target to consider in (pre)clinical trials. Administering the HSP90 inhibitor 17-DMAG to AID-GFPtg mice at doses similar to those tested in human patients [39, 41] reduced the expression of AID protein in GC B cells from the spleen and Peyer’s patches. At 20 mg/kg, 17-DMAG was tolerated for up to 20 days without affecting splenic lymphocyte populations or the expression of B220 and surface IgM, indicating that no major immunosuppression through B cell or T cell depletion occurs. Furthermore, the decrease in AID-GFP protein was accompanied by a significant reduction in isotype switching of the antibody response elicited after immunization. The small number of B cells that normally express AID in immunized mice is insufficient to test the effect of HSP90 inhibitors on endogenous AID by western blot. However, 17-DMAG reduced the titers of total and high-affinity antigen-specific antibodies in C57BL6/J mice to a similar extent than in AID-GFPtg mice. Our results demonstrate that HSP90 inhibition can be used to blunt the production of switched antibodies in vivo, and that a substantial part of that effect is through destabilizing AID. This demonstration that AID is a target of HSP90 inhibition should be considered when anticipating possible side effects and interactions of HSP90 inhibitors in the clinic; although we posit that it would rather be one of their benefits. AID destabilization could also have therapeutic applications for autoimmune diseases. In fact, HSP90 inhibitors reduced the levels of anti-dsDNA antibodies and proteinuria, a consequence of antibody deposition in the kidneys in the MRLlpr/lpr mouse model of SLE [47]. Our results strongly suggest that part of the mechanism behind this action is AID destabilization. Indeed, the effect of HSP90 inhibitors on MRLlpr/lpr mice [47] mimics the effect of genetically ablating AID [6] or reducing AID dosage by using Aicda+/− mice in the MRLlpr/lpr background [8]. Since complete AID deficiency is also associated to autoimmune manifestations because of defects in tolerance [48], a partial reduction of AID levels could balance the benefits of reducing AID without causing additional pathologies.
We analyzed AID in cancer cells in mice as well as in human samples. Cancer cells rely on HSP90 to stabilize mutated and overexpressed oncoproteins [49], can overexpress HSP90 [37] and may express a form of HSP90 more sensitive to inhibition [50]. For these reasons, HSP90 inhibitors constitute a promising therapy for multiple cancers [36, 37]. Continued AID expression in lymphoma and leukemia can contribute to disease progression and hence be a relevant target of HSP90 inhibitors [25]. Indeed, AID expression correlates with poor outcome in CLL [19, 22-24], B-ALL [17] and CML [16]. Here, we show that 17-DMAG increases survival in a B-ALL mouse model in which AID clearly confers increased aggressiveness. Aicda−/− leukemia was also susceptible to the inhibitors, which was not unexpected because they would destabilize multiple HSP90 clients that are important for cell proliferation [49, 51]. However, we expected that AID+ leukemia might be more affected than Aicda−/− leukemia. Although this was the case in our experiment, the difference between the groups was modest. In hindsight, the kinetics of this B-ALL model are probably too fast to reveal large differential effects. Chronic models, probably in combination with a selectable marker of AID action, such as imatinib resistance [16], would need to be developed to further test our proposed strategy. We verified Aicda induction in BM cells due to enforced expression of BCR-ABL1, but AID levels were too low to be detected by western blot in the leukemic cells. Nevertheless, an effect on AID could be indirectly inferred from the development of masses that were found in mice bearing either Aicda−/− leukemia or WT leukemia treated with 17-DMAG, but not in untreated mice with WT leukemia. Of course this result is not conclusive but it does suggest that 17-DMAG can change the quality of the leukemia, making it more benign, in a similar way to AID-deficiency.
To extend our analysis to human AID we analyzed primary and tissue culture cells representing the spectrum of AID expression in humans. Despite inter-individual variation we show that AID protein levels are sensitive to similar doses of HSP90 inhibitors in normal B cells and BL cell lines, and perhaps less in CLL cell samples. This might be ascribed to variable HSP90 amounts, as it has been shown to happen in lymphomas [52] and CLL cells, which express >3-fold more HSP90 protein than WT leukocytes [53]. We also show that HSP90 inhibition abolishes cytokine-induced EMT in the epithelial breast cancer cell line ZR75.1 by preventing the upregulation of genes that are crucial for the cells to gain mobility and invasiveness. While HSP90 inhibition surely affects additional targets in these cells, it is again very suggestive that treatment with 17-AAG mirrors the effect achieved by AID knockdown [30].
In conclusion, the fact that HSP90 inhibitors had similar quantitative and qualitative effects to AID ablation in multiple settings supports our hypothesis that a substantial part of their effect is achieved by destabilizing AID. It is possible that HSP90 inhibitors at low doses are effective in chronically reducing AID levels, thereby delaying its pathogenicity in relevant chronic diseases, while minimizing toxicity issues of the current regimes [37]. For example HSP90 inhibition might help reducing switched pathogenic antibodies in SLE patients [48]. In CML patients AID underpins resistance to the BCR-ABL1 inhibitor imatinib [16] and adjunctive therapy with HSP90 inhibitors could delay this ability of AID, as we have shown to be possible in vitro [33]. Adjunctive therapy could also reduce clonal diversity in CLL patients and help chemotherapy [25]. In this sense, retrospective analysis of clinical trials of HSP90 inhibitors for CML and CLL [40, 54], cross-referencing response to AID status, could be informative. Our demonstration that HSP90 inhibitors can decrease AID levels in vivo as well as in human cancer samples, suggest a possible mechanism of action to explain in part the clinical utility of these drugs.
Material and Methods
Mice
C57BL6/J and NRG mice were from Jackson labs (Bar Harbor, ME). AID-GFPtg mice [42], a gift of Dr R Casellas (NCI, Bethesda, MD) and Aicda−/− mice [1], a gift of Dr T Honjo (Kyoto University, Japan), were in C57BL6/J background. Mice were kept under specific pathogens free conditions. All experiments were approved by the Animal protection committee at the IRCM, protocol 2011-24, according to the guidelines of the Canadian Council on Animal Care.
HSP90 inhibitors
17-AAG (LC laboratories A-6880) dissolved in DMSO and 17-DMAG (LC laboratories D-3440) dissolved in water were aliquoted and stored at −20°C. Mice were administered 17-DMAG in 0.2 mL of water (or water control) by gavage using a 22G feeding needle. Immunized mice were treated with 20 mg/kg, from day 3 post-immunization daily, until sacrificed (day 11 p.i.) for measuring antibody responses. Transplanted NRG mice were treated from day 5 post-transplantation with 20 mg/kg for 20 days, monitored daily until reaching an endpoint. Human cells were treated as indicated in Results.
Measuring antibody responses
Mice were immunized with NP18-CGG (Biosearch Technologies) in Imject Alum adjuvant (Thermo Scientific) intraperitoneally. Antibody titers were measured by ELISA as described [44]. Briefly, ELISA plates were coated with 100 μl of 50 μg/mL NP26-BSA, for capturing total anti-NP antibodies, or NP4-BSA, for capturing high affinity anti-NP antibodies. Serial dilutions of mouse serum were loaded in duplicate wells and incubated overnight at 4°C. IgG1 was detected using 50 μl biotin-anti-IgG1 for 2 h followed by 50 μl of HRP-streptavidin for 1 h and visualized using 2,2′-Azino-bis(3-ethybenzothiazoline-6-sulfonic acid) (Sigma), and OD read at 405 nm.
B-ALL mouse model
A retroviral vector encoding BCR-ABL1p210-ires-GFP was transfected into the ecotropic packaging cell line PLAT-E and 24 h later supplemented with 10 mM sodium butyrate (Sigma) to augment viral production. The supernatant was collected 24 h later, concentrated using Vivaspin 20 (GE Healthcare) and used to infect BM cells. BM cells were isolated from the long bones of C57BL6/J or Aicda−/− mice and cultured in StemSpan medium (StemCell technologies) for 48 h with 50 ng/mL Flt3L and 10 ng/mL of rIL-7 (Preprotech). Pelleted BM cells were resuspended in 0.2 mL of concentrated viral supernatant containing 8 μg/mL polybrene (Sigma) and incubated for 2 h before adding 1 mL of StemSpan medium with cytokines, spinning for 30 min at 600 x g and then incubating overnight in 6-well plates. One million cells were then injected in the tail vein of sublethally irradiated (5 Gy) NRG mice. The visible efficiency of infection at the time of injection was typically 5%. Mice were monitored daily and sacrificed when moribund or reaching one of the endpoints (paralysis, rapid loss of weight, ataxia, lack of cleaning, general posture, visible masses). Cells from the BM, blood, spleen, brain and visible masses were isolated using a 70 μM filter and analyzed by flow cytometry or cultured for 48 h in complete IMDM media at 2×106/mL in 6 well plates for RNA isolation.
Human cells and samples
Peripheral blood was collected from healthy donors at the IRCM and B cells purified from PBMCs as described [33]. Human tonsils were obtained from donors undergoing preprogrammed tonsilectomy at the otorhinolaryngology service at the Ste-Justine Hospital (Montreal). Lymphocytes were obtained by crushing the tonsil through a 70 μm filter, washed with RPMI and purified using Lymphoprep (Axis-Shield). All work was approved by the ethics committees at Ste-Justine Hospital and at IRCM (certificate 2011-18). CLL samples were obtained from patients followed at the Hospital Maciel (Montevideo) that have signed an informed consent in accordance with the ethical regulations from Uruguay and the Helsinki Declaration. PBMCs were isolated by centrifugation on Ficoll-Hypaque (Sigma Aldrich, USA) and cryopreserved in liquid nitrogen. Purified B cells and CLL samples were activated with 5 ng/mL human IL-4 (Peprotech) and 5 μg/mL recombinant sCD40L (Peprotech) for 72 h before treating with 2 mM 17-AAG for 24 h. Human BL cell lines were a gift of Dr M Neuberger (Cambridge, UK) and cultured in complete RPMI medium.
RT-PCR
RNA isolated using TRIzol (Life Technologies) was reverse transceibed with ProtoScript™ M-MuLV Taq RT-PCR kit (New england Biolabs). End-point PCR to amplify 389 bp of mouse AID from cDNA was performed for 35 cycles [94°C 20 sec, 55°C 2 min, 68°C 1 min] using Taq DNA polymerase. Quantitative PCR using SYBR select master mix (Applied Biosystems) was performed and analyzed in a ViiA™ 7 machine and software (Life technologies). Mouse AID was amplified using primers OJ844 (GCCACCTTCGCAACAAGTCT) and OJ845 (CCGGGCACAGTCATAGCAC) for a 137 bp product for 40 cycles [95°C 15 sec, 60°C 1min] and normalized to Hprt, amplified with OJ1008 (CCTTCATGACATCTCGAGCAAGT) and OJ1009 (CCGAGGATTTGGAAAAAGTGTT). A 133 bp product of AICDA mRNA from BL B-cell lines was amplified using OJ1225 (GCAATAAGAACGGCTGCCAC) and OJ1226 (ACATGTCGGGCACAGTCGTA), normalized to GAPDH amplified with primers OJ1010 (TGACAACTTTGGTATCGTGGAAGG) and OJ1011 (AGGGATGATGTTCTGGAGAGCC), as above. AID mRNA in stimulated PBMCs from CLL patients was measured in a Corbette Rotor Gene 6000 Real-Time PCR machine using primers fwd (GAGGCAAGAAGACACTCTGG) and rev (GCGGTCCTCACAGAAGTAG) for 40 cycles [95°C 10 sec, 60°C 30 sec, 72°C 30 sec] and normalized to GAPDH. Relative expression levels were obtained by calculating the ΔΔCt values using ΔCt of Ramos cell line as a calibrator.
EMT analysis
ZR75.1 cells stably expressing an shRNA targeting human AID or scrambled control have been described [30]. ZR75.1 cells were incubated with 1μM 17-AAG (Sellek Chemicals) for 72 h in basal conditions or for 72 h prior to and during the induction of the EMT. Total RNA was reversed transcribed using Superscript III (Invitrogen) and mRNA levels of AID and EMT factors analyzed as described [30].
Flow cytometry and western blot
Lymphocytes were stained with anti-B220-allophycocyanin and anti-CD3-phycoerythrin (BD pharmingen). Leukemic cells from NRG mice were stained with anti-CD19-biotin (BD Biosciences) followed by anti-biotin- phycoerythrin-Vio770 (Miltenyi Biotech) and/or anti-B220-allophycocyanin (BD Biosciences). Dead cells were excluded using PI. Results were acquired using a BD LSR I (BD biosciences) and analyzed using FlowJo. For intracellular detection of human AID PBMCs or BL cells were fixed with PBS 4% paraformaldehyde and permeabilized with PBS 0.5% saponin 4% of FBS before adding mAb anti-AID (clone EK2 5G9, Cell Signaling Technology) followed by anti-Rat IgG Alexa Fluor 488 (Cell Signaling). Negative isotype controls were performed accordingly. In western blots, endogenous human AID was detected using the same antibody. Endogenous mouse AID was detected using mAb anti-AID (ebioscience, cat#14-5959-82 at 1:250 in 2.5% BSA). Densitometry was performed using ImageJ (NIH).
Supplementary Material
Acknowledgements
We thank R Casellas, T Honjo and K Podsypanina, for reagents, Dr P Arcand from the otorhinolaryngology service at Ste-Justine Hospital and Dr P Larochelle and nurse M Gauthier from IRCM clinic for facilitating the access to tonsil and blood samples. We thank M CaWThorn, M Laprise, M-C Lavallée and J Dussureault for technical assistance with mice. This work was funded by a grant from The Cancer Research Society to JMDN and National Institutes of Health Grant 5K22CA163969-02 and a Pink Pumpkin Patch Grant to DPM. JMDN holds a Canada Research Chair tier 2.
Abbreviations
- 17-AAG
17-(Allylamino)-17-demethoxygeldanamycin
- 17-DMAG
17-(Dimethylaminoethylamino)-17-demethoxygeldanamycin
- AID
activation induced deaminase.
- B-ALL
B-cell acute lymphoblastic leukemia.
- BL
Burkitt’s lymphoma.
- CLL
chronic lymphocytic leukemia.
- CML
chronic myelogenous leukemia.
- CSR
class switch recombination.
- NP-CGG
4-Hydroxy-3-nitrophenylacetyl-Chicken Gamma Globulin.
- EMT
epithelial to mesenchymal transition
- SHM
somatic hypermutation.
- SLE
systemic lupus erythematosus.
- HSP90
heat shock protein 90 kDa
Footnotes
Conflict of interest
The authors declare no financial or commercial conflicts of interest.
References
- 1.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 2.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu. Rev. Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. DOI: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 3.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. DOI: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 4.Stavnezer J, Guikema JEJ, Schrader CE. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. DOI:10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu H-C, Wu Y, Yang P, Wu Q, Job G, Chen J, Wang J, et al. Overexpression of activation-induced cytidine deaminase in B cells is associated with production of highly pathogenic autoantibodies. J. Immunol. 2007;178:5357–5365. doi: 10.4049/jimmunol.178.8.5357. [DOI] [PubMed] [Google Scholar]
- 6.Jiang C, Foley J, Clayton N, Kissling G, Jokinen M, Herbert R, Diaz M. Abrogation of lupus nephritis in activation-induced deaminase-deficient MRL/lpr mice. J. Immunol. 2007;178:7422–7431. doi: 10.4049/jimmunol.178.11.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zan H, Zhang J, Ardeshna S, Xu Z, Park S-R, Casali P. Lupus-prone MRL/faslpr/lpr mice display increased AID expression and extensive DNA lesions, comprising deletions and insertions, in the immunoglobulin locus: concurrent upregulation of somatic hypermutation and class switch DNA recombination. Autoimmunity. 2009;42:89–103. doi: 10.1080/08916930802629554. DOI: 10.1080/08916930802629554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang C, Zhao ML, Diaz M. Activation-induced deaminase heterozygous MRL/lpr mice are delayed in the production of high-affinity pathogenic antibodies and in the development of lupus nephritis. Immunology. 2009;126:102–113. doi: 10.1111/j.1365-2567.2008.02882.x. DOI: 10.1111/j.1365-2567.2008.02882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Hsu HC, Chen J, Grizzle WE, Chatham WW, Stockard CR, Wu Q, et al. Increased expression of activation-induced cytidine deaminase is associated with anti-CCP and rheumatoid factor in rheumatoid arthritis. Scand. J. Immunol. 2009;70:309–316. doi: 10.1111/j.1365-3083.2009.02302.x. DOI: 10.1111/j.1365-3083.2009.02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White CA, Seth Hawkins J, Pone EJ, Yu ES, Al-Qahtani A, Mai T, Zan H, et al. AID dysregulation in lupus-prone MRL/Fas(lpr/lpr) mice increases class switch DNA recombination and promotes interchromosomal c-Myc/IgH loci translocations: modulation by HoxC4. Autoimmunity. 2011;44:585–598. doi: 10.3109/08916934.2011.577128. DOI: 10.3109/08916934.2011.577128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okazaki I-M, Hiai H, Kakazu N, Yamada S, Muramatsu M, Kinoshita K, Honjo T. Constitutive expression of AID leads to tumorigenesis. J. Exp. Med. 2003;197:1173–1181. doi: 10.1084/jem.20030275. DOI: 10.1084/jem.20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbiani DF, Bunting S, Feldhahn N, Bothmer A, Camps J, Deroubaix S, McBride KM, et al. AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Mol. Cell. 2009;36:631–641. doi: 10.1016/j.molcel.2009.11.007. DOI: 10.1016/j.molcel.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M, Duke JL, Richter DJ, Vinuesa CG, Goodnow CC, Kleinstein SH, Schatz DG. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–845. doi: 10.1038/nature06547. DOI: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- 14.Ramiro AR, Jankovic M, Eisenreich TR, Difilippantonio S, Chen-Kiang S, Muramatsu M, Honjo T, et al. AID is required for c-myc/IgH chromosome translocations in vivo. Cell. 2004;118:431–438. doi: 10.1016/j.cell.2004.08.006. DOI: 10.1016/j.cell.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Pasqualucci L, Bhagat G, Jankovic M, Compagno M, Smith P, Muramatsu M, Honjo T, et al. AID is required for germinal center-derived lymphomagenesis. Nat Genet. 2008;40:108–112. doi: 10.1038/ng.2007.35. DOI: 10.1038/ng.2007.35. [DOI] [PubMed] [Google Scholar]
- 16.Klemm L, Duy C, Iacobucci I, Kuchen S, Levetzow von G, Feldhahn N, Henke N, et al. The B Cell Mutator AID Promotes B Lymphoid Blast Crisis and Drug Resistance in Chronic Myeloid Leukemia. Cancer Cell. 2009;16:232–245. doi: 10.1016/j.ccr.2009.07.030. DOI: 10.1016/j.ccr.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruber TA, Chang MS, Sposto R, Muschen M. Activation-Induced Cytidine Deaminase Accelerates Clonal Evolution in BCR-ABL1-Driven B-Cell Lineage Acute Lymphoblastic Leukemia. Cancer Research. 2010;70:7411–7420. doi: 10.1158/0008-5472.CAN-10-1438. DOI: 10.1158/0008-5472.CAN-10-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldhahn N, Henke N, Melchior K, Duy C, Soh BN, Klein F, Levetzow von G, et al. Activation-induced cytidine deaminase acts as a mutator in BCR-ABL1-transformed acute lymphoblastic leukemia cells. J. Exp. Med. 2007;204:1157–1166. doi: 10.1084/jem.20062662. DOI: 10.1084/jem.20062662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oppezzo P, Vuillier F, Vasconcelos Y, Dumas G, Magnac C, Payelle-Brogard B, Pritsch O, et al. Chronic lymphocytic leukemia B cells expressing AID display dissociation between class switch recombination and somatic hypermutation. Blood. 2003;101:4029–4032. doi: 10.1182/blood-2002-10-3175. DOI: 10.1182/blood-2002-10-3175. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy H, Wierda WG, Barron LL, Cromwell CC, Wang J, Coombes KR, Rangel R, et al. High expression of activation-induced cytidine deaminase (AID) and splice variants is a distinctive feature of poor-prognosis chronic lymphocytic leukemia. Blood. 2003;101:4903–4908. doi: 10.1182/blood-2002-09-2906. DOI: 10.1182/blood-2002-09-2906. [DOI] [PubMed] [Google Scholar]
- 21.Albesiano E, Messmer BT, Damle RN, Allen SL, Rai KR, Chiorazzi N. Activation-induced cytidine deaminase in chronic lymphocytic leukemia B cells: expression as multiple forms in a dynamic, variably sized fraction of the clone. Blood. 2003;102:3333–3339. doi: 10.1182/blood-2003-05-1585. DOI: 10.1182/blood-2003-05-1585. [DOI] [PubMed] [Google Scholar]
- 22.Palacios F, Moreno P, Morande P, Abreu C, Correa A, Porro V, Landoni AI, et al. High expression of AID and active class switch recombination might account for a more aggressive disease in unmutated CLL patients: link with an activated microenvironment in CLL disease. Blood. 2010;115:4488–4496. doi: 10.1182/blood-2009-12-257758. DOI: 10.1182/blood-2009-12-257758. [DOI] [PubMed] [Google Scholar]
- 23.Huemer M, Rebhandl S, Zaborsky N, Gassner FJ, Hainzl S, Weiss L, Hebenstreit D, et al. AID induces intraclonal diversity and genomic damage in CD86(+) cells in chronic lymphocytic leukemia. Eur. J. Immunol. 2014 doi: 10.1002/eji.201344421. DOI: 10.1002/eji.201344421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patten PEM, Chu CC, Albesiano E, Damle RN, Yan X-J, Kim D, Zhang L, et al. IGHV-unmutated and IGHV-mutated chronic lymphocytic leukemia cells produce activation-induced deaminase protein with a full range of biologic functions. Blood. 2012;120:4802–4811. doi: 10.1182/blood-2012-08-449744. DOI: 10.1182/blood-2012-08-449744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montamat-Sicotte D, Palacios F, Di Noia JM, Oppezzo P. Origins and Consequences of AID Expression in Lymphoid Neoplasms. Current Immunological Reviews. 2013;9:72–85. [Google Scholar]
- 26.Marusawa H, Takai A, Chiba T. Role of activation-induced cytidine deaminase in inflammation-associated cancer development. Adv. Immunol. 2011;111:109–141. doi: 10.1016/B978-0-12-385991-4.00003-9. DOI: 10.1016/B978-0-12-385991-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 27.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, Bignell GR, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. DOI: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramiro AR, Barreto VM. Activation-induced cytidine deaminase and active cytidine demethylation. Trends Biochem. Sci. 2015 doi: 10.1016/j.tibs.2015.01.006. DOI:10.1016/j.tibs.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Isobe T, Song S-NJ, Tiwari P, Ito H, Yamaguchi Y, Yoshizaki K. Activation-induced cytidine deaminase auto-activates and triggers aberrant gene expression. FEBS Lett. 2013 doi: 10.1016/j.febslet.2013.06.028. DOI: 10.1016/j.febslet.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Muñoz DP, Lee EL, Takayama S, Coppé J-P, Heo S-J, Boffelli D, Di Noia JM, et al. Activation-induced cytidine deaminase (AID) is necessary for the epithelial-mesenchymal transition in mammary epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E2977–86. doi: 10.1073/pnas.1301021110. DOI: 10.1073/pnas.1301021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orthwein A, Di Noia JM. Activation induced deaminase: how much and where? Semin. Immunol. 2012;24:246–254. doi: 10.1016/j.smim.2012.05.001. DOI: 10.1016/j.smim.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Vuong BQ, Chaudhuri J. Combinatorial mechanisms regulating AID-dependent DNA deamination: interacting proteins and post-translational modifications. Semin. Immunol. 2012;24:264–272. doi: 10.1016/j.smim.2012.05.006. DOI: 10.1016/j.smim.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orthwein A, Patenaude A-M, Affar EB, Lamarre A, Young JC, Di Noia JM. Regulation of activation-induced deaminase stability and antibody gene diversification by Hsp90. J. Exp. Med. 2010;207:2751–2765. doi: 10.1084/jem.20101321. DOI: 10.1084/jem.20101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasqualucci L, Guglielmino R, Houldsworth J, Mohr J, Aoufouchi S, Polakiewicz R, Chaganti RSK, et al. Expression of the AID protein in normal and neoplastic B cells. Blood. 2004;104:3318–3325. doi: 10.1182/blood-2004-04-1558. DOI: 10.1182/blood-2004-04-1558. [DOI] [PubMed] [Google Scholar]
- 35.Orthwein A, Zahn A, Methot SP, Godin D, Conticello SG, Terada K, Di Noia JM. Optimal functional levels of activation-induced deaminase specifically require the Hsp40 DnaJa1. EMBO J. 2012;31:679–691. doi: 10.1038/emboj.2011.417. DOI: 10.1038/emboj.2011.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taldone T, Gozman A, Maharaj R, Chiosis G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol. 2008;8:370–374. doi: 10.1016/j.coph.2008.06.015. DOI: 10.1016/j.coph.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin. Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. DOI: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egorin MJ, Lagattuta TF, Hamburger DR, Covey JM, White KD, Musser SM, Eiseman JL. Pharmacokinetics, tissue distribution, and metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (NSC 707545) in CD2F1 mice and Fischer 344 rats. Cancer Chemother Pharmacol. 2002;49:7–19. doi: 10.1007/s00280-001-0380-8. DOI: 10.1007/s00280-001-0380-8. [DOI] [PubMed] [Google Scholar]
- 39.Kummar S, Gutierrez ME, Gardner ER, Chen X, Figg WD, Zajac-Kaye M, Chen M, et al. Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies. Eur. J. Cancer. 2010;46:340–347. doi: 10.1016/j.ejca.2009.10.026. DOI: 10.1016/j.ejca.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hertlein E, Wagner AJ, Jones J, Lin TS, Maddocks KJ, Towns WH, Goettl VM, et al. 17-DMAG targets the nuclear factor-kappaB family of proteins to induce apoptosis in chronic lymphocytic leukemia: clinical implications of HSP90 inhibition. Blood. 2010;116:45–53. doi: 10.1182/blood-2010-01-263756. DOI: 10.1182/blood-2010-01-263756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, Arkenau H-T, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin. Cancer Res. 2011;17:1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. DOI: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crouch EE, Li Z, Takizawa M, Fichtner-Feigl S, Gourzi P, Montano C, Feigenbaum L, et al. Regulation of AID expression in the immune response. J. Exp. Med. 2007;204:1145–1156. doi: 10.1084/jem.20061952. DOI: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teng G, Hakimpour P, Landgraf P, Rice A, Tuschl T, Casellas R, Papavasiliou FN. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity. 2008;28:621–629. doi: 10.1016/j.immuni.2008.03.015. DOI: 10.1016/j.immuni.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zahn A, Daugan M, Safavi S, Godin D, Cheong C, Lamarre A, Di Noia JM. Separation of Function between Isotype Switching and Affinity Maturation In Vivo during Acute Immune Responses and Circulating Autoantibodies in UNG-Deficient Mice. J. Immunol. 2013;190:5949–5960. doi: 10.4049/jimmunol.1202711. DOI: 10.4049/jimmunol.1202711. [DOI] [PubMed] [Google Scholar]
- 45.Cumano A, Rajewsky K. Structure of primary anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in normal and idiotypically suppressed C57BL/6 mice. Eur. J. Immunol. 1985;15:512–520. doi: 10.1002/eji.1830150517. DOI: 10.1002/eji.1830150517. [DOI] [PubMed] [Google Scholar]
- 46.Dedeoglu F, Horwitz B, Chaudhuri J, Alt FW, Geha RS. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFkappaB. Int. Immunol. 2004;16:395–404. doi: 10.1093/intimm/dxh042. DOI: 10.1093/intimm/dxh042. [DOI] [PubMed] [Google Scholar]
- 47.Shimp SK, Chafin CB, Regna NL, Hammond SE, Read MA, Caudell DL, Rylander M, et al. Heat shock protein 90 inhibition by 17-DMAG lessens disease in the MRL/lpr mouse model of systemic lupus erythematosus. Cell. Mol. Immunol. 2012;9:255–266. doi: 10.1038/cmi.2012.5. DOI: 10.1038/cmi.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diaz M. Activation-induced deaminase in immunity and autoimmunity: introduction. Autoimmunity. 2013;46:81–82. doi: 10.3109/08916934.2013.768370. DOI: 10.3109/08916934.2013.768370. [DOI] [PubMed] [Google Scholar]
- 49.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. DOI: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 50.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. DOI: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 51.McClellan AJ, Xia Y, Deutschbauer AM, Davis RW, Gerstein M, Frydman J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell. 2007;131:121–135. doi: 10.1016/j.cell.2007.07.036. DOI: 10.1016/j.cell.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 52.Valbuena JR, Rassidakis GZ, Lin P, Atwell C, Georgakis GV, Younes A, Jones D, et al. Expression of heat-shock protein-90 in non-Hodgkin’s lymphomas. Mod. Pathol. 2005;18:1343–1349. doi: 10.1038/modpathol.3800459. DOI: 10.1038/modpathol.3800459. [DOI] [PubMed] [Google Scholar]
- 53.Dempsey NC, Leoni F, Ireland HE, Hoyle C, Williams JHH. Differential heat shock protein localization in chronic lymphocytic leukemia. J. Leukoc. Biol. 2010;87:467–476. doi: 10.1189/jlb.0709502. DOI: 10.1189/jlb.0709502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng C, Brain J, Hu Y, Goodrich A, Kong L, Grayzel D, Pak R, et al. Inhibition of heat shock protein 90 prolongs survival of mice with BCR-ABL-T315I-induced leukemia and suppresses leukemic stem cells. Blood. 2007;110:678–685. doi: 10.1182/blood-2006-10-054098. DOI: 10.1182/blood-2006-10-054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.