SUMMARY
Epithelial cells undergo dynamic polarity changes as organs pattern, but the relationship between epithelial polarity and cell fate is poorly understood. Using the developing lung as a model, we have found that distinct alterations in apical-basal polarity dictate airway epithelial differentiation. We demonstrate that Crb3, a Crumbs isoform that determines epithelial apical domain identity, is required for airway differentiation by controlling the localization of the transcriptional regulator Yap. We show that Crb3 promotes interaction between Yap and the Hippo pathway kinases Lats1/2 at apical cell junctions to induce Yap phosphorylation and cytoplasmic retention, which drives cell differentiation. Loss of Crb3 in developing mouse airways, or in isolated adult airway progenitors, results in unrestricted nuclear Yap activity and consequent cell differentiation defects. Our findings demonstrate that polarity-dependent cues control airway cell differentiation, offering important molecular insight into organ patterning.
Graphical Abstract
INTRODUCTION
The epithelium lining the lung undergoes dramatic morphological changes as it develops, ultimately giving rise to specialized cells within a network of branched airways that transport air to gas-exchanging alveoli. Signals that instruct lung epithelial patterning have been identified, but how these fate decisions are coordinated with lung morphogenesis is poorly understood (Hogan et al., 2014; Morrisey and Hogan, 2010). Structural features associated with the epithelium relate to lung patterning: the early distal epithelium that gives rise to alveolar lineages is composed of cuboidal cells, whereas the epithelium in the proximal airways exhibits a columnar pseudostratified morphology. Additionally, as the proximal airways develop, the positioning of cells within the maturing tubules correlate with cell specification; luminal cells differentiate and specialize, whereas basal cells take on progenitor properties (Rock et al., 2009).
Recent work has demonstrated an essential role for the transcriptional regulator Yes-associated protein (Yap) in the distal-proximal patterning and terminal differentiation of the embryonic and adult mouse lung epithelium (Mahoney et al., 2014; Zhao et al., 2014). Yap localization is dynamically controlled as the lung epithelium develops, which dictates its cell fate-regulating activity. Nuclear Yap is required for proximal airway progenitor specification, and a subsequent shift of Yap from the nucleus to the cytoplasm is associated with proximal airway maturation. Nuclear Yap activity also promotes airway basal progenitor identity, with removal of nuclear Yap driving airway cell differentiation (Mahoney et al., 2014; Zhao et al., 2014). Precise control of Yap localization is therefore important for directing airway epithelial specification and homeostasis. The Hippo pathway has emerged as a major regulator of Yap localization, with the core pathway kinases, Lats1 and Lats2 (Lats1/2), promoting the direct phosphorylation of Yap on conserved serine residues, which induce cytoplasmic sequestration and degradation of Yap (Dong et al., 2007; Zhao et al., 2010). These modifications have been implicated in the regulation of Yap activity in the lung (Lange et al., 2014; Lin et al., 2015; Mahoney et al., 2014; Zhao et al., 2014). However, how these kinases are regulated with respect to organ morphogenesis and patterning is unclear.
Proteins important for generating and maintaining polarity direct Yap localization (Genevet and Tapon, 2011). In particular, proteins that make up the evolutionary conserved Crumbs complex, which is known to specify the apical domain of epithelial cells (Pocha and Knust, 2013), have important roles in controlling Hippo pathway activity to promote the cytoplasmic localization of Yap (Chen et al., 2010; Ling et al., 2010; Robinson et al., 2010; Varelas et al., 2010). Here we describe that in developing mouse airway cells the cytoplasmic localization of Yap correlates precisely with the expression and asymmetric distribution of Crb3, the major Crumbs isoform expressed in the lung (Lemmers et al., 2004). We show that apical recruitment of Crb3 controls apical-basal polarity in airway epithelial cells, induces binding of Yap to activated Lats1/2 kinases at apical junctions to promote phosphorylation and cytoplasmic sequestration of Yap, and consequently initiates airway progenitor differentiation. We also show that loss of Crb3 results in the aberrant accumulation of nuclear Yap and subsequent prevention of airway epithelial cell differentiation. These findings indicate that apical-basal polarity cues control the localization of Yap in mammalian development, acting as essential mediators of cell fate during organogenesis.
RESULTS
Apical-basal polarity regulators are coordinated with changes in the localization of Yap during the proximal patterning of the lung epithelium
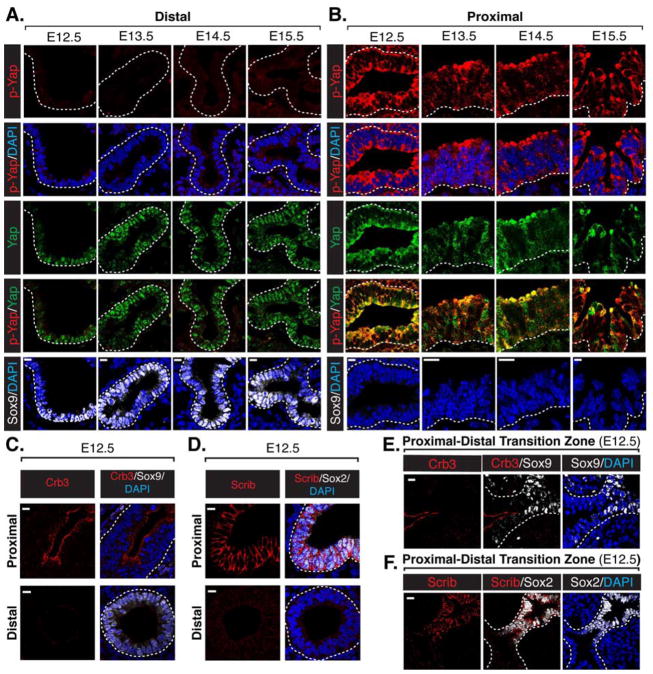
The Hippo pathway effector Yap controls the patterning of lung epithelial progenitors, with distinct intracellular localization changes dictating Yap function in these cells (Mahoney et al., 2014; Zhao et al., 2014). Phosphorylation of Yap on a conserved Serine residue (S112 in mouse Yap, homologous to S127 in human Yap; herein referred to as p-YapS112) promotes sequestration of Yap in the cytoplasm (Basu et al., 2003; Dong et al., 2007). To gain insight into Yap regulation in the lung epithelium we characterized the pattern of p-YapS112 modifications with respect to total Yap. Early developing lungs were obtained from mouse embryos (E12.5–E15.5; the pseudoglandular stage) and immunostained for p-YapS112 and total Yap (antibody specificity was validated using Yap-null lung epithelium, Figures S1A). Minimal p-YapS112 was observed in Sox9-positive distal epithelial progenitors, which are cells that exhibit nuclear-localized total Yap (Figure 1A). In contrast, high levels of p-YapS112 were observed in proximal airway epithelial cells (Figure 1B), implicating Yap phosphorylation in the development of proximal epithelial cells of the lung.
Figure 1. Apical-localized p-YapS112 correlates with polarized Crb3 and Scrib localization as the lung epithelium patterns the proximal domain.
(A, B) Immunofluorescence analysis of E12.5–E15.5 mouse lungs examining the levels and localization of Yap (green) and p-YapS112 (red) in the (A) distal and (B) proximal epithelium. The presence of Sox9 (white) served as a marker of distal epithelial cells. (C) Increased Crb3 levels localized to the apical domain are observed specifically in the Sox9-negative proximal region of E12.5 lung epithelium. (D) Scrib levels increase and localize to the basal-lateral surface of E12.5 Sox2-positive proximal lung epithelium. (E, F) High resolution imaging of the transition zone between the Sox9-positive distal to Sox2-positive proximal compartments in the developing lung epithelium reveals an increase in the levels of (E) apical-localized Crb3, and (F) basal-lateral-localized Scrib immediately following the boundary between the Sox2 and Sox9 domains. Of note, this transition zone is precisely where Yap shifts localization from the nucleus to the cytoplasm (Mahoney et al., 2014). DAPI was used to mark the nuclei (blue) in all images, and for clarity the basal surface of the epithelium is outlined with a white dotted line. Scale bar = 10μm.
Interestingly, our microscopy analysis revealed prominent accumulation of Yap and p-YapS112 at the apical domain of proximal epithelial cells (Figure 1B, S1B and S1C). Given that apical-basal polarity-regulators have been shown to direct Hippo pathway activity (Genevet and Tapon, 2011), we hypothesized that these proteins might direct Yap localization, and therefore characterized their expression and localization pattern in the developing lung epithelium. We started by analyzing the Crumbs transmembrane proteins (Crb1-3 in mammals), which are evolutionary conserved factors that define epithelial apical domain identity (Pocha and Knust, 2013). We found that Crb3, which is the primary Crumbs isoform expressed in the lung epithelium (Lemmers et al., 2004), is highly expressed and is restricted to the apical domain in the developing Sox2-positive/Sox9-negative proximal epithelial cells of E12.5 to E15.5 lungs (Figure 1C, S1B and S1D). In contrast, Crb3 levels were almost undetectable in the Sox2-negative/Sox9-positive distal compartment. The proximal expression and polarization of Crb3 strikingly correlated with a loss of Yap from the nucleus. A similar distal-proximal relationship was observed for polarity-regulator Scribble (Scrib), which establishes the basal-lateral domains via a mutually exclusive relationship with Crumbs (Rodriguez-Boulan and Macara, 2014). High levels of basal-lateral-localized Scrib were observed in the Sox2-positive/Sox9-negative proximal domain, and very low levels of cortical membrane-localized Scrib were found in the distal compartment of E12.5–E15.5 lungs (Figures 1D, S1C and S1E). Notably, increased Crb3 and Scrib expression and polarization was observed upstream from the distal-proximal transition zone in the developing lung epithelium (Figure 1E and 1F), which is precisely the area where Yap shifts localization from the nucleus to the cytoplasm (Mahoney et al., 2014). We did not, however, observe any differences in the expression of the adherens junction protein E-cadherin in all proximal and distal regions examined, and when comparing proximal and distal epithelium, E-cadherin exhibited a similar basal-lateral localization pattern (Figure S1F). Our observations therefore indicated that while cell adhesion and aspects of polarity (i.e. adherens junctions) exist throughout the entire developing lung epithelium, specific apical-basal polarity cues (i.e. apical Crb3 and basal-lateral Scrib) are established following the distal-proximal transition zone and characterize the proximal compartment. Given the key roles for Yap in patterning of the lung, our findings suggested that these apical-basal polarity cues might actively direct Yap localization to control lung patterning.
The apical-basal distribution of Crb3 and Scrib marks proximal airway epithelial cell differentiation
At approximately E14.5, proximal airway epithelial progenitors are specified into multi-ciliated and secretory cells (Hogan et al., 2014; Tsao et al., 2009). Epithelial cells of the developing trachea and proximal airways of the lung also give rise to a population of progenitor cells, marked by Krt5 and p63 expression, which are positioned on the basal side of the pseudostratified layer and play key roles in injury-repair of the post-natal lung (Rock et al., 2009). Nuclear Yap activity promotes expansion and maintains the progenitor state of this basal cell population, and a shift in Yap from the nucleus to the cytoplasm instructs their differentiation (Mahoney et al., 2014; Zhao et al., 2014). We therefore investigated whether the polarization of Crb3 or Scrib is linked to the localization of Yap and differentiation status of these airway epithelial cells.
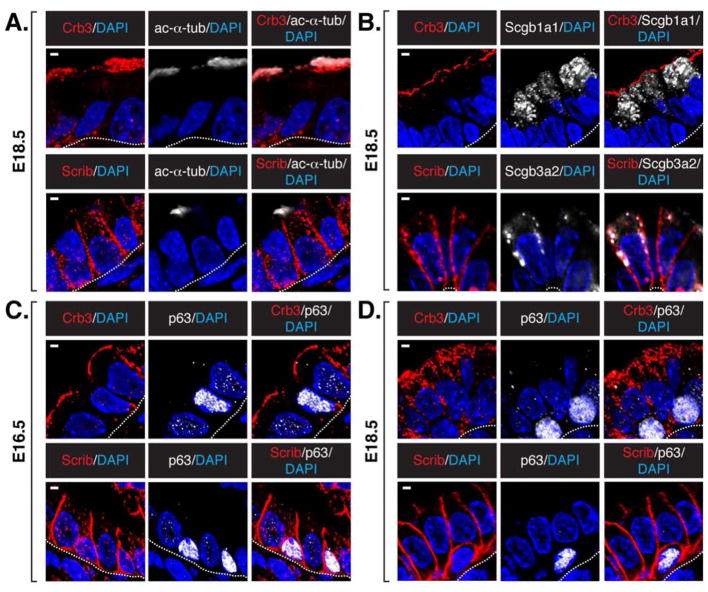
Our analysis of E16.5–E18.5 proximal epithelial cells revealed that Crb3 localizes across the apical surface and Scrib localizes to the basal-lateral membrane in differentiated ciliated cells (Figure 2A) and secretory cells (Figure 2B). Crb3 and Scrib localization was very different in p63-positive basal cells: Crb3 was nearly absent, whereas Scrib was distributed in a cortical pattern at the cell membrane (Figure 2C and 2D). E-cadherin was abundant in basal progenitors, but like Scrib displayed a cortical membrane pattern of localization (Figure S2). These observations indicated that basal cells lack aspects of apical-basal polarity, which correlates with differentiation. Interestingly, cells lacking polarized Crb3 and Scrib were observed with the emergence of p63-positive cells even in regions where these cells have yet to establish their basal positioning in the tissue (Figure 2C and 2D), suggesting that these apical-basal polarity cues are related to, but are not strictly dependent on, the organization of pseudostratified tissue structure.
Figure 2. Apical Crb3 and basal-lateral Scrib mark differentiating cells in proximal lung epithelium.
Crb3 and Scrib levels were examined by immunofluorescence microscopy in E18.5 mouse lung epithelium, and their pattern of localization was determined in (A) ciliated cells marked by apical acetylated α-tubulin, and (B) secretory cells marked by Scgb1a1 or Scgb1a2 expression. Crb3 and Scrib levels and localization were correlated with p63 expression in (C) E16.5 and (D) E18.5 proximal lung epithelium. Note that p63-positive cells lack Crb3, and display a cortical localization of Scrib. DAPI was used to mark the nuclei (blue) in all images, and the basal surface of the epithelium is outlined with a white dotted line. Scale bar = 2μm.
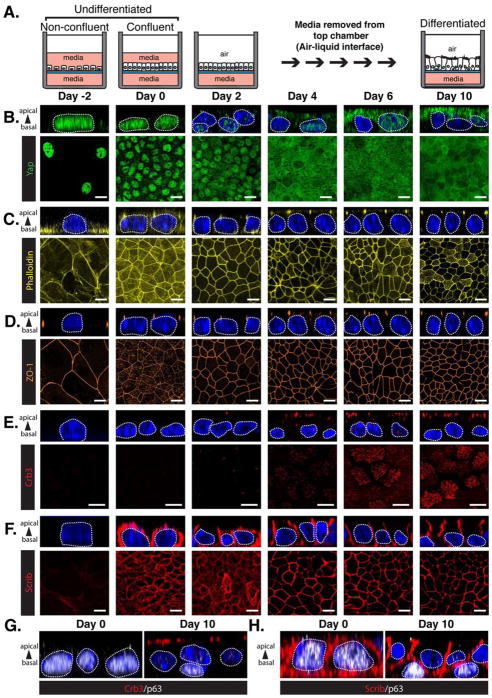
Basal airway progenitors can be isolated from adult murine tracheas and induced to differentiate in air-liquid interface (ALI) cultures (Figure 3A) (You et al., 2002). Analysis of Yap localization during ALI-mediated differentiation of basal progenitors revealed a shift of nuclear Yap in progenitors to an apical cytoplasmic enrichment of Yap upon cell differentiation (Figure 3B). Dramatic actin cytoskeleton remodeling was also observed, with cytoplasmic and membrane-associated stress-fibers found in expanding progenitors that transform into an organized apical adhesion belt as the cells differentiated (Figure 3C). The tight junction-associated protein Zo-1 (Figure 3D) and the adherens junction proteins E-cadherin and α-catenin (Figures S3A and S3B) were expressed in undifferentiated progenitors and exhibited junctional localization as cells established cell contact in their undifferentiated state, with this localization remaining unchanged with differentiation. Crb3 expression followed a similar pattern to what we observed in vivo, with undifferentiated progenitors exhibiting almost undetectable levels of Crb3, and an increase in apical Crb3 levels observed with cell differentiation (Figure 3E). Apical localization of Crb3 was observed in cells positioned on the luminal domain, with prominent staining observed at cilia in multi-ciliated cells, similar to observations from prior in vitro studies in other contexts (Fan et al., 2004; Lemmers et al., 2002). The apical distribution of Crb3 correlated precisely with apical Yap enrichment (Figure 3B and 3E). Scrib levels and polarization followed a comparable relationship with cell fate; Scrib was almost undetectable during progenitor cell expansion in vitro, increased in levels with cell confluence to distribute in a cortical-membrane pattern in the undifferentiated state, and then polarized to the basal-lateral domain with cell differentiation (Figure 3F). Close inspection of airway epithelial cells differentiated for 10 days in ALI cultures revealed that the cells positioned on the basal side of the epithelium, which maintained p63-positive and nuclear Yap status, also lacked the expression of Crb3, and exhibited a cortical membrane Scrib localization pattern (Figure 3G and 3H). These results indicated that, like in vivo, polarization of Crb3 and Scrib into apical-basal domains correlates with cytoplasmic Yap localization and airway epithelial cell differentiation, and that the polarization of these proteins is evidently distinct from adherens and tight junction formation.
Figure 3. Apical-Basal Polarity cues correlate with the differentiation of adult airway progenitors.
(A) Diagram depicting the differentiation of epithelial progenitors isolated from adult mouse tracheas following cultured in an air-liquid interface (ALI). (B–F) Immunofluorescence confocal miscopy was performed to examine the levels and localization of (B) Yap, (C) F-actin, (D) Crb3, and (E) Scrib in mouse airway basal progenitor cells induced to differentiate in ALI cultures. X-Y views (bottom panels) and the Z-plane apical-basal views (top panels) for each are shown. Basal progenitors, marked by the presence of p63, were examined by immunofluorescence confocal microscopy for the levels and localization of (F) Crb3 and (G) Scrib, and the Z-plane apical-basal view for each is shown. DAPI was used to mark the nuclei (blue) in all images, and in all Z-plane images the nuclei are also outlined with a thin white dotted line. Scale bar = 10μm.
Loss of Crb3 prevents the differentiation of airway epithelial progenitors
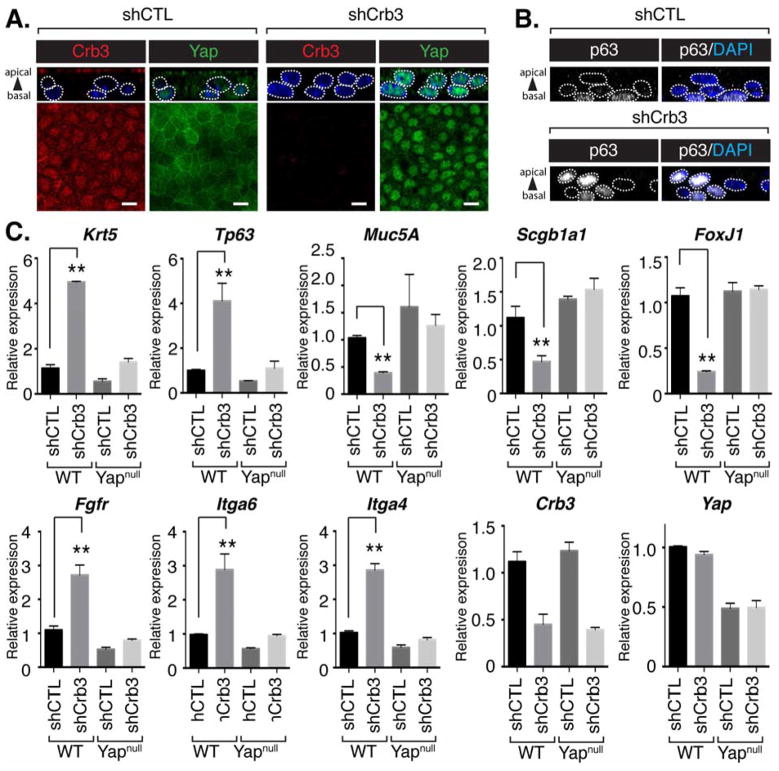
Given the correlation between apical-localized Crb3 and Yap, and airway epithelial differentiation, we next tested if Crb3 directs cell fate by controlling Yap localization. We initially used lentiviruses to transduce the expression of control shRNA or shRNA that targets Crb3 in basal progenitors isolated from adult mouse airways. Progenitors expressing control shRNA exhibited obvious localization of Yap to the cytoplasmic apical domain following differentiation in ALI cultures. In contrast, Crb3-depleted cultures retained substantial nuclear Yap even after six days of ALI culture (Figure 4A), and these cells maintained p63-positive status, and organized aberrantly into luminal positions (Figure 4B). To better understand the consequences of Crb3 depletion, we isolated RNA from cells transduced with control shRNA or shRNA targeting Crb3 and examined the expression of progenitor and differentiation markers by real-time quantitative PCR (q-PCR). This analysis clearly showed that depletion of Crb3 maintained high expression levels of the progenitor markers p63 and Krt5, and prevented the expression of the differentiation markers Scgb1a1, FoxJ1, and Muc5ac. The changes in expression of airway fate markers following Crb3 depletion were dependent on Yap, as knockdown of Crb3 did not affect the ability of Yap-null airway progenitors to differentiate (Figure 4C).
Figure 4. Depletion of Crb3 prevents the differentiation of adult airway epithelial progenitors.
Lentiviruses were used to transduce control shRNA (shCTL) or shRNA targeting Crb3 in airway progenitor cells isolated from mouse tracheas, and these cells were subsequently cultured in ALI conditions to induce differentiation. (A) Crb3 and Yap, as well as (B) p63 levels and localization were examined after 6 days of ALI culture by immunofluorescence confocal microscopy. The X-Y view (bottom panels) and the Z-plane apical-basal view (top panels) in (A) are shown, whereas only the Z-plane in (B) is shown. DAPI marked nuclei are outlined with a white dotted line in the Z-plane images. Scale bar = 10μm. (C) Samples obtained from shCTL-or shCrb3-expressing WT or Yap-null airway progenitors cultured for 6 days in ALI cultures were examined by qPCR for expression levels of Crb3, basal progenitor cell markers Tp63 and Krt5, as well as the differentiation markers FoxJ1 (ciliated cells), Scgb1a1 (secretory cells), and Muc5ac (goblet cells) (n=3, average +SEM, ** p<0.0001).
Nuclear Yap binds to and regulates the transcriptional activity of p63 (Zhao et al., 2014), which prompted us to also investigate the expression of Yap-p63-regulated genes. Depletion of Crb3 in ALI-cultured airway progenitors strongly induced the expression Yap-p63-regulated genes, including Fgfr, Itga6, and Itgb4. The induction of these genes was dependent on Yap, as parallel analysis of Crb3-depletion in Yap-null airway progenitors did not show significant expression differences (Figure 4C). Intriguingly, expression of canonical gene targets of Yap described in other contexts, such as Ctgf and Cyr61, was minimally affected by the depletion of Crb3 or by the deletion of Yap (Figure S4A), suggesting that in airway progenitors nuclear Yap directs transcriptional events distinct from those described in other contexts. Given that nuclear Yap directs TGFβ-induced transcriptional processes (Hiemer et al., 2014; Mahoney et al., 2014; Varelas et al., 2010), we also tested whether airway progenitors were more sensitive to TGFβ-induced signals following Crb3 depletion. Indeed, we found that Yap-p63-regulated target genes were significantly induced with TGFβ treatment, and that this induction was further enhanced following Crb3 depletion (Figure S4B). Taken together our observations indicated that Crb3 restricts nuclear Yap activity to drive airway progenitor cell differentiation.
We extended our analysis to also test whether loss of polarity can impact Yap localization and activity in differentiated airway epithelial cells. For this, we disrupted adherens junctions by chelating calcium-mediated junctional interactions in airway cells that were previously cultured in ALI conditions for 10 days. A loss of adherens junction proteins was observed following calcium depletion, as was a striking loss of Crb3 levels, and an accumulation of Yap in the nucleus of these cells (Figure S4C). In parallel to the increased nuclear Yap localization we observed a significant increase in the expression of Yap-p63-regulated genes (Figure S4D), suggesting that loss of junctions and polarity in differentiated airway cells is sufficient to induce nuclear Yap activity.
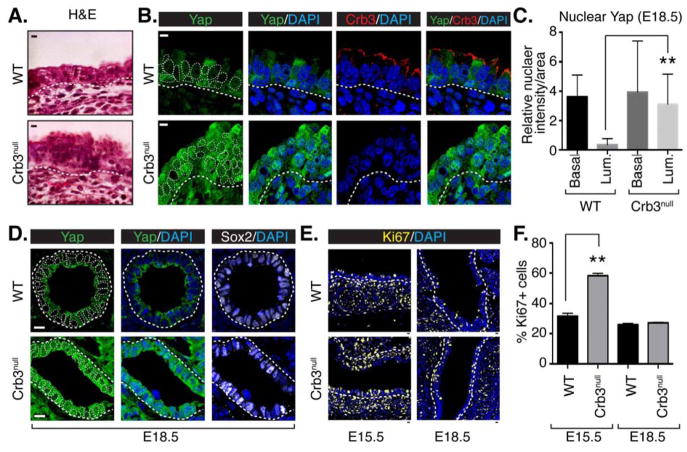
To substantiate the relationship between apical-basal polarity and Yap in vivo, we generated mice in which loxP sites flanked the Crb3 gene (Crb3-loxP/loxP). We conditionally deleted Crb3 in the foregut endoderm, and consequently in the earliest stages of pulmonary epithelial development, by crossing Crb3-loxP/loxP mice with mice expressing Cre recombinase from the Sonic hedgehog promoter (Shh-Cre) (Harris et al., 2006) (Figure S5A). Deletion of both Crb3 alleles in Crb3-loxP/loxP;Shh-Cre/+ (herein referred to as Crb3-null) was confirmed by qPCR analysis, showing a complete loss of Crb3 mRNA (Figure S5B). Crb3 deletion resulted in lethality at birth, with the likely reason being respiratory distress, similar to what has been described with the germline deletion of Crb3 (Whiteman et al., 2014). Examination of Crb3-null lungs at different developmental stages showed no gross morphological defects compared to wild type counterparts. However, examination of tissue sections by hematoxylin and eosin staining (H&E) from the lung and trachea of Crb3-null embryos revealed substantial epithelial organization differences, with obvious stratification of the tracheal epithelium (Figure 5A). Analysis of Crb3-null tissues by immunofluorescence microscopy showed a complete loss of Crb3 protein, further validating Crb3 deletion and also the specificity of our antibody (Figure 5B). When compared to their wild type equivalents, the Crb3-null epithelium showed an accumulation of Yap in the nucleus of luminal cells of the upper proximal airways (Figure 5B and 5C), and in Sox2-positive intrapulmonary airways (Figure 5D), which are cells that normally exhibit exclusively cytoplasmic Yap localization. Analysis of cells undergoing apoptosis, as assessed by activated Caspase-3, showed negligible numbers of apoptotic cells in both wild type and Crb3-null airways (data not shown). However, analysis of Ki67 expression indicated a burst of proliferation in E15.5 Crb3-null airways, which was brought back to wild type levels by E18.5 (Figure 5E and 5F). Such defects in the Crb3-null airway epithelium are consistent with increased nuclear Yap activity, as increased nuclear Yap levels are capable of driving airway epithelial cell proliferation (Zhao et al., 2014).
Figure 5. Deletion of Crb3 induces nuclear Yap localization in proximal airways.
(A) E18.5 proximal airway sections from wild type (WT) and Crb3-null mice were examined by H&E staining. (B) Proximal airways from the tracheas of WT and Crb3-null mice were analyzed by immunofluorescence microscopy for Crb3 and Yap. (C) The relative levels of nuclear Yap in cells positioned at the basal or luminal side of the epithelium in E18.5 WT and Crb3-null proximal airways was quantitated by determining the Yap pixel intensity/area of DAPI-stained nuclei using Image J software (n=50 from three separate experiments, average +SEM, ** p<0.0001). (D) Intrapulmonary proximal airways were analyzed by immunofluorescence microscopy for Yap and Sox2. (E) Proximal airways from E15.5 and E18.5 WT and Crb3-null lungs were examined for the presence of the proliferation marker Ki67, and (F) the percentage of these respective cells was quantitated (n= 160 to 300 cells from each group, average from three experiments +SEM, ** p<0.0001). The basal epithelial surface is outlined with a white dotted line in all images, and DAPI marked nuclei in the first panels are outlined with a thin white dotted line. Scale bar = 10μm.
The large majority of cells within Crb3-null airways also exhibited a loss of apical-basal polarity, as these cells showed cortical membrane localization of E-cadherin (Figure S5C), α-catenin (Figure S5D), and Scrib (Figure S5E and S5F), concurrent with loss of Zo-1 (Figure S5E), and phospho-Ezrin/Radixin/Moesin (p-ERM) (Figure S5F). However, there was a small population of cells (approximately 5%) at the most luminal side of the Crb3-null airway epithelium that exhibited aspects of apical-basal polarity (Figures S5C–F), suggesting that some cells may activate compensatory mechanisms in the absence of Crb3. Analysis of RNA from Crb3-null tissues (Figure S5B) and Crb3-depleted airway progenitors cultured in ALI conditions (Figure S5G) indicated that Crb2 levels are elevated in cells lacking Crb3, providing a potential candidate for such compensation.
An ectopic increase in nuclear Yap levels in mature proximal airway cells is sufficient to dedifferentiate these cells and have them adopt progenitor identities (Zhao et al., 2014). We therefore rationalized that the Crb3-null epithelium may be abnormally specified, and analyzed the expression pattern of various cell fate makers in different compartments of the lung and trachea (Figure S6A). No obvious differences were observed in the patterning of Sox9- and Sox2-expressing cells that make up the distal and proximal epithelial compartments in the developing lung, respectively (Figure S6B). However, analysis of markers of differentiation for the Sox2+ proximal airways showed striking defects in cell differentiation, as developing intrapulmonary Crb3-null airways lacked cells expressing the secretory markers Scgb1a1 or Scgb3a2, and apically-localized acetylated-α-tubulin, which marks ciliated cells (Figure S6C and S6D). Expression of the goblet cell marker Muc5Ac was not detected in developing Crb3-null airways, similar to that observed in wild type airways (Figure S6C).
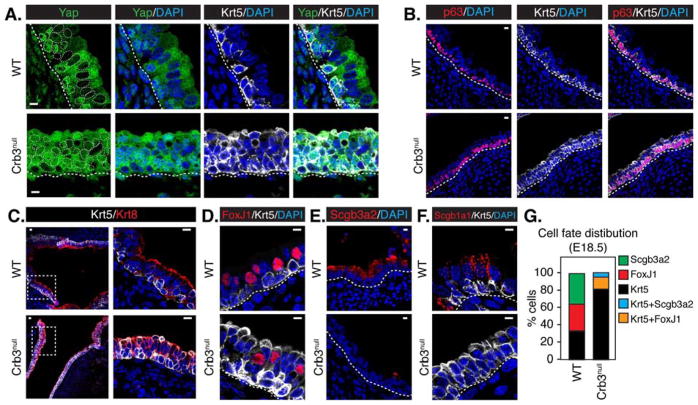
Analysis of the Crb3-null tracheas showed remarkable expansion of Krt5-expressing cells, with almost all luminal cells expressing this basal-progenitor marker (Figure 6A, 6B, 6D, 6F and S6E). The number of cells expressing p63 was also increased in Crb3-null airways compared to wild type. However, in stratified regions these cells were maintained only on the basal domain (Figure 6B), despite the presence of Krt5 in the luminal cells. The luminal Krt5-positive cells also expressed the luminal marker, Krt8 (Figure 6C), suggesting that loss of Crb3 induces an intermediate progenitor state, much like that observed following ectopic nuclear Yap expression in airways (Zhao et al., 2014). Additional marker analysis showed that very few of the Krt5-positive cells also expressed terminal markers, such as multi-ciliated cell marker Foxj1 (Figure 6D and S6F), the early secretory marker Scgb3a2 (Figure 6E), and the later marker of the secretory lineage, Scgb1a1 (Figure 6F). Quantitation revealed that out of the Krt5-positive cells in the Crb3-null airways, ~14% of them co-expressed FoxJ1 and ~5% co-expressed Scgb3a2 (Figure 6G). Krt5/FoxJ1 or Krt5/Scgb3a2 double-positive cells were never observed in wild type airways, but have been observed following ectopic expression of nuclear Yap (Zhao et al., 2014). Thus, our data strongly suggest that the defective maturation of the Crb3-null epithelium is a result of persistent nuclear Yap activity.
Figure 6. Deletion of Crb3 leads to an aberrant accumulation of undifferentiated basal progenitors in the developing airway.
E18.5 proximal airway sections from the tracheas of wild type (WT) and Crb3-null tissues were examined by immunofluorescence microscopy for (A) Krt5 and Yap, (B) Krt5 and p63, (C) Krt5 and Krt8, (D) Krt5 and FoxJ1, (E) Scgb3a2, and (F) Krt5 and Scgb1a1. The basal surface of the epithelium is outlined with a white dotted line, and DAPI marked nuclei in the first panels in experiment (A) are outlined with a thin white dotted line. Scale bar = 10μm. (G) The number of cells expressing the indicated cell fate markers was quantitated and is shown as a percentage of the total population (n=488 from WT, and n=456 from Crb3-null; total from three separate experiments).
The Hippo pathway Kinases Lats1/2 Interact with Yap at the apical domain of differentiated epithelial cells
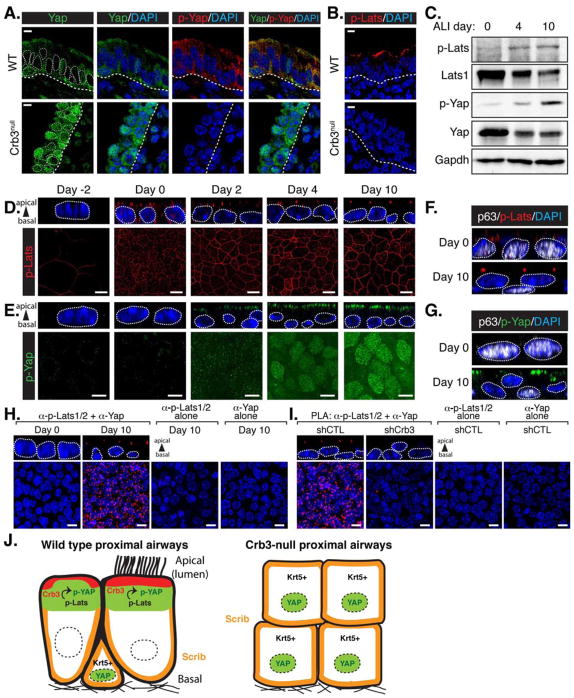
Given the importance of p-YapS112 in controlling Yap localization, and the prior relationship we observed between this modification and the apical domain of proximal airway epithelial cells, we investigated whether p-YapS112 was affected in Crb3-null airways. We observed a complete loss of p-YapS112 in Crb3-null airways, indicating an accumulation of nuclear hypo-phosphorylated Yap (Figure 7A). Given this observation, we speculated that the activity of Lats1/2 might be coupled to the acquisition of apical-basal polarity cues as airway cells differentiated. Phosphorylation of Lats1 and Lats2 on Thr1079 and Thr1041, respectively, promotes their activation (Chan et al., 2005). Analysis of p-Lats1/2T1079/T1041 levels revealed strong apical enrichment in the epithelial cells of proximal airways of wild type mice, but a complete absence of activated Lats1/2 in Crb3-null tissues (Figure 7B). These data suggested that Lats1/2 activation is linked to the apical epithelial domain, and is compromised in cells lacking Crb3.
Figure 7. Activated Lats1/2 kinases associate with Yap at the apical membrane in differentiated airway epithelial cells.
(A) E18.5 proximal airway sections from wild type and Crb3-null mice were examined by immunofluorescence microscopy for total Yap and p-YapS112 (p-Yap), and (B) for p-Lats1/2T1079/T1041 (p-Lats). The basal surface of the epithelium is outlined with a thick white dotted line, and DAPI marked nuclei in the first panels are outlined with a thin white dotted line. Scale bar = 10μm. (C) Cell lysates isolated from undifferentiated Day 0 airway progenitor cells, and ALI-cultured Day 4 and Day 10 differentiated cells were examined by immunoblotting using the indicated antibodies. (D, E) Immunofluorescence confocal microscopy was used to examine the levels and localization of activated (D) p-Lats and (E) p-Yap at different stages of differentiation in ALI-cultured airway progenitors. X-Y images (bottom panels) and the Z-plane apical-basal view (top panels) for each are shown. (F) Disorganized pLats, and (G) a reduction in p-Yap levels, was observed in p63-positive basal airway progenitors cultured under ALI conditions for 0 or 10 days. Z-plane apical-basal view for each is shown. (H) In situ Proximity ligation assay (PLA) followed by confocal microscopy was performed using the indicated antibodies on airway progenitor cells cultured under ALI conditions for the indicated days. Red dots indicate endogenous interactions. (I) In situ PLA followed by confocal microscopy was performed using the indicated antibodies on airway progenitor cells transduced to express shCTL or shCrb3 and grown for 6 days in ALI cultures. For the experiments in (H) and (I) a compressed image of the X-Y stacks is shown to highlight all the PLA spots, as is the Z-plane apical-basal view to highlight the polarization of these spots. DAPI was used to mark the nuclei (blue), and in all Z-plane images the nuclei are also outlined with a thin white dotted line. Scale bar = 10μm for all images. (J) Model for how cell polarity integrates with Hippo pathway signaling to control cell fate in the proximal airways.
To explore the relationship between cell polarity, Lats1/2 regulation, and cell fate, we returned to the in vitro differentiation of isolated basal airway progenitors. Immunoblot analysis of cell lysates obtained from ALI-cultured airway progenitors showed an increase in the levels of p-Lats1/2T1079/T1041, which correlated with p-YapS112, as the cells differentiated (Figure 7C). Immunofluorescence analysis revealed increases in endogenous p-Lats1/2T1079/T1041 levels as cells differentiated (Figure 7D), which temporally correlated with increases in p-YapS112 (Figure 7E), and the shift of Yap from the nucleus to the cytoplasm (Figure 3B). Similar to our in vivo observations, we also found that activated Lats1/2 (Figure 7D), as well as p-YapS112 (Figure 7E and S7A), accumulated at the apical regions of differentiated cells. Moreover, basal p63-positive progenitors exhibited some p-Lats1/2T1079/T1041, albeit at lower levels, but this activated Lats1/2 displayed non-polar localization (Figure 7F), and correlated with low levels of p-YapS112 (Figure 7G). Given that p-Lats1/2T1079/T1041 was observed in cells with nuclear hypo-phosphorylated Yap, we hypothesized that mutual apical recruitment may induce the binding of p-Lats1/2T1079/T1041 to Yap. We tested this possibility by examining the localized interaction of activated Lats1/2 and Yap using an in situ proximity ligation assay (PLA), which is a technique that can visualize the localization and association of endogenous protein complexes (proteins localized within 40 nm of each other) by microscopy (Koos et al., 2013). PLA analysis revealed that interactions between activated Lats1/2 and Yap were only observed following differentiation of ALI-cultured airway progenitors, and that these interactions were found at the apical junctions of the differentiated cells (Figure 7H). To test whether Crb3 mediates apical Lats1/2 binding to Yap, we used lentiviruses to transduce the expression of control shRNA or shRNA targeting Crb3 in isolated airway progenitors that were differentiated in vitro using ALI cultures. Using PLA methods we detected activated Lats1/2 and Yap in close proximity in differentiated cells treated with control shRNA, but not in Crb3-depleted cultures (Figure 7I). Thus, Crb3 is required to promote the phosphorylation of Lats1/2, bridge interactions between Lats1/2 and Yap, and thereby induce Yap phosphorylation and cytoplasmic localization. These molecular events have critical roles in promoting cell differentiation, highlighting the apical epithelial domain as an important signaling hub in airway development.
DISCUSSION
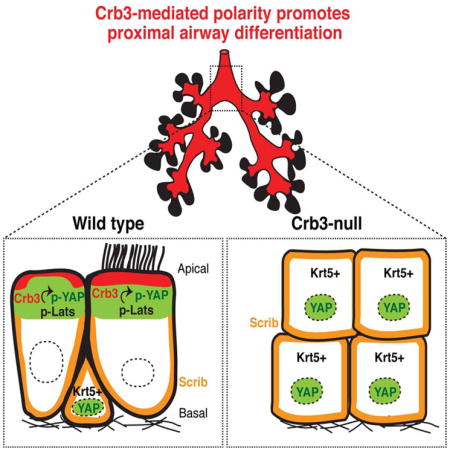
The findings presented here reveal a precise coordination between apical-basal polarity and cell fate in the airway epithelium. Specifically, we have observed that the transmembrane Crb3 protein, an important mediator of the apical epithelial domain, controls cell maturation in the proximal lungs and trachea. We propose that Crb3 does so by mediating the polarization and apical scaffolding of the Lats1/2 kinases with the transcriptional regulator Yap to promote phosphorylation and subsequent restriction of Yap from the nucleus. Yap localization has critical roles in directing epithelial progenitor fate, and therefore, Crb3-mediated control of Yap provides a mechanism to integrate morphogenic changes with differentiation in the airway epithelium (see model in Figure 7J).
Our observations provide evidence that Crb3 acts as a major regulator of Yap localization in the lung epithelium, and offer clues into how Crb3 acts in this role. Most compelling is the correlation between Crb3 expression and the restriction of Yap from the nucleus in the developing airways. Our data show that developing distal epithelial compartments, which exhibit high nuclear Yap levels, express very low Crb3 levels. Interestingly, however, despite low Crb3 levels these distal compartments maintain aspects of epithelial polarity, including having organized adherens junctions marked by E-cadherin. We found that as airways proximalize Crb3 expression increases, and does so precisely in the regions where Yap leaves the nucleus. Similar correlation was observed in vitro during airway progenitor differentiation in ALI cultures where we found that the expression and asymmetric distribution of Crb3 occurred subsequent to adherens and tight junction formation, but precisely matched the restriction of Yap from the nucleus. Therefore, Crb3 appears to organize a specific type of polarity within airway epithelial cells that is required for Hippo pathway-mediated Yap control.
At the molecular level Crb3 promotes apical domain identity, in part by assembling an evolutionary conserved protein complex at the apical membrane (Pocha and Knust, 2013). Studies from Drosophila have indicated that this complex consists of proteins that directly interact with the Yap homolog Yorkie, such as the FERM-domain protein Expanded, and suggest that Crumbs scaffolds these proteins to promote Hippo pathway activity and possibly directly sequester Yorkie to restrict its nuclear activity (Chen et al., 2010; Ling et al., 2010; Robinson et al., 2010). Our observations suggest that similar mechanisms exist in the lung epithelium, and implicate this regulatory mechanism as an essential determinant of cell differentiation. Defining the precise roles of Crb3 in Yap regulation, however, is complicated by the general dysregulation of other polarity proteins in Crb3-null airways. Moreover, we found that loss of polarity resulting from the disruption of E-cadherin-mediated adhesion also increased nuclear Yap accumulation, indicating that the loss apical polarity by other means also affects Yap regulation, which is consistent with prior work in other tissues (Schlegelmilch et al., 2011; Silvis et al., 2011). Therefore, it is likely that other proteins or environmental factors that affect Crb3 localization and/or the apical domain will influence Yap localization and alter airway epithelial function.
Notably, our study of Crb3-null lungs revealed striking cell fate defects, most obvious of which was the inability for the Sox2-positive population to differentiate into mature airway cells. However, we did not observe defects in the ability for distal Sox9-positive progenitors to pattern into Sox2-positive cells. These observations are consistent with cell fate defects resulting from increased nuclear Yap activity, as nuclear Yap is capable of patterning distal to proximal progenitor fate, but prevents terminal differentiation of proximal progenitors. Based on these data we propose that establishment of apical-basal polarity immediately following the distal-proximal “transition zone” serves to direct cytoplasmic Yap in proximal airways, and that this shift in Yap localization is critical for epithelial progenitor maturation that eventually gives rise to the luminal lineages of the airways.
Our observations reveal that differences in epithelial cell polarity also direct the signals required for progenitor identity in basal cells that arise later in airway development and persist into adulthood. In developing upper airways, we observed that p63-positive cells always lacked polarized Crb3 and Scrib localization and exhibited nuclear Yap. Also, deletion of Crb3 resulted in an aberrant expression of the progenitor marker Krt5 in luminal epithelial cells found in proximal airways. These luminal cells mirrored those that undergo dedifferentiation from ectopic nuclear Yap expression (Zhao et al., 2014), with cells losing their identity and exhibiting both progenitor markers and luminal differentiation markers. Thus, luminal cell populations in Crb3-null airways likely represent intermediates between the differentiated and basal progenitor state. Such immature airway epithelial cells have been described in other studies (Mori et al., 2015; Rock et al., 2011), and therefore our work offers potential insight into their origin. Notably, cells co-expressing Krt5 and p63 were only observed in the basal layer of Crb3-null airways, suggesting that while nuclear Yap can direct p63-regulated transcriptional events to promote the status of basal progenitor cells (Zhao et al., 2014), it is likely additional signals, possibly those from the basal matrix, crosstalk with nuclear Yap to facilitate this primitive state.
Mechanisms establishing polarity in airways are unknown, and likely involve a multitude of inputs from various factors. The Grainyhead-like transcription factors are candidate regulators of genes encoding polarity proteins in the airways, as are implicated in airway epithelial fate (Gao et al., 2013; Varma et al., 2012) and are known to induce polarity protein levels in other contexts (Werth et al., 2010). Indeed, Scrib has been identified a target of Grainyhead-like 2 in lung epithelial cells (Gao et al., 2013), and Scrib fidelity is critical for airway epithelial integrity (Yates et al., 2013). Establishing the mutually exclusive relationship between Scrib and Crb3 may therefore be an initial event driving airway polarity changes. Other attractive mechanisms impacting cell polarity and Yap activity include local mechanical differences and signals from the underlying matrix that arise during morphogenesis of the tubules. Mechanical cues that affect actin cytoskeleton dynamics are known to associate with those controlling Yap localization (Dupont et al., 2011), and thus these signals may have key roles in directing Yap and cell fate in the airways. However, further work is required to understand whether and how these signals are related.
Our analysis of how Crb3 and cell polarity integrates with cell fate has implications beyond development and likely plays key roles in epithelial regeneration and disease progression. It is almost certain that epithelial tissue damage results in aberrant cell polarity, and consequent altered downstream signals in damaged epithelium may therefore influence defective cell fate events often observed in diseases (Kotton and Morrisey, 2014). Given the described roles of Yap activity in tissue overgrowth and cancer (Harvey et al., 2013), and the fact that loss of epithelial polarity is a hallmark of carcinoma progression (Hanahan and Weinberg, 2011), it is also likely that loss of polarity-mediated control of Yap is linked to its roles in lung cancer progression (Lau et al., 2014; Shao et al., 2014). Indeed, our analysis shows increased proliferation of airway epithelium lacking Crb3, leading to a stratified morphology resembling pre-malignant epithelium.
Taken together, our data demonstrate a direct and functional link between apical-basal polarization and Yap in the lung epithelium, offering novel molecular insights into the control of airway epithelial patterning. Since Yap is emerging as an essential mediator of cell fate in the development of many organs (Varelas, 2014), it is likely that regulation of Yap localization by cell polarity cues will provide a general molecular mechanism that links organ morphogenesis and patterning.
EXPERIMENTAL PROCEDURES
Mice
Developmental and adult expression studies in mice were performed using CD1 or C57BL/6 mice. To conditionally delete the Crb3 gene in vivo, we created a Crb3-loxP/loxP mouse line at the Mouse Biology Program at the University of California, Davis. This was achieved by designing a targeting construct that introduced loxP sites flanking the entirety of Crb3 coding sequence (002,003,004,005,201 in Ensembl). Positive selection markers accompanied each loxP site (Neomycin [Neo] and Puromycin [Puro]), ensuring no recombination occurred in the floxed region following selection in embryonic stem cells. We designed the construct so that frt sites flanked the Neo cassette and rox sites flanked the Puro cassette, allowing future selective deletion of these regions. ES cells containing the loxP-flanked Crb3 gene were injected in blastocysts of C57BL/6 mice to generate chimeric mice. Chimeric mice were bred to germline transmission and then mated to Act-Flp mice to remove the Neo-selection cassette, and then Act-Dre to remove the Puro-selection cassette. For deletion of Crb3 in mice, we crossed Crb3-loxP/loxP mice with Crb3-loxP/+; Shh-Cre/+ mice, enabling the expression of Cre recombinase and subsequent deletion of all exons (exons 2–6) of Crb3 in the developing endoderm (see Figure S3 for design and targeting outline). Genotyping of the Crb3 deleted mice was conducted using the primers and protocol described in the Supplementary Experimental Procedures. Yap-loxP/loxP mice were generously provided by Dr. Fernando Camargo (Harvard University), and are previously described (Schlegelmilch et al., 2011). All animal experiments were done in accordance with protocols approved by Boston University School of Medicine (IACUC protocol: AN-15304).
Air Liquid Interface (ALI) and Lentivirus
Recombinant lentiviruses were generated by transfecting of HEK-293T cells with a lentiviral transfer vector, the packaging plasmid psPAX2, and pMD2G-VSVG envelope DNA. The pLKO1-puro transfer vector was used to drive the expression of scrambled control shRNA (shCTL; sense sequence: GGGCAAGACGAGCGGGAAG), shRNA targeting Crb3 (shCrb3; sense sequence: CGGACCCUUUCACAAAUAGCA), or shRNA targeting Lats1 and Lats2 (shLats1/2; sense sequence: ATGGACAAGTCTATGTTTGT). Recombinant viruses were harvested by collecting media from transfected cells and subsequently concentrated by ultracentrifugation and tittered based the ability to deliver Puromycin resistance to cells. Transduction of isolated airway progenitors and differentiation of these cells using ALI cultures was achieved as previously described (You et al., 2002). Briefly, primary airway progenitors were isolated from the tracheas of adult mice and expanded in vitro on 0.4 μm Transwell filters. Lentiviral-mediated transduction was achieved by infecting freshly plated progenitor cultures with 1×107–108 units of active viral particles, culturing the cells for 48 hours, and then selecting transduced cells with 1μg/ml Puromycin. Upon confluence the progenitors were induced to differentiate by exposing the apical layer to air. For deletion of Yap in airway progenitors, cells were isolated from Yap-loxP/loxP mice and transduced with a lentivirus expressing Cre recombinase from an EF1a-promoter (generous gift from Dr. Darrell Kotton, Boston University).
Real-Time PCR
For quantitative real-time PCR, RNA was extracted with the RNeasy kit (QIAGEN) and reverse transcribed using iScript enzymes (BioRad). TaqMan reactions were performed using Universal Master Mix II, with UNG (Applied Biosystems). TaqMan primers (Applied Biosystems) were used for some experiments, including those examining the following genes: Gapdh (Mm99999915_g1), Krt5 (Mm01305291_g1), Tp63 (Mm00495788_m1), FoxJ1 (Mm01267279_m1), Scgb1a1 (Mm00442046_m1), and Muc5ac (Mm01276718_m1). Analysis other genes was monitored using Fast SYBR Green Master Mix (Applied Biosystems; 4385612) with the primers listed in the Supplementary Experimental Procedures. All real-time qPCR reactions were carried out in a ViiA7 Real-Time PCR System (Applied Biosystems) and analyzed using the ddCT method.
Calcium Depletion
Air Liquid Interface cultures were differentiated for 10 days under normal conditions. On day 10, media was removed and the cells washed three times with PBS. Fresh media was added to the bottom chamber and chelation buffer was added to the top of the cells (12 mM EGTA in 10 mM HEPES, pH 7.4) at 37°C for 20 minutes as previously described (Vladar and Stearns, 2007). After visual conformation of junction disruption, both the media and chelation buffer were removed. Cells were then washed five times to remove remaining buffer, fresh media was added to the bottom chamber, and cells were returned to the air liquid interface condition. Cultures were monitored visually every hour for four hours to ensure viability and junction disruption before lysing or fixing for analysis.
Immunofluorescence microscopy and Proximity Ligation Assay
Embryonic and adult lungs were fixed overnight in 4% PFA (Electron Microscopy Sciences; 15710) and processed for paraffin embedding. Staining was performed using a standard dewaxing and hydration protocol, followed by a microwave-assisted antigen retrieval step using a low pH buffer (Vector Labs; H-3300). H&E staining was done using Mayer’s hematoxylin (Sigma; MHS16) and Eosin Y alcoholic (Sigma; HT110116) following the manufacturer’s protocol. Air Liquid Interface inserts were fixed in 4% PFA and then blocked/permeabilized in 0.2% Triton X-100/0.1% BSA/PBS. Cells were then incubated with primary and then secondary antibody incubation in a 0.1% Triton X-100/0.2% BSA/PBS buffer. Primary antibodies used and their dilutions are described in Supplementary Experimental Procedures. Secondary antibodies used were conjugated to Alexa Fluor-488, -555, -568, or -647 (Life Technologies). PLA was carried out as per manufacturer’s protocol (Sigma; DUO92102). Briefly, ALI inserts were treated with primary antibodies (mouse Yap and rabbit phospho-Lats1/2) using the above immunofluorescence microscopy protocol, followed by incubation with the PLA probe set (anti rabbit PLUS strand/anti mouse MINUS strand) at 37°C for 1 hour. Ligation of probes in close proximity was carried out at 37°C for 30 minutes followed by a 100 minute amplification step. All processed slides were mounted using ProLong Gold antifade reagent (Life Technologies; P36930) and images were captured using a confocal microscope system (Carl Zeiss; LSM 710).
Supplementary Material
Acknowledgments
We would like to thank Dr. Shioko Kimura (NIH) for the Scgb3a2 antibody, Dr. Darrell Kotton for the EF1a-Cre plasmid, and Dr. Fernando Camargo for the Yap-loxP/loxP mice. We would also like to thank Xingbin Ai, Matthew Layne, and Laertis Ikonomou for valuable discussions and comments. This work was funded by grants from the March of Dimes Foundation (1-FY14-219 to X.V.) and from the NIH-National Heart Lung and Blood Institute (R01 HL124392-01 to X.V. and R01 HL105971-01 to W.V.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, Halder G. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A. 2010;107:15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, Hurd EA, Domino SE, Margolis B. Polarity proteins control ciliogenesis via kinesin motor interactions. Current biology : CB. 2004;14:1451–1461. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Gao X, Vockley CM, Pauli F, Newberry KM, Xue Y, Randell SH, Reddy TE, Hogan BL. Evidence for multiple roles for grainyheadlike 2 in the establishment and maintenance of human mucociliary airway epithelium. Proc Natl Acad Sci U S A. 2013;110:9356–9361. doi: 10.1073/pnas.1307589110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem J. 2011;436:213–224. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci U S A. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor beta-induced tumorigenic phenotypes in breast cancer cells. The Journal of biological chemistry. 2014;289:13461–13474. doi: 10.1074/jbc.M113.529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos B, Andersson L, Clausson CM, Grannas K, Klaesson A, Cane G, Soderberg O. Analysis of Protein Interactions in situ by Proximity Ligation Assays. Curr Top Microbiol Immunol. 2013 doi: 10.1007/82_2013_334. [DOI] [PubMed] [Google Scholar]
- Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nature medicine. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. Journal of molecular cell biology. 2014 doi: 10.1093/jmcb/mju046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AN, Curtis SJ, Fillmore CM, Rowbotham SP, Mohseni M, Wagner DE, Beede AM, Montoro DT, Sinkevicius KW, Walton ZE, et al. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. The EMBO journal. 2014;33:468–481. doi: 10.1002/embj.201386082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers C, Medina E, Delgrossi MH, Michel D, Arsanto JP, Le Bivic A. hINADl/PATJ, a homolog of discs lost, interacts with crumbs and localizes to tight junctions in human epithelial cells. The Journal of biological chemistry. 2002;277:25408–25415. doi: 10.1074/jbc.M202196200. [DOI] [PubMed] [Google Scholar]
- Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, Arsanto JP, Le Bivic A. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell. 2004;15:1324–1333. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yao E, Chuang PT. A conserved MST1/2-YAP axis mediates Hippo signaling during lung growthc. Developmental biology. 2015 doi: 10.1016/j.ydbio.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci U S A. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Developmental cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Mahoney JE, Stupnikov MR, Paez-Cortez JR, Szymaniak AD, Varelas X, Herrick DB, Schwob J, Zhang H, Cardoso WV. Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development. 2015;142:258–267. doi: 10.1242/dev.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Developmental cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocha SM, Knust E. Complexities of Crumbs function and regulation in tissue morphogenesis. Current biology : CB. 2013;23:R289–293. doi: 10.1016/j.cub.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Current biology : CB. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15:225–242. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Developmental cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Varma S, Cao Y, Tagne JB, Lakshminarayanan M, Li J, Friedman TB, Morell RJ, Warburton D, Kotton DN, Ramirez MI. The transcription factors Grainyhead-like 2 and NK2-homeobox 1 form a regulatory loop that coordinates lung epithelial cell morphogenesis and differentiation. The Journal of biological chemistry. 2012;287:37282–37295. doi: 10.1074/jbc.M112.408401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar EK, Stearns T. Molecular characterization of centriole assembly in ciliated epithelial cells. J Cell Biol. 2007;178:31–42. doi: 10.1083/jcb.200703064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth M, Walentin K, Aue A, Schonheit J, Wuebken A, Pode-Shakked N, Vilianovitch L, Erdmann B, Dekel B, Bader M, et al. The transcription factor grainyhead-like 2 regulates the molecular composition of the epithelial apical junctional complex. Development. 2010;137:3835–3845. doi: 10.1242/dev.055483. [DOI] [PubMed] [Google Scholar]
- Whiteman EL, Fan S, Harder JL, Walton KD, Liu CJ, Soofi A, Fogg VC, Hershenson MB, Dressler GR, Deutsch GH, et al. Crumbs3 is essential for proper epithelial development and viability. Molecular and cellular biology. 2014;34:43–56. doi: 10.1128/MCB.00999-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates LL, Schnatwinkel C, Hazelwood L, Chessum L, Paudyal A, Hilton H, Romero MR, Wilde J, Bogani D, Sanderson J, et al. Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Developmental biology. 2013;373:267–280. doi: 10.1016/j.ydbio.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes & development. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M, Nunna N, Hariri LP, et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Developmental cell. 2014;30:151–165. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.