Abstract
Objective
We estimate the prevalence of hearing-aid use in Iceland and identify sex-specific factors associated with use.
Design
Population-based cohort study.
Study sample
A total of 5172 age, gene/environment susceptibility - Reykjavik study (AGES-RS) participants, aged 67 to 96 years (mean age 76.5 years), who completed air-conduction and pure-tone audiometry.
Results
Hearing-aid use was reported by 23.0% of men and 15.9% of women in the cohort, although among participants with at least moderate hearing loss in the better ear (pure-tone average [PTA] of thresholds at 0.5, 1, 2, and 4 kHz ≥ 35 dB hearing level [HL]) it was 49.9% and did not differ by sex. Self-reported hearing loss was the strongest predictor of hearing-aid use in men [OR: 2.68 (95% CI: 1.77, 4.08)] and women [OR: 3.07 (95% CI: 1.94, 4.86)], followed by hearing loss severity based on audiometry. Having diabetes or osteoarthritis were significant positive predictors of use in men, whereas greater physical activity and unimpaired cognitive status were important in women.
Conclusions
Hearing-aid use was comparable in Icelandic men and women with moderate or greater hearing loss. Self-recognition of hearing loss was the factor most predictive of hearing-aid use; other influential factors differed for men and women.
Keywords: Age-related hearing loss, hearing impairment, hearing aids, older persons, sex differences
Age-related hearing loss (ARHL) begins in middle age, and by old age it is highly prevalent (Davis, 1989; Stevens et al, 2011). ARHL has been associated with a wide range of adverse health outcomes, including depression, reduced physical functioning, impaired cognitive ability, and mortality (Li et al, 2014; Reuben et al, 1999; Keller et al, 1999; Lin et al, 2011; Michikawa et al, 2009; Laforge et al, 1992; Agrawal et al, 2011; Fisher et al, 2014; Dalton et al, 2003). Hearing aids are the primary rehabilitative strategy for those diagnosed with moderate-to-severe hearing loss, and individuals who utilize them report better quality of life with increased social interaction, independence, and activity levels, less depression, and improved general health (Bridges & Bentler, 1998; Appollonio et al, 1996; Mulrow et al, 1992). Hearing-aid use has also been shown to be independently associated with increased survival among those with ARHL in the Age, Gene/Environment Susceptibility - Reykjavik Study (AGES-RS) cohort (Fisher et al, 2014). Unfortunately, ARHL often goes undiagnosed, and when diagnosed, individuals frequently do not acquire hearing aids (Chien & Lin, 2012; Lee et al, 1991; Popelka et al, 1998; Hartley et al, 2010; Gopinath et al, 2011; Bainbridge & Ramachandran, 2014; Nash et al, 2013).
The topic of hearing-aid use is gaining attention, given reports of increases in life expectancy and the demographic shift towards old age with the anticipated increase in ARHL prevalence. Continuing advances in hearing-aid technology are improving their design, functionality, and ease of use. While a number of studies have examined factors associated with the acquisition, acceptance, and use of hearing aids, (Hartley et al, 2010; Gopinath et al, 2011; Bainbridge & Ramachandran, 2014; Nash et al, 2013; Fischer et al, 2011) most lack conclusive findings. To our knowledge, no studies have reported sex-specific predictors or addressed the possible impact of co-existing health conditions on hearing-aid use.
In many parts of the world, hearing screening is not routinely offered, and when hearing loss is identified, a major deterrent to acquiring a hearing aid is cost (Gopinath et al, 2011; Kochkin, 1993). The provision of health care in Iceland, particularly for hearing screening and access to hearing aids for those who could benefit, is such that cost to the patient is of lesser importance. The current paper estimates the prevalence of hearing-aid use among older men and women in the well-characterized AGES-RS cohort and seeks to identify sex-specific factors that positively predispose participants with ARHL to use hearing aids.
Methods
Study population
Participants in the AGES-RS (Harris et al, 2007), a population-based cohort study designed to investigate genetic and environmental risk factors of health, disease, and disability in older adults, were sampled from the earlier Reykjavik Study (N = 30 795 with 11 549 alive in 2002) initiated by the Icelandic Heart Association (IHA) in 1967. Of the 5764 participants (aged 67 years and older) who were examined as part of the AGES-RS between 2002 and 2006, 5183 (89.9%) completed the hearing examination. Eleven of these participants were excluded due to insufficient hearing or hearing-aid use data, resulting in 5172 individuals for the analysis.
In adherence to the Tenets of the Declaration of Helsinki, the AGES-RS was approved by the Icelandic National Bioethics Committee (VSN: 00-063), the Icelandic Data Protection Authority, Iceland, and by the Institutional Review Board for the National Institute of Aging, National Institutes of Health, USA. Written informed consent was acquired from all participants.
Hearing examination
Air-conduction, pure-tone audiometry was performed, using a standardized protocol, in a sound-treated booth with an Interacoustics AD229e microprocessor audiometer (Interacoustics A/S, DK-5610, Assens, Denmark) and standard TDH-39P supra-aural audiometric headphones or E.A.R. tone 3A insert earphones (MEDI, Benicia, USA). Hearing thresholds at frequencies from 0.5 to 8 kHz (0.5, 1, 2, 3, 4, 6, and 8 kHz with a repeat threshold test at 1 kHz to ensure reliability) were determined for each ear. Masking was not used when significant inter-ear differences were found; however, retests were performed using insert earphones to maximize the inter-aural attenuation. Hearing impairment (HI) was defined as a pure-tone average (PTA) of four frequencies (0.5, 1, 2, and 4 kHz) in the better ear (BE) of at least 20 decibels (dB) hearing level (HL). This definition is consistent with at least mild hearing loss as determined by the Global Burden of Disease (GBD) hearing loss expert group (Stevens et al, 2011). HI was also examined using the BE L-PTA of three low or middle frequencies (0.5, 1, and 2 kHz) and the BE H-PTA of four high frequencies (3, 4, 6, and 8 kHz), respectively. Severity of HI in the BE was further categorized as none, unilateral HI only (BE PTA < 20 dB HL; worse ear PTA ≥ 35 dB HL), mild (20 – 34.9 dB HL), moderate (35 – 49.9 dB HL), moderately severe (50 – 64.9 dB HL), and severe-to-profound (65 + dB HL).
Assessment of potential explanatory factors
Potential predictors of hearing-aid use, including demographic and lifestyle characteristics and medical and hearing health history, were captured at baseline clinic visits during detailed in-person interviews and clinical examinations. Utilization of hearing aids was determined based on the subject ’ s response to the following question: “Do you wear a hearing aid?” Education was dichotomized as secondary school and higher versus less than secondary school completion. Smoking status was categorized as never smoked, former smoker, or current smoker. Body mass index (BMI) was calculated as weight (kg) divided by height (metres) squared. Diabetes mellitus was defined as a self-reported history of diabetes, use of glucose-modifying medications, or fasting blood glucose of ≥ 7.0 mmol/L. Hypertension was defined as a self-reported history of hypertension, use of antihypertensive drugs, or blood pressure ≥ 140/90 mm Hg. Self-reported health status was rated as poor, fair, good, very good, or excellent, and subsequently categorized for this analysis as poor or better. Criteria for depressive symptomology were based on a score of six or greater on the 15-item geriatric depression scale. Cognitive status was determined by review of a series of cognitive examinations and classified as normal, mild cognitive impairment (MCI), or demented by a consensus panel using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) guidelines and, subsequently, dichotomized for this analysis as unimpaired (normal) or impaired (MCI or demented). Physical activity level, during lifetime, was defined as moderate or greater frequency (approximately equivalent to more than four hours per week of moderate or vigorous activity) or less. A walking disability was determined from self-report of difficulty walking or use of walking aids. Activities of daily living, including dressing, bathing, transferring, eating, and walking, were summarized using a composite score, ranging from zero to five with one point assigned when the activity could not be completed independently. Responses to questions on leisure activities were summarized to characterize the number of days per month an individual participated, stratified into mental activities or social activities and ‘overall’ (both mental and social). Individuals were asked to bring to the clinic all prescribed medications including over-the-counter drugs; the number of medications was tallied. A clinical cardiovascular event was recorded if an individual had a hospital-documented myocardial infarction, coronary artery bypass, or angioplasty. Hand osteoarthritis (OA) was determined from digital photographs of the hands with categories: none/absent, doubtful OA, mild definite OA, moderate definite OA, and severe definite OA, with definite hand OA comprising the latter three groups (Jonsson et al, 2012). Moderate vision impairment was defined as a presenting visual acuity of 20/50 or worse but better than 20/200 in the better eye; severe vision impairment was defined as a presenting visual acuity of 20/200 or worse in the better eye. Other health conditions and personal characteristics used in the analysis came from self-reported responses to questions asked during the baseline clinic interview.
Statistical analysis
Participant characteristics were described using means and standard deviations for continuous variables and percentages and counts for categorical variables. Analyses of baseline characteristics by hearing-aid use, adjusted for age and sex, were completed using analysis of covariance (ANCOVA) and logistic regression. Prevalence of hearing-aid use was calculated by age and severity of hearing loss for the entire cohort and for men and women separately. All characteristics were considered as potential explanatory variables in stepwise age- and sex-adjusted logistic regression models; the minimum Akaike information criteria (AIC) corresponding to an implied significance level of P < 0.22 was used to determine which variables would be retained. The final analytic models included age, sex, BMI, diabetes, cognitive status, physical activity level, activities of daily living, leisure activities, number of medications, self-reported history of angina, hand osteoarthritis, low- and high-frequency audiometric HI (BE L-PTA or H-PTA ≥ 20 dB HL), self-reported hearing loss, and self-reported history of repeated ear infections as covariates (these variables are also listed in table footnotes). Multivariable logistic regression, adjusting for these selected explanatory factors, was then used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) estimating the odds of hearing-aid use for each factor in participants with ARHL. Since level of hearing impairment differed between men and women, analyses were stratified by sex in order to capture sex-specific differences for predictors of hearing aid-use. Additional analyses examining interactions between age and severity of hearing impairment along with other explanatory factors on hearing-aid use were attempted and, where sample sizes allowed, findings were reported. We also looked specifically at whether there was any interaction between sex and L-PTA or H-PTA among those with hearing impairment. Two-sided tests and a 5% significance level were employed. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, North Carolina, USA).
Results
Hearing-aid use was reported by 19.0% (N = 981) of participants who completed the audiometric examination; 52.1% of whom were men. Table 1 presents baseline participant characteristics stratified by hearing-aid use for men, women, and ‘all’ (combined sex). In analyses adjusted only for age and sex, compared to individuals without hearing aids, all measures related to hearing, including greater severity of measured HI, self-reported hearing loss, noise exposure, tinnitus, self-reported history of repeated ear infections, ear disease, or ear surgeries, and self-reported difficulties due to hearing were significantly more likely in participants with hearing aids. Individuals who reporting using hearing aids were also significantly older (79.5 ± 5.4 vs. 75.7 ± 5.3 years, p < 0.01) and more likely to have a walking disability, use a greater number of medications, and participate in more leisure activities than participants who did not utilize hearing aids. Low-frequency hearing loss did not differ between men and women after adjusting for age, although hearing – aid users were, not surprisingly, significantly more likely to have worse low-frequency hearing compared to those without hearing aids. In contrast, men did, after age adjustment, have significantly worse hearing in the high frequency range compared to women, regardless of whether or not they were a hearing-aid user.
Table 1.
Characteristics by Hearing Aid Use in Men and Women (N = 5172).
| Characteristic | Use Hearing Aids (N = 981, 19.0%) | Do Not Use Hearing Aids (N = 4191, 81.0%) | |||||
|---|---|---|---|---|---|---|---|
| All Participants | Men (N = 511, 52.1%) |
Women (N =
470, 47.9%) |
All Participants | Men (N = 1709, 40.8%) |
Women (N = 2482, 59.2%) |
p-valuea | |
| Mean Age in years | 79.5 (5.4) | 79.0 (5.3) | 80.0 (5.6) | 75.7 (5.3) | 75.9 (5.2) | 75.7 (5.3) | <0.01 |
| Education Level, Completed Secondary or More | 73.5% (711) | 80.9% (406) | 65.6% (305) | 77.1% (3214) | 84.9% (1440) | 71.8% (1774) | 0.44 |
| Former Smoker | 48.4% (475) | 58.5% (299) | 37.5% (176) | 44.1% (1847) | 59.5% (1016) | 33.5% (831) | 0.15 |
| Current Smoker | 10.6% (104) | 11.9% (61) | 9.2% (43) | 12.6% (528) | 11.5% (196) | 13.4% (332) | 0.57 |
| Mean BMI | 26.4 (4.0) | 26.4 (3.5) | 26.5 (4.4) | 27.2 (4.5) | 27.0 (3.9) | 27.4 (4.9) | 0.06 |
| Diabetes | 14.0% (137) | 17.8% (91) | 9.8% (46) | 11.5% (480) | 14.6% (249) | 9.3% (231) | 0.08 |
| Hypertension | 84.1% (825) | 82.4% (421) | 86.0% (404) | 80.1% (3355) | 79.8% (1364) | 80.3% (1991) | 0.65 |
| Self-Reported Health Status, Poor | 6.5% (63) | 5.5% (28) | 7.5% (35) | 5.5% (232) | 3.9% (67) | 6.7% (165) | 0.41 |
| Depressive Symptomology | 9.0% (83) | 8.1% (39) | 10.0% (44) | 6.9% (275) | 5.8% (94) | 7.7% (181) | 0.09 |
| Cognitive Status, Impaired | 22.6% (217) | 24.9% (124) | 20.1% (93) | 13.0% (540) | 15.3% (259) | 11.4% (281) | 0.17 |
| Physical Activity, Moderate or Greater | 31.2% (279) | 32.8% (152) | 29.5% (127) | 32.3% (1254) | 36.6% (590) | 29.1% (664) | 0.45 |
| Self-Reported History of Dizziness | 6.6% (60) | 4.4% (21) | 9.1% (39) | 7.4% (295) | 5.2% (85) | 8.9% (210) | 0.67 |
| Self-Reported History of Imbalance/Unsteadiness | 34.8% (319) | 30.2% (146) | 40.1% (173) | 29.2% (1168) | 25.4% (415) | 31.8% (753) | 0.66 |
| Walking Disability | 26.4% (259) | 23.1% (118) | 30.0% (141) | 16.2% (678) | 14.0% (238) | 17.8% (440) | 0.04 |
| Self-Reported History of Falls | 19.5% (191) | 13.9% (71) | 25.6% (120) | 17.7% (740) | 14.8% (252) | 19.7% (488) | 0.55 |
| Mean Activities of Daily Living Composite Score | 0.6 (1.1) | 0.4 (0.9) | 0.7 (1.2) | 0.5 (1.0) | 0.4 (0.8) | 0.5 (1.0) | 0.38 |
| Mean Leisure Activities Summary Measure | 4.9 (3.5) | 4.3 (3.4) | 5.5 (3.5) | 4.9 (3.5) | 4.5 (3.6) | 5.2 (3.4) | <0.01 |
| Mean Leisure Activities (Social) Summary Measure | 3.6 (3.6) | 3.2 (3.5) | 4.0 (3.6) | 3.4 (3.5) | 3.2 (3.5) | 3.6 (3.5) | 0.21 |
| Mean Leisure Activities (Mental) Summary Measure | 6.5 (6.0) | 5.6 (5.9) | 7.3 (6.0) | 6.8 (5.9) | 6.2 (6.1) | 7.2 (5.7) | 0.01 |
| Mean Number of Medications | 4.6 (3.1) | 4.5 (3.0) | 4.8 (3.2) | 4.0 (2.8) | 3.8 (2.8) | 4.1 (2.8) | <0.01 |
| Baseline History by Self-Report | |||||||
| Arthritis | 37.6% (360) | 26.8% (133) | 49.4% (227) | 39.0% (1608) | 24.8% (416) | 48.9% (1192) | 0.53 |
| Angina | 16.4% (156) | 20.3% (101) | 12.1% (55) | 13.9% (571) | 17.5% (292) | 11.4% (279) | 0.34 |
| Cardiovascular Disease | 28.6% (280) | 38.6% (197) | 17.7% (83) | 22.2% (931) | 31.4% (535) | 16.0% (396) | 0.09 |
| Record of Clinical Cardiovascular Event | 18.3% (178) | 27.4% (139) | 8.4% (39) | 14.7% (609) | 24.8% (420) | 7.7% (189) | 0.36 |
| Hand Osteoarthritisb | 47.3% (443) | 46.9% (226) | 47.7% (217) | 45.7% (1865) | 41.9% (693) | 48.4% (1172) | 0.5 |
| Mild | 17.6% (165) | 20.3% (98) | 14.7% (67) | 20.4% (831) | 20.8% (344) | 20.1% (487) | |
| Moderate | 14.2% (133) | 15.8% (76) | 12.5% (57) | 13.1% (536) | 13.5% (224) | 12.9% (312) | |
| Severe | 15.5% (145) | 10.8% (52) | 20.4% (93) | 12.2% (498) | 7.6% (125) | 15.4% (373) | |
| Level of Vision Impairment (Better Eye)c | 0.65 | ||||||
| Moderate | 18.1% (169) | 18.8% (92) | 17.2% (77) | 12.9% (517) | 12.4% (203) | 13.3% (314) | |
| Severe (includes blind) | 2.8% (26) | 2.9% (14) | 2.7% (12) | 2.2% (87) | 2.2% (36) | 2.2% (51) | |
| Mean PTA Valued | 46.4 (12.7) | 46.6 (13.0) | 46.2 (12.3) | 25.4 (10.5) | 27.4 (10.7) | 24.1 (10.2) | <0.01 |
| Mean L-PTA Valuee | 40.8 (13.8) | 39.6 (14.4) | 42.1 (13.0) | 20.3 (9.8) | 20.1 (10.1) | 20.4 (9.5) | <0.01 |
| Mean H-PTA Valuef | 66.5 (13.1) | 69.5 (12.6) | 63.4 (13.0) | 45.2 (16.3) | 51.5 (15.9) | 40.8 (15.1) | <0.01 |
| Level of Hearing Impairment (Better Ear)g | <0.01 | ||||||
| None, Mild, or Unilateral only | 14.5% (142) | 16.2% (83) | 12.6% (59) | 79.9% (3347) | 74.6% (1275) | 83.5% (2072) | |
| Moderate | 50.0% (490) | 46.0% (235) | 54.3% (255) | 18.4% (772) | 22.5% (385) | 15.6% (387) | |
| Moderately Severe | 28.2% (277) | 29.6% (151) | 26.8% (126) | 1.6% (68) | 2.8% (47) | 0.9% (21) | |
| Severe (includes profound and deaf) | 7.3% (72) | 8.2% (42) | 6.4% (30) | 0.1% (4) | 0.1% (2) | 0.1% (2) | |
| Self-Reported Hearing Loss | 47.5% (465) | 47.8% (244) | 47.1% (221) | 3.9% (165) | 4.9% (83) | 3.3% (82) | <0.01 |
| Self-Reported Tinnitus | 18.3% (178) | 18.9% (96) | 17.6% (82) | 11.8% (493) | 13.4% (227) | 10.8% (266) | <0.01 |
| Self-Reported Frequent Tinnitush | 15.5% (152) | 16.6% (85) | 14.3% (67) | 9.8% (412) | 11.2% (191) | 8.9% (221) | <0.01 |
| Self-Reported History of Repeated Ear Infections | 22.2% (182) | 18.6% (78) | 25.9% (104) | 12.9% (448) | 10.9% (155) | 14.3% (293) | <0.01 |
| Self-Reported Ear Operation | 6.4% (62) | 8.0% (41) | 4.5% (21) | 2.7% (114) | 2.5% (43) | 2.9% (71) | <0.01 |
| Self-Reported Noise Exposure, High | 45.9% (443) | 65.1% (327) | 25.0% (116) | 33.5% (1394) | 50.4% (853) | 21.9% (541) | <0.01 |
| Self-Reported Diagnosis of Ear Disease | 9.6% (91) | 9.7% (48) | 9.4% (43) | 8.5% (352) | 7.4% (125) | 9.3% (227) | <0.01 |
| Self-Reported Difficulties due to Hearing | |||||||
| Difficulty Meeting New People | 49.8% (464) | 50.4% (243) | 49.1% (221) | 11.2% (427) | 12.9% (203) | 10.0% (224) | <0.01 |
| Difficult to Visit Friends, Relatives, Neighbors | 35.9% (334) | 36.8% (177) | 34.9% (157) | 6.9% (264) | 8.2% (129) | 6.0% (135) | <0.01 |
| Problems Listening to TV or Radio | 18.5% (172) | 18.1% (87) | 18.9% (85) | 3.3% (124) | 4.0% (63) | 2.7% (61) | <0.01 |
Data are presented as % (N) or mean (standard deviation).
Comparison Results of the comparison between all hearing aid users and all non-users overall, adjusted for each characteristic after adjustment for age and sex. (in the case of age, after adjustment for sex).
Hand osteoarthritis determined from digital photographs.
Moderate vision impairment was defined as a presenting visual acuity of 20/50 or worse but better than 20/200 in the better eye; severe vision impairment was defined as a presenting visual acuity of 20/200 or worse in the better eye.
Pure tone audiometry (PTA) value is the average of the audiometric thresholds at 0.5, 1, 2, and 4 kHz frequencies in the better ear.
Pure tone audiometry (PTA) value – low frequency is the average of the audiometric thresholds at 0.5, 1, and 2 kHz frequencies in the better ear.
Pure tone audiometry (PTA) value – high frequency is the average of the audiometric thresholds at 3, 4, 6, and 8 kHz frequencies in the better ear.
HI was defined as moderate (35–49.9 dB HL), moderately severe (50–64.9 dB HL), and severe (65 + dB HL).
Daily or almost always.
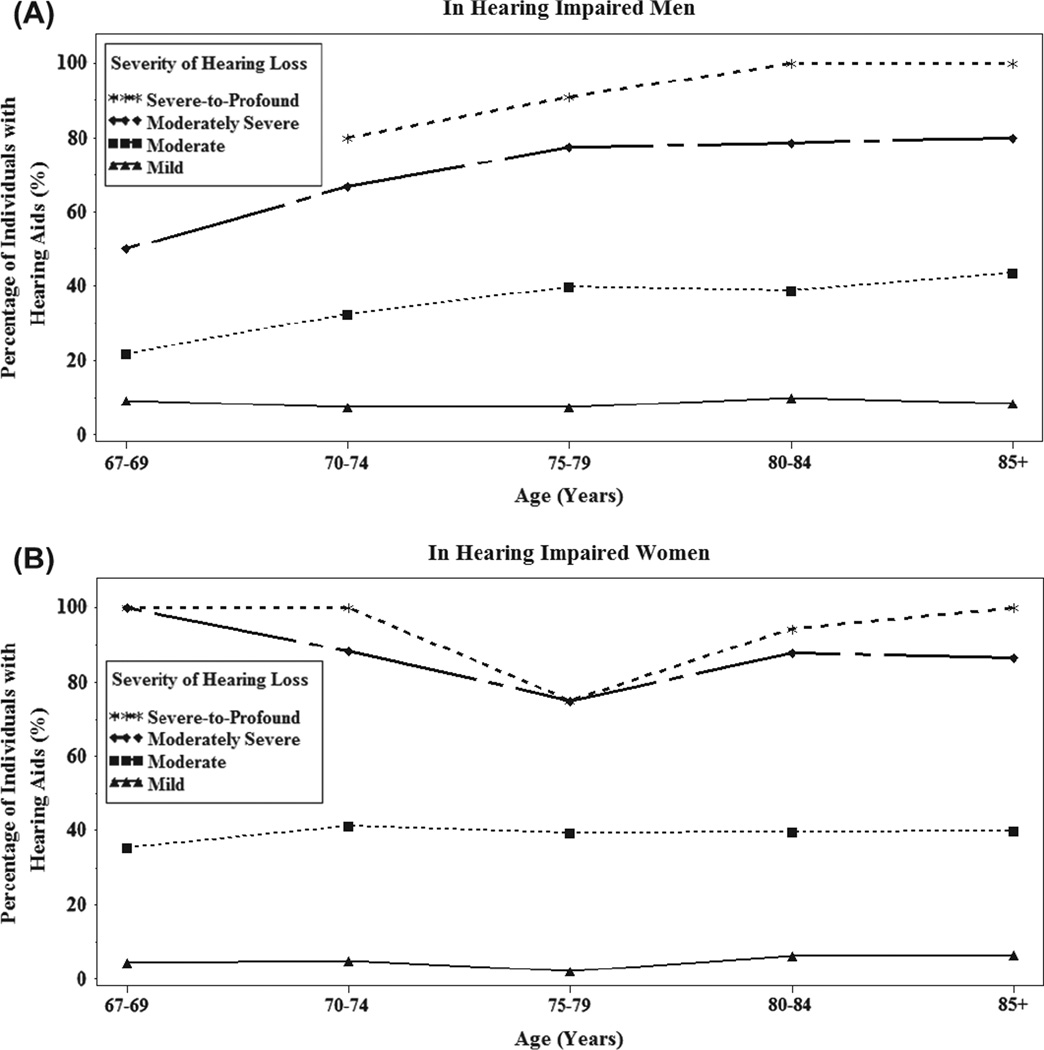
The prevalence of hearing-aid use varied by age and severity of hearing loss in both men and women (Figure 1). Not unexpectedly, younger participants and individuals with less hearing loss reported using hearing aids less frequently than their older and more hearing-impaired peers (Table 2). Men reported higher levels of hearing-aid use than women for every age category and at lower levels of hearing loss (Table 2; p < 0.01). Individuals with only unilateral hearing loss were the least likely to obtain a hearing aid; only 12 of 615 (2.0%) reported hearing-aid use. Additionally, younger individuals and those with milder impairment had more factors significantly associated with acquiring and use of hearing aids than their older, more hearing-impaired counterparts, but these factors varied across age and severity groups with higher degree of measured HI and self-reporting hearing loss being the only consistent predictors of hearing-aid use in stratified analyses (results not shown). Among those with moderate HI or worse, 49.9% used hearing aids and the percentage did not differ significantly between men (49.7%) and women (50.1%).
Figure 1.
Prevalence of hearing-aid use in hearing-impaired men and women by age and severity of hearing loss in the better ear. Hearing loss in the better ear was defined as mild (20 – 34.9 dB HL), moderate (35 – 49.9 dB HL), moderately severe (50 – 64.9 dB HL), and severe-to-profound (65 + dB HL).
Table 2.
Prevalence of hearing-aid use by age and severity of hearing impairment.
| All participants | Men | Women | ||||
|---|---|---|---|---|---|---|
| Characteristic | Participants at risk, N |
Prevalence % (N) |
Participants at risk, N |
Prevalence % (N) |
Participants at risk, N |
Prevalence % (N) |
| Overall | 5172 | 19.0% (981) | 2220 | 23.0% (511) | 2952 | 15.9% (470) |
| Age (years) | ||||||
| 67–69 | 526 | 5.5% (29) | 192 | 7.3% (14) | 334 | 4.5% (15) |
| 70–74 | 1554 | 11.1% (172) | 669 | 13.9% (93) | 885 | 8.9% (79) |
| 75–79 | 1502 | 16.9% (254) | 680 | 22.9% (156) | 822 | 11.9% (98) |
| 80–84 | 1164 | 29.4% (342) | 489 | 33.5% (164) | 675 | 26.4% (178) |
| 85+ | 426 | 43.2% (184) | 190 | 44.2% (84) | 236 | 42.4% (100) |
| Severity of hearing | ||||||
| impairment in better eara | ||||||
| None | 702 | 0.0% (0) | 184 | 0.0% (0) | 518 | 0.0% (0) |
| Unilateral HI only | 615 | 2.0% (12) | 227 | 3.1% (7) | 388 | 1.3% (5) |
| Mild | 2172 | 6.0% (130) | 947 | 8.0% (76) | 1225 | 4.4% (54) |
| Moderate | 1262 | 38.8% (490) | 620 | 37.9% (235) | 642 | 39.7% (255) |
| Moderately severe | 345 | 80.3% (277) | 198 | 76.3% (151) | 147 | 85.7% (126) |
| Severe-to-profound | 76 | 94.7% (72) | 44 | 95.5% (42) | 32 | 93.8% (30) |
HI was defined as none (no HI in either ear), unilateral HI only (BE PTA <20 dB HL and worse ear PTA ≥ 35 dB HL), mild (20–34.9 dB HL), moderate (35–49.9 dB HL), moderately severe (50–64.9 dB HL), and severe-to-profound (65 + dB HL).
In multivariable logistic regression analyses of data from men and women combined, lower BMI, normal cognitive status, greater number of mental and social leisure activities, higher number of medications used, more severe low- and high-frequency HI, and self-reported hearing loss were statistically significant factors associated with utilization of hearing aids (Table 3). Age was not a significant factor after inclusion of these other predictors in the multivariable model.
Table 3.
Multivariate results associated with hearing-aid use in participants with any hearing impairment.
| Overall (N = 3855) | Men (N = 1809) | Women (N = 2046) | ||||
|---|---|---|---|---|---|---|
| Characteristic | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age, per SD | 1.03 (0.90, 1.17) | 0.67 | 1.00 (0.84, 1.19) | 0.99 | 1.03 (0.84, 1.25) | 0.80 |
| Sex, female vs. male | 0.81 (0.63, 1.06) | 0.13 | -- | -- | -- | -- |
| BMI, per SD | 0.83 (0.73, 0.94) | <0.01 | 0.81 (0.69, 0.96) | 0.01 | 0.85 (0.70, 1.03) | 0.10 |
| Diabetes, yes vs. no | 1.35 (0.95, 1.90) | 0.09 | 1.69 (1.09, 2.60) | 0.02 | 0.88 (0.48, 1.59) | 0.68 |
| Cognitive status, impaired vs. unimpaired | 0.66 (0.48, 0.91) | 0.01 | 0.77 (0.50, 1.20) | 0.25 | 0.53 (0.32, 0.86) | 0.01 |
| Physical activity, moderate or greater, yes vs. no | 1.19 (0.92, 1.53) | 0.18 | 0.88 (0.63, 1.25) | 0.48 | 1.65 (1.12, 2.43) | 0.01 |
| Activities of daily living, per SD | 0.94 (0.83, 1.06) | 0.31 | 0.98 (0.83, 1.16) | 0.79 | 0.89 (0.74, 1.07) | 0.23 |
| Leisure activities: mental, per SD | 1.15 (1.02, 1.30) | 0.02 | 1.18 (1.00, 1.39) | 0.05 | 1.13 (0.94, 1.35) | 0.19 |
| Leisure activities: social, per SD | 1.13 (1.00, 1.27) | 0.04 | 1.17 (1.00, 1.37) | 0.05 | 1.08 (0.90, 1.30) | 0.39 |
| Number of medications, per SD | 1.16 (1.02, 1.33) | 0.02 | 1.12 (0.94, 1.34) | 0.19 | 1.22 (1.00, 1.49) | 0.05 |
| Self-reported history of angina, yes vs. no | 0.75 (0.53, 1.07) | 0.11 | 0.93 (0.59, 1.45) | 0.74 | 0.53 (0.30, 0.95) | 0.03 |
| Moderate or severe hand osteoarthritis, yes vs. no | 1.28 (0.98, 1.66) | 0.07 | 1.49 (1.03, 2.18) | 0.04 | 1.07 (0.73, 1.56) | 0.74 |
| PTA value - low frequencya, per 5 dB HL increment | 1.78 (1.66, 1.92) | <0.01 | 1.61 (1.47, 1.77) | <0.01 | 2.08 (1.85, 2.35) | <0.01 |
| PTA value - high frequencyb, per 5 dB HL increment | 1.20 (1.13, 1.27) | <0.01 | 1.16 (1.08, 1.25) | <0.01 | 1.25 (1.15, 1.37) | <0.01 |
| Self-reported hearing loss, yes vs. no | 2.76 (2.04, 3.75) | <0.01 | 2.68 (1.77, 4.08) | <0.01 | 3.07 (1.94, 4.86) | <0.01 |
| Self-reported repeated ear infections, yes vs. no | 1.27 (0.94, 1.73) | 0.12 | 1.12 (0.71, 1.77) | 0.63 | 1.26 (0.81, 1.94) | 0.31 |
-- indicates variable not included in model; SD = standard deviation; OR = odds ratio; CI = confidence interval; BMI = body mass index. All p-values <0.05 are shown in bold print.
Pure-tone audiometry (PTA) value – low frequency is the average of the audiometric thresholds at 0.5, 1, and 2 kHz frequencies in the better ear.
Pure-tone audiometry (PTA) value – high frequency is the average of the audiometric thresholds at 3, 4, 6, and 8 kHz frequencies in the better ear. Models included all of the following variables: age, sex (in overall model), BMI, diabetes, cognitive status, physical activity, activities of daily living, leisure activities (mental), leisure activities (social), number of medications, self-reported history of angina, moderate or severe hand osteoarthritis, PTA threshold (low frequency) in better ear, PTA threshold (high frequency) in better ear, self-reported hearing loss, and self-reported repeated ear infections.
Greater severity of measured HI and self-reporting hearing loss were factors common to both men and women. Other specific factors associated with hearing-aid use differed for men and women. Diabetes, leisure activities, lower BMI, and the presence of hand osteoarthritis were significant factors associated with hearing-aid use in men whereas normal cognitive status, increased physical activity, and no (self-reported) history of angina were significant factors associated with hearing-aid use in women (Table 3). Interactions for L-PTA with sex and H-PTA with sex in the overall model were not statistically significant (p = 0.24 and p = 0.21, respectively) and were not retained.
Discussion
Health care in Iceland is, by law, universal and comprehensive, delivered almost exclusively in regional public health-care institutions, such as the National University Hospital of Iceland, which serves the greater Reykjavik area. Hearing aids are subsidized by the healthcare system and, in some cases, are available free, depending on the level of hearing impairment. Despite cost-controlled access to care, only half of those with at least moderate hearing impairment were using hearing aids. Men and women with at least moderate HI were equally likely to utilize hearing aids. The percentage of hearing-aid use observed in this cohort is higher than rates reported by several previous studies in comparisons made using the same criteria for hearing impairment (Chien & Lin, 2012; Lee et al, 1991; Popelka et al, 1998; Hartley et al, 2010; Bainbridge & Ramachandran, 2014; Nash et al, 2013; Johansson & Arlinger, 2003; Uimonen et al, 1999). This may be due, in part, to ease of access to hearing health-care in Iceland, social cohesion of the population encouraging interaction, and a cultural willingness to consider electronics as a way to improve quality of life. Even with access to hearing health care and the potential for its significant personal benefit, there remains a sizable unmet need in men and women for rehabilitative intervention for ARHL.
Other studies using PTA ≥ 35 dB HL reported lower rates of hearing-aid use. Findings from the U.S. NHANES and a population-based study in Sweden both reported one in three with ARHL used hearing aids (Bainbridge & Ramachandran, 2014; Johansson & Arlinger, 2003) compared to our rate of one in two. In Finland, 41% of those with PTA > 30 dB HL used a hearing aid (Uimonen et al, 1999). Several additional studies utilized a PTA > 25 dB HL threshold. Hearing-aid use was reported by 14.6% of Beaver Dam participants with HI (20.7% admitted to ever using hearing aids) (Popelka et al, 1998). Similarly, among NHANES participants ages 50 years and older, hearing aids were utilized by 14.2% of participants (Chien & Lin, 2012). Other studies report still lower rates of hearing-aid use, including use less than 10% in a U.S. study in Hispanics (Lee et al, 1991) and 11% of participants ages 49 to 99 years (mean age 67 years) with measured HI in a Blue Mountains Hearing Study (Hartley et al, 2010). Applying a PTA > 25 dB HL threshold to the current study equates to a use rate of 33% in Iceland, notably higher than all other population studies, and even higher than the Beaver Dam offspring study, which, among those with PTA > 40 dB HL in the worse ear, found that 22.5% of participants used hearing aids (Nash et al, 2013). Among AGES-RS participants with PTA ≥ 50 dB HL, 83% used a hearing aid.
The current study also investigated which factors influenced utilization. In other populations of comparable age, men reported using hearing aids more often than women (Chien & Lin, 2012; Popelka et al, 1998; Nash et al, 2013); however, no sex differences in utilization among those with moderate or greater hearing impairment were found in the current study. Hearing-aid use was directly related to the severity of measured HI, with the highest utilization in men and women with more severe hearing loss, consistent with earlier studies (Chien & Lin, 2012; Lee et al, 1991; Popelka et al, 1998; Gopinath et al, 2011; Nash et al, 2013). In multivariable analyses, age was not a significant factor, whereas severity of measured hearing loss and perception of hearing ability were the most consistent and significant factors associated with hearing-aid use in men and in women, corroborating results from community-based studies and targeted investigations probing help-seeking factors in hearing-impaired individuals (Hartley et al, 2010; Gopinath et al, 2011; Garstecki & Erler, 1998; Southall, Gagne & Leroux, 2006; Solheim, 2011; Meyer et al, 2014; Laplante-Levesque et al, 2012).
Other determinants of hearing-aid use found to be important in the current study, particularly among people with milder hearing loss, included indicators of regular utilization of health care (i.e. diabetes, number of medications used, osteoarthritis of the hands) and an active lifestyle (e.g. lower BMI, normal cognitive status, higher levels of physical activity, greater number of leisure activities, and no [self-reported] history of angina). The importance of these factors differed between men and women, with health-care utilization a more important predictor of hearing-aid utilization in men whereas an active lifestyle was associated with hearing-aid use in women. For individuals with moderately-severe or worse HI (BE PTA of 50 + dB HL), only severity of HI and self-reported hearing loss were significant determinants of hearing aid utilization (results not shown). We did not observe any sex difference in rates of hearing-aid use nor could we attribute sex differences in predictors of hearing-aid use to specific differences between men and women as to whether their hearing loss was predominantly in the low frequency or high frequency range.
These results provide evidence, at a community level, in support of results from studies of hearing-impaired individuals indicating that non-audiologic factors play a fundamental role in the early adoption of hearing rehabilitation and receptivity to hearing health care would be greater if delivered in a more integrated manner within the health-care setting (Meyer et al, 2014; Laplante-Levesque et al, 2012; McMahon et al, 2013). Other studies on determinants of hearing-aid acquisition and use have reported that education, occupation, and income disparities were significantly associated with hearing-aid use (Popelka et al, 1998; Bainbridge & Ramachandran, 2014; Nash et al, 2013; Fischer et al, 2011). To the extent that it was possible to discern such differences in the Icelandic population, none of these factors were significant, perhaps indicative of the social welfare and health-care system in Iceland.
The benefits of hearing-aid use are many, including the obvious improvements in hearing which, in turn, supports social interaction. Results from an analysis on sensory impairment in association with mortality in this same cohort found those with HI, alone or in combination with visual impairment, had a higher risk of dying, but, surprisingly, hearing-aid use mitigated some of the increased risk (Fisher et al, 2014). The reasons for this are unclear, but suggest there may be additional physiologic justification for encouraging the adoption of hearing aids.
Strengths of the current study include a large cohort of older individuals, followed longitudinally since 1967 who continue to demonstrate a high participation rate, standardized audiometric procedures for measuring hearing loss, sufficient sample size with a high degree of HI in which to study hearing-aid utilization, and an extensive profile of participant characteristics allowing us to discern which external factors, including coexisting health conditions, most influenced hearing-aid use, separately for men and women. The provision of health care in Iceland also offered an advantage in that personal income and access to care provided a more level background from which to investigate hearing-aid utilization without overwhelming cost concerns. The study is not without limitations and these include the cross-sectional design of the study with a single measurement of demographic and health variables. This cohort did not collect any data on the pattern of referral for a hearing aid, the type(s) of hearing aids selected, whether the device was obtained from the health care system or privately (including outside of Iceland), or the final cost to the participant after subtracting the subsidy. Additionally, there was no direct assessment of psychosocial factors influencing use, and no data assessing the frequency and length of use among those wearing hearing aids. The exclusively Caucasian Nordic cohort may also limit comparisons with other racial or ethnic groups.
Our findings suggest that men and women tend to be equally inclined to acquire hearing aids when their hearing deteriorates and they both perceive and are willing to articulate their hearing loss. Men who access the health-care system due to coexisting health conditions may be more likely to have hearing loss detected and may be positively inclined towards using a hearing aid whereas an active lifestyle appears to be a significant motivator for women. Yet, only half of those who could likely benefit have adopted hearing aids. These results suggest that routine hearing examinations integrated into health care for older persons, in combination with a discussion of the benefits of hearing aids (for improved communication, increased independence, greater well-being and, as previous results show, the possibility of promoting health in other non-hearing functional domains), should increase hearing-aid acquisition and use, provided that cost is not an overriding barrier. Studies measuring the effectiveness and cost benefit of hearing aids to improve health outcomes and maintain quality of life in older persons with coexisting health conditions could provide additional motivation for men and women to increase hearing-aid use.
Acknowledgements
The authors thank the AGES-RS participants; without whom the study would not be possible. Sources of funding: This work was supported by the Intramural Research Programs of the National Institute of Aging ZIAAG007380) and the National Eye Institute (ZIAEY000401), and the National Institute on Deafness and Other Communication Disorders (IAA Y2-DC-1004-02), National Institutes of Health (N01-AG-12100), Bethesda, Maryland, USA; the Icelandic Heart Association, Kopavogur, Iceland; the Icelandic Parliament, Reykjavik, Iceland; the University of Iceland Research Fund, Reykjavik, Iceland; and the Helga Jonsdottir and Sigurlidi Kristjansson Research Fund, Reykjavik, Iceland. The sponsors had no role in the design, methods, subject recruitment, data collection, data analysis, or preparation of the paper.
Abbreviations
- AGES-RS
Age, gene/environment susceptibility - Reykjavik study
- ARHL
Age-related hearing loss
- BE
Better ear
- dB HL
Decibels hearing level
- HA
Hearing aids
- HI
Hearing impairment
- PTA
Pure-tone average
Footnotes
Declaration of interest: The authors have no proprietary or commercial interest in any materials discussed in this article. No financial disclosures were reported by the authors of this paper.
References
- Agrawal N, Kalaivani M, Gupta SK, Misra P, Anand K, et al. Association of blindness and hearing impairment with mortality in a cohort of elderly persons in a rural area. Indian J Community Med. 2011;36:208–212. doi: 10.4103/0970-0218.86522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appollonio I, Carabellese C, Frattola L, Trabucchi M. Effects of sensory aids on the quality of life and mortality of elderly people: A multivariate analysis. Age Ageing. 1996;25:89–96. doi: 10.1093/ageing/25.2.89. [DOI] [PubMed] [Google Scholar]
- Bainbridge KE, Ramachandran V. Hearing-aid use among older United States adults: The National Health and Nutrition Examination Survey, 2005–2006 and 2009–2010. Ear Hear. 2014;35:289–294. doi: 10.1097/01.aud.0000441036.40169.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges JA, Bentler RA. Related hearing-aid use to well-being among older adults. The Hearing Journal. 1998;51:39–44. [Google Scholar]
- Chien W, Lin FR. Prevalence of hearing-aid use among older adults in the United States. Arch Intern Med. 2012;172:292–293. doi: 10.1001/archinternmed.2011.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, et al. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43:661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- Davis AC. The prevalence of hearing impairment and reported hearing disability among adults in Great Britain. Int J Epidemiol. 1989;18:911–917. doi: 10.1093/ije/18.4.911. [DOI] [PubMed] [Google Scholar]
- Fischer ME, Cruickshanks KJ, Wiley TL, Klein BE, Klein R, et al. Determinants of hearing-aid acquisition in older adults. Am J Public Health. 2011;101:1449–1455. doi: 10.2105/AJPH.2010.300078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D, Li CM, Chiu MS, Themann CL, Petersen H, et al. Impairments in hearing and vision impact on mortality in older persons: The AGES-Reykjavik study. Age Ageing. 2014;43:69–76. doi: 10.1093/ageing/aft122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garstecki DC, Erler SF. Hearing loss, control, and demographic factors influencing hearing-aid use among older adults. JSLHR. 1998;41:527–537. doi: 10.1044/jslhr.4103.527. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Schneider J, Hartley D, Teber E, McMahon CM, et al. Incidence and predictors of hearing-aid use and ownership among older adults with hearing loss. Ann Epidemiol. 2011;21:497–506. doi: 10.1016/j.annepidem.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, et al. Age, gene/environment susceptibility - Reykjavik study: Multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley D, Rochtchina E, Newall P, Golding M, Mitchell P. Use of hearing aids and assistive listening devices in an older Australian population. J Am Acad Audiol. 2010;21:642–653. doi: 10.3766/jaaa.21.10.4. [DOI] [PubMed] [Google Scholar]
- Johansson MS, Arlinger SD. Prevalence of hearing impairment in a population in Sweden. Int J Audiol. 2003;42:18–28. doi: 10.3109/14992020309056081. [DOI] [PubMed] [Google Scholar]
- Jonsson H, Helgadottir GP, Aspelund T, Sverrisdottir JE, Eiriksdottir G, et al. The use of digital photographs for the diagnosis of hand osteoarthritis: The AGES-Reykjavik study. BMC Musculoskeletal Disorders. 2012;13:20. doi: 10.1186/1471-2474-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller BK, Morton JL, Thomas VS, Potter JF. The effect of visual and hearing impairment on functional status. J Am Geriatr Soc. 1999;47:1319–1325. doi: 10.1111/j.1532-5415.1999.tb07432.x. [DOI] [PubMed] [Google Scholar]
- Kochkin S. MarkeTrak III: Why 20 million in U.S. don’t use hearing aids for their hearing loss. Hear J. 1993;46:20–27. [Google Scholar]
- Laforge RG, Spector WD, Sternberg J. The relationship of vision and hearing impairment to one-year mortality and functional decline. J Aging Health. 1992;4:126–148. [Google Scholar]
- Laplante-Levesque A, Knudsen LV, Preminger JE, Jones L, Nielsen C, et al. Hearing help-seeking and rehabilitation: Perspectives of adults with hearing impairment. Int J Audiol. 2012;51:93–102. doi: 10.3109/14992027.2011.606284. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Carlson DL, Lee HM, Ray LA, Markides KS. Hearing loss and hearing-aid use in Hispanic adults: Results from the Hispanic Health and Nutrition Examination Survey. Am J Public Health. 1991;81:1471–1474. doi: 10.2105/ajph.81.11.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CM, Zhang X, Hoffman HJ, Cotch MF, Themann CL, et al. Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005–2010. JAMA Otolaryngol Head Neck Surg. 2014;140:293–302. doi: 10.1001/jamaoto.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, et al. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25:763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CM, Gopinath B, Schneider J, Reath J, Hickson L, et al. The need for improved detection and management of adult-onset hearing loss in Australia. Int J Otolaryngol, 2013. 2013:308509. doi: 10.1155/2013/308509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Hickson L, Lovelock K, Lampert M, Khan A. An investigation of factors that influence help-seeking for hearing impairment in older adults. Int J Audiol. 2014;53(Suppl 1):S3–S17. doi: 10.3109/14992027.2013.839888. [DOI] [PubMed] [Google Scholar]
- Michikawa T, Nishiwaki Y, Kikuchi Y, Nakano M, Iwasawa S, et al. Gender-specific associations of vision and hearing impairments with adverse health outcomes in older Japanese: A population-based cohort study. BMC Geriatrics. 2009;9:50. doi: 10.1186/1471-2318-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrow CD, Tuley MR, Aguilar C. Sustained benefits of hearing aids. J Speech Hear Res. 1992;35:1402–1405. doi: 10.1044/jshr.3506.1402. [DOI] [PubMed] [Google Scholar]
- Nash SD, Cruickshanks KJ, Huang GH, Klein BE, Klein R, et al. Unmet hearing health care needs: The Beaver Dam offspring study. Am J Public Health. 2013;103:1134–1139. doi: 10.2105/AJPH.2012.301031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popelka MM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, et al. Low prevalence of hearing-aid use among older adults with hearing loss: The epidemiology of hearing loss study. J Am Geriatr Soc. 1998;46:1075–1078. doi: 10.1111/j.1532-5415.1998.tb06643.x. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Mui S, Damesyn M, Moore AA, Greendale GA. The prognostic value of sensory impairment in older persons. J Am Geriatr Soc. 1999;47:930–935. doi: 10.1111/j.1532-5415.1999.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Solheim J. Preconceptions and expectations of older adults about getting hearing aids. J Multidisciplinary Healthcare. 2011;4:1–8. doi: 10.2147/JMDH.S14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall K, Gagne JP, Leroux T. Factors that influence the use of assistance technologies by older adults who have a hearing loss. Int J Audiol. 2006;45:252–259. doi: 10.1080/14992020500258586. [DOI] [PubMed] [Google Scholar]
- Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, et al. Global and regional hearing impairment prevalence: An analysis of 42 studies in 29 countries. Eur J Public Health. 2013;23:146–152. doi: 10.1093/eurpub/ckr176. [DOI] [PubMed] [Google Scholar]
- Uimonen S, Huttunen K, Jounio-Ervasti K, Sorri M. Do we know the real need for hearing rehabilitation at the population level? Hearing impairments in the 5- to 75-year old cross-sectional Finnish population. Br J Audiol. 1999;33:53–59. doi: 10.3109/03005364000000099. [DOI] [PubMed] [Google Scholar]